See the article by Miller et al. pp. 53–62.
Mutations in isocitrate dehydrogenase 1 (IDH1) and, to a lesser extent, IDH2 are hallmarks of lower-grade gliomas. IDH enzymes normally catalyze the decarboxylation of isocitrate to alpha-ketoglutarate (α-KG), but the hotspot recurrent mutations in IDH confer a neomorphic enzymatic activity that leads to overproduction of 2-hydroxyglutarate (2-HG) via NADPH-dependent reduction of α-KG.1 A distinctive outcome of 2-HG accumulation is the genome-wide DNA hypermethylation observed in IDH mutant tumors.2 In addition to its role in promoting epigenetic alterations, mutant IDH1 affects multiple metabolic pathways such as the citric acid cycle, glucose, glutamine, and lipid metabolism.3 To gain insights into these metabolic alterations and to determine whether they create metabolic vulnerabilities in IDH mutant tumors, further investigations are warranted. In this issue of Neuro-Oncology, Miller et al shed light on a metabolic Achilles’ heel in IDH mutant gliomas and demonstrate that sirtuin 1 (SIRT1) activation leads to augmented NAD+ depletion, reduced growth, and increased cytotoxicity when combined with nicotinamide phosphoribosyltransferase inhibitors (NAMPTi) in IDH mutant cells (Fig. 1).4
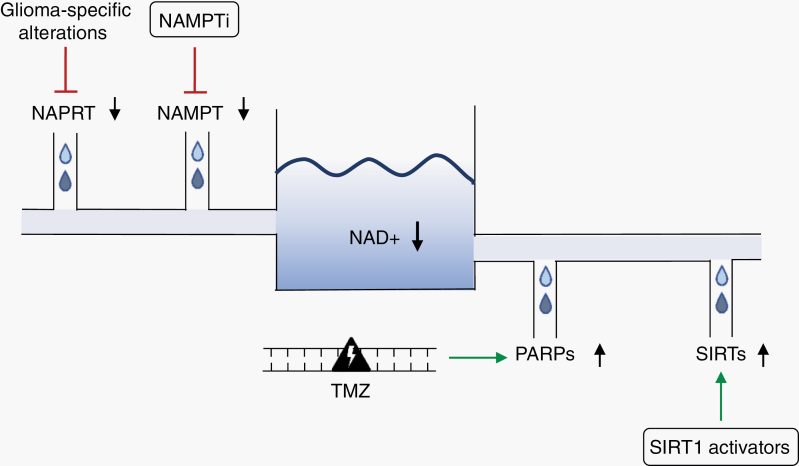
Fig. 1.
Overview of strategies to decrease NAD+ levels in gliomas. NAD+ levels can be reduced by inhibition of the NAD+ biosynthetic enzyme NAPRT or the NAD+ salvage enzyme NAMPT, and by activation of NAD+ consuming enzymes such as PARPs (eg, after TMZ treatment) and SIRTs (eg, by SIRT1 activators). Abbreviations: NAD+, nicotinamide adenine dinucleotide; NAPRT, nicotinic acid phosphoribosyltransferase; NAMPT, nicotinamide phosphoribosyltransferase; NAMPTi, nicotinamide phosphoribosyltransferase inhibitor; TMZ, temozolomide; PARP, poly (ADP-ribose) polymerase (PARP); SIRT, sirtuin.
The study follows on previous findings by the authors, which described low basal levels of the essential coenzyme NAD+ in IDH mutant gliomas.5 Using an unbiased metabolic screen and small molecule inhibitors targeting the salvage NAD+ synthesis enzyme NAMPT, the authors discovered a profound susceptibility of IDH1 mutant cancers to NAD+ depletion, which they attributed to 2-HG-mediated downregulation of the NAD+ biosynthetic enzyme nicotinic acid phosphoribosyltransferase (NAPRT).5 NAD+, a critical molecule for cell signaling and survival, is a substrate for several key enzymes including, poly(ADP-ribose) polymerases (PARPs) and SIRTs. In a subsequent study, the authors showed that temozolomide treatment led to PARP activation and NAD+ consumption, critically depleting NAD+ levels and creating metabolic stress that potentiated NAMPT inhibition.6 Although NAMPTi show promising efficacy in preclinical models of IDH1 mutant tumors, their toxic profiles have dampened drug discovery efforts. To overcome current limitations associated with NAMPTi, in the current issue, Miller and colleagues explore alternative approaches to exploit the NAD+ sensitivity inherent to mutant IDH gliomas. Consequently, they identified SIRT1 activation as a relatively non-toxic means to modulate cellular NAD+ pools to target IDH mutant tumors. SIRTS are a family of NAD+ dependent protein deacetylases (SIRT1–7) that play a major regulatory role in almost all cellular functions, and enhanced SIRT activity leads to accelerated NAD+ consumption.
Using IDH mutant cells, the authors show that a small molecule SIRT inhibitor, Ex-527, or SIRT1 deletion using CRISPR/Cas9 gene-editing decreased sensitivity to NAMPT inhibition. These results suggest that SIRT1-dependent NAD+ consumption synergizes with NAMPTi to reduce NAD+ levels. Further supporting these results, tetracycline-inducible SIRT1 overexpression increased sensitivity to NAMPT inhibition, and combination of SIRT1 overexpression and NAMPTi decreased cell viability. Collectively, these results indicate that sensitivity to NAMPTi is influenced by the activity of SIRT1 in IDH mutant cancer cells.
Pharmacologically, SIRT1 activation can be facilitated by small-molecule SIRT1-activating compounds (STACs). To this end, the authors asked whether STACs may have preclinical efficacy in IDH mutant gliomas. Consistent with their findings using genetic systems, treatment of IDH mutant glioma cells with STACs (i) decreased proliferation and viability, and (ii) increased sensitivity to NAMPTi. Importantly, low doses of the NAMPTi FK866 led to increased sensitivity when combined with STACs, a promising finding given the toxicities associated with NAMPT inhibition. Taken together, SIRT1 activation by a STAC leads to increased NAD+ consumption and resulting cytotoxicity in IDH mutant cells. STACs, which have been developed and primarily investigated in the context of longevity studies, were shown to be well tolerated and relatively nontoxic in clinical trials. Therefore, targeting IDH mutant gliomas with STACs either as a stand-alone treatment or in combination with a low-dose NAMPTi is a tantalizing possibility for IDH mutant gliomas, and potentially other tumors that harbor IDH mutations.
Miller et al observed that the effect of SIRT activation was less prominent in IDH wildtype gliomas than in IDH mutant gliomas. However, it remains to be investigated systematically whether also IDH wildtype gliomas could benefit from the combination of NAMPTi with STACs. Vulnerability to NAD+ depletion seems to arise from genetic alterations and epigenomic remodeling and is dependent on the NAD+ biosynthetic pathways used by the cell of origin.7 Generally, the brain shows very low expression of NAPRT,7 which is in line with the lack of NAPRT expression in IDH mutant gliomas5 as well as in IDH wildtype glioblastomas.8 Moreover, NAD+ depletion leads to metabolic catastrophe in pediatric high grade diffuse midline gliomas including diffuse intrinsic pontine ‘gliomas’ (DIPG), which do not harbor IDH mutations. Specifically, in DIPG, mutation of PPM1D (protein phosphatase Mg2+/Mn2+ dependent 1D) leads to epigenetic silencing of NAPRT, thus sensitizing the tumors to NAMPTi.9 Moreover, NAD+ depletion also contributes to the cytotoxic effects of the newly identified combination treatment for DIPG,10 panobinostat and marizomib, further underscoring the importance of NAD+ in these tumors. Further studies will shed light on the types of brain tumors that could benefit from a combination of NAD+ depleting therapies including but not limited to SIRT activation and NAMPT inhibition. In the future, the rational design of NAD+ metabolism targeting strategies may lead to novel combinatorial pharmacologic approaches to modulate NAD+ metabolism in gliomas.
Although challenges to successful translation remain, the in vitro data described by Miller et al is highly encouraging. Further studies using preclinical mouse models of IDH mutant gliomas are required to evaluate the in vivo efficacy of STACs on tumor growth and to design optimal combinatorial strategies. In addition, studies are needed to determine whether metabolic adaptation or resistance to NAD+ depletion strategies occur, for example through activation of alternative sources for NAD+ biosynthesis. Overall, the compelling preclinical evidence provided by Miller et al should motivate efforts to translate these promising therapeutic strategies into clinical trials for the treatment of IDH mutant gliomas and possibly other brain tumors.
Acknowledgment
The text is the sole product of the authors. No third party had input or gave support to its writing.
Funding
C.A.O and S.T. acknowledge funding by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project-ID 404521405, SFB 1389 - UNITE Glioblastoma.
References
- 1. Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Noushmehr H, Weisenberger DJ, Diefes K, et al. ; Cancer Genome Atlas Research Network Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17(5):510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parker SJ, Metallo CM. Metabolic consequences of oncogenic IDH mutations. Pharmacol Ther. 2015;152:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller JJ, Fink A, Banagis JA, et al. Sirtuin activation targets IDH-mutant tumors [published online ahead of print July 26, 2020]. Neuro Oncol. 2021;23(1):53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tateishi K, Wakimoto H, Iafrate AJ, et al. Extreme vulnerability of IDH1 mutant cancers to NAD+ Depletion. Cancer Cell. 2015;28(6):773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tateishi K, Higuchi F, Miller JJ, et al. The alkylating chemotherapeutic temozolomide induces metabolic stress in IDH1-mutant cancers and potentiates NAD+ depletion-mediated cytotoxicity. Cancer Res. 2017;77(15):4102–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chowdhry S, Zanca C, Rajkumar U, et al. NAD metabolic dependency in cancer is shaped by gene amplification and enhancer remodelling. Nature. 2019;569(7757):570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watson M, Roulston A, Bélec L, et al. The small molecule GMX1778 is a potent inhibitor of NAD+ biosynthesis: strategy for enhanced therapy in nicotinic acid phosphoribosyltransferase 1-deficient tumors. Mol Cell Biol. 2009;29(21):5872–5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fons NR, Sundaram RK, Breuer GA, et al. PPM1D mutations silence NAPRT gene expression and confer NAMPT inhibitor sensitivity in glioma. Nat Commun. 2019;10(1):3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin GL, Wilson KM, Ceribelli M, et al. Therapeutic strategies for diffuse midline glioma from high-throughput combination drug screening. Sci Transl Med. 2019;11(519):eaaw0064. [DOI] [PMC free article] [PubMed] [Google Scholar]