ABSTRACT
Subcutaneous abdominal adipose tissue (SAT), is the largest fat depot and major provider of free fatty acids to the liver. Abdominal fat is indirectly (via increased levels of low-grade inflammation) correlated with many of the adverse health effects of obesity. Although exercise is one of the most prominent components of obesity management, its effects on SAT are still unclear. The aim of this study was to investigate the independent effects of aerobic training (AT) and resistance training (RT) modalities and combined exercise modalities on SAT in adults. PubMed, SCOPUS, and Google Scholar were searched to find relevant publications up to November 2018. The effect sizes were represented as weighted mean difference (WMD) and 95% CIs. Between-study heterogeneity was examined using the I2 test. Overall, 43 identified trials that enrolled 3552 subjects (2684 women) were included. After removal of outliers, combining effect sizes indicated a significant effect of AT (WMD: −13.05 cm2; 95% CI: −18.52, −7.57; P < 0.001), RT (WMD: −5.39 cm2; 95% CI: −9.66, −1.12; P = 0.01), and combined exercise training (CExT; WMD: −28.82 cm2; 95% CI: −30.83, −26.81; P < 0.001) on SAT relative to control groups. Pooled effect sizes demonstrated a significant effect of AT on SAT compared with a CExT group (WMD: 11.07 cm2; 95% CI: 1.81, 20.33; P = 0.01). However, when comparing the AT and RT groups, no significant difference was seen in SAT (WMD: −0.73 cm2; 95% CI: −4.50, 3.04; P = 0.70). Meta-analysis of relevant trials indicated that AT, RT, and CExT lead to SAT reduction. Aerobic exercise was shown to produce greater efficacy in decreasing SAT.
Keywords: aerobic exercise, resistance training, combined exercise, subcutaneous abdominal fat, and meta-analysis
Introduction
The prevalence of obesity and chronic disease has been increasing at an alarming rate (1). It is now widely recognized that increased deposition of abdominal fat has been implicated as a key etiology in the development and progression of various chronic conditions such as obesity, diabetes, cardiovascular diseases, and some cancers (2). Abdominal fat has been suggested to be associated with these chronic diseases through the release of cytokines and bioactive mediators (3). Although persons with abdominal obesity appear to develop metabolic syndrome more frequently than individuals with peripheral body fat distribution, the site of abdominal fat accumulation (subcutaneous compared with visceral) is potentially important and still a matter of debate (4–6). Over recent years, most attention has focused on visceral adipose tissue (VAT) (7); however, it should be kept in mind that subcutaneous abdominal adipose tissue (SAT) accounts for ∼80% of abdominal fat and is the major provider of free fatty acids to the liver (8, 9). In a meta-analysis that included 89 studies, the effect of different exercise on visceral and subcutaneous fat loss was investigated. Based on the included studies, it indicated a greater decrease in SAT compared with VAT (7) across all of the strategies (10). Currently, in addition to exercising, other approaches for the removal of these tissues, including bariatric or liposuction surgeries, have attracted more and more people. In addition, nowadays, more individuals are using the aggressive method for the removal of these tissues, the cost-benefit consequences of which are really prominent in terms of economic aspects, although their effect on human life is still unclear (11, 12). Also, reduction in body fat through aggressive procedures (liposuction) had no effect on improving metabolic parameters (13).
Many other methods to prevent obesity such as dietary interventions have been investigated over the past decades as well (14). It is now well known that a minimal loss of 5–10% of the initial body weight (especially abdominal fat) is effective in improving risk profiles of diseases related to obesity (15, 16). However, a 20% reduction in body fat (or 40% of subcutaneous fat) through an aggressive procedure (liposuction) had no effect on improving metabolic parameters. This can be due to specific differences between visceral and subcutaneous fat or a fundamental problem of energy imbalance (13, 17). Along with dietary modifications, exercise seems to be another important and central component of weight-loss programs. Exercise-induced weight-loss interventions can affect the amount of abdominal fat and thereby prevent the onset of chronic diseases. Also, despite effects on body fat, regular exercise has many health benefits, including reduced blood pressure and blood lipids, improved diabetes, and other metabolic health outcomes independent of body fat loss (18–21). In the past years, a great deal of attention has been focused on the impact of aerobic exercise with or without caloric restriction in subjects with obesity (16, 19, 20, 22, 23). On the other hand, there has been considerable recent interest regarding the effects of resistance exercise on metabolic profile and weight loss in individuals with obesity (23–26). Although many studies compared the effect of resistance exercise and aerobic exercise on abdominal fat (24, 27, 28), it is still not clear which of these exercise modalities is better for the reduction of SAT (27, 29). Thus, our main objective in this study was to systematically review the present evidence on the effects of aerobic exercise (aerobic training; AT), resistance training (RT), and a combination of these modalities (combined exercise training; CExT) on SAT reduction, and to summarize the available findings in a meta-analysis.
Methods
This study was carried out based on the guidelines of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) statement.
Search strategy
We performed a literature search using the online databases of Medline (PubMed) and Scopus for relevant publications up to November 2018. To find relevant publications, the following medical subject headings (MeSH) and non-MeSH keywords were searched by 2 independent investigators (HY and KD) as follows: (Subcutaneous Fat, Subcutaneous Adipose Tissue, Abdominal Subcutaneous Fats, Adipose Tissue, Abdominal Subcutaneous, Abdominal Fat, Abdominal Adipose Tissue) and (Exercise, Physical Activity, Physical Exercise, Acute Exercise, Aerobic Exercise, endurance training, cardio training or Exercise Tolerance, Resistance Training, Strength Training, Weight-Lifting Strengthening Program, progressive training, weight training). We also searched systematic reviews from the above-mentioned databases and hand checked reference lists to identify studies that might have been missed. Unpublished studies were excluded, as well as duplicate citations.
Selection of studies
After removal of duplications, the search results were evaluated by 1 investigator (HY). Then, retained studies were retrieved and reviewed by 2 investigators. Any disagreement between the 2 researchers was resolved by discussion or by a third person (30).
Inclusion and exclusion criteria for studies
In our meta-analysis, eligible publications were included based on the following criteria: 1) all studies assessing the effects of exercise on abdominal fat area; 2) studies that used a randomized controlled clinical trial design; 3) those studies that only investigated the effect of intervention on abdominal fat area (cm2) but not other indicators of fat tissue, such as its volume (cm3), thickness (31), and weight (kg); 4) computed tomography (CT) or MRI was used for quantification of SAT; 5) human studies with a minimum follow-up period of 4 wk; and 6) manuscripts published in the English language.
Studies that met the following criteria were excluded: 1) participants <18 y, 2) non–original research (letters, review articles, and meta-analysis), 3) not enough information was available, 4) studies on specific diseases (e.g., spinal cord injury and AIDS), and 5) the other methods used for quantification of SAT (such as ultrasound or DXA).
Data extraction
The following data of interest from each individual study were extracted: first author, year of publication, study population, sample size, age, sex, weight, and BMI (Table 1); exercise details (nutritional intervention, exercise frequency, intensity, session duration and intervention duration) and subcutaneous fat quantification (type of measurement technique and region) (Table 2). For 3 studies that presented data graphically, means and SDs were extracted using the GetData Graph Digitizer 2.24 (Fedorov 2008).
TABLE 1.
Summary demographic characteristics of the studies examining the effects of AT, RT, and CExT modalities on subcutaneous abdominal fat1
| First author (reference); year | Study population | % Male | % Female | Sample size, n | Mean age, y | Mean BMI, kg/m2 | Mean weight, kg |
|---|---|---|---|---|---|---|---|
| Bacchi et al. (27); 2013 | Sedentary subjects with type 2 diabetes | AT: 71RT: 70 | AT: 29RT: 30 | AT: 14RT: 17 | AT: 55.6RT: 56 | AT: 30.5RT: 28.8 | NR |
| Binder et al. (32); 2005 | Older sedentary with physical frailty | 0 | 100 | C: 38RT: 53 | C: 83RT: 83 | C: 26RT: 27 | C: 71RT: 77 |
| Boudou et al. (33); 2003 | Middle-aged men with type 2 diabetes | 100 | 0 | C: 8AT: 8 | C: 47.9AT: 42.9 | C: 30.85AT: 28.3 | C: 86.9AT: 90.4 |
| Brochu et al. (34); 2009 | Postmenopausal women | 0 | 100 | C: 71RT: 36 | C: 58RT: 57.2 | C: 32.2RT: 32.6 | C: 83.6RT: 84.1 |
| Brown et al. (35); 2016 | Women with high risk for breast cancer | 39 | 61 | C: 13AT: 14 | NR | C: 29.2AT: 29.5 | C: 83.7AT: 86.2 |
| Brown et al. (35); 2016 | Women with high risk for breast cancer | 39 | 61 | C: 13AT: 12 | NR | C: 29.2AT: 32.5 | C: 83.7AT: 92.2 |
| Carr et al. (36); 2005 | Subjects with impaired glucose tolerance | C: 17AT: 12 | C: 83AT: 88 | C: 32AT: 30 | C: 57.2AT: 55.7 | C: 26.6AT: 25.7 | C: 69.7AT: 66.5 |
| Choi et al. (37); 2012 | Korean women | 0 | 100 | C: 15AT: 15 | C: 45.4AT: 45.4 | C: 26.1AT: 25.6 | C: 63AT: 64.4 |
| Choi et al. (37); 2012 | Korean women | 0 | 100 | C: 15AT: 15 | C: 45.4AT: 45.4 | C: 26.1AT: 25.1 | C: 63AT: 63.2 |
| Choi et al. (37); 2012 | Patients with type 2 diabetes | 0 | 100 | C: 37AT: 38 | C: 55AT: 53.8 | C: 26.8AT: 26.8 | C: 64.9AT: 64.6 |
| Christiansen et al. (38); 2009 | Obese men and women | 50 | 50 | C: 19AT: 21 | C: 35.6AT: 37.5 | C: 35.3AT: 34.2 | C: 107.8AT: 105.8 |
| Cuff et al. (23); 2003 | Obese postmenopausal women with type 2 diabetes | 0 | 100 | C: 9CExT: 10AT: 9 | C: 60CExT: 63.4AT: 59.4 | C: 36.7CExT: 33.3AT: 32.5 | C: 95.6CExT: 89.5AT: 81.2 |
| DiPietro et al. (39); 1998 | Healthy older men and women | NR | NR | C: 7AT: 9 | C: 73AT: 72 | C: 26.8AT: 27.5 | C: 69AT: 65 |
| Donnelly et al. (40); 2003 | Sedentary overweight and moderately obese | 0 | 100 | C: 18AT: 25 | C: 21AT: 24 | C: 29.3AT: 28.7 | C: 79.9AT: 77 |
| Donnelly et al. (40); 2003 | Sedentary overweight and moderately obese | 100 | 0 | C: 15AT: 16 | C: 24AT: 22 | C: 29AT: 29.7 | C: 94.1AT: 94 |
| Drapeau et al. (41); 2011 | Overweight and obese postmenopausal women | 0 | 100 | C: 22RT: 24 | C: 58.5RT: 58 | C: 32.1RT: 32.9 | C: 82.5RT: 84.4 |
| Fisher et al. (29); 2011 | Healthy premenopausal women | 0 | 100 | C: 29AT: 43RT: 54 | NR | C: 28AT: 28RT: 28 | C: 78AT: 77RT: 78 |
| Fisher et al. (29); 2011 | Healthy premenopausal women | 0 | 100 | C: 24AT: 32RT: 41 | NR | C: 28AT: 28RT: 28 | C: 79AT: 75RT: 78 |
| Friedenreich et al. (42); 2011 | Normal-weight to obese postmenopausal women | 0 | 100 | C: 160AT: 160 | C: 60.6AT: 61.2 | C: 29.2AT: 29.1 | C: 76.3AT: 75.6 |
| García-Unciti et al. (43); 2012 | Obese women | 0 | 100 | C: 9RT: 13 | C: 50.2RT: 48.6 | C: 35RT: 35 | C: 88.9RT: 90.2 |
| Hays et al. (44); 2006 | Older men and women | 40 | 60 | C: 11AT: 11 | C: 67.5AT: 64.8 | C: 31AT: 30.8 | C: 89.6AT: 82.9 |
| Henríquez et al. (45); 2017 | Sedentary women | 0 | 100 | AT: 18RT: 16 | AT: 58RT: 55 | AT: 30.6RT: 30.8 | AT: 75RT: 77.1 |
| Houghton et al. (46); 2017 | Patients with sedentary lifestyles and NASH (nonalcoholic steatohepatitis) | NR | NR | C: 12CExT: 12 | C: 51CExT: 54 | C: 33CExT: 33 | C: 94CExT: 90 |
| Houghton et al. (46); 2017 | Patients who were overweight or obese and consuming alcohol | NR | NR | C: 14CExT: 13 | C: 56CExT: 51 | C: 31CExT: 33 | NR |
| Hunter et al. (47); 2010 | Healthy premenopausal women | 0 | 100 | C: 30AT: 18RT: 21 | C: 34.8AT: 34.7RT: 34.1 | C: 23.9AT: 23.5RT: 23.9 | C: 65AT: 62.8RT: 66 |
| Ibáñez et al. (48); 2010 | Obese women | 0 | 100 | C: 12RT: 13 | C: 51.4RT: 48.6 | C: 34.6RT: 35 | C: 88RT: 90.2 |
| Irving et al. (72); 2008 | Obese women | 0 | 100 | C: 7AT: 11 | C: 51AT: 51 | C: 32.7AT: 34.7 | C: 89.6AT: 97.2 |
| Irving et al. (72); 2008 | Obese women | 0 | 100 | C: 7AT: 9 | C: 51AT: 51 | C: 32.7AT: 34.7 | C: 89.6AT: 93.5 |
| Irving et al. (49); 2009 | Obese adults with the metabolic syndrome | C: 40AT: 24 | C: 60AT: 76 | C: 10AT: 13 | C: 49.2AT: 49.2 | C: 32AT: 35.5 | C: 95.9AT: 101.7 |
| Irving et al. (49); 2009 | Obese adults with the metabolic syndrome | C: 40AT: 28 | C: 60AT: 72 | C: 10AT: 11 | C: 49.2AT: 49 | C: 32AT: 34.2 | C: 95.9AT: 97.7 |
| Irwin et al. (50); 2003 | Overweight postmenopausal women | 0 | 100 | C: 86CExT: 87 | C: 60.6CExT: 61 | C: 30.6CExT: 30.5 | C: 81.7CExT: 81.6 |
| Janssen et al. (51); 2002 | Obese premenopausal women | 0 | 100 | C: 13AT: 11RT: 14 | C: 40.1AT: 37.5RT: 34.8 | C: 33.7AT: 36RT: 31.6 | C: 90.8AT: 99.9RT: 86.1 |
| Janssen and Ross (52); 1999 | Upper-body-obese men | 100 | 0 | C: 10AT: 10RT: 10 | C: 45.6AT: 47.4RT: 37.9 | C: 31.6AT: 33RT: 33.6 | C: 98.1AT: 101.9RT: 109.1 |
| Janssen and Ross (52); 1999 | Upper-body-obese women | 0 | 100 | C: 10AT: 10RT: 10 | C: 39.6AT: 39RT: 37.3 | C: 34.5AT: 35.5RT: 32.5 | C: 92.9AT: 98.3RT: 87.2 |
| Johnson et al. (53); 2009 | Obese sedentary | NR | NR | C: 7AT: 12 | C: 47.3AT: 49.1 | C: 31.1AT: 32.2 | C: 98.8AT: 94.4 |
| Kim et al. (73); 2008 | Women with metabolic syndrome | 0 | 100 | C: 10CExT: 10 | NR | C: 25.7CExT: 26.1 | C: 58.33CExT: 58.13 |
| Koo et al. (54); 2010 | Women with type 2 diabetes | 0 | 100 | C: 18AT: 13 | C: 57AT: 59 | C: 28.5AT: 25.5 | C: 66AT: 64 |
| Koo et al. (54); 2010 | Women with type 2 diabetes | 0 | 100 | C: 19AT: 14 | C: 57AT: 53 | C: 27.1AT: 29.4 | C: 67.4AT: 69.4 |
| Lee et al. (55); 2012 | Overweight or obese premenopausal women | 0 | 100 | C: 7AT: 8 | C: 38.3AT: 41.6 | C: 27.3AT: 27.4 | C: 70.3AT: 67.3 |
| Lee et al. (55); 2012 | Overweight or obese premenopausal women | 0 | 100 | C: 7AT: 7 | C: 38.3AT: 41.7 | C: 27.3AT: 25.4 | C: 70.3AT: 65.2 |
| McTiernan et al. (56); 2007 | Sedentary | 0 | 100 | C: 51AT: 49 | C: 53.7AT: 54.4 | C: 28.5AT: 28.9 | C: 77.9AT: 78 |
| McTiernan et al. (56); 2007 | Sedentary | 100 | 0 | C: 51AT: 51 | C: 56.6AT: 56.2 | C: 30.1AT: 29.7 | C: 97.4AT: 94.8 |
| Moghadasi et al. (57); 2012 | Sedentary overweight and obese men | 100 | 0 | C: 8AT: 8 | C: 41.18AT: 41.18 | C: 32.03AT: 32.08 | C: 90.47AT: 87.86 |
| Mourier et al. (58); 1997 | Non–insulin-dependent diabetes (NIDDM) | NR | NR | C: 11AT: 10 | C: 46AT: 45 | C: 30.1AT: 30.4 | C: 84.4AT: 85.3 |
| Poehlman et al. (28); 2000 | Premenopausal women | 0 | 100 | C: 20AT: 14RT: 17 | C: 28AT: 29RT: 28 | C: 22AT: 22RT: 22 | C: 60AT: 59RT: 58 |
| Ryan et al. (59); 2011 | Patients with impaired glucose tolerance and obese postmenopausal women | 0 | 100 | C: 17AT: 16 | C: 65AT: 62 | C: 32.7AT: 34.8 | C: 84.4AT: 91.4 |
| Ryan et al. (59); 2011 | Obese postmenopausal women | 0 | 100 | C: 29AT: 33 | C: 60AT: 59 | C: 32.8AT: 30.6 | C: 88.3AT: 81.4 |
| Schmitz et al. (60); 2007 | Overweight and obese women | 0 | 100 | C: 67RT: 71 | C: 36RT: 36 | C: 29.4RT: 29.4 | C: 80.7RT: 81.6 |
| Schmitz et al. (60); 2007 | Overweight and obese women | 0 | 100 | C: 63RT: 70 | C: 36RT: 36 | C: 29.4RT: 29.4 | C: 80.7RT: 81.6 |
| Shojaee-Moradie et al. (61); 2007 | Sedentary healthy men | 100 | 0 | C: 7AT: 10 | C: 55AT: 47 | C: 27.6AT: 27.6 | C: 84.1AT: 87.4 |
| Sigal et al. (31); 2007 | Adults with type 2 diabetes | C: 65CExT: 62AT: 65RT: 62 | C: 35CExT: 38AT: 35RT: 38 | C: 63CExT: 64AT: 60RT: 63 | C: 54.8CExT: 53.5AT: 53.9RT: 54.7 | C: 35CExT: 35AT: 35.6RT: 34.1 | C: 101.3CExT: 101.9AT: 103.5RT: 99.1 |
| Slentz et al. (62); 2011 | Sedentary men and women | CExT: 43AT: 45RT: 42 | CExT: 57AT: 55RT: 58 | CExT: 44AT: 48RT: 52 | CExT: 46.9AT: 49.5RT: 49.7 | CExT: 30.7AT: 30.4RT: 30.5 | CExT: 90.4AT: 88.5RT: 88.6 |
| Slentz et al. (63); 2005 | Overweight or mildly obese with moderate lipid abnormalities | C:49AT:55 | C: 51AT: 45 | C: 47AT: 40 | C: 52.3AT: 54 | C: 29.8AT: 29.8 | NR |
| Slentz et al. (63); 2005 | Overweight or mildly obese with moderate lipid abnormalities | C: 49AT: 50 | C: 51AT: 50 | C: 47AT: 46 | C: 52.3AT: 53 | C: 29.8AT: 29.7 | NR |
| Slentz et al. (63); 2005 | Overweight or mildly obese with moderate lipid abnormalities | C: 49AT: 54 | C: 51AT: 46 | C:47AT:42 | C: 52.3AT: 51.5 | C: 29.8AT: 29.1 | NR |
| Sun et al. (64); 2018 | Healthy elderly men | 100 | 0 | C: 10AT: 10 | C: 69AT: 72 | C: 22.6AT: 22.7 | C: 61.6AT: 62.8 |
| Zhang et al. (65); 2017 | Obese young women | 0 | 100 | C: 13AT: 15 | C: 20.8AT: 20.9 | NR | C: 67.5AT: 68.5 |
| Zhang et al. (65); 2017 | Obese young women | 0 | 100 | C: 13AT: 15 | C: 20.8AT: 21.5 | NR | C: 67.5AT: 67.3 |
All data are reported as means. AT, aerobic training; C, controls; CExT, combined exercise training; NR, not reported; RT, resistance training.
TABLE 2.
Summary exercise details of the studies examining the effects of AT, RT, and CExT modalities on subcutaneous abdominal fat1
| First author (reference); year | Nutritional intervention2 (%) | Weight change, kg | Intensity | Frequency, d/wk | Session duration | Intervention duration | Measure | Region | Jadad score |
|---|---|---|---|---|---|---|---|---|---|
| Bacchi et al. (27); 2013 | According to standard recommendations for people with type 2 diabetes | NR | AT: 60–65% HRRRT: 70–80% 1-RM | 3 | 60 min | 17 wk | CT | L4-L5 | 5 |
| Binder et al. (32); 2005 | Supplemental calcium and vitamin D | C: 0.0RT: 0.0 | RT: 65–100% 1-RM | 2–3 | 60–90 min | 26 wk | MRI | L4-L5 | 5 |
| Boudou et al. (33); 2003 | Not change their diet (50% CHO, 30% fat, 20% PRO) | C: -1.65AT: -1.9 | AT: 50–85% VO2peak | 1–2 | C: 20 minAT: 45 min | 8 wk | MRI | L4-L5 | 2 |
| Brochu et al. (34); 2009 | Reduce their caloric intake by 500–800 kthcal/d(55% CHO; 30% fat; 15% PRO) | C: -5.1RT: -5.8 | RT: 65–80% 1 RM | 3 | NR | 26 wk | CT | L4-L5 | 3 |
| Brown et al. (35); 2016 | Not change their diet | C: 1.04AT: -0.49 | AT: 50–70% max HR | NR | 150 min/wk | 26 wk | CT | Whole body | 5 |
| Brown et al. (35); 2016 | Not change their diet | C: 10.4AT: -0.31 | AT: 50– 70% max HR | NR | 300 min/wk | 26 wk | CT | Whole body | 5 |
| Carr et al. (36); 2005 | AHA isocaloric diet(30% fat, 7% SFA, 200 mg cholesterol, 55% CHO, 15% PRO) | C: -0.9AT: -3 | AT: 70% HRR | 3 | 60 min | 104 wk | CT | Umbilical | 3 |
| Carr et al. (36); 2005 | AHA isocaloric diet(30% fat, 7% SFA, 200 mg cholesterol, 55% CHO, 15% PRO) | C: 0.6AT: -1.8 | AT: maximal V̇O2max | 3 | 60 min | 26 wk | CT | Umbilical | 3 |
| Choi et al. (37); 2012 | Not change their diet(70% CHO, 12% fat, 18% PRO) | C: 1.5AT: -2.1 | AT: 40–50% of VO2max | 3 | Expended 300–400 kcal/session | 12 wk | CT | L4-L5 | 5 |
| Choi et al. (37); 2012 | Not change their diet(70% CHO, 12% fat, 18% PRO) | C: 1.5AT: -2.8 | AT: 70–75% VO2max | 3 | 400 kcal/d | 12 wk | CT | L4-L5 | 5 |
| Choi et al. (37); 2012 | Not change their diet | C: -1AT: -1 | NR | 5 | 60 min | 12 wk | CT | NR | 5 |
| Christiansen et al. (38); 2009 | Reduce their caloric intake by 600 and 800 kcal/d (55% CHO, 30% fat, 15% PRO) | C: -12.3AT: -12.3 | AT: 70% HRR | 3 | 60–75 min | 12 wk | MRI | L5-L4 | 3 |
| Cuff et al. (23); 2003 | Not change their diet | C: 2CExT: -2.9AT: -1.2 | AT: 60–75% HRRRT: 12 1-RM | 3 | 75 min | 16 wk | CT | L4-L5 | 2 |
| DiPietro et al. (39); 1998 | NR | C: 0AT: -1 | AT: 55–75% HR max | 4 | 60 min | 13 wk | CT | L4-L5 | 2 |
| Donnelly et al. (40); 2003 | Not change their diet | C: -0.5AT: -5.2 | AT: 60–75% HRR | NR | 20–45 min | 26 wk | CT | L4-L5 | 4 |
| Donnelly et al. (40); 2003 | Not change their diet | C: 2.9AT: 0.6 | AT: 60–75% HRR | NR | 20–45 min | 26 wk | CT | L4-L5 | 3 |
| Drapeau et al. (41); 2011 | Reduce their caloric intake by 500 to 800 kcal/d (55% CHO, 30% fat, 15% PRO) | C: -7.7RT: 8.2 | Maximum 1-RM | weekly on 3 non-consecutive days | NR | 26 wk | CT | L4-L5 | 3 |
| Fisher et al. (29); 2011 | (55% CHO, 22% fat, 23% PRO) | C: -12AT: -12RT: -12 | AT: 65–80% max HRRT: 60–80% 1-RM | 3 | 50 min | 8 wk | CT | L4-L5 | 3 |
| Fisher et al. (29); 2011 | Reduce their caloric intake by 800 kcal/d (58–62% CHO, 20–22% fat, 18–22% PRO) | C:-7AT: -6RT: -12 | AT: 65–80% max HRRT: 60–80% 1-RM | 2–3 | 30–40 min | 52 wk | CT | L4-L5 | 2 |
| Friedenreich et al. (42); 2011 | Not change their diet | C: -0.5AT: -2.3 | AT: 85% HR | 5 | 45 min | 52 wk | CT | Umbilical | 5 |
| García-Unciti et al. (43); 2012 | Reduce their caloric intake by 500 kcal/d (55% CHO, 30% fat, 15% PRO) | C: -0.1RT: -7.1 | RT: 50–80% 1-RM | 2 | 45–60 min | 16 wk | MRI | L4-L5 | 2 |
| Hays et al. (44); 2006 | Not change their diet(63% CHO, 18% fat, 19% PRO, 26/1000 g/kcal fiber) | C: -3AT: -4.3 | AT: 80% VO2peak | 4 | 45 min | 12 wk | CT | L4-L5 | 3 |
| Henríquez et al. (45); 2017 | Diet providing 80% of the total EE(5% CHO, 25% fat, 25% PRO) | AT: -3.2RT: -3.1 | AT: 60–65% VO2maxRT: 20–30% 1-RM | 3 | 40 min | 26 wk | CT | Lumbar spine L3 | 2 |
| Houghton et al. (46); 2017 | NR | C: 1CExT: 1 | CExT: 6–20 RPE | 3 | 45–60min | 12 wk | MRI | NR | 3 |
| Houghton et al. (46); 2017 | NR | C: 0CExT: 0 | CExT: 6–20 RPE | 3 | 45–60min | 12wk | MRI | NR | 3 |
| Hunter et al. (47); 2010 | Reduce their caloric intake by 800 kcal/d (58–62% CHO, 20–22% fat, 18–22% PRO) | C: 6.4AT: 3.1RT: 3.9 | AT: 67–80% HR maxRT: 80% 1-RM | 2–3 | 20–40 min | 52 wk | CT | L4-L5 | 2 |
| Ibáñez et al. (48); 2010 | Reduce their caloric intake by 500 kcal/d(55% CHO, 30% fat, 15% PRO) | C: -5.7RT: -7.1 | RT: 50–80% 1-RM | 2 | 45–60 min | 16 wk | MRI | Abdominal | 1 |
| Irving et al. (72); 2008 | NR | C: -2.4AT: -2.7 | AT: 15–17 RPE | 2–3 | Expended 300–400 kcal/session | 16 wk | CT | L4-L5 | 5 |
| Irving et al. (72); 2008 | NR | C: -2.4AT: -2.7 | AT: 15–17 RPE | 2–3 | 16 wk | CT | L4-L5 | 5 | |
| Irving et al. (49); 2009 | (55% CHO, 30% fat, 15% PRO) | C: -0.9AT: -2.1 | NR | 5 | Expended 300–400 kcal/session | 16 wk | CT | L4–L5 | 2 |
| Irving et al. (49); 2009 | (55% CHO, 30% fat, 15% PRO) | C: -0.9AT: -3.5 | NR | 5 | Expended 300–400 kcal/session | 16 wk | CT | L4–L5 | 2 |
| Irwin et al. (50); 2003 | Not change their diet | C: 0.1CExT: -1.3 | AT: 40–75% HR maxRT: 2 sets of 10 reps | NR | 45 min | 52 wk | CT | L4-L5 | 5 |
| Janssen et al. (51); 2002 | Reduce their caloric intake by 1000 kcal/d(limit dietary fat intake to <30%) | C: -10AT: -11.1RT: -10 | AT: 50–85% HR maxRT: 1 set of 8–12 reps | AT: 5RT: 3 | AT: 15–60 minRT: 30 min | 16 wk | MRI | L4-L5 | 2 |
| Janssen and Ross (52); 1999 | Reduce their caloric intake by 1000 kcal/d(limit dietary fat intake to <30%) | C: -11.7AT: -11.4RT: -12.7 | AT: 50–85% HR maxRT: 1 set of 8–12 reps | AT: 5RT: 3 | AT: 15–60 minRT: 30 min | 16wk | MRI | L4-L5 | 5 |
| Janssen and Ross (52); 1999 | Reduce their caloric intake by 1000 kcal/d(limit dietary fat intake to <30%) | C: -10.7AT: -11.5RT: -10 | AT: 50–85% HR maxRT: 1 set of 8–12 reps | AT: 5RT: 3 | AT: 15–60 minRT: 30 min | 16 wk | MRI | L4-L5 | 5 |
| Johnson et al. (53); 2009 | Not change their diet(60% CHO, 20% fat, 20% PRO) | C: -0.2AT: -0.3 | AT: 50–70% VO2peak | 3 | 30–45 min | 4 wk | MRI | L4-L5 | 5 |
| Kim et al. (73); 2008 | NR | C: 0.57CExT: -0.3 | AT: 40–75% HRRRT: 75% 1-RM | 4 | 60 min | 12 wk | CT | L4 vertebrae close to umbilicus | 3 |
| Koo et al. (54); 2010 | Hypocaloric diet(30 kcal/kg/day of ideal body weight) | C: -0.2AT: -1.6 | NR | 7 | 120 min | 12 wk | CT | L4-L5 | 2 |
| Koo et al. (54); 2010 | Reduce energy intake to 1200 kcal/d(50–55% CHO, 20–25% fat, 15–20% PRO) | C: -5AT: -4.6 | NR | 7 | 120 min | 12 wk | CT | L4-L5 | 2 |
| Lee et al. (55); 2012 | Not change their diet | C: -1.1AT: -1.7 | AT: 50% V̇O2max | 3–5 | Expended 14.2–23.6 kcal/kg/wk | 14 wk | CT | L4-L5 | 4 |
| Lee et al. (55); 2012 | Not change their diet | C: -1.1AT: -2.8 | AT: 70% VO2max | 3–5 | Expended 14.2–23.6 kcal/kg/wk | 14 wk | CT | L4-L5 | 4 |
| McTiernan et al. (56); 2007 | Not change their diet | C: -0.1AT: -1.8 | AT: 60–85% HR max | 6 | 60 min | 52 wk | CT | L4-L5 | 5 |
| McTiernan et al. (56); 2007 | Not change their diet | C: 0.7AT: -1.4 | AT: 60–85% HR max | 6 | 60 min | 52 wk | CT | L4-L5 | 5 |
| Moghadasi et al. (57); 2012 | Not change their diet | C: 0.19AT: -2.74 | AT: 75–80% VO2max | 4 | 45 min | 12 wk | MRI | L4-L5 | 2 |
| Mourier et al. (58); 1997 | Not change their diet;50% of C and AT group BCAA supplemented (PRO: 0.6 g/kg/d) | C: -0.2AT: -1.5 | AT: 75–85% VO2peak | 3 | 20–45 min | 2-wk pretraining and 8-wk training | MRI | Umbilical | 2 |
| Poehlman et al. (28); 2000 | NR | C: 1AT: 0RT: 2 | AT: 50–95% HR maxRT: 80% 1-RM | 3 | 25–60 min | 26 wk | CT | L4-L5 | 3 |
| Ryan et al. (59); 2011 | Reduce their caloric intake by 500 kcal/d | C: -7.4AT: -6.8 | AT: >85% HRR | 3 | 45 min | 26 wk | CT | L4-L5 | 1 |
| Ryan et al. (59); 2011 | Reduce their caloric intake by 500 kcal/d | C: -6.4AT: -6.9 | AT: >85% HRR | 3 | 45 min | 26 wk | CT | L4-L5 | 1 |
| Schmitz et al. (60); 2007 | Not change their diet | C: 0.88RT: 1.17 | RT: 1–2 sets of 8–10 reps | 2 | 30–45 min | 52 wk | CT | L2-L3 | 5 |
| Schmitz et al. (60); 2007 | Not change their diet | C: 2.24RT: 1.72 | RT: 1–2 sets of 8–10 reps | 2 | 30–45 min | 104 wk | CT | L2-L3 | 5 |
| Shojaee-Moradie et al. (61); 2007 | Not change their diet | C: -0.8AT: 0.2 | AT: 60–85% V̇O2max | 3 | 20 min | 6 wk | CT | L4-L5 | 2 |
| Sigal et al. (31); 2007 | Not change their diet | C: -0.3CExT: -2.6AT: -2.6RT: -1.1 | AT: 60–75% max HRRT: 2–3 sets of 7–9 reps | 3 | 20–40 min | 26 wk | CT | L4-L5 | 5 |
| Slentz et al. (62); 2011 | Not change their diet | CExT: -2.1AT: -2RT: 0.7 | AT: 75% peak V̇O2RT: 72 sets/wk | 3 | Expended 14–23 kcal/kg/wk | 16 wk | CT | L4 pedicle | 4 |
| Slentz et al. (63); 2005 | Not change their diet | C: -1AT: -0.7 | AT: 40–55% V̇O2max | 3–4 | Expended 14 kcal/kg/wk | 34 wk | CT | L4 pedicle | 2 |
| Slentz et al. (63); 2005 | Not change their diet | C: 1AT: -0.8 | AT: 65–80% V̇O2max | 3 | Expended 14 kcal/kg/wk | 34 wk | CT | L4 pedicle | 2 |
| Slentz et al. (63); 2005 | Not change their diet | C: 1AT: -2.6 | AT: 65–80% V̇O2max | 3–4 | Expended 23 kcal/kg/wk | 34 wk | CT | L4 pedicle | 2 |
| Sun et al. (64); 2018 | NR | C: 0.5AT: 0.3 | AT: 60–75% V̇O2max | 3 | 30–45 min | 5 wk | MRI | Umbilical | 2 |
| Zhang et al. (65); 2017 | Not change their diet | C: 0.1AT: -3.4 | AT: 60% V̇O2max | 4 | 300 kJ in sessions | 13 wk | CT | L4-L5 | 3 |
| Zhang et al. (65); 2017 | Not change their diet | C: 0.1AT: -3.3 | AT: 90% V̇O2max | 4 | 300 kJ in sessions | 12 wk | CT | L4-L5 | 3 |
AT, aerobic training; BCAA, branched-chain amino acid; C, controls; CExT, combined exercise training; CHO, carbohydrate; CT, computed tomography; EE: energy expenditure; HR, heart rate; HRR, heart rate reserve; max, maximum; NR, not reported; PRO, protein; reps: repetition; RM, maximal repetition; RPE: Rating of perceived exertion; RT: resistance training; V̇O2max, maximum oxygen consumption; VO2peak, peak oxygen consumption; 1-RM, 1-repetition maximum.
Percentage of total energy intake.
Assessment of study quality
Study quality was assessed by a modified Jadad scale, in which the total score ranges from 0 to 5 points based on the following criteria: 1) randomization present, 2) method of randomization, 3) double blinding performed, 4) method of double blinding, and 5) reports of dropouts and withdrawals. In the current study, trials scored 1 point for each area addressed in the study design with a possible score of 0 to 3 (highest level of quality). In exercise studies, it is impossible to blind the people to the intervention. As a result, scores of 5 are impossible to achieve, hence we defined high-quality publications as those that had a Jadad score of ≥3 (Table 2).
Data synthesis and statistical analysis
This analysis was conducted using Stata software version 14 (StataCorp LP). Random- and fixed-effects models were utilized to obtain pooled estimates of training impacts on SAT, using weighted mean difference (WMD). Studies that reported ≥2 interventions of different exercise intensity were entered as separate studies. Also, studies that reported separate results for male and female subjects were also entered separately. We performed 5 analyses to compare the effect of 1) AT compared with control, 2) RT compared with control, 3) CExT compared with control, 4) AT compared with RT, and 5) CExT compared with AT on SAT change. The mean change (66) in SAT from baseline was used to calculate the mean difference (with 95% CI) between the intervention and control groups. Some studies provided an SEM from which we calculated the SD (66) according to the formula (SD = SEM × square root of N). Then we calculated the SD of the mean difference as follows: SD change = square root [(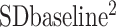 +
+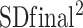 ) − (2 × R × SD baseline × SD final)]. The SD of mean differences was calculated using a correlation coefficient “R” of 0.9 (67). When the publications revealed medians and ranges or 95% CIs, the mean (66) was calculated by the method of Hozo et al. (68). The between-trial WMD and 95% CI were calculated. Between-study heterogeneity was examined using the I2 test. To assess the influence of each study on the overall mean difference, we used a sensitivity analysis by the jack-knife approach, which involves systematically removing and replacing each study. Publication bias was assessed by visual evaluation of the funnel plot and Egger's test. P values <0.05 were considered significant.
) − (2 × R × SD baseline × SD final)]. The SD of mean differences was calculated using a correlation coefficient “R” of 0.9 (67). When the publications revealed medians and ranges or 95% CIs, the mean (66) was calculated by the method of Hozo et al. (68). The between-trial WMD and 95% CI were calculated. Between-study heterogeneity was examined using the I2 test. To assess the influence of each study on the overall mean difference, we used a sensitivity analysis by the jack-knife approach, which involves systematically removing and replacing each study. Publication bias was assessed by visual evaluation of the funnel plot and Egger's test. P values <0.05 were considered significant.
Results
Included studies
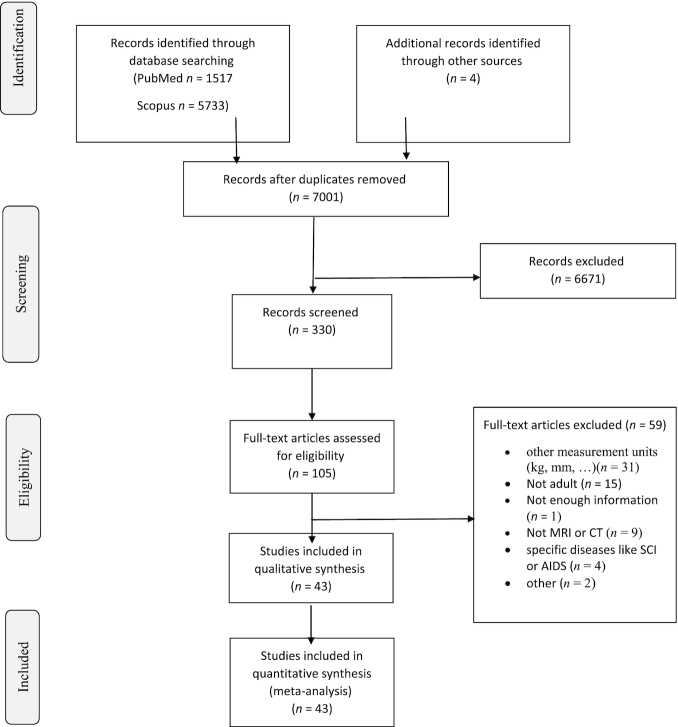
A total of 7250 studies were identified through the PubMed and Scopus databases, and after removal of duplicates a total of 6997 studies remained. Furthermore, we found 4 more studies from the reference lists of the manuscripts retrieved. After elimination of articles based on the eligibility criteria, 43 articles remained (Figure 1).
FIGURE 1.
Summary of search strategy and selection process based on included and excluded studies. CT, computed tomography; SCI, spinal cord injury.
Study characteristics
A total of 3552 individuals (with a mean age range of 20.8–83 y) had been enrolled in the trials, which included 761 men and 2684 women and gender was not reported for 107 subjects. Of the 59 studies (43 articles) in the meta-analysis, 33 studies were exclusively conducted in women, 7 studies were exclusively conducted in men, and 14 studies were conducted in both men and women, whereas sex was not reported in 5 studies. The mean BMI (kg/m2) of participants ranged from 22 to 36.7. Thirteen studies were specifically conducted in participants with obesity, 12 studies conducted in subjects who were either overweight or had obesity, 11 studies conducted in participants with type 2 diabetes, 3 studies in people with metabolic syndrome, and other studies conducted in women with a high risk of breast cancer and patients with hepatic impairment (Table 1). The quality score of studies included in this meta-analysis ranged from 1 to 3. Ninety trials were categorized as low-quality publications (Jadad score <3) and 44 trials were categorized as high quality (Jadad score ≥3). All studies were randomized trials, but 14 of these studies did not explain the randomization procedure. All studies except for 4 reported details concerning the number of participants who dropped out.
Details of AT
AT interventions ranged from 4 to 52 wk. The frequency of AT was from 1 to 7 d/wk. The frequency of AT was most commonly 3 d/wk (19 of 48 studies) followed by 5 d/wk (7 of 48 studies). For the AT group, the sessions were from 15 to 120 min (but some studies reported energy expended per training session rather than time) at intensities between 40% and 95% of peak aerobic capacity. AT intensities have been reported as percentage of heart rate reserve (HRR) or a percentage of maximal heart rate (HR), or maximum oxygen consumption (VO2max).
Details of RT
RT interventions ranged from 8 to 104 wk. The frequency of RT was from 2 to 4 d/wk, but most frequently 3 d/wk (10 of 18 studies) followed by 2 d/wk (4 of 18 studies). For the RT group, the sessions were from 20 to 90 min. Moreover, the intensity of RT, which was quantified as a percentage of 1-repetition maximum (1-RM), ranged between 20% and 100% of 1-RM. Five studies prescribed 1 or 2 sets of 8–12 repetitions until volitional fatigue and 1 study prescribed 2 to 3 sets of 7–9 repetitions.
Details of CExT
CExT interventions ranged from 12 to 52 wk. The frequency of CExT was 3 d/wk. For the CExT group, the sessions were from 20 to 75 min. CExT is a combination of RT and AT that their intensity is defined as follows: AT: HRR or HR or VO2max; RT: 1-RM. In addition, Rating of Perceived Exertion (69) was used to express intensity.
Details of diets
Twenty-nine of the studies stated that diet was not changed, 22 studies required the participants to follow a prescribed diet, and 8 studies did not report on food intake. Some of the studies asked participants to reduce caloric intake to 500–1200 kcal/d. The contribution of each macronutrient to daily energy intake was as follows: in the range of 50%–70% for carbohydrate, in the range of 12%–30% for fat, in the range of 10%–25% for protein. However, the most commonly prescribed macronutrient combination was 55% carbohydrate, 30% fat, and 15% protein.
Outcomes
AT versus control
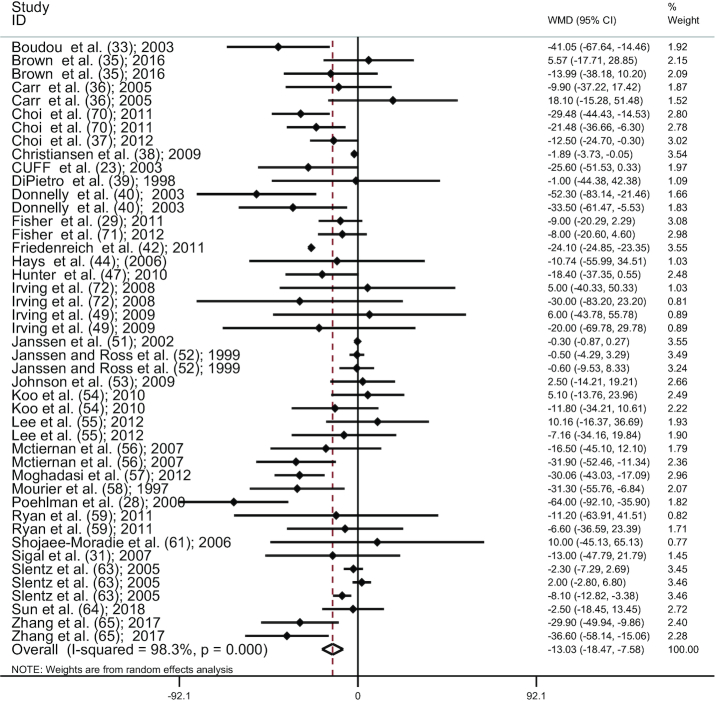
Combining effect sizes of 45 studies (23, 28, 29, 31, 33, 35–40, 42, 44, 47, 49, 51–59, 61,63–65, 70–72), AT significantly reduced SAT compared with controls (WMD: −13.02 cm2; 95% CI: −18.47, −7.58; P < 0.001) (Figure 2), with a considerable between-study heterogeneity (I2 = 98.3%, P < 0.001). The results of the influence analysis did not change the significance level of our finding after systematic removal of each study. There was no evidence of publication bias, by the funnel plot shape. In addition, the lack of bias was confirmed by the Egger's test (P = 0.732).
Figure 2.
Forest plot for AT studies (n = 45). Graph depicts WMDs and 95% CIs for individual studies and the pooled estimate. AT, aerobic training; WMD, weighted mean difference.
RT versus control
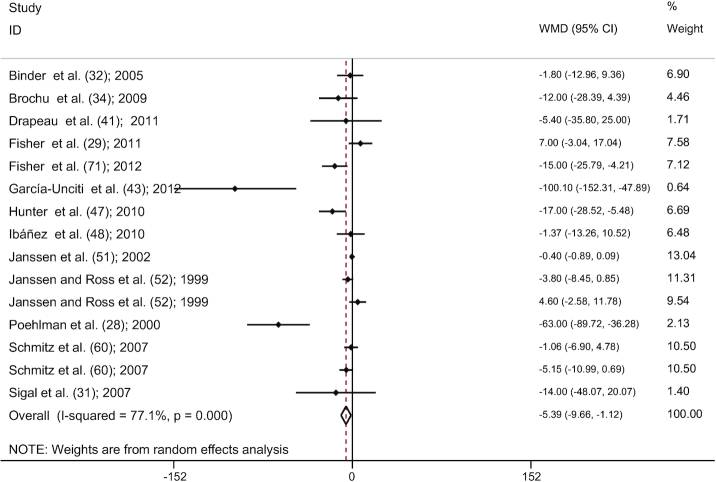
Combining findings of 15 studies (28, 29, 31, 32, 34, 41, 43, 47, 48, 51, 52, 60, 71), a significant reduction in SAT was detected after RT compared with controls (WMD: −5.39 cm2; 95% CI: −9.66, −1.12; P = 0.013 ), with a significant between-study heterogeneity (I2 = 77.1%, P < 0.001) (Figure 3). The results of the influence analysis did not change the significance level of our finding after systematic removal of each study. There was a significant statistical evidence of publication bias among studies by visual interpretation of the funnel plot shapes and significance in Egger's linear regression test (P = 0.021).
FIGURE 3.
Forest plot for RT studies (n = 15). Graph depicts WMDs and 95% CIs for individual studies and the pooled estimate. RT, resistance training; WMD, weighted mean difference.
CExT versus control
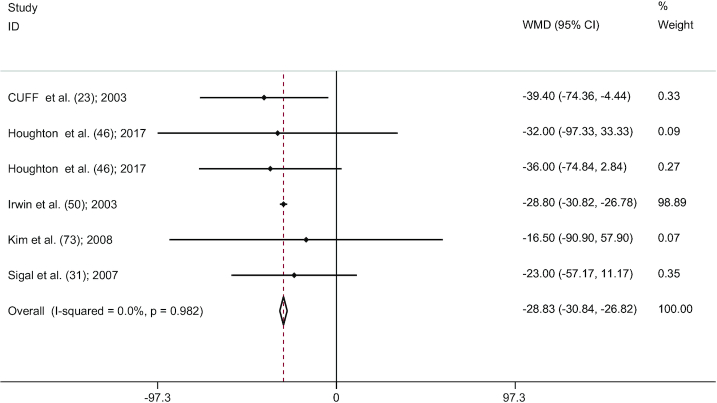
There was a significant pooled effect size (6 studies) (23, 31, 46, 50, 73) for the comparison between CExT and control (WMD: −28.82 cm2; 95% CI: −30.83, −26.81; P < 0.001). Significant heterogeneity between studies was not found (I2 = 0.0%, P = 0.982) (Figure 4). According to the results of the influence analysis, 2 studies (31, 50) were outliers. Despite the removal of the outliers, after re-analysis via fixed-effects model, a significant pooled effect size was maintained (WMD: −31.34 cm2; 95% CI: −50.40, −12.28; P = 0.001). We found no evidence of publication bias after visual interpretation of the funnel plot shape and Egger's linear regression test (P = 0.700).
FIGURE 4.
Forest plot for CExT studies (n = 6). Graph depicts WMDs and 95% CIs for individual studies and the pooled estimate. CExT, combined exercise training; WMD, weighted mean difference.
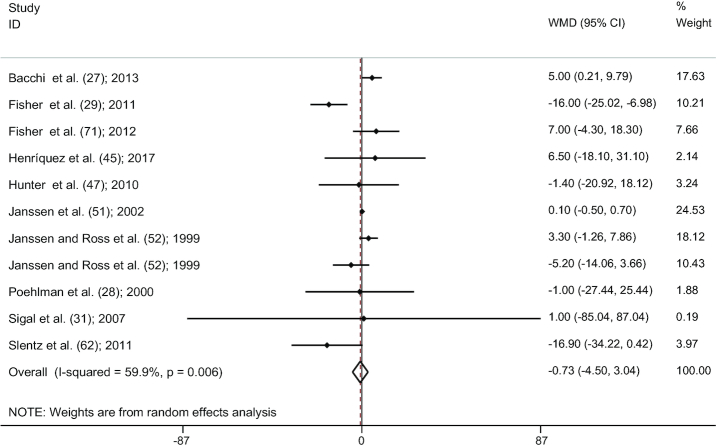
AT versus RT
Pooling effect sizes of 11 studies (27–29, 31, 45, 47,51, 52, 62, 71), no significant reduction was seen in SAT comparing AT with RT groups (WMD: −0.73; 95% CI: −4.49, 3.03; P = 0.704). However, we observed a significant heterogeneity between studies (I2 = 59.9%, P = 0.006) (Figure 5). The results of the influence analysis illustrated no change in the result after the systematic removal of each study. There was no significant evidence of publication bias among studies by visual evaluation of the funnel plot shape and Egger's test (P = 0.704).
FIGURE 5.
Forest plot for the comparison between AT and RT (11 studies). Graph depicts WMDs and 95% CIs for individual studies and the pooled estimate. AT, aerobic training; RT, resistance training; WMD, weighted mean difference.
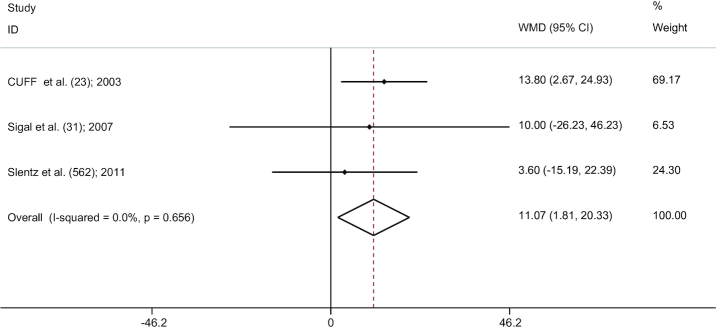
AT versus CExT
There was a significant pooled effect size (3 studies) (23, 31, 62) for the comparison between AT and CExT (WMD: 11.07; 95% CI: 1.81, 20.33; P = 0.019) (Figure 6). In fact, this present study depicted that combined training decreased subcutaneous abdominal fat more than AT. In addition, the results of the influence analysis showed that, even with the removal of 1 study, the significance of this finding remained. Furthermore, we did not observe any significant heterogeneity between studies (I2 = 0.0%, P = 0.656), or any evidence of publication bias by the funnel plot shape and Egger's test (P = 0.218).
FIGURE 6.
Forest plot for the comparison between AT and CExT (3 studies). Graph depicts WMDs and 95% CIs for individual studies and the pooled estimate. AT, aerobic training; CExT, combined exercise training; WMD, weighted mean difference.
Meta-regression
Meta-regression was used to assess the association between SAT reduction and change in weight and duration of intervention in all of the exercise groups (AT, RT, and CExT). There was no significant association between SAT reduction and weight loss (slope: −1.10; 95% CI: −2.87, 0.66; P = 0.21) and duration of the intervention (slope: −1.03; 95% CI: −2.91, 0.83; P = 0.2).
Discussion
This systematic review and meta-analysis investigated the independent effects of AT and RT modalities along with CExT modality on SAT in adults. Our results illustrated that AT, RT, and CExT regimes are all effective in lowering SAT compared with no intervention. However, in studies where AT and RT were directly compared, statistical significance was not observed. Moreover, in studies where AT and CExT were directly evaluated, we found statistically significant differences between them. Combining both AT and RT resulted in lowering of SAT more than AT or RT alone.
Central abdominal fat, which includes subcutaneous and visceral fat, has been shown to be a risk factor for chronic disease (74). Although persons with abdominal obesity appear to develop metabolic syndrome more frequently than individuals with peripheral body fat distribution, the impact of the site of abdominal fat accumulation (subcutaneous compared with visceral) is still a matter of debate (2, 4–6). Since the mass of subcutaneous abdominal fat is greater, it contributes more than visceral fat to circulating free fatty acids (4).
A recent systematic review study by Merlotti et al. (10) found that decrease in SAT was greater than VAT when measured as area (cm2), volume (cm3), and weight (kg); however percentage decrease in VAT was greater than percentage decrease in SAT. Contrary to our findings, a meta-analysis has reported that AT reduces visceral fat compared with a control group, while RT and CExT had no significant effect (75). Since the studies were conducted using different measurement indicators (studies with adipose tissue volume, thickness, and weight), it is possible that their results vary due to this issue. Also, González-Ruiz et al. (76) showed that AT alone led to a slightly greater reduction in VAT and SAT in a pediatric population, but RT and CExT did not demonstrate significant reductions. It is conceivable that these results might be due to different measurement units (kilograms, liters, millimeters, and cubic centimeters), which were assessed in this regard. In line with our findings, a meta-analysis interestingly showed that dietary intervention and aerobic and resistance exercise training are effective in weight loss and in improving glucose tolerance (77). In fact, the results of that study (77) supported RT for weight loss. In addition, another meta-analysis showed that AT plus RT (CExT) improves body composition (fat mass, body weight, and BMI) (78).
Previous studies have found that exercise increases the secretion of lipolytic hormones (such as growth hormone) stimulating lipolysis in adipose tissues such as SAT (79, 80). In fact, growth hormone directly via hormone-sensitive lipase leads to stimulation of adipose tissue (81, 82). Although chronic AT can cause prolonged increases in 24-h growth hormone release, RT induces acute increases in growth hormone secretion (82). Studies also revealed that CExT produced more enhancement of growth hormone than did AT (83). In the same vein, this is consistent with our finding of a significant reduction in SAT following AT, RT, and CExT. This is also consistent with our observation that CExT is better than AT for reducing SAT.
The present study has some limitations that should be considered. Although most of the populations examined had diabetes or obesity, there were 2 studies that included women with high risk of breast cancer and others with known metabolic disease. Moreover, significant heterogeneity between studies could be due to differences in exercise prescription (intensity, frequency, session duration, intervention duration, and modality). Since participants usually are aware of whether they are training or not, it is obviously difficult to blind the people to the intervention. Therefore, the maximum possible quality score was 3 points (Jadad score of ≤3). The variables were not sufficient to compare RT and CExT. Other potential confounders included activity performed outside of the interventions, differences in kind of exercise (walking, running, stair stepping, and bicycling), and differences in dietary intake. Although this study has limitations, it provides useful information for the management and treatment of obesity. In this meta-analysis, strong and important evidence indicates the effectiveness of exercise therapy for reducing SAT. These findings suggest that not only AT but also other types of exercise are beneficial for obesity prevention. In addition, the combination of these types of exercise is able to produce better results than using them separately. In conclusion, CExT should be considered instead of AT or RT alone to produce greater reductions in SAT.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—HY and KD: designed the research; HY and RE: conducted the research; HY: analyzed data; HY and JA-S: wrote the paper; KD and JRS: revised and improved the grammar; HY and KD: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: AT, aerobic training; CExT, combined exercise training; HR, heart rate; HRR, heart rate reserve; MeSH, medical subject heading; RT, resistance training; SAT, subcutaneous abdominal adipose tissue; WMD, weighted mean difference; VAT, visceral adipose tissue; VO2max, maximum oxygen consumption; 1-RM, 1-repetition maximum.
Contributor Information
Habib Yarizadeh, Students' Scientific Center, Tehran University of Medical Sciences, Tehran, Iran; Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran.
Reza Eftekhar, Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran.
Javad Anjom-Shoae, Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran.
John R Speakman, School of Biological Sciences, University of Aberdeen, Aberdeen, United Kingdom; State Key Laboratory of Molecular Developmental Biology, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China.
Kurosh Djafarian, Clinical Nutrition Department, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran.
References
- 1. Chukwuonye II, Chuku A, John C, Ohagwu KA, Imoh ME, Isa SE, Ogah OS, Oviasu EJD. Prevalence of overweight and obesity in adult Nigerians—a systematic review. Diabetes Metab Syndr Obes. 2013;6:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Friedenreich CM, Orenstein M. Physical activity and cancer prevention: etiologic evidence and biological mechanisms.J Nutr. 2002;132(11):3456S–64S. [DOI] [PubMed] [Google Scholar]
- 3. Van Gaal LF, Mertens IL, Christophe EJN. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875. [DOI] [PubMed] [Google Scholar]
- 4. Cnop M, Landchild MJ, Vidal J, Havel PJ, Knowles NG, Carr DR, Wang F, Hull RL, Boyko EJ, Retzlaff B. The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations: distinct metabolic effects of two fat compartments. Diabetes. 2002;51(4):1005–15. [DOI] [PubMed] [Google Scholar]
- 5. Wajchenberg Br Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21(6):697–738. [DOI] [PubMed] [Google Scholar]
- 6. Freedland E Role of a critical visceral adipose tissue threshold (CVATT) in metabolic syndrome: implications for controlling dietary carbohydrates: a review. Nutr Metab (Lond). 2004;1(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haas MC, Bodner EV, Brown CJ, Bryan D, Buys DR, Keita AD, Flagg LA, Goss A, Gower B, Hovater Met al. Calorie restriction in overweight seniors: response of older adults to a dieting study: the CROSSROADS randomized controlled clinical trial. J Nutr Gerontol Geriatr. 2014;33(4):376–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ibrahim MR Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11(1):11–8. [DOI] [PubMed] [Google Scholar]
- 9. Boden G, Shulman GJE. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and β‐cell dysfunction. Eur J Clin Invest. 2002;32:14–23. [DOI] [PubMed] [Google Scholar]
- 10. Merlotti C, Ceriani V, Morabito A, Pontiroli AoO. Subcutaneous fat loss is greater than visceral fat loss with diet and exercise, weight-loss promoting drugs and bariatric surgery: a critical review and meta-analysis. Int J Obes. 2017;41(5):672. [DOI] [PubMed] [Google Scholar]
- 11. Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52. [DOI] [PubMed] [Google Scholar]
- 12. Pollack SC Liposuction of the abdomen: the basics. Int J Obes. 1999;17(4):823–34. [DOI] [PubMed] [Google Scholar]
- 13. Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156(1-2):20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buttar HS, Li T, Ravi NJE, Cardiology C. Prevention of cardiovascular diseases: role of exercise, dietary interventions, obesity and smoking cessation. Exp Clin Cardiol. 2005;10(4):229. [PMC free article] [PubMed] [Google Scholar]
- 15. Brochu M, Tchernof A, Turner AN, Ades PA, Poehlman E. Is there a threshold of visceral fat loss that improves the metabolic profile in obese postmenopausal women?. Metabolism. 2003;52(5):599–604. [DOI] [PubMed] [Google Scholar]
- 16. Lau DC, Douketis JD, Morrison KM, Hramiak IM, Sharma AM, Ur E. Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary]. Can Med Assoc J. 2007;176(8):S1–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, Mohammed BS. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med. 2004;350(25):2549–57. [DOI] [PubMed] [Google Scholar]
- 18. Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, Janssen IJA. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men: a randomized, controlled trial. Ann Intern Med. 2000;133(2):92–103. [DOI] [PubMed] [Google Scholar]
- 19. Carroll S, Miilunpalo MJ. What is the relationship between exercise and metabolic abnormalities?. Sports Med. 2004;34(6):371–418. [DOI] [PubMed] [Google Scholar]
- 20. Plaisance EP, Grandjean PM. Physical activity and high-sensitivity C-reactive protein. Sports Med. 2006;36(5):443–58. [DOI] [PubMed] [Google Scholar]
- 21. Dekker MJ, Lee S, Hudson R, Kilpatrick K, Graham TE, Ross R, Robinson LE. An exercise intervention without weight loss decreases circulating interleukin-6 in lean and obese men with and without type 2 diabetes mellitus. Metabolism. 2007;56(3):332–8. [DOI] [PubMed] [Google Scholar]
- 22. Asikainen T-M, Kukkonen-Harjula K, Miilunpalo SJSM. Exercise for health for early postmenopausal women. Sports Med. 2004;34(11):753–78. [DOI] [PubMed] [Google Scholar]
- 23. Cuff DJ, Meneilly GS, Martin A, Ignaszewski A, Tildesley HD, Frohlich JC. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care. 2003;26(11):2977–82. [DOI] [PubMed] [Google Scholar]
- 24. Fagard R Exercise is good for your blood pressure: effects of endurance training and resistance training. Clin Exp Pharmacol Physiol. 2006;33(9):853–6. [DOI] [PubMed] [Google Scholar]
- 25. Hansen D, Dendale P, Berger J, van Loon LJ, Meeusen R. The effects of exercise training on fat-mass loss in obese patients during energy intake restriction. Sports Med. 2007;37(1):31–46. [DOI] [PubMed] [Google Scholar]
- 26. Winett RA, Carpinelli RM. Potential health-related benefits of resistance training. Prev Med. 2001;33(5):503–13. [DOI] [PubMed] [Google Scholar]
- 27. Bacchi E, Negri C, Targher G, Faccioli N, Lanza M, Zoppini G, Zanolin E, Schena F, Bonora E, Moghetti PJH. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 randomized trial). Hepatology. 2013;58(4):1287–95. [DOI] [PubMed] [Google Scholar]
- 28. Poehlman ET, Dvorak RV, DeNino WF, Brochu M, Ades PA. Effects of resistance training and endurance training on insulin sensitivity in nonobese, young women: a controlled randomized trial. J Clin Endocrinol Metab. 2000;85(7):2463–8. [DOI] [PubMed] [Google Scholar]
- 29. Fisher G, Hyatt TC, Hunter GR, Oster RA, Desmond RA, Gower B. Effect of diet with and without exercise training on markers of inflammation and fat distribution in overweight women. Obesity. 2011;19(6):1131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang T, Zheng Y, Hruby A, Williamson DA, Bray GA, Shen Y, Sacks FM, Qi L. Dietary protein modifies the effect of the MC4R genotype on 2-year changes in appetite and food craving: the POUNDS Lost Trial. J Nutr. 2017;147(3):439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sigal RJ, Kenny GP, Boulé NG, Wells GA, Prud'homme D, Fortier M, Reid RD, Tulloch H, Coyle D, Phillips PJA. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(6):357–69. [DOI] [PubMed] [Google Scholar]
- 32. Binder EF, Yarasheski KE, Steger-May K, Sinacore DR, Brown M, Schechtman KB, Holloszy JO. Effects of progressive resistance training on body composition in frail older adults: results of a randomized, controlled trial. J Gerontol. 2005;60(11):1425–31. [DOI] [PubMed] [Google Scholar]
- 33. Boudou P, Sobngwi E, Mauvais-Jarvis F, Vexiau P, Gautier JoE. Absence of exercise-induced variations in adiponectin levels despite decreased abdominal adiposity and improved insulin sensitivity in type 2 diabetic men. Eur J Nutr. 2003;149(5):421–4. [DOI] [PubMed] [Google Scholar]
- 34. Brochu M, Malita MF, Messier V, Doucet E, Strychar I, Lavoie J-M, Prud'homme D, Rabasa-Lhoret R. Resistance training does not contribute to improving the metabolic profile after a 6-month weight loss program in overweight and obese postmenopausal women. J Clin Endocrinol Metab. 2009;94(9):3226–33. [DOI] [PubMed] [Google Scholar]
- 35. Brown JC, Kontos D, Schnall M, Wu S, Schmitz K. The dose-response effects of aerobic exercise on body composition and breast tissue among women at high risk for breast cancer: a randomized trial. Br J Nutr. 2016;9(7):581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carr DB, Utzschneider KM, Boyko EJ, Asberry PJ, Hull RL, Kodama K, Callahan HS, Matthys CC, Leonetti DL, Schwartz R. A reduced-fat diet and aerobic exercise in Japanese Americans with impaired glucose tolerance decreases intra-abdominal fat and improves insulin sensitivity but not β-cell function. Diabetes. 2005;54(2):340–7. [DOI] [PubMed] [Google Scholar]
- 37. Choi KM, Han KA, Ahn HJ, Hwang SY, Hong HC, Choi HY, Yang SJ, Yoo HJ, Baik SH, Choi DoCEet al. Effects of exercise on sRAGE levels and cardiometabolic risk factors in patients with type 2 diabetes: a randomized controlled trial. Int J Res. 2012;97(10):3751–8. [DOI] [PubMed] [Google Scholar]
- 38. Christiansen T, Paulsen SK, Bruun JM, Overgaard K, Ringgaard S, Pedersen SB, Positano V, Richelsen BJE. Comparable reduction of the visceral adipose tissue depot after a diet-induced weight loss with or without aerobic exercise in obese subjects: a 12-week randomized intervention study. J Clin Endocrinol Metab. 2009;160(5):759–67. [DOI] [PubMed] [Google Scholar]
- 39. DiPietro L, Seeman TE, Stachenfeld NS, Katz LD, Nadel EAGS. Moderate‐intensity aerobic training improves glucose tolerance in aging independent of abdominal adiposity. J Am Geriatr Soc. 1998;46(7):875–9. [DOI] [PubMed] [Google Scholar]
- 40. Donnelly JE, Hill JO, Jacobsen DJ, Potteiger J, Sullivan DK, Johnson SL, Heelan K, Hise M, Fennessey PV, Sonko BJA. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: the Midwest Exercise Trial. Arch Intern Med. 2003;163(11):1343–50. [DOI] [PubMed] [Google Scholar]
- 41. Drapeau S, Doucet É, Rabasa-Lhoret R, Brochu M, Prud'homme D, Imbeault P. Improvement in insulin sensitivity by weight loss does not affect hyperinsulinemia-mediated reduction in total and high molecular weight adiponectin: a MONET study. Appl Physiol Nutr Metab. 2011;36(2):191–200. [DOI] [PubMed] [Google Scholar]
- 42. Friedenreich CM, Woolcott C, McTiernan A, Terry T, Brant R, Ballard-Barbash R, Irwin M, Jones C, Boyd N, Yaffe MoO. Adiposity changes after a 1-year aerobic exercise intervention among postmenopausal women: a randomized controlled trial. Int J Res. 2011;35(3):427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. García-Unciti M, Izquierdo M, Idoate F, Gorostiaga E, Grijalba A, Ortega-Delgado F, Martínez-Labari C, Moreno-Navarrete JM, Forga L, Fernández-Real JoNet al. Weight-loss diet alone or combined with progressive resistance training induces changes in association between the cardiometabolic risk profile and abdominal fat depots. Ann Nutr Metab. 2012;61(4):296–304. [DOI] [PubMed] [Google Scholar]
- 44. Hays NP, Starling RD, Sullivan DH, Fluckey JD, Coker RH, Williams RH, Evans WJ. Effects of an ad libitum, high carbohydrate diet and aerobic exercise training on insulin action and muscle metabolism in older men and women. J Gerontol. 2006;61(3):299–304. [DOI] [PubMed] [Google Scholar]
- 45. Henríquez S, Monsalves-Alvarez M, Jimenez T, Barrera G, Hirsch S, de la Maza MP, Leiva L, Rodriguez JM, Silva C, Bunout DoSet al. Effects of two training modalities on body fat and insulin resistance in postmenopausal women. J Strength Cond Res. 2017;31(11):2955–64. [DOI] [PubMed] [Google Scholar]
- 46. Houghton D, Thoma C, Hallsworth K, Cassidy S, Hardy T, Burt AD, Tiniakos D, Hollingsworth KG, Taylor R, Day Cet al. Exercise reduces liver lipids and visceral adiposity in patients with nonalcoholic steatohepatitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2017;15(1):96–102.. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hunter GR, Brock DW, Byrne NM, Chandler‐Laney PC, Del Corral P, Gower B. Exercise training prevents regain of visceral fat for 1 year following weight loss. Obesity. 2010;18(4):690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ibáñez J, Izquierdo M, Martínez‐Labari C, Ortega F, Grijalba A, Forga L, Idoate F, García‐Unciti M, Fernández‐Real JM, Gorostiaga E. Resistance training improves cardiovascular risk factors in obese women despite a significative decrease in serum adiponectin levels. Obesity. 2010;18(3):535–41. [DOI] [PubMed] [Google Scholar]
- 49. Irving BA, Weltman J, Patrie JT, Davis CK, Brock DW, Swift D, Barrett EJ, Gaesser GA, Weltman A. Effects of exercise training intensity on nocturnal growth hormone secretion in obese adults with the metabolic syndrome. Med Sci Sports Exerc. 2009;94(6):1979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Irwin ML, Yasui Y, Ulrich CM, Bowen D, Rudolph RE, Schwartz RS, Yukawa M, Aiello E, Potter JD, McTiernan AJJ. Effect of exercise on total and intra-abdominal body fat in postmenopausal women: a randomized controlled trial. JAMA. 2003;289(3):323–30. [DOI] [PubMed] [Google Scholar]
- 51. Janssen I, Fortier A, Hudson R, Ross RJ. Effects of an energy-restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care. 2002;25(3):431–8. [DOI] [PubMed] [Google Scholar]
- 52. Janssen I, Ross RJI. Effects of sex on the change in visceral, subcutaneous adipose tissue and skeletal muscle in response to weight loss. Int J Obes. 1999;23(10):1035. [DOI] [PubMed] [Google Scholar]
- 53. Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, George JJH. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50(4):1105–12. [DOI] [PubMed] [Google Scholar]
- 54. Koo B, Han K, Ahn H, Jung J, Kim H, Min K. The effects of total energy expenditure from all levels of physical activity vs. physical activity energy expenditure from moderate‐to‐vigorous activity on visceral fat and insulin sensitivity in obese type 2 diabetic women. Diabet Med. 2010;27(9):1088–92. [DOI] [PubMed] [Google Scholar]
- 55. Lee M-G, Park K-S, Kim D-U, Choi S-M, Kim H-J. Effects of high-intensity exercise training on body composition, abdominal fat loss, and cardiorespiratory fitness in middle-aged Korean females. Appl Physiol Nutr Metab. 2012;37(6):1019–27. [DOI] [PubMed] [Google Scholar]
- 56. McTiernan A, Sorensen B, Irwin ML, Morgan A, Yasui Y, Rudolph RE, Surawicz C, Lampe JW, Lampe PD, Ayub KJO. Exercise effect on weight and body fat in men and women. Obesity. 2007;15(6):1496–512. [DOI] [PubMed] [Google Scholar]
- 57. Moghadasi M, Mohebbi H, Rahmani-Nia F, Hassan-Nia S, Noroozi H, Pirooznia NJE. High-intensity endurance training improves adiponectin mRNA and plasma concentrations. Obesity. 2012;112(4):1207–14. [DOI] [PubMed] [Google Scholar]
- 58. Mourier A, Gautier J-F, De Kerviler E, Bigard AX, Villette J-M, Garnier J-P, Duvallet A, Guezennec CY, Cathelineau G. . Mobilization of visceral adipose tissue related to the improvement in insulin sensitivity in response to physical training in NIDDM: effects of branched-chain amino acid supplements. Diabetes Care. 1997;20(3):385–91. [DOI] [PubMed] [Google Scholar]
- 59. Ryan AS, Ortmeyer HK, Sorkin J. Exercise with calorie restriction improves insulin sensitivity and glycogen synthase activity in obese postmenopausal women with impaired glucose tolerance. Am J Physiol Cell Physiol. 2011;302(1):E145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schmitz KH, Hannan PJ, Stovitz SD, Bryan CJ, Warren M, Jensen M. Strength training and adiposity in premenopausal women: Strong, Healthy, and Empowered Study. Am J Clin Nutr. 2007;86(3):566–72. [DOI] [PubMed] [Google Scholar]
- 61. Shojaee-Moradie F, Baynes K, Pentecost C, Bell J, Thomas E, Jackson N, Stolinski M, Whyte M, Lovell D, Bowes SJD. Exercise training reduces fatty acid availability and improves the insulin sensitivity of glucose metabolism. Diabetologia. 2007;50(2):404–13. [DOI] [PubMed] [Google Scholar]
- 62. Slentz CA, Bateman LA, Willis LH, Shields AT, Tanner CJ, Piner LW, Hawk VH, Muehlbauer MJ, Samsa GP, Nelson RoP-Eet al. Effects of aerobic vs. resistance training on visceral and liver fat stores, liver enzymes, and insulin resistance by HOMA in overweight adults from STRRIDE AT/RT. Am J Physiol Endocrinol Metab. 2011;301(5):E1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Slentz CA, Aiken LB, Houmard JA, Bales CW, Johnson JL, Tanner CJ, Duscha BD, Kraus WE. Inactivity, exercise, and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol. 2005;99(4):1613–8. [DOI] [PubMed] [Google Scholar]
- 64. Sun X, Cao Z-B, Tanisawa K, Taniguchi H, Kubo T, Higuchi MJE. Effects of chronic endurance exercise training on serum 25 (OH) D concentrations in elderly Japanese men. Endocrine. 2018;59(2):330–7. [DOI] [PubMed] [Google Scholar]
- 65. Zhang H, Tong TK, Qiu W, Zhang X, Zhou S, Liu Y, He YJJ. Comparable effects of high-intensity interval training and prolonged continuous exercise training on abdominal visceral fat reduction in obese young women. J Diabetes Res. 2017;2017:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Haufe S, Engeli S, Budziarek P, Utz W, Schulz-Menger J, Hermsdorf M, Wiesner S, Otto C, Fuhrmann JC, Luft FCet al. Determinants of exercise-induced fat oxidation in obese women and men. Horm Metab Res. 2010;42(3):215–21. [DOI] [PubMed] [Google Scholar]
- 67. Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1. 0. The Cochrane Collaboration West Sussex, England: John Wiley & Sons Ltd; 2011. [Google Scholar]
- 68. Hozo SP, Djulbegovic B, Hozo IJB. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mojtahedi MC, Thorpe MP, Karampinos DC, Johnson CL, Layman DK, Georgiadis JG, Evans EM. The effects of a higher protein intake during energy restriction on changes in body composition and physical function in older women. J Gerontol A Biol Sci. 2011;66(11):1218–25. [DOI] [PubMed] [Google Scholar]
- 70. Cho J-K, Lee S-H, Lee J-Y, Kang H-SJI. Randomized controlled trial of training intensity in adiposity. Int J Sports Med. 2011;32(06):468–75. [DOI] [PubMed] [Google Scholar]
- 71. Fisher G, Hunter GR, Gower BoAP. Physiology and pathophysiology of physical inactivity: aerobic exercise training conserves insulin sensitivity for 1 yr following weight loss in overweight women. J Appl Physiol. 2012;112(4):688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Irving BA, Davis CK, Brock DW, Weltman JY, Swift D, Barrett EJ, Gaesser GA, Weltman A. Effect of exercise training intensity on abdominal visceral fat and body composition. J Clin Endocrinol Metab. 2008;40(11):1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kim E, Park S, Kwon Y. The effects of combined exercise on functional fitness and risk factors of metabolic syndrome in the older women. Jpn J Phys Fitness Sports Med. 2008;57(2):207–16. [Google Scholar]
- 74. Larsson B, Svärdsudd K, Welin L, Wilhelmsen L, Björntorp P, Tibblin G. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. BMJ. 1984;288(6428):1401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ismail I, Keating S, Baker M, Johnson NA. A systematic review and meta‐analysis of the effect of aerobic vs. resistance exercise training on visceral fat. Obes Rev. 2012;13(1):68–91. [DOI] [PubMed] [Google Scholar]
- 76. González-Ruiz K, Ramirez-Velez R, Correa-Bautista JE, Peterson MD, Garcia-Hermoso A. The effects of exercise on abdominal fat and liver enzymes in pediatric obesity: a systematic review and meta-analysis. Child Obes. 2017;13(4):272–82. [DOI] [PubMed] [Google Scholar]
- 77. Aguiar EJ, Morgan PJ, Collins CE, Plotnikoff RC, Callister R. Efficacy of interventions that include diet, aerobic and resistance training components for type 2 diabetes prevention: a systematic review with meta-analysis. Int J Behav Nutr Phys Act. 2014;11(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. García-Hermoso A, Sánchez-López M, Martínez-Vizcaíno VJP. Effects of aerobic plus resistance exercise on body composition related variables in pediatric obesity: a systematic review and meta-analysis of randomized controlled trials. J Hum Kinet. 2015;27(4):431–40. [DOI] [PubMed] [Google Scholar]
- 79. Bredella MA, Karastergiou K, Bos SA, Gerweck AV, Torriani M, Fried SK, Miller K. GH administration decreases subcutaneous abdominal adipocyte size in men with abdominal obesity. Growth Horm IGF Res. 2017;35:17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Birzniece V, Nelson AE, Ho KKY. Growth hormone and physical performance. Trends Endocrinol Metab. 2011;22(5):171–8. [DOI] [PubMed] [Google Scholar]
- 81. Franco C, Brandberg J, Lönn L, Andersson Br, Bengtsson B-A, Johannsson G. Growth hormone treatment reduces abdominal visceral fat in postmenopausal women with abdominal obesity: a 12-month placebo-controlled trial. J Clin Endocrinol Metab. 2005;90(3):1466–74. [DOI] [PubMed] [Google Scholar]
- 82. Wideman L, Weltman JY, Hartman ML, Veldhuis JD, Weltman AJ. Growth hormone release during acute and chronic aerobic and resistance exercise. Sports Med. 2002;32(15):987–1004. [DOI] [PubMed] [Google Scholar]
- 83. Seo D-I, Jun T-W, Park K-S, Chang H, So W-Y, Song WJI. 12 Weeks of combined exercise is better than aerobic exercise for increasing growth hormone in middle-aged women. J Hum Kinet. 2010;20(1):21–6. [DOI] [PubMed] [Google Scholar]