Abstract
BACKGROUND
Frailty indices may represent useful decision support tools to optimize modifiable drivers of quality and cost in neurosurgical care. However, classic indices are cumbersome to calculate and frequently require unavailable data. Recently, a more lean 5-factor modified frailty index (mFI-5) was introduced, but it has not yet been rigorously applied to brain tumor patients.
OBJECTIVE
To investigate the predictive value of the mFI-5 on length of stay (LOS), complications, and charges in surgical brain tumor patients.
METHODS
We retrospectively reviewed data for brain tumor patients who underwent primary surgery from 2017 to 2018. Bivariate (ANOVA) and multivariate (logistic and linear regression) analyses assessed the predictive power of the mFI-5 on postoperative outcomes.
RESULTS
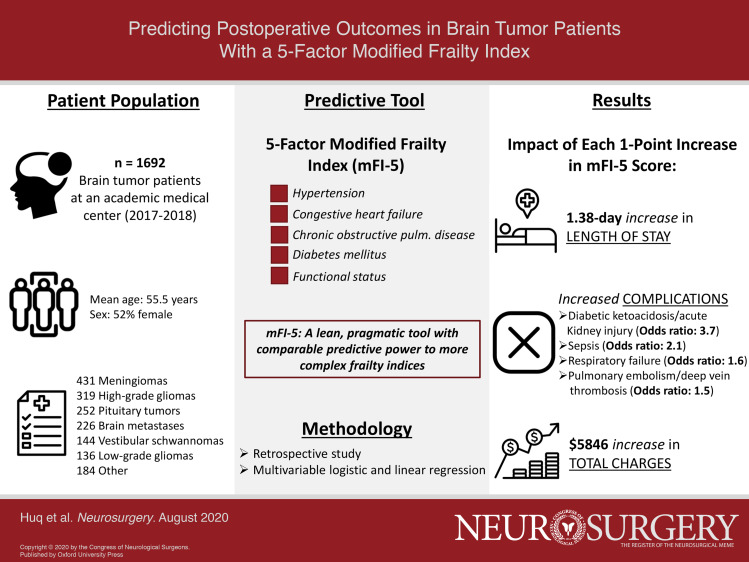
Our cohort included 1692 patients with a mean age of 55.5 yr and mFI-5 of 0.80. Mean intensive care unit (ICU) and total LOS were 1.69 and 5.24 d, respectively. Mean pulmonary embolism (PE)/deep vein thrombosis (DVT), physiological/metabolic derangement, respiratory failure, and sepsis rates were 7.2%, 1.1%, 1.6%, and 1.7%, respectively. Mean total charges were $42 331. On multivariate analysis, each additional point on the mFI-5 was associated with a 0.32- and 1.38-d increase in ICU and total LOS, respectively; increased odds of PE/DVT (odds ratio (OR): 1.50), physiological/metabolic derangement (OR: 3.66), respiratory failure (OR: 1.55), and sepsis (OR: 2.12); and an increase in total charges of $5846.
CONCLUSION
The mFI-5 is a pragmatic and actionable tool which predicts LOS, complications, and charges in brain tumor patients. It may guide future efforts to risk-stratify patients with subsequent impact on postoperative outcomes.
Keywords: Frailty, Brain tumor, Oncology, Cost effectiveness
Graphical Abstract
Graphical Abstract.
ABBREVIATIONS
- ASA
American Society of Anesthesiologists
- CCI
Charlson Comorbidity Index
- CI
confidence interval
- Coef
coefficient of linear/logistic regression equation
- CSHA-FI
Canadian Study of Health and Aging Frailty Index
- DVT
deep vein thrombosis
- ERAS
enhanced recovery after surgery
- mFI-5
modified frailty index
- ICU
intensive care unit
- LOS
length of stay
- NSQIP
National Surgical Quality Improvement Program
- OR
odds ratio
- PE
pulmonary embolism
- UTI
urinary tract infection
Neurosurgical interventions are among the most expensive types of medical care in a healthcare landscape increasingly focused on cost and value.1 It is essential for our field to optimize modifiable drivers of quality and cost in order to provide high-value care. Recent efforts have begun to investigate cost effectiveness across multiple aspects of neurosurgery in order to meet this objective.2-10 However, the neurosurgical literature on cost and value, particularly in cranial neurosurgery, is relatively sparse in comparison to that of other specialties.2,11,12 There is a clear need for clinically pragmatic and actionable tools to predict and modify costs of neurosurgical care.
One solution may involve tools that measure frailty, a state involving loss of physiological reserves with increased vulnerability to stressors and adverse patient outcomes.13 Various indices have been developed to measure frailty and stratify patients – particularly the elderly– based on risk. These include the 70-item frailty index developed from the Canadian Study of Health and Aging (CSHA-FI), the Johns Hopkins Adjusted Clinical Groups, Charlson Comorbidity Index (CCI), and 11-factor modified frailty index (mFI-11) (Table 1), the latter which was constructed by mapping key variables from the CSHA-FI to available data in the National Surgical Quality Improvement Program (NSQIP) database.
TABLE 1.
Comparison of mFI-5, mFI-11, and CCI Components
| mFI-5 | mFI-11 | CCI (weight) |
|---|---|---|
| Functional status | Functional status | AIDS (6) |
| Diabetes | Diabetes | Solid tumor (metastatic) (6) |
| COPD | COPD/pneumonia | Liver disease (moderate/severe) (3) |
| CHF | CHF | Hemiplegia (2) |
| HTN | HTN | Solid tumor (localized) (2) |
| MI | Lymphoma (2) | |
| Prior PCI/angina | Leukemia (2) | |
| PVD/ischemic rest pain | CKD (moderate/severe) (2) | |
| Impaired sensorium | Diabetes (end-organ damage) (2) | |
| CVA/TIA | Diabetes (uncomplicated) (1) | |
| CVA with deficit | Liver disease (mild) (1) | |
| PUD (1) | ||
| CTD (1) | ||
| COPD (1) | ||
| Dementia (1) | ||
| CVA/TIA (1) | ||
| PVD (1) | ||
| CHF (1) | ||
| MI (1) |
COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; HTN, hypertension; PCI, percutaneous coronary intervention; MI, myocardial infarction; TIA, transient ischemic attack; CVA, cerebrovascular accident; AIDS, acquired immunodeficiency syndrome; CKD, chronic kidney disease; PUD, peptic ulcer disease; CTD, connective tissue disease.
Definitions: Functional status = dependent/requiring assistance with activities of daily living.
While classically used for risk stratification purposes and as predictors of morbidity and mortality, these frailty indices have also been shown to predict key financial outcomes in a number of patient populations.14-16 These include some neurosurgical studies involving brain tumors, intracerebral hemorrhage, and spine surgery.6,17-21 However, these older frailty indices are cumbersome to calculate within existing clinical workflows, and variables comprising them are frequently unavailable or unreliable in large datasets.22 These barriers, among others, have prevented their widespread adoption. There is therefore a clinical need for a more pragmatic tool with predictive power in anticipating clinical and financial outcomes.
Recently, a 5-factor modified frailty index (mFI-5) was introduced using the NSQIP database23 (Table 1). Unlike existing frailty indices, the mFI-5 uses a small number of variables that are readily available in the patient history, including functional status (partially or totally dependent), history of diabetes, history of COPD, history of congestive heart failure, and hypertension requiring medication. The mFI-5 has been shown to predict key clinical and economic outcomes in the orthopedic literature.24,25 More recently, it has been explored as a predictor of adverse events and mortality in the neurosurgical literature.26-28 However, to our knowledge, the mFI-5 has not been applied in the context of financial and economically relevant clinical outcomes in brain tumor patients. We sought to address this unmet need by examining the predictive power of the mFI-5 upon key clinical and financial outcomes (including total length of stay (LOS), intensive care unit (ICU) LOS, complications, charges, and 30-d readmissions) in this patient population.
METHODS
Patient Selection and Data Collection
Our cohort included 1692 adult patients who underwent primary surgery for brain tumors at a single institution between January 1, 2017 and December 31, 2018. We extracted data from an institutional database using International Statistical Classification of Diseases and Related Health Problems 10th revision (ICD-10) codes. Demographic and clinical variables collected included age, sex, race, ethnicity, American Society of Anesthesiologists (ASA) classification, and brain tumor diagnosis. Brain tumor diagnoses were verified via manual chart review of electronic medical records. Outcome variables included total LOS, ICU LOS, complications (collected using ICD-10 codes as described previously29), and 30-d readmissions. Financial outcome variables including pharmacy, imaging, and total charges were provided by the Center for Clinical Data Analysis at our institution. Each patient's mFI-5 score was calculated as described previously.23
Statistical Analysis
For bivariate analyses, patients were stratified into 3 categories based on mFI-5 score: zero frailty (mFI-5 = 0), some frailty (mFI-5 = 1), and significant frailty (mFI-5 ≥ 2). Clinical and financial outcomes in each of the 3 mFI-5 groups (mFI-5 = 0, mFI-5 = 1, mFI-5 ≥ 2) were presented as mean ± standard deviation and compared using one-way analysis of variance (ANOVA), chi squared, or Fisher's exact tests where appropriate. Multivariate analysis assessing the predictive value of the mFI-5 (assessed linearly with groups of mFI = 0, 1, 2, 3, or 4) on outcomes of interest was performed using logistic regression models for binary outcomes (complications, 30-d readmissions) and linear regression models for continuous outcomes (LOS, charges). All multivariate models adjusted for age, sex, race, ethnicity, ASA classification, and diagnosis. Regression outputs were presented as odds ratios (ORs) for logistic regression models and the coefficient of the regression equation for linear regression models. P values less than 0.05 were considered to be statistically significant. All statistics were performed in Stata version 15 (StataCorp LLC, College Station, Texas).
Ethical Considerations
Our Institutional Review Board (IRB00209855) reviewed our study protocol and approved the waiver of informed patient consent for this study.
Reporting Guidelines
We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines for this study.
RESULTS
Patient Demographics
Our cohort included 1692 patients with mean age of 55.5 ± 15.2 yr, mFI-5 score of 0.80 ± 0.84, and ASA of 2.63 ± 0.59 (Table 2). The majority of patients were female (52%), Caucasian (70%), and not Hispanic/Latino (95%). The most common brain tumor diagnosis was meningioma (25%), followed by high-grade glioma (19%), pituitary tumor (15%), metastasis (13%), vestibular schwannoma (9%), and low-grade glioma (8%). Other tumor diagnoses were rare and grouped together as “other” (11%).
TABLE 2.
Demographics of 1692 Patients, Stratified by mFI-5 = 0, 1, or ≥2
| mFI-5 score | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 + | |||
| Characteristic | Mean | n (%) | n (%) | n (%) | n (%) |
| Age (years) | 55.5 ± 15.2 | 48.4 ± 14.5 | 59.2 ± 13.5 | 64.1 ± 12.7 | |
| mFI-5 | 0.80 ± 0.84 | 1692 (100%) | 729 (43%) | 643 (38%) | 320 (19%) |
| Sex | |||||
| Males | 807 (48%) | 322 (40%) | 323 (40%) | 162 (20%) | |
| Females | 885 (52%) | 407 (46%) | 320 (36%) | 158 (18%) | |
| Race | |||||
| Caucasian | 1189 (70%) | 534 (45%) | 451 (38%) | 204 (17%) | |
| African American | 289 (17%) | 75 (26%) | 132 (46%) | 82 (28%) | |
| Asian | 94 (6%) | 52 (55%) | 26 (28%) | 16 (17%) | |
| Other | 120 (7%) | 68 (57%) | 34 (28%) | 18 (15%) | |
| Ethnicity | |||||
| Hispanic/Latino | 79 (5%) | 42 (53%) | 31 (39%) | 6 (8%) | |
| Not Hispanic/Latino | 1613 (95%) | 687 (43%) | 612 (38%) | 314 (20%) | |
| Brain Tumor | |||||
| Meningioma | 431 (25%) | 164 (38%) | 183 (42%) | 84 (19%) | |
| High-grade glioma | 319 (19%) | 141 (44%) | 117 (37%) | 61 (19%) | |
| Pituitary tumor | 252 (15%) | 98 (39%) | 99 (39%) | 55 (22%) | |
| Brain metastasis | 226 (13%) | 79 (35%) | 83 (37%) | 64 (28%) | |
| Vestibular schwannoma | 144 (9%) | 71 (49%) | 59 (41%) | 14 (10%) | |
| Low-grade glioma | 136 (8%) | 83 (61%) | 40 (29%) | 13 (10%) | |
| Other | 184 (11%) | 93 (51%) | 62 (34%) | 29 (16%) | |
A total of 43% of patients were not frail (mFI-5 = 0), 38% had 1 measure of frailty (mFI-5 = 1), and 19% had multiple measures of frailty (mFI-5 ≥ 2). Increasing frailty was associated with a step-wise increase in age (P < .0001). In our cohort, the frailest patients (mFI-5 ≥ 2) most frequently carried diagnoses of meningioma, brain metastasis, and high-grade glioma (Table 2). A full breakdown of patient demographic information stratified by mFI-5 score is provided in Table 2.
Bivariate Analysis of mFI-5 Predictive Value on LOS, Complications, Charges, and 30-Day Readmissions
Globally, our cohort had a mean ICU and total LOS of 1.69 and 5.24 d, respectively (Table 3). Mean complication rates were as follows: pulmonary embolism (PE)/deep vein thrombosis (DVT) – 7.2%, physiological and metabolic derangement (diabetic ketoacidosis, acute kidney injury) – 1.1%, respiratory failure – 1.6%, sepsis – 1.7%, urinary tract infection (UTI) – 0.5%, and wound infection – 1.4% (Table 3). Mean pharmacy, imaging, and total charges were $2319, $2304, and $42 331, respectively. Mean 30-d readmission rate was 6.9%.
TABLE 3.
Bivariate Analyses
| mFI-5 score | |||||
|---|---|---|---|---|---|
| Mean ± SD | 0 | 1 | 2+ | P value | |
| LOS (days) | |||||
| ICU | 1.69 ± 3.52 | 1.33 ± 1.03 | 1.84 ± 5.18 | 2.17 ± 2.86 | .0011 |
| Total | 5.24 ± 6.84 | 4.00 ± 4.87 | 5.27 ± 6.88 | 8.00 ± 9.34 | <.0001 |
| Complications | |||||
| PE or DVT | 122 (7.2%) | 32 (4.4%) | 49 (7.6%) | 41 (12.8%) | <.001 |
| Physiological and metabolic derangement | 19 (1.1%) | 2 (0.3%) | 3 (0.5%) | 14 (4.4%) | <.001 |
| Respiratory failure | 27 (1.6%) | 5 (0.7%) | 11 (1.7%) | 11 (3.4%) | .005 |
| Sepsis | 28 (1.7%) | 5 (0.7%) | 10 (1.6%) | 13 (4.1%) | .001 |
| UTI | 8 (0.5%) | 2 (0.3%) | 4 (0.6%) | 2 (0.6%) | .579 |
| Wound infection | 24 (1.4%) | 8 (1.1%) | 10 (1.6%) | 6 (1.9%) | .526 |
| Charges ($) | |||||
| Pharmacy | 2319 ± 5686 | 2024 ± 5952 | 2292 ± 5440 | 3046 ± 5503 | .0272 |
| Imaging | 2304 ± 2457 | 1950 ± 1674 | 2336 ± 2524 | 3045 ± 3457 | <.0001 |
| Total | 42 331 ± 30 710 | 37 505 ± 23 308 | 42 789 ± 31 212 | 52 402 ± 40 539 | <.0001 |
| 30-d readmit (%) | 6.9 ± 25.3 | 5.1 ± 22.0 | 7.5 ± 26.3 | 9.7 ± 29.6 | .0182 |
LOS, length of stay; ICU, intensive care unit; PE, pulmonary embolism; DVT, deep vein thrombosis; UTI, urinary tract infection. LOS, charges, postoperative complications, and readmissions for 1692 patients, stratified by mFI-5 = 0, 1, or ≥2. Physiological and metabolic derangement = diabetic ketoacidosis, acute kidney injury. P values represent chi squared or Fisher's exact test.
We performed bivariate analyses assessing differences in these key clinical and economic outcomes by level of frailty. Globally, we found significant step-wise differences in all outcome variables between patients with 3 different levels of frailty (zero frailty (mFI-5 = 0), some frailty (mFI-5 = 1), and significant frailty (mFI-5 ≥ 2)) (Table 3).
More specifically, Mean ICU LOS for patients with mFI-5 = 0, mFI = 1, and mFI = 5 ≥ 2 was 1.33, 1.84, and 2.17, respectively (P = .0011). Total LOS followed the same trend: mean total LOS for mFI-5 = 0, mFI = 1, and mFI = 5 ≥ 2 groups was 4.00, 5.27, and 8.00 d, respectively (P < .0001). Similarly, complication rates increased with increasing mFI-5 score for PE/DVT (4.4%, 7.6%, and 12.8%, respectively), physiological and metabolic decline (0.3%, 0.5%, and 4.4%, respectively), respiratory failure (0.7%, 1.7%, and 3.4%, respectively), and sepsis (0.7%, 1.6%, and 4.1%, respectively), but not for UTI (0.3%, 0.6%, and 0.6%, respectively) or wound infection (1.1%, 1.6%, and 1.9%, respectively).
Pharmacy charges for patients with mFI-5 = 0, mFI-5 = 1, and mFI-5 ≥ 2 were $2024, $2292, and $3046, respectively (P = .0272). Imaging charges were $1950, $2336 and $3045, respectively (P < .001). Total charges were $37 505, $42 789, and $52 402, respectively (P < .001). A 30-d readmission rate for the 3 groups were 5.1%, 7.5%, and 9.7%, respectively (P = .0182).
Multivariate Analysis of mFI-5 Predictive Value on LOS, Complications, Charges, and 30-Day Readmissions
We next performed logistic and linear regression modeling to investigate the predictive value of the mFI-5 on these key outcomes when adjusting for age, race, ethnicity, sex, ASA classification, and diagnosis. This analysis revealed increased odds for key outcome variables associated with each additional point on the mFI-5 score (Table 4). ICU and total LOS increased by 0.32 (95% CI 0.09-0.55, P = .007) and 1.38 (95% CI 0.96-1.80, P < .001) d with each 1-point increase in mFI-5, respectively. Increasing mFI-5 score was similarly associated with increased odds of complications including PE/DVT (OR 1.50, 95% CI 1.20-1.89, P < .001), physiological/metabolic derangement (OR 3.66, 95% CI 2.13-6.28, P < .001), respiratory failure (OR 1.55, 95% CI 1.01-2.40, P = .047), and sepsis (OR 2.12, 95% CI 1.39-3.24, P < .001) but not UTI or wound infection. Additionally, total and imaging charges increased by $5846 (95% CI $3971-$7721, P < .001) and $416 (95% CI $265-$568, P < .001) with each 1-point increase in mFI-5 score, respectively, while pharmacy charges had a nonsignificant increase of $356 (95% CI -$6-$717, P = .054). mFI-5 was not a statistically significant predictor of 30-d readmissions (OR 1.24, 95% CI 0.97-1.58, P = .080).
TABLE 4.
Multivariate Analyses
| Variable | OR/Coef | 95% CI | P value |
|---|---|---|---|
| LOS (days) | |||
| ICU | 0.32 | 0.09-0.55 | .007 |
| Total | 1.38 | 0.96-1.80 | <.001 |
| Complications | |||
| PE or DVT | 1.50 | 1.20-1.89 | <.001 |
| Physiological/metabolic derangement | 3.66 | 2.13-6.28 | <.001 |
| Respiratory failure | 1.55 | 1.01-2.40 | .047 |
| Sepsis | 2.12 | 1.39-3.24 | <.001 |
| Charges ($) | |||
| Pharmacy | 356 | -6-717 | .054 |
| Imaging | 416 | 265-568 | <.001 |
| Total | 5846 | 3971-7721 | <.001 |
| 30 d readmit (%) | 1.24 | 0.97-1.58 | .080 |
LOS, length of stay; ICU, intensive care unit; OR, odds ratio; Coef, coefficient of logistic regression equation (represents increase in LOS or $ per point increase in mFI-5 score); PE, pulmonary embolism; DVT, deep vein thrombosis.
Logistic and linear regression models demonstrating the predictive value of mFI-5 on total and ICU LOS, complications, charges, and 30-d readmissions. Variables included in model: Age, race, ethnicity, sex, American Society of Anesthesiologists (ASA) score, diagnosis.
Physiological/metabolic derangement = diabetic ketoacidosis, acute kidney injury.
DISCUSSION
Rationale for Study
With a renewed focus on high-value care, there is a need for practical, actionable tools to predict and modify the costs of neurosurgical intervention. Frailty indices offer a potential solution to this problem, as they can identify patients at risk of adverse clinical and financial outcomes. However, classically used frailty indices such as the CCI, mFI-11, and CSHA-FI have not been widely adopted in clinical practice, likely due in part to their cumbersome nature and dependence on data that are often difficult to obtain. The mFI-5 has counteracted this problem and demonstrated promise as a streamlined tool with predictive power similar to its predecessors.23 It uses readily available patient data that are often already obtained within existing clinical workflows, such that neurosurgeons may rapidly understand a patient's frailty status and plan to counsel accordingly even before the patient presents for initial consultation. While early studies have demonstrated the potential of the mFI-5,23-28 this new tool has not been widely explored in neurosurgery and to our knowledge has not been assessed as a predictor of financial and economically-relevant clinical outcomes in brain tumor patients. The present study assessed the predictive power of the mFI-5 on these outcomes in brain tumor patients.
Key Results
We found that the mFI-5 significantly predicted LOS, complications, charges, and 30-d readmissions in brain tumor patients in a step-wise fashion in bivariate analyses. Multivariate analysis demonstrated that each additional point on the mFI-5 was associated with a 0.32 d increase in ICU LOS; 1.38 d increase in total LOS; increased odds of PE/DVT (OR 1.50), physiological/metabolic derangement (OR 3.66), respiratory failure (OR 1.55), and sepsis (OR 2.12); increases in imaging and total charges of $416 and $5846, respectively, and a nonsignificant trend toward increases in pharmacy charges of $356 (P = .054); and a nonsignificant increase in 30-d readmissions (OR 1.24, P = .080). More generally, these data reflect our own clinical experience regarding healthcare utilization and necessary resources to care for frail brain tumor patients.
Results from Similar Studies
Our results align with prior work demonstrating the predictive value of frailty indices on clinical and financial outcomes in surgical patients. In one study applying the mFI-11 to elderly patients with glioblastoma, the frailest patients had a median LOS 2 d longer and overall complication rate 24.6% higher than their less frail counterparts.18 Similarly, another group applying the Hopkins Frailty score to brain tumor patients found a mean LOS 2.18 d longer and overall complication rate 12.3% higher in frail patients than nonfrail patients.20 In elderly patients with metastatic brain tumors, each point on the CCI was found to be associated with a 0.52 d increase in LOS and $1710.61 increase in hospital charges.21 We note a grossly similar magnitude and direction in change in our own data.
Our data are also consistent with similar efforts undertaken in other surgical specialties. For example, in patients undergoing total shoulder arthroplasty, each additional point on the mFI-5 was associated with a step-wise increase in LOS and complications (OR: 1.601) and increased odds of 30-d readmission (OR: 1.450).24 Another orthopedic study analyzing both revision total knee and hip arthroplasty cohorts showed that the mFI-11 was associated with increased LOS, increased odds of serious medical complications, and increased odds of readmission.25 In the cardiac surgery literature, the Fried score and Short Performance Physical Battery score (both measures of frailty) have been shown to predict increased hospitalization costs and readmission in patients undergoing coronary artery bypass grafting and heart valve surgery.11 Similar efforts in general surgery have shown that the Clinical Frailty Scale predicted costs in elderly patients undergoing emergent abdominal operations.12
Globally, our data applying the mFI-5 to a large brain tumor patient cohort reflect the common theme that frailty is associated with increased LOS, complications, and charges in surgical patients; however, similar to the regressions in the original mFI-5 paper,23 the mFI-5 was not a strong predictor of 30-d readmissions in our brain tumor patient cohort.
Applications of mFI-5 to Brain Tumor Patient Management and Risk Stratification
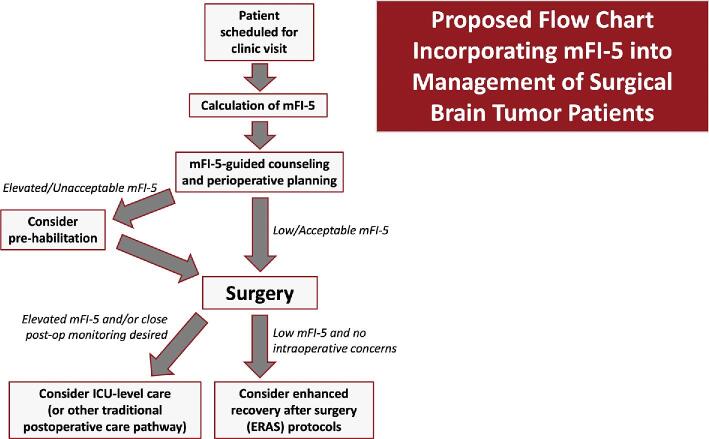
We postulate that the mFI-5 could be used as an integral component of strategies to improve the quality and value of neurosurgical care for brain tumor patients by facilitating thoughtful patient stratification and perioperative management (Figure). Conceptually, this may involve 2 general strategies: preoperative prehabilitation in frail patients and streamlined postoperative care pathways (ie, reduced ICU time for nonfrail patients). While not well-studied in brain tumor patients, the concept of prehabilitation – beginning rehabilitation before surgery in order to enhance functional status, increase reserves, and hasten postoperative recovery – is well-described in the general surgical literature.16 In patients undergoing major abdominal surgery, for instance, prehabilitation was shown to decrease complications and reduce hospital charges by $21 946 per patient.30 Prehabilitation similarly decreased costs and hastened achievement of key postoperative milestones in patients undergoing lumbar spine surgery.31 We recognize that similar efforts may be difficult for highly malignant tumors like glioblastoma which require operation soon after diagnosis; however, opportunities for prehabilitation in frail patients with slower-growing tumors may warrant further investigation as a potential means of reducing LOS, avoiding complications, and controlling charges.
FIGURE.
Patient flow chart (based on retrospective evaluation of predictive value of mFI-5 on postoperative outcomes). We propose that future prospective studies consider this perioperative care pathway as a potential strategy to improve the quality and value of neurosurgical care for brain tumor patients.
While standard practice for brain tumor patients at many institutions involves conservative, ICU-level care to avoid missing postoperative complications, we note that the mFI-5 could potentially be used to guide decision-making related to enhanced recovery after surgery (ERAS) protocols and reduced ICU time for select patients. ERAS pathways emphasizing early mobilization (as well as early feeding and appropriate pain control) have become commonplace in other surgical specialties as well as spine surgery; the evidence suggests that ERAS protocols may lead to decreased complications and LOS, which may have beneficial downstream effects on cost.32,33 While this is largely unchartered territory for brain tumor patients, early studies have suggested that ERAS protocols in elective craniotomies may decrease LOS without an increase in complication rates34; they may also improve patient satisfaction.35 We hypothesize that the field will move toward this direction for appropriately selected patients, guided by clinical tools such as the mFI-5.
Similarly, it is common practice for brain tumor patients to be automatically admitted to ICU-level care in order to detect and manage potentially severe complications in the postoperative period. However, the clinical utility and cost effectiveness of this practice have been questioned, with several studies showing that routine postoperative ICU admission may not benefit all such patients and could unnecessarily increase costs.36 Once they have recovered in the postanesthesia care unit, appropriately selected patients could perhaps instead be sent to the neurosurgical ward/floor or “stepdown” unit. One study found that changing an institutional policy from “ICU, unless” to “no ICU, unless” for supratentorial tumor craniotomies was safe, well-liked by patients, and cost-effective, with savings of $1950 per case37; the management of these patients on the neurosurgical ward resembled that of other ERAS protocols. Several other studies have reported similarly positive clinical and financial outcomes, with some showing reduction in LOS and significant cost savings with floor-level care rather than automatic ICU-level care.38,39 More studies will be needed to determine which patients can be safely managed in lower acuity settings postoperatively. The mFI-5 could be a practical, evidence-based tool to guide these efforts. Given the preponderance of evidence in the literature, this work may prove to be a safe and suitable strategy for providing high-value neurosurgical care.
Finally, in addition to guiding clinical decision-making, we note that the mFI-5 may also serve as a useful risk adjustment tool for hospital quality- and reimbursement-related metrics for brain tumor patients, given its demonstrated impact on LOS, complications, and charges.
Ultimately, we propose that the mFI-5, coupled with surgeons’ clinical judgment, has significant potential to improve the quality and value of neurosurgical care for brain tumor patients. Its sleek, standardized nature would allow for straightforward incorporation into clinical workflows; benefits could be numerous, including identification of patients warranting further preoperative optimization as well as guidance in selecting appropriate postoperative care pathways. These efforts may ultimately improve the quality of neurosurgical care while simultaneously reducing costs.
Limitations
We recognize several limitations of our study. This was a retrospective, single institution study; prospective randomized studies would provide stronger evidence to support our claims. We used a database largely built based on ICD-10 coding, which is at times unreliable40; to address this, we sought to verify the integrity of our data through manual chart review of key demographic and clinical data. Our main financial outcomes are reported as hospital charges, which may not fully reflect true costs. However, the use of charges instead of costs is consistent with reported and derived data in similar studies and several large databases.7,21,41 Despite these limitations, our study is the first of its kind to describe a pragmatic, well-validated tool with the potential to improve both clinical and financial outcomes in brain tumor patients.
CONCLUSION
The current healthcare landscape has driven investigation into tools and practices that may improve the quality and cost of neurosurgical care. The mFI-5 represents a lean, powerful tool that has previously been shown to predict key clinical and financial outcomes in surgical patients. We performed the first study investigating the predictive power of the mFI-5 on these outcomes in surgical brain tumor patients. Our data show that the mFI-5 has strong predictive value related to LOS, complications, and charges in a large brain tumor cohort. While further studies are needed to validate its potential impact in neurosurgery, we postulate that the mFI-5 could be used as an integral component of strategies to improve the quality and value of neurosurgical care for brain tumor patients by facilitating thoughtful patient stratification and perioperative management.
Disclosures
The authors acknowledge assistance for clinical data coordination and retrieval from the Core for Clinical Research Data Acquisition, supported in part by the Johns Hopkins Institute for Clinical and Translational Research (UL1TR001079). The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Acknowledgments
We thank the Johns Hopkins Neuro-Oncology Surgical Outcomes Laboratory for helpful comments and edits.
Contributor Information
Sakibul Huq, Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Adham M Khalafallah, Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Adrian E Jimenez, Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Abhishek Gami, Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Shravika Lam, Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Miguel A Ruiz-Cardozo, Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Leonardo A P Oliveira, Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Debraj Mukherjee, Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, Maryland.
The Naica Mine in Chihuahua, Mexico is a lead, zinc, and silver mine that contains huge selenite (gypsum) crystals. The largest crystals are 4 feet in diameter and 50 feet long. Depicted here, you can see the scale against the miner in the lower right corner. The caves of the mine sit above magma chambers which are thought to pushed hydrothermal fluids up to create the selenite crystals. Because of the magma the cave is inhospitably hot. The peak underground temperature is 136° F (58° C) with 99% humidity. This environment makes it impossible for unprotected researchers to spend more than 10 minutes in the caverns. In 2015 the mines were closed and some chambers allowed to flood to continue crystal growth. Other minerals found in the mines include galena, sphalerite, calcite, and fluorite. Image by By Alexander Van Driessche - Gaianauta received this from Alexander Van Driessche via Email., CC BY 3.0, https://commons.wikimedia.org/w/index.php?curid=23231964. Information from Wikipedia.com.
REFERENCES
- 1. Cowan JA Jr, Chandler WF. Changing trends in the use and costs of procedures performed by neurosurgeons in the United States. Clin Neurosurg. 2006;54:209-211. [PubMed] [Google Scholar]
- 2. Zygourakis CC, Kahn JG. Cost-effectiveness research in neurosurgery. Neurosurg Clin N Am. 2015;26(2):189-196. [DOI] [PubMed] [Google Scholar]
- 3. DeWitt JC, Jordan JT, Frosch MP et al. Cost-effectiveness of IDH testing in diffuse gliomas according to the 2016 WHO classification of tumors of the central nervous system recommendations. Neuro Oncol. 2017;19(12):1640-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eseonu CI, Rincon-Torroella J, ReFaey K, Quinones-Hinojosa A. The cost of brain surgery: awake vs asleep craniotomy for perirolandic region tumors. Neurosurgery. 2017;81(2):307-314. [DOI] [PubMed] [Google Scholar]
- 5. Moghavem N, Morrison D, Ratliff JK, Hernandez-Boussard T. Cranial neurosurgical 30-day readmissions by clinical indication. J Neurosurg. 2015;123(1):189-197. [DOI] [PubMed] [Google Scholar]
- 6. Dasenbrock HH, Liu KX, Devine CA et al. Length of hospital stay after craniotomy for tumor: a national surgical quality improvement program analysis. Neurosurg Focus. 2015;39(6):E12. [DOI] [PubMed] [Google Scholar]
- 7. Zygourakis CC, Liu CY, Yoon S et al. Analysis of cost variation in craniotomy for tumor using 2 national databases. Neurosurgery. 2017;81(6):972-979. [DOI] [PubMed] [Google Scholar]
- 8. Sherrod BA, Gamboa NT, Wilkerson C et al. Effect of patient age on glioblastoma perioperative treatment costs: a value driven outcome database analysis. J Neurooncol. 2019;143(3):465-473. [DOI] [PubMed] [Google Scholar]
- 9. Bekelis K, Gottlieb DJ, Su Y, Lanzino G, Lawton MT, MacKenzie TA. Medicare expenditures for elderly patients undergoing surgical clipping or endovascular intervention for subarachnoid hemorrhage. J Neurosurg. 2017;126(3):805-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rolston JD, Han SJ, Lau CY, Berger MS, Parsa AT. Frequency and predictors of complications in neurological surgery: national trends from 2006 to 2011. J Neurosurg. 2014;120(3):736-745. [DOI] [PubMed] [Google Scholar]
- 11. Goldfarb M, Bendayan M, Rudski LG et al. Cost of cardiac surgery in frail compared with nonfrail older adults. Can J Cardiol. 2017;33(8):1020-1026. [DOI] [PubMed] [Google Scholar]
- 12. Eamer GJ, Clement F, Holroyd-Leduc J, Wagg A, Padwal R, Khadaroo RG. Frailty predicts increased costs in emergent general surgery patients: a prospective cohort cost analysis. Surgery. 2019;166(1):82-87. [DOI] [PubMed] [Google Scholar]
- 13. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McIsaac DI, Beaule PE, Bryson GL, Van Walraven C. The impact of frailty on outcomes and healthcare resource usage after total joint arthroplasty. Bone Joint J. 2016;98-b(6):799-805. [DOI] [PubMed] [Google Scholar]
- 15. Robinson TN, Wu DS, Stiegmann GV, Moss M. Frailty predicts increased hospital and six-month healthcare cost following colorectal surgery in older adults. Am J Surg. 2011;202(5):511-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilkes JG, Evans JL, Prato BS, Hess SA, MacGillivray DC, Fitzgerald TL. Frailty cost: economic impact of frailty in the elective surgical patient. J Am Coll Surg. 2019;228(6):861-870. [DOI] [PubMed] [Google Scholar]
- 17. Ali R, Schwalb JM, Nerenz DR, Antoine HJ, Rubinfeld I. Use of the modified frailty index to predict 30-day morbidity and mortality from spine surgery. J Neurosurg Spine. 2016;25(4):537-541. [DOI] [PubMed] [Google Scholar]
- 18. Cloney M, D’Amico R, Lebovic J et al. Frailty in geriatric glioblastoma patients: a predictor of operative morbidity and outcome. World Neurosurg. 2016;89:362-367. [DOI] [PubMed] [Google Scholar]
- 19. Imaoka Y, Kawano T, Hashiguchi A et al. Modified frailty index predicts postoperative outcomes of spontaneous intracerebral hemorrhage. Clin Neurol Neurosurg. 2018;175:137-143. [DOI] [PubMed] [Google Scholar]
- 20. Harland TA, Wang M, Gunaydin D et al. Frailty as a predictor of neurosurgical outcomes in brain tumor patients. World Neurosurg. 2020;133:e813-e818. [DOI] [PubMed] [Google Scholar]
- 21. Grossman R, Mukherjee D, Chang DC et al. Predictors of inpatient death and complications among postoperative elderly patients with metastatic brain tumors. Ann Surg Oncol. 2011;18(2):521-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gani F, Canner JK, Pawlik TM. Use of the modified frailty index in the American College of Surgeons National Surgical Improvement Program database. JAMA Surg. 2017;152(2):205-207. [DOI] [PubMed] [Google Scholar]
- 23. Subramaniam S, Aalberg JJ, Soriano RP, Divino CM. New 5-factor modified frailty index using American College of Surgeons NSQIP data. J Am Coll Surg. 2018;226(2):173-181.e8. [DOI] [PubMed] [Google Scholar]
- 24. Traven SA, McGurk KM, Reeves RA, Walton ZJ, Woolf SK, Slone HS. Modified frailty index predicts medical complications, length of stay, readmission, and mortality following total shoulder arthroplasty. J Shoulder Elbow Surg. 2019;28(10):1854-1860. [DOI] [PubMed] [Google Scholar]
- 25. Traven SA, Reeves RA, Slone HS, Walton ZJ. Frailty predicts medical complications, length of stay, readmission, and mortality in revision hip and knee arthroplasty. J Arthroplasty. 2019;34(7):1412-1416. [DOI] [PubMed] [Google Scholar]
- 26. Youngerman BE, Neugut AI, Yang J, Hershman DL, Wright JD, Bruce JN. The modified frailty index and 30-day adverse events in oncologic neurosurgery. J Neurooncol. 2018;136(1):197-206. [DOI] [PubMed] [Google Scholar]
- 27. Weaver DJ, Malik AT, Jain N, Yu E, Kim J, Khan SN. The modified 5-Item frailty index: a concise and useful tool for assessing the impact of frailty on postoperative morbidity following elective posterior lumbar fusions. World Neurosurg. 2019;124:e626-e632. [DOI] [PubMed] [Google Scholar]
- 28. Leven DM, Lee NJ, Kothari P et al. Frailty index is a significant predictor of complications and mortality after surgery for adult spinal deformity. Spine. 2016;41(23):E1394-E1401. [DOI] [PubMed] [Google Scholar]
- 29. Nuno M, Carico C, Mukherjee D et al. Association between in-hospital adverse events and mortality for patients with brain tumors. J Neurosurg. 2015;123(5):1247-1255. [DOI] [PubMed] [Google Scholar]
- 30. Howard R, Yin YS, McCandless L, Wang S, Englesbe M, Machado-Aranda D. Taking control of your surgery: impact of a prehabilitation program on major abdominal surgery. J Am Coll Surg. 2019;228(1):72-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nielsen PR, Andreasen J, Asmussen M, Tonnesen H. Costs and quality of life for prehabilitation and early rehabilitation after surgery of the lumbar spine. BMC Health Serv Res. 2008;8(1):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Epstein NE. A review article on the benefits of early mobilization following spinal surgery and other medical/surgical procedures. Surg Neurol Int. 2014;5(4):S66-S73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zakaria HM, Bazydlo M, Schultz L et al. Ambulation on postoperative day #0 is associated with decreased morbidity and adverse events after elective lumbar spine surgery: analysis from the Michigan Spine Surgery Improvement Collaborative (MSSIC). Neurosurgery. published online: December 12, 2019. (doi:10.1093/neuros/nyz501). [DOI] [PubMed] [Google Scholar]
- 34. Wang Y, Liu B, Zhao T et al. Safety and efficacy of a novel neurosurgical enhanced recovery after surgery protocol for elective craniotomy: a prospective randomized controlled trial. J Neurosurg. 2019;130(5):1409-1788. [DOI] [PubMed] [Google Scholar]
- 35. Liu B, Liu S, Wang Y et al. Neurosurgical enhanced recovery after surgery (ERAS) programme for elective craniotomies: are patients satisfied with their experiences? A quantitative and qualitative analysis. BMJ Open. 2019;9(11):e028706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Almeida CC, Boone MD, Laviv Y, Kasper BS, Chen CC, Kasper EM. The utility of routine intensive care admission for patients undergoing intracranial neurosurgical procedures: a systematic review. Neurocrit Care. 2018;28(1):35-42. [DOI] [PubMed] [Google Scholar]
- 37. Laan MT, Roelofs S, Van Huet I, Adang EMM, Bartels R. Selective intensive care unit admission after adult supratentorial tumor craniotomy: complications, length of stay, and costs. Neurosurgery. 2020;86(1):E54-E59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Florman JE, Cushing D, Keller LA, Rughani AI. A protocol for postoperative admission of elective craniotomy patients to a non-ICU or step-down setting. J Neurosurg. 2017;127(6):1392-1397. [DOI] [PubMed] [Google Scholar]
- 39. Osorio JA, Safaee MM, Viner J et al. Cost-effectiveness development for the postoperative care of craniotomy patients: a safe transitions pathway in neurological surgery. Neurosurg Focus. 2018;44(5):E19. [DOI] [PubMed] [Google Scholar]
- 40. Woodworth GF, Baird CJ, Garces-Ambrossi G, Tonascia J, Tamargo RJ. Inaccuracy of the administrative database. Neurosurgery. 2009;65(2):251-257; discussion 256-257. [DOI] [PubMed] [Google Scholar]
- 41. Little AS, Chapple K. Predictors of resource utilization in transsphenoidal surgery for Cushing disease. J Neurosurg. 2013;119(2):504-511. [DOI] [PubMed] [Google Scholar]