Abstract
Two experiments were conducted to evaluate dose–response and supplemental effects of whey permeate on growth performance and intestinal health of nursery pigs. In experiment (exp.) 1, 1,080 pigs weaned at 6.24 kg body weight (BW) were allotted to five treatments (eight pens/treatment) with increasing levels of whey permeate in three phases (from 10% to 30%, 3% to 23%, and 0% to 9% for phase 1, 2, and 3, respectively) fed until 11 kg BW and then fed a common phase 4 diet (0% whey permeate) until 25 kg BW in a 48-d feeding trial. Feed intake and BW were measured at the end of each phase. In exp. 2, 1,200 nursery pigs at 7.50 kg BW were allotted to six treatments (10 pens/treatment) with increasing levels of whey permeate from 0% to 18.75% fed until 11 kg BW. Feed intake and BW were measured during 11 d. Six pigs per treatment (1 per pens) were euthanized to collect the jejunum to evaluate tumor necrosis factor-alpha, interleukin-8 (IL-8), transforming growth factor-beta 1, mucin 2, histomorphology, digestive enzyme activity, crypt cell proliferation rate, and jejunal mucosa-associated microbiota. Data were analyzed using contrasts in the MIXED procedure and a broken-line analysis using the NLIN procedure of SAS. In exp. 1, increasing whey permeate had a quadratic effect (P < 0.05) on feed efficiency (G:F; maximum: 1.35 at 18.3%) in phase 1. Increasing whey permeate linearly increased (P < 0.05) average daily gain (ADG; 292 to 327 g/d) and G:F (0.96 to 1.04) of pigs in phase 2. In exp. 2, increasing whey permeate linearly increased (P < 0.05) ADG (349 to 414 g/d) and G:F (0.78 to 0.85) and linearly increased (P < 0.05) crypt cell proliferation rate (27.8% to 37.0%). The breakpoint from a broken-line analysis was obtained at 13.6% whey permeate for maximal G:F. Increasing whey permeate tended to change IL-8 (quadratic, P = 0.052; maximum: 223 pg/mg at 10.9%), to decrease Firmicutes:Bacteroidetes (P = 0.073, 1.59 to 1.13), to increase (P = 0.089) Bifidobacteriaceae (0.73% to 1.11%), and to decrease Enterobacteriaceae (P = 0.091, 1.04% to 0.52%) and Streptococcaceae (P = 0.094, 1.50% to 0.71%) in the jejunal mucosa. In conclusion, dietary inclusion of whey permeate increased the growth of nursery pigs from 7 to 11 kg BW. Pigs grew most efficiently with 13.6% whey permeate. Improvement in growth performance is partly attributed to stimulating intestinal immune response and enterocyte proliferation with positive changes in jejunal mucosa-associated microbiota in nursery pigs.
Keywords: growth performance, intestinal health, microbiome, nursery pig, whey permeate
Introduction
Proper nutritional management for newly weaned pigs is vital for successful swine production (Kim, 2017). In particular, animal proteins and milk coproducts have been used in nursery diets to improve the growth performance and health of young pigs (Tokach et al., 1989; Mahan, 1993; Kim et al., 2006). Milk coproducts have been widely used as sources of lactose and milk proteins in nursery diets globally. Previous studies have shown that the nursery feeds with milk coproducts were beneficial for the growth of nursery pigs (Mahan et al., 2004; Cromwell et al., 2008; Naranjo et al., 2010). However, economic concerns could limit the use of milk coproducts in nursery feeds.
Whey permeate is a coproduct obtained after removing whey proteins and other solids through physical filtration of liquid whey (Menchik et al., 2019). Whey permeate, therefore, contains a higher proportion of lactose than other milk coproducts (NRC, 2012). Previous studies also showed that various types of milk oligosaccharides are present in whey permeate (Barile et al., 2009; Dallas et al., 2014), which possess functional properties maintaining the intestinal health of neonates (Tran et al., 2012; Pacheco et al., 2015; Li et al., 2019).
Lactose is more digestible and palatable than starch from cereal grains for newly weaned pigs (Mahan, 1992). Newborn piglets rely on milk from sows during lactation, and thus their digestive tracts are adapted to lactose digestion upon weaning. The primary function of lactose is to produce energy as a carbon source through cellular respiration (Cromwell et al., 2008). Previous studies showed that supplementation of lactose in nursery feeds positively improved the growth performance of nursery pigs, but the growth responses to lactose were gradually disappeared with maturity of pigs (Mahan et al., 2004; Cromwell et al., 2008; Gahan et al., 2009).
Milk oligosaccharides have been well documented for the beneficial prebiotic effects (Bering, 2018; Ramani et al., 2018) preventing intestinal dysfunction and aiding in the development of the brain of infants (Bode, 2012; Moukarzel and Bode, 2017). One possible mechanism of milk oligosaccharides for these effects could be their binding to pathogenic bacteria in the small intestine (Morrow et al., 2005) inhibiting possible attachment of pathogenic bacteria and their toxins to enterocytes (El-Hawiet et al., 2015; Nguyen et al., 2016). Another possible mode of action could be an increase of Bifidobacterium and Lactobacilli in the small intestine producing short-chain fatty acids (Garrido et al., 2013; Yu et al., 2013) as energy sources for enterocytes and to reduce the lumen pH (Fukuda et al., 2011; Kim, 2018).
Based on previous findings, it is hypothesized that whey permeate as a source of lactose and milk oligosaccharides may enhance the growth performance of nursery pigs by improving intestinal health, and this enhancement would be related to dose–response of whey permeate. To test the hypothesis, the objective of this study was to evaluate dose–response and supplemental effects of whey permeate on the intestinal health and growth performance of nursery pigs.
Materials and Methods
The procedure of this study was reviewed and approved by North Carolina State University Animal Care and Use Committee (Raleigh, NC). Two experiments were conducted at commercial pig facilities. Whey permeate was obtained from Agri-Mark, Inc. (Middlebury, Vermont, USA).
Experimental design, animals, and diets
In experiment (exp.) 1, 1,080 pigs (6.2 ± 0.3 kg body weight [BW]) were weaned at 21 d of age and allotted to five dietary treatments in a randomized complete block design using the location of pens in the barn as a blocking criterion. There were eight pens per treatment with 27 pigs per pen. Experimental diets (Table 1) were mixed at Pipestone Grow Finish (Pipestone, MN, USA). Whey permeate was supplemented by adjusted ratios with corn, choice white grease, and crystalline amino acids to meet the nutrient requirements suggested by NRC (2012). Feeding programs with varied whey permeate supplementations were A = 10%, 3%, 0%; B = 15%, 8%, 1%; C = 20%, 13%, 4%; D = 25%, 18%, 6%; and E = 30%, 23%, 9% whey permeate for phase 1 (day 0 to 7), 2 (day 7 to 14), 3 (day 14 to 20), respectively, and a common diet (0%) for phase 4 (day 20 to 48). Phase 1 and 2 diets were formulated in a pellet form and phase 3 and 4 diets were in a mash form. Feed disappearance and BW were measured at the end of each phase (phase 1 at day 7, phase 2 at day 14, phase 3 at day 20, and phase 4 at day 48) after weaning to calculate average daily gain (ADG), average daily feed intake (ADFI), and feed efficiency (G:F). Mortality and morbidity were monitored and recorded daily. Morbidity was evaluated as “the number of pigs under unnormal conditions such as disability, poor health, or death indicated by weight loss during the overall period” divided by “the number of pigs initially assigned to each pens” as previously described by Guo et al. (2015). Pigs were provided with feed and water ad libitum.
Table 1.
Composition of experimental diets for exp. 11
| Whey permeate, % | ||||||
|---|---|---|---|---|---|---|
| Phase 12 | Phase 22 | Phase 32 | ||||
| Item | 10.00 | 30.00 | 2.50 | 22.50 | 0.00 | 8.75 |
| Ingredient, % | ||||||
| Corn, yellow | 29.58 | 10.50 | 43.20 | 22.00 | 49.97 | 40.70 |
| Soybean meal, 48% CP | 15.00 | 15.00 | 21.00 | 21.00 | 29.00 | 29.00 |
| Whey permeate | 10.00 | 30.00 | 2.50 | 22.50 | — | 8.75 |
| Corn DDGS3 | 5.00 | 5.00 | 7.50 | 7.50 | 10.00 | 10.00 |
| Specialty protein4 | 7.50 | 7.50 | 5.95 | 8.42 | 2.44 | 3.52 |
| Choice white grease | 3.59 | 3.11 | 3.17 | 2.59 | 3.24 | 2.99 |
| Blood plasma | 3.00 | 3.00 | — | — | — | — |
| l-Lys HCl | 0.43 | 0.46 | 0.57 | 0.51 | 0.49 | 0.46 |
| l-Trp | 0.02 | 0.03 | 0.04 | 0.04 | 0.03 | 0.03 |
| dl-Met | 0.23 | 0.29 | 0.25 | 0.28 | 0.18 | 0.19 |
| l-Thr | 0.18 | 0.21 | 0.23 | 0.23 | 0.17 | 0.17 |
| l-Ile | 0.07 | 0.10 | 0.07 | 0.07 | 0.03 | 0.03 |
| l-Val | — | 0.03 | 0.06 | 0.06 | 0.02 | 0.02 |
| Others5 | 25.40 | 24.77 | 15.46 | 14.80 | 4.43 | 4.14 |
| Calculated composition | ||||||
| ME, kcal/kg | 3,375 | 3,375 | 3,285 | 3,285 | 3,233 | 3,233 |
| Standardized ileal digestible Lys, % | 1.40 | 1.40 | 1.40 | 1.40 | 1.35 | 1.35 |
| Lactose, % | 8.00 | 24.00 | 2.00 | 18.00 | 0.00 | 7.00 |
| Milk oligosaccharides6, % | 0.013 | 0.039 | 0.003 | 0.029 | 0.000 | 0.011 |
1Detailed nutrient composition was proprietary information of Pipestone (MN, USA). Only the composition of feeds relevant to the study is provided in this table. All feeds provided nutrients meeting the requirements suggested by NRC (2012).
2Phase 1 was from day 0 to 7, phase 2 was from day 7 to 14, and phase 3 was from day 14 to 20 of postweaning.
3Distillers dried grains with solubles.
4The source of specialty protein was proprietary information of Pipestone.
5Others include oats, salt, Ca, P, Zn, vitamins, and trace minerals. Oats were fixed across treatments by phase, and the mineral and vitamin sources are used to balance the nutrient composition of the diets.
6Data based on Mehra et al. (2014) and Altmann et al. (2015).
In exp. 2, 1,200 newly weaned pigs at 21 d of age fed a common diet with 20.9% crude protein (CP) and 13.1% lactose until 7.5 kg BW. Pigs at 7.5 ± 1.4 kg BW were, then, allotted to six dietary treatments in a randomized complete block design using the initial BW (heavy, medium, and light) as a blocking criterion. Dietary treatments were increasing levels of whey permeate (0%, 3.75%, 7.50%, 11.25%, 15.00%, and 18.75%) mainly replacing corn with minor adjustment with poultry fat, and supplemental amino acids to maintain the same essential nutrients meeting the nutrient requirements suggested by NRC (2012). There were 10 pens per treatment with 20 pigs per pen. Experimental diets (Table 2) were formulated at the North Carolina State University Feed Education Unit (Raleigh, NC, USA). Experimental diets were fed for 11 d. All diets were formulated in pellet form. Feed disappearance and BW were measured at the beginning and end of experiment to calculate ADG, ADFI, and G:F. Fecal scores of each pen were recorded based on a 1 to 5 scale (1: watery and 5: firm) by visual observation of fresh feces from day 2 at 2-d intervals. Pigs were provided with feed and water ad libitum.
Table 2.
Composition of experimental diets for exp. 2
| Whey permeate, % | ||||||
|---|---|---|---|---|---|---|
| Item | 0 | 3.75 | 7.50 | 11.25 | 15.00 | 18.75 |
| Ingredient, % | ||||||
| Corn, yellow | 60.59 | 56.89 | 53.14 | 49.39 | 45.63 | 41.84 |
| Soybean meal, 48% CP | 23.00 | 23.00 | 23.00 | 23.00 | 23.00 | 23.00 |
| Whey permeate | — | 3.75 | 7.50 | 11.25 | 15.00 | 18.75 |
| Blood plasma | 3.80 | 3.80 | 3.80 | 3.80 | 3.80 | 3.80 |
| Poultry meal | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 |
| Fish meal | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Poultry fat | 1.20 | 1.30 | 1.40 | 1.50 | 1.60 | 1.70 |
| Dicalcium phosphate | 0.60 | 0.43 | 0.31 | 0.19 | 0.08 | 0.00 |
| Limestone | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 |
| l-Lys HCl | 0.24 | 0.25 | 0.25 | 0.26 | 0.27 | 0.27 |
| dl-Met | 0.09 | 0.10 | 0.11 | 0.12 | 0.13 | 0.14 |
| l-Thr | 0.03 | 0.03 | 0.04 | 0.04 | 0.04 | 0.05 |
| Salt | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 |
| Vitamin permix1 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| Trace mineral permix2 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Zinc oxide | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Calculated composition | ||||||
| DM, % | 89.95 | 90.04 | 90.18 | 90.32 | 90.47 | 90.62 |
| ME, kcal/kg | 3,411 | 3,413 | 3,413 | 3,413 | 3,414 | 3,413 |
| Lactose, % | 0.00 | 3.00 | 6.00 | 9.00 | 12.00 | 15.00 |
| Milk oligosaccharides3, % | 0.000 | 0.005 | 0.010 | 0.015 | 0.020 | 0.024 |
| SID4 Lys, % | 1.35 | 1.35 | 1.35 | 1.35 | 1.35 | 1.35 |
| SID Cys + Met, % | 0.74 | 0.74 | 0.74 | 0.74 | 0.74 | 0.74 |
| SID Trp, % | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| SID Thr, % | 0.79 | 0.79 | 0.79 | 0.79 | 0.79 | 0.79 |
| Ca, % | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.81 |
| STTD P5, % | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Analyzed composition | ||||||
| DM, % | 90.68 | 91.22 | 91.87 | 91.89 | 92.25 | 93.27 |
| CP, % | 23.61 | 24.02 | 24.15 | 24.15 | 23.88 | 23.15 |
| Lactose, %6 | 0.14 | 2.10 | 4.68 | 7.92 | 9.94 | 12.84 |
1The vitamin premix provided per kilogram of complete diet: 6,614 IU of vitamin A as vitamin A acetate, 992 IU of vitamin D3, 19.8 IU of vitamin E, 2.64 mg of vitamin K as menadione sodium bisulfate, 0.03 mg of vitamin B12, 4.63 mg of riboflavin, 18.52 mg of d-pantothenic acid as calcium pantothenate, 24.96 mg of niacin, and 0.07 mg of biotin.
2The trace mineral premix provided per kilogram of complete diet: 33 mg of Mn as manganous oxide, 110 mg of Fe as ferrous sulfate, 110 mg of Zn as zinc sulfate, 16.5 mg of Cu as copper sulfate, 0.30 mg of I as ethylenediamine dihydroiodide, and 0.30 mg of Se as sodium selenite.
3Data based on Mehra et al. (2014) and Altmann et al. (2015).
4Standardized ileal digestible.
5Standardized total tract digestible phosphorus.
6Lactose contents were analyzed by the University of Missouri AESCL Analytical Services (Columbia, MO).
In both exps., whey permeate was supplemented by altering ratios among corn, crystalline amino acids, dicalcium phosphate, and poultry fat in each diet to meet the nutrient requirements suggested by NRC (2012). The experimental diets were sampled and sent to the North Carolina Department of Agriculture (Raleigh, NC, USA) to analyze dry matter (DM) and CP in the diets. Whey permeate and experimental diets were also sent to the University of Missouri Agriculture Experiment Station Chemical Laboratory (AESCL) to analyze the lactose content. Whey permeate was also sent to the University of Georgia Complex Carbohydrate Research Center (CCRC) to analyze the milk oligosaccharides content by high-performance anion-exchange chromatography-pulsed amperometric detection.
Sample collection
At the end of feeding in exp. 2, 36 pigs representing the median BW of each pen (36 pens total, 6 pens per treatment) were selected and euthanized by the penetration of a captive bolt followed by exsanguination. After euthanasia, jejunal mucosa and jejunal tissues were collected. Jejunal tissues (15 cm) were obtained and flushed with saline solution to remove digesta. The first 10 cm was used to collect jejunal mucosa by scraping of the mucosal layer in the jejunum using a glass microscope slide, and the remaining 5 cm was stored in 10% formalin buffer for histological evaluation. Jejunal mucosa samples were used to measure tumor necrosis factor-alpha (TNF-α) and interleukin-8 (IL-8), transforming growth factor beta-1 (TGF-β1), and mucin-2 protein (MUC2) as indicators of immune response.
Histology and immunohistochemistry
Jejunal tissue was fixed in 10% formalin buffer for 3 wk, then rinsed three times with deionized water, stored in 70% ethanol buffer, and sent to Histology Laboratory of North Carolina State University (Raleigh, NC, USA) for hematoxylin and eosin staining as well as immunohistochemical staining with the Ki67+ antibody. A total of 15 villi and crypts in each slide were selected to measure villus height (VH), crypt depth (CD), and percent of Ki67+ enterocyte using the microscope (Olympus CX31 microscope, Tokyo, Japan). The ratio of VH to CD (VH:CD) was then calculated.
Immune status
Jejunal mucosa was grounded using a homogenizer (Tissuemiser, Thermo Fisher Scientific Inc., Rockford, IL, USA) on ice in 2 mL phosphate-buffered saline for 30 s. Sample preparation for analysis was followed as previously described by Chen et al. (2017). Homogenized samples were centrifuged at 14,000 × g for 30 min, and the supernatant was collected. Protein contents in the supernatants were measured using Pierce BCA Protein Assay Kit (23225#, Thermo Fisher Scientific Inc. Rockford, IL, USA) to determine the concentrations of TNF-α, IL-8, TGF-β1, and MUC2 per milligram of protein in the jejunal mucosa. The concentration of TNF-α and IL-8 in jejunal mucosa was measured by ELISA (R&D Systems, Minneapolis, MN) following Jang and Kim (2019) and Holanda and Kim (2020). The concentration of TGF-β1 and MUC2 in jejunal mucosa was measured by ELISA (MyBioSource, San Diego, CA) according to Jang et al. (2020).
Digestive enzyme activities
Activities of sucrase, maltase, and lactase in the jejunal tissues were determined by a colorimetric method using assay kits (MyBioSource Inc., San Diego, CA). One enzyme unit of sucrase was defined as the 1 μg of sucrase hydrolyzing activity per 1 mg tissue protein per minute. One enzyme unit of maltase was defined as the amount of maltase activity per milligram of tissue protein producing 5.55 mmol/L of glucose which reacted with the substrate at 37 °C for 20 min. One enzyme unit of lactase activity was defined as 1 nmol of lactase hydrolyzing activity per 1 mg of protein producing 5.55 mmol/L of glucose which reacted with the substrate at 37 °C for 15 min.
Microbiome sequencing
The deoxyribonucleic acid (DNA) was extracted from 250 mg of jejunal mucosa samples using a commercial DNA extraction kit (DNA Stool MiniKit, QIAGEN, Germany). Extracted DNA samples were sent to Mako Medical Laboratories (Raleigh, NC, USA) and prepared for template preparation on the Ion Chef instrument, and sequencing was performed on the Ion S5 system. Variable regions V2, V3, V4, V6, V7, V8, and V9 of the 16S ribosomal ribonucleic acid (rRNA) gene were amplified with the Ion 16S Metagenomics Kit (Thermo Fisher Scientific, Waltham, MA). Libraries were prepared from the amplified target regions with the Ion Xpress Plus Fragment Library Kit (Thermo Fisher Scientific, Waltham, MA). The IonCode Barcode Adapters 1–384 Kit (Thermo Fisher Scientific, Waltham, MA) was used for barcoding and multiplexing of the prepared libraries. The libraries were quantified with the Ion Universal Library Quantitation Kit (Thermo Fisher Scientific, Waltham, MA), and samples were diluted to equal concentration and pooled into multiplexed libraries for template preparation. Template preparation and chip loading were performed using the Ion Chef instrument, and sequencing was performed on the Ion S5 system with the Ion 520 & Ion 530 Kit-Chef (Thermo Fisher Scientific, Waltham, MA) and the Ion 530 Chip Kit-4 Reactions (Thermo Fisher Scientific, Waltham, MA). The 16s rRNA sequences were processed using the Torrent Suite Software (version 5.2.2) to produce unaligned bam files for further analysis. For data analysis, GreenGenes and MiSeq databases were used to identify a taxonomic identification. Alpha-diversity and relative abundance of bacteria were analyzed by the Ion Reporter Software Suite (version 5.2) of bioinformatics analysis tools. All samples had a depth of sequencing coverage > 1,000×. Sample preparation and analysis settings were performed according to the manufacturer’s recommended protocols and analysis settings.
Statistical analysis
Data from both experiments were analyzed using the MIXED procedure of SAS 9.4 (SAS Inst. Inc., Cary, NC). The effect of whey permeate supplementation was analyzed using the polynomial contrast statements with coefficients for unequally spaced treatments of both experiments by the Proc IML procedure of SAS 9.4. In exp.2, the growth data were analyzed using the NLIN procedure of SAS, followed by previous studies (Robbins et al., 2006; Shen et al., 2012) for a broken-line analysis to determine an optimal whey permeate level and daily whey permeate intake for G:F. The G:F was plotted based on whey permeate supplementation and average daily lactose intake of pigs in each pen. Fecal score was analyzed with Kruskal–Wallis test using the NPAR1WAY procedure of SAS. Statistical significance and tendency were considered at P < 0.05 and 0.05 ≤ P < 0.10, respectively.
Results
Whey Permeate
Whey permeate used in this study contained 76.3% lactose and 0.4% milk oligosaccharides.
Exp. 1
The initial BW of pigs at the beginning of exp. 1 was 6.2 ± 0.3 kg, and there was no difference among treatments (Table 3). Increasing whey permeate supplementation had quadratic effect (P < 0.05) on ADFI (minimum: 71 g/d at 20.0% whey permeate) and G:F (maximum: 1.35 at 18.3% whey permeate) during phase 1. Increasing whey permeate supplementation tended to linearly increase (P = 0.092) the BW (8.9 to 9.2 kg), whereas linearly increased (P < 0.05) ADG (292 to 327 g/d) and G:F (0.96 to 1.04) of pigs during phase 2. Increasing whey permeate supplementation tended to linearly decrease (P = 0.052) G:F (0.78 to 0.74) of pigs during phase 3. Increasing whey permeate supplementation linearly increased (P < 0.05) ADG (238 to 256 g/d) and G:F (0.87 to 0.90) of pigs during the entire period (phase 1 to 3).
Table 3.
Growth performance of nursery pigs fed diets with whey permeate in exp. 1
| Whey permeate, %1 | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | A 10/3/0 |
B 15/8/1 |
C 20/13/4 |
D 25/18/6 |
E 30/23/9 |
SEM | Linear | Quad. | A vs. others |
| BW, kg | |||||||||
| Day 0 | 6.2 | 6.2 | 6.2 | 6.2 | 6.2 | 0.1 | 0.973 | 0.995 | 1.000 |
| Day 7 | 6.8 | 6.8 | 6.9 | 6.9 | 6.9 | 0.1 | 0.452 | 0.831 | 0.551 |
| Day 14 | 8.9 | 9.0 | 9.2 | 9.1 | 9.2 | 0.1 | 0.092 | 0.629 | 0.124 |
| Day 20 | 11.0 | 11.2 | 11.1 | 11.3 | 11.4 | 0.2 | 0.113 | 0.936 | 0.140 |
| Day 48 | 25.3 | 25.9 | 26.2 | 26.2 | 26.0 | 0.5 | 0.266 | 0.363 | 0.180 |
| ADG, g/d | |||||||||
| Phase 1 | 83 | 86 | 98 | 95 | 99 | 8 | 0.107 | 0.661 | 0.200 |
| Phase 2 | 292 | 309 | 319 | 311 | 327 | 8 | 0.008 | 0.489 | 0.011 |
| Phase 3 | 355 | 369 | 330 | 368 | 356 | 14 | 0.953 | 0.555 | 0.971 |
| Phase 1 to 3 | 238 | 249 | 245 | 252 | 256 | 4 | 0.011 | 0.901 | 0.017 |
| Phase 42 | 596 | 610 | 626 | 620 | 612 | 17 | 0.448 | 0.308 | 0.280 |
| Overall | 397 | 408 | 415 | 415 | 413 | 9 | 0.195 | 0.301 | 0.122 |
| ADFI, g/d | |||||||||
| Phase 1 | 90 | 73 | 74 | 71 | 89 | 8 | 0.898 | 0.027 | 0.143 |
| Phase 2 | 306 | 308 | 319 | 317 | 315 | 9 | 0.324 | 0.507 | 0.359 |
| Phase 3 | 453 | 474 | 441 | 482 | 481 | 17 | 0.256 | 0.547 | 0.397 |
| Phase 1 to 3 | 274 | 276 | 270 | 280 | 286 | 6 | 0.168 | 0.226 | 0.604 |
| Phase 4 | 720 | 739 | 760 | 762 | 752 | 23 | 0.237 | 0.372 | 0.675 |
| Overall | 536 | 545 | 554 | 561 | 554 | 14 | 0.220 | 0.474 | 0.247 |
| G:F | |||||||||
| Phase 1 | 0.93 | 1.22 | 1.33 | 1.35 | 1.17 | 0.07 | 0.011 | 0.001 | 0.001 |
| Phase 2 | 0.96 | 1.01 | 1.00 | 0.98 | 1.04 | 0.01 | 0.013 | 0.965 | 0.011 |
| Phase 3 | 0.78 | 0.78 | 0.74 | 0.77 | 0.74 | 0.01 | 0.052 | 0.874 | 0.181 |
| Phase 1 to 3 | 0.87 | 0.90 | 0.91 | 0.90 | 0.90 | 0.01 | 0.081 | 0.023 | 0.005 |
| Phase 4 | 0.83 | 0.83 | 0.82 | 0.81 | 0.81 | 0.01 | 0.178 | 0.984 | 0.398 |
| Overall | 0.74 | 0.75 | 0.75 | 0.74 | 0.74 | 0.01 | 0.624 | 0.590 | 0.719 |
| Mortality, % | 2.78 | 1.85 | 2.31 | 0.93 | 2.32 | 1.10 | 0.609 | 0.498 | 0.460 |
| Morbidity, % | 3.70 | 6.02 | 4.17 | 2.31 | 5.56 | 1.54 | 1.000 | 0.751 | 0.642 |
1The whey permeate treatment programs were composed of A = 10%, 3%, 0%; B = 15%, 8%, 1%; C = 20%, 13%, 4%; D = 25%, 18%, 6%; and E = 30%, 23%, 9% whey permeate in the experimental diets for phase 1 (day 0 to 7), phase 2 (day 7 to 14), and phase 3 (day 14 to 20), respectively.
2A common diet without lactose was fed for phase 4 (day 20 to 48).
Exp. 2
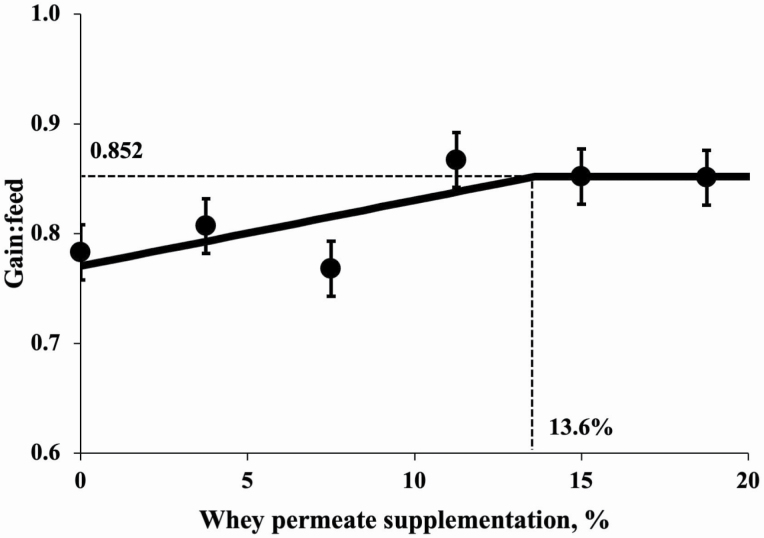
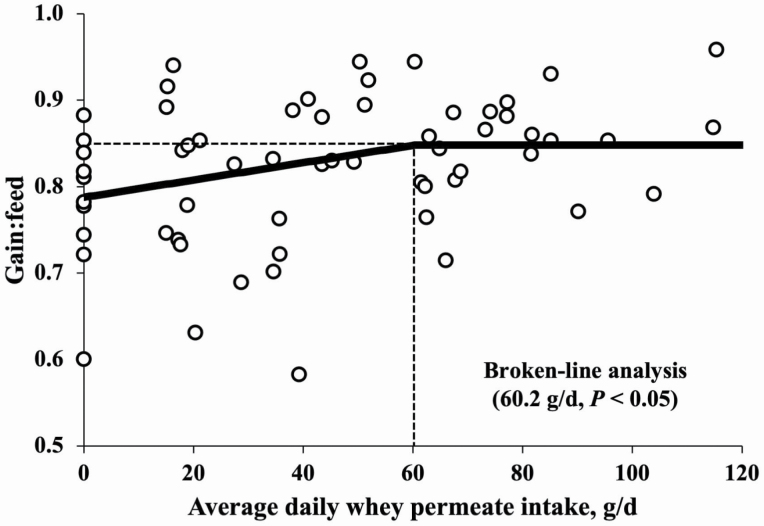
The initial BW of pigs at the beginning of exp. 2 was 7.5 ± 1.4 kg, and there was no difference among treatments (Table 4). Increasing whey permeate supplementation from 0% to 18.75% linearly increased (P < 0.05) BW of pigs. Increasing whey permeate supplementation from 0% to 18.75% linearly increased (P < 0.05) ADG (349 to 414 g/d) during pig BW 7 to 11 kg. Increasing whey permeate supplementation from 0% to 18.75% tended to linearly increase (P = 0.062) ADFI of pigs during pig BW 7 to 11 kg. Increasing whey permeate supplementation from 0% to 18.75% linearly increased (P < 0.05) G:F of pigs during pig BW 7 to 11 kg. Increasing whey permeate supplementation from 0% to 18.75% did not affect fecal scores during pig BW 7 to 11 kg. Collected fecal scores averaged 3.5 based on a 1 to 5 scale indicating a normal soft to dry condition. According to broken-line analysis, G:F was affected by increasing whey permeate levels in the diets (Figure 1). A broken-line analysis (P = 0.022) showed that G:F was increased until whey permeate supplementation was increased from 0 to 13.60%. The G:F was also affected by lactose intake (Figure 2). A broken-line analysis (P = 0.027) showed that G:F was increased until daily whey permeate intake was increased from 0 to 60.2 g/d.
Table 4.
Growth performance of nursery pigs fed diets with whey permeate in exp.2
| Whey permeate1, % | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | 0 | 3.75 | 7.50 | 11.25 | 15.00 | 18.75 | SEM | Linear | Quad. | 0% vs. others |
| BW, kg | ||||||||||
| Day 0 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 0.7 | 0.928 | 0.997 | 0.958 |
| Day 11 | 11.3 | 11.6 | 11.3 | 11.9 | 11.9 | 12.1 | 1.0 | 0.019 | 0.781 | 0.150 |
| ADG, g/d | ||||||||||
| Day 0 to 112 | 349 | 369 | 346 | 401 | 399 | 414 | 29 | 0.001 | 0.689 | 0.035 |
| ADFI, g/d | ||||||||||
| Day 0 to 11 | 442 | 461 | 452 | 465 | 468 | 484 | 32 | 0.062 | 0.792 | 0.161 |
| G:F | ||||||||||
| Day 0 to 11 | 0.78 | 0.81 | 0.77 | 0.87 | 0.85 | 0.85 | 0.03 | 0.006 | 0.893 | 0.084 |
| Fecal score2 | Kruskal–Wallis test3 | |||||||||
| Day 0 to 11 | 3.47 | 3.48 | 3.51 | 3.63 | 3.26 | 3.53 | 0.14 | 0.635 | ||
1Treatments were the supplemental levels of whey permeate in the diets.
2Fecal score is measured from day 2 at 2-d intervals based on a 1 to 5 scale: 1 (watery diarrhea) to 5 (dried feces).
3Fecal score was analyzed with Kruskal–Wallis test by using the procedure of NPAR1WAY in SAS 9.4 (SAS Inst. Inc., Cary, NC).
Figure 1.
Changes in G:F of pigs fed diets with whey permeate using a broken-line analysis. The breakpoint (one-slope broken-line model) was 13.6% whey permeate supplementation when G:F was 0.852 (P < 0.05). One-slope broken-line model; the equation for G:F is y = 0.852 – 0.006 × z1; if whey permeate supplementation is ≥ breakpoint, then z1 = 0; if whey permeate supplementation is < breakpoint, then z1 = breakpoint − whey permeate supplementation.
Figure 2.
Changes in G:F of pigs fed diets with whey permeate using a broken-line analysis. Average daily whey permeate intake (g/d) was calculated based on the supplemental levels of whey permeate and ADFI. The breakpoint (one-slope broken-line model) was 60.2 g/d average daily whey permeate intake when G:F was 0.848 (P < 0.05). One-slope broken-line model; the equation for G:F is y = 0.848 – 0.001 × z1; if whey permeate supplementation is ≥ breakpoint, then z1 = 0; if whey permeate supplementation is < breakpoint, then z1 = breakpoint − whey permeate supplementation.
There was no difference in the VH and the VH:CD of the jejunum by whey permeate supplementation (Table 5). Increasing whey permeate supplementation from 0% to 18.75% was tended to linearly decrease (P = 0.082) crypt depth (268 to 251 μm) in the jejunum. Increasing whey permeate supplementation from 0% to 18.75% linearly increased (P < 0.05) crypt cell proliferation (27.8% to 37.0%) in the jejunum. Increasing whey permeate supplementation did not affect TNF-α, TGF-β1, and MUC2 in the jejunal mucosa. Increasing whey permeate supplementation from 0% to 18.75% tended to have quadratic effect (P = 0.052) on IL-8 (maximum: 223 pg/mg of protein at 10.89% whey permeate) in jejunal mucosa. Whey permeate supplementation increased (P < 0.05) IL-8 in the jejunal mucosa when compared with no addition of whey permeate. Increasing whey permeate supplementation from 0% to 18.75% did not affect sucrase activity in the jejunum, but increasing whey permeate supplementation from 0% to 18.75% linearly decreased (P < 0.05) lactase activity (15.84 to 6.60 U/mg of protein) in the jejunum. Increasing whey permeate supplementation from 0% to 18.75% decreased lactase activity (P < 0.05) and tended to decrease maltase activity (P = 0.077) in the jejunum compared with pigs fed diets with no added whey permeate.
Table 5.
Histomorphology, crypt cell proliferation, immune response, mucin protein, and digestive enzyme activity in the jejunum of nursery pigs fed with whey permeate in exp. 2
| Whey permeate1, % | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | 0 | 3.75 | 7.50 | 11.25 | 15.00 | 18.75 | SEM | Linear | Quad. | 0% vs. others |
| Histomorphology | ||||||||||
| Villus height, μm | 548 | 531 | 571 | 531 | 547 | 500 | 32 | 0.359 | 0.391 | 0.701 |
| Villus width2, μm | 98 | 98 | 99 | 100 | 95 | 105 | 4 | 0.483 | 0.747 | 0.377 |
| Crypt depth, μm | 268 | 255 | 265 | 253 | 247 | 251 | 8 | 0.082 | 0.725 | 0.121 |
| VH:CD | 2.04 | 2.08 | 2.15 | 2.11 | 2.23 | 1.99 | 0.12 | 0.868 | 0.256 | 0.569 |
| Crypt cell proliferation, % | 27.8 | 29.1 | 31.8 | 31.6 | 34.2 | 37.0 | 3.0 | 0.012 | 0.762 | 0.113 |
| Immune response | ||||||||||
| TNF-α, pg/mg | 0.80 | 0.81 | 0.72 | 0.72 | 0.74 | 0.91 | 0.16 | 0.359 | 0.754 | 0.924 |
| IL-8, pg/mg | 170 | 214 | 223 | 203 | 214 | 202 | 14 | 0.272 | 0.052 | 0.015 |
| TGF-β1, pg/mg | 4.40 | 3.10 | 3.88 | 3.64 | 4.18 | 2.86 | 0.75 | 0.387 | 0.857 | 0.225 |
| MUC2, U/mg | 0.52 | 0.43 | 0.43 | 0.55 | 0.53 | 0.39 | 0.07 | 0.700 | 0.656 | 0.456 |
| Digestive enzyme activity | ||||||||||
| Sucrase, U/mg | 20.4 | 21.4 | 19.1 | 16.5 | 20.7 | 18.8 | 2.0 | 0.413 | 0.514 | 0.587 |
| Maltase, U/mg | 87.1 | 70.4 | 56.5 | 46.8 | 92.4 | 51.4 | 12.3 | 0.222 | 0.288 | 0.077 |
| Lactase, U/mg | 15.8 | 11.7 | 9.0 | 9.3 | 4.9 | 6.6 | 2.8 | 0.020 | 0.441 | 0.033 |
1Treatments were the supplemental levels of whey permeate in the diets.
2Villus width is the average of the three lengths (tip, middle, and base of villus).
Increasing whey permeate supplementation from 0% to 18.75% did not affect the alpha-diversity estimates of microbiota in the jejunal mucosa (Table 6). Whey permeate supplementation tended to decrease (P = 0.073) Firmicutes to Bacteroidetes ratio (F:B) in the jejunal mucosa compared with no addition of whey permeate. Increasing whey permeate supplementation from 0% to 18.75% tended to linearly increase Bifidobacteriaceae (P = 0.089) and decrease Enterobacteriaceae (P = 0.091) and Streptococcaceae (P = 0.094) in jejunal mucosa. Increasing whey permeate supplementation from 0% to 18.75% have quadratic effects on Lactobacillaceae (P < 0.05; maximum: 9.14% at 12.91% whey permeate) and Rhodobacteraceae (P < 0.05; minimum: 2.54% at 8.85% whey permeate) and tended to have quadratic effects on Corynebacteriaceae (P = 0.090; maximum: 0.87% at 10.48% whey permeate) in jejunal mucosa. Whey permeate supplementation decreased (P < 0.05) Enterobacteriaceae in the jejunum mucosa compared with no addition of whey permeate.
Table 6.
Diversity and relative abundance of bacteria in the jejunal mucosa of nursery pigs fed diets with increasing levels of whey permeate in exp. 2
| Whey permeate1, % | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | 0 | 3.75 | 7.50 | 11.25 | 15.00 | 18.75 | SEM | Linear | Quad. | 0% vs. others |
| Diversity | ||||||||||
| Chao1 | 97.4 | 132.2 | 104.2 | 103.2 | 107.0 | 92.1 | 21.4 | 0.569 | 0.543 | 0.663 |
| Shannon | 3.14 | 3.28 | 3.47 | 2.54 | 3.36 | 3.00 | 0.60 | 0.784 | 0.993 | 0.984 |
| Simpson | 0.69 | 0.69 | 0.76 | 0.63 | 0.76 | 0.73 | 0.08 | 0.678 | 0.920 | 0.774 |
| Phylum level | ||||||||||
| Actinobacteria | 5.65 | 4.21 | 5.97 | 5.23 | 6.24 | 5.28 | 0.91 | 0.595 | 0.934 | 0.753 |
| Bacteroidetes | 21.92 | 21.93 | 19.59 | 23.07 | 21.69 | 22.55 | 2.19 | 0.779 | 0.729 | 0.954 |
| Firmicutes | 31.39 | 25.64 | 33.15 | 26.65 | 28.13 | 30.04 | 5.05 | 0.498 | 0.537 | 0.598 |
| Proteobacteria | 17.11 | 17.75 | 17.71 | 15.63 | 18.3 | 21.06 | 2.49 | 0.363 | 0.366 | 0.722 |
| F:B ratio | 1.59 | 1.19 | 1.35 | 1.25 | 1.22 | 1.13 | 0.17 | 0.144 | 0.650 | 0.073 |
| Family level | ||||||||||
| Acetobacteraceae | 4.16 | 3.34 | 3.31 | 4.33 | 4.19 | 5.91 | 1.06 | 0.199 | 0.270 | 0.965 |
| Bacillaceae | 0.92 | 0.95 | 0.84 | 0.97 | 1.89 | 0.67 | 0.27 | 0.475 | 0.417 | 0.638 |
| Bifidobacteriaceae | 0.73 | 0.55 | 0.83 | 0.81 | 0.97 | 1.11 | 0.22 | 0.089 | 0.599 | 0.607 |
| Clostridiaceae | 2.20 | 1.31 | 1.66 | 2.11 | 2.58 | 1.27 | 0.47 | 0.922 | 0.695 | 0.375 |
| Corynebacteriaceae | 0.40 | 0.96 | 0.71 | 0.69 | 1.11 | 0.43 | 0.28 | 0.742 | 0.090 | 0.131 |
| Enterobacteriaceae | 1.04 | 0.66 | 0.62 | 0.49 | 0.51 | 0.52 | 0.20 | 0.091 | 0.266 | 0.036 |
| Helicobacteraceae | 8.37 | 9.11 | 6.47 | 9.02 | 7.62 | 7.31 | 2.63 | 0.723 | 0.990 | 0.862 |
| Lachnospiraceae | 4.85 | 4.09 | 4.27 | 4.79 | 4.79 | 2.69 | 1.09 | 0.341 | 0.660 | 0.480 |
| Lactobacillaceae | 6.94 | 7.03 | 12.66 | 12.81 | 9.19 | 8.82 | 1.48 | 0.182 | 0.010 | 0.052 |
| Methylobacteriaceae | 1.10 | 2.15 | 2.23 | 1.94 | 1.82 | 1.55 | 0.46 | 0.810 | 0.114 | 0.139 |
| Prevotellaceae | 7.25 | 7.99 | 7.08 | 7.08 | 6.68 | 6.73 | 4.75 | 0.298 | 0.586 | 0.772 |
| Propionibacteriaceae | 1.77 | 1.39 | 2.27 | 1.85 | 2.41 | 1.74 | 0.71 | 0.687 | 0.685 | 0.840 |
| Rhodobacteraceae | 5.92 | 2.61 | 2.64 | 3.08 | 4.35 | 5.83 | 1.19 | 0.609 | 0.015 | 0.101 |
| Ruminococcaceae | 2.44 | 2.20 | 1.75 | 2.43 | 2.42 | 0.98 | 0.61 | 0.271 | 0.488 | 0.476 |
| Streptococcaceae | 1.50 | 1.87 | 0.82 | 0.92 | 0.67 | 0.71 | 0.51 | 0.094 | 0.743 | 0.380 |
1Treatments were the inclusion levels of whey permeate in the diets.
Discussion
Milk coproducts such as whey powder and whey permeate have been essential feedstuffs in feeding nursery pigs (Mahan et al., 2004; Cromwell et al., 2008). Lactose is the major component in whey permeate, whereas various oligosaccharides are also included as minor but with functional properties (Bode, 2012; Bering, 2018; Ramani et al., 2018). Lactose is the major carbohydrate responsible for the growth of nursery pigs (Mahan, 1992; Nessmith et al., 1997; Pierce et al., 2007). Milk oligosaccharides include 3′sialyl-lactose, 6′-sialyl-lactose, and 6′-sialyl-lactosamine (Dallas et al., 2014), which are shown to have prebiotic effects modulating intestinal bacteria and frequency of diarrhea (Hester et al., 2013; Li et al., 2014) and also have immunomodulatory activities in pigs (Comstock et al., 2014, 2017). Whey permeate was used in this study as a source of lactose and milk oligosaccharides. The lactose composition in whey permeate in this study was 76.3%, and it was slightly lower than NRC (2012) but higher than lactose in other milk coproducts, such as skim milk powder (47.8%), whey powder (72.9%), and whey protein concentrate (5%) described in NRC (2012). Previous studies showed that whey permeate could be also a valuable source of milk oligosaccharides (Barile et al., 2009; Dallas et al., 2014; Lee et al., 2015). Previous studies showed that liquid whey permeate from skimmed bovine milk had an average of 71 mg/L milk oligosaccharides (Mehra et al., 2014; Altmann et al., 2015). Considering whey permeate contains between 5% and 6% solids (Illanes, 2016), milk oligosaccharides could be obtained approximately at 0.12% to 0.14% from whey permeate on a dry basis. Whey permeate also contained 0.4% milk oligosaccharides, and it was slightly higher than the calculated composition of milk oligosaccharides in whey permeate (0.12% to 0.14%) based on the previous studies (Mehra et al., 2014; Altmann et al., 2015).
Beneficial effects of lactose and milk oligosaccharides on the intestinal health are well documented in previous studies showing that supplementation of lactose positively affected growth performance and fecal microbiota in the nursery pigs (Mahan et al., 2004; Tran et al., 2012) and milk oligosaccharides stimulated intestinal mucosal immunity with increased natural killer cell and T-cell and affected infant gut microbiome (Li et al., 2014; Comstock et al., 2017; Ramani et al., 2018). In this study, exp.1 shows that increasing whey permeate supplementation improved the growth performance of nursery pigs from weaning at 6.2 to 11 kg BW. Experiment 2 shows that the supplementation of whey permeate in nursery diets may have beneficial impacts on the intestinal health by increased IL-8 and crypt cell proliferation with positive changes in jejunal mucosa-associated microbiota including increased Bifidobacteriaceae and Lactobacillaceae and decreased Enterobacteriaceae.
Nursery pigs are situated in stressful conditions with intestinal challenges and growth retardation during the initial postweaning period (Rhouma et al., 2017; Duarte et al., 2019; Jang and Kim, 2019). The results from exp. 1 showed that the supplementation of whey permeate increased G:F greater than 1.00 during the initial weeks after weaning. It could indicate that whey permeate would help newly weaned pigs to increase water intake to prevent dehydration caused by weaning stress. According to Rhouma et al. (2017), weaning stress could cause low water intake and electrolyte imbalance by dehydration in weaned pigs, including diarrhea. Horn et al. (2014) also reported that reduced water intake showed increased cortisol levels as a stress marker in the serum of newly weaned pigs. Therefore, supplementation of whey permeate in nursery feeds would help pigs to recover from weaning stress by increasing the feed intake during the initial weeks after weaning. It is also warranted to further investigate whether lactose in whey permeate or solid form diets has effects on water consumption of newly weaned pigs.
In addition, two experiments conducted in this study showed a consistent improvement of growth by 7.6% in exp. 1 and 18.8% in exp. 2, when the levels of whey permeate were increased to an average of 16.4% and 18.6%, respectively, in diets fed to nursery pigs from weaning to 11 kg BW. These results are supported by the previous study showing the growth improvement by 25.9% when the levels of whey permeate were increased to 21.5% in diets fed to nursery pigs (Gahan et al., 2009). According to Cromwell et al. (2008), the inclusion of whey permeate at 9.4% in nursery diets maximized growth responses of pigs at 11 to 25 kg from day 14 to 28 postweaning. Few studies used whey permeate as a lactose source to evaluate the supplemental effects of lactose on growth performance in nursery pigs (Gahan et al., 2009; Naranjo et al., 2010; O′Doherty et al., 2010). However, this study further investigated an optimal inclusion level (13.6%) or daily intake (60.2 g) of whey permeate for G:F of pigs at 7 to 11 kg BW in commercial farms using a broken-line analysis (Robbins et al., 2006; Shen et al., 2012).
Reasons for increased growth of nursery pigs with dietary supplementation of whey permeate could be explained by its benefits of effective energy provision and enhanced appetite (Graham et al., 1981; Tokach et al., 1989; Mahan, 1993; Nessmith et al., 1997). However, the beneficial effects of milk oligosaccharides on the intestinal health cannot be also ignored (Zivkovic and Barile, 2011; Hester et al., 2013; Yu et al., 2013; Li et al., 2014). Based on the data from this study, it can be speculated that the supplementation of whey permeate could affect the intestinal health of nursery pigs with potential activation of immune response and improved enterocyte proliferation. A properly functioning immune system can robustly and quickly respond to various challenges via immune responses within the intestinal barrier of nursery pigs. The activation of immune responses aids in the prevention of the systemic infection and excess inflammation. The IL-8 is the major pro-inflammatory cytokine induced by intestinal epithelial cells and the significant mediator of immune reaction to stimulate the activation of neutrophils (Kucharzik et al., 2005; Andrews et al., 2018). The IL-8 can also recruit other immune cells related to the adaptive immune systems such as T-lymphocytes and dendritic cells (Andrews et al., 2018). In a previous study, human milk factors were shown to stimulate the secretion of IL-8 from fetal enterocytes pretreated with epidermal growth factor (Claud et al., 2003). Previous in vitro studies also showed that lactose supplementation increased IL-8 production by stimulation of the innate immune system in the intestinal epithelial cells (Rusu et al., 2010; Ustunol and Wong, 2010). In addition, milk oligosaccharides could activate the immune cells as well as the adaptive immune response (Fuhrer et al., 2010; Kurakevich et al., 2013). Furthermore, this study illustrated that whey permeate increased enterocyte proliferation in the jejunal crypts of nursery pigs. These findings are in accordance with previous studies that have shown that milk components could stimulate intestinal enterocyte proliferation in neonatal pigs (Reznikov et al., 2014; Li et al., 2018). Wang et al. (2020) also reported that milk oligosaccharides could activate the epidermal growth factor receptor by phosphorylation increasing cell proliferation in the mouse intestinal epithelium and Caco-2 cells. This study indicates that whey permeate could help to protect and maintain the intestinal health of nursery pigs by stimulating the intestinal immune response and enterocyte proliferation.
Lactase is a digestive enzyme hydrolyzing lactose to glucose and galactose. It has been known that lactase activity declines with the age of pigs upon birth (Ekstrom et al., 1975). The mechanisms for the decrease in lactase activity are not clearly elucidated, although the diets, hormonal interactions, or post-translation modification could be considered as major regulators (Kelly et al., 1991). This study showed that the supplementation of whey permeate decreased lactase activity in the jejunum of nursery pigs. Previous studies suggest that the decline in lactase activity could be related to lumen pH and immune activation (Gray et al., 1969; Smith et al., 1988). The reduction in lactase activity with the supplementation of whey permeate could be associated with a reduction of pH in the intestine by microbial fermentation producing volatile fatty acids (VFA) and lactic acid from lactose and milk oligosaccharides (Montalto et al., 2006; Fukuda et al., 2011; Kim, 2018). According to Gahan et al. (2009), the fecal pH of nursery pigs was linearly decreased by supplementation of lactose. Another possible mechanism is that the immune activation by milk oligosaccharides could be related to the reduction of lactase activity in the jejunum. The results showed that the supplementation of whey permeate increased the concentration of IL-8 in the jejunum with decreased activities of maltase and lactase compared with the pigs fed diets without whey permeate. Previous studies showed that immune activation induced a drastic decline in intestinal disaccharidase activities (Pié et al., 2004; Liu et al., 2009; Solaymani-Mohammadi and Singer, 2011).
It has been well documented that weaning stress could induce an imbalance of intestinal microbiota with increased opportunistic pathogens, leading to intestinal inflammatory response (Castillo et al., 2006; Su et al., 2008). This study observed relative abundance of microbiome that are associated with jejunal mucosa. The results showed an increased relative abundance of Bifidobacteriaceae and Lactobacillaceae, potentially beneficial bacterium (Adhikari et al., 2019), whereas showed a reduced relative abundance of Enterobacteriaceae, potential opportunistic pathogenic group (Schierack et al., 2007). This study also showed that the supplementation of whey permeate tended to reduce the F:B, an index of the microbial balance and intestinal inflammatory response (Vemuri et al., 2018; Rinninella et al., 2019). Milk oligosaccharides in whey permeate may act as prebiotics in the intestine of nursery pigs by directly binding to membrane components of pathogens (Fuhrer et al., 2010; Kurakevich et al., 2013) or inhibiting pathogens from binding to intestinal epithelial cells (Smilowitz et al., 2014; Triantis et al., 2018). Lactose and milk oligosaccharides in whey permeate could be fermented reducing the lumen pH of the small intestine (Montalto et al., 2006; Fukuda et al., 2011; Kim, 2018), resulting in intestinal environments favorable to potentially beneficial bacteria (Pierce et al., 2007; Tran et al., 2012). Therefore, the supplementation of whey permeate could enhance the balance of gut-associated microbiome with prebiotics effects of lactose and milk oligosaccharides.
Feeding lactose more than tolerance could cause excessive lactose fermentation in the intestinal lumen, resulting in decreased short-chain fatty acids production with a reduction of luminal pH (Montalto et al., 2006; Fukuda et al., 2011; Kim, 2018). It may also lead to intestinal barrier dysfunction due to energy deficiency in the intestinal epithelium (Venema, 2012). Therefore, excessive lactose may cause intestinal barrier dysfunction, leading to translocation of potential pathogens (Ten Bruggencate et al., 2005; Bischoff et al., 2014; Szilagyi and Ishayek 2018). Previous studies showed that the fermentation of undigested carbohydrates affects the intestinal barrier functions (Flint et al., 2012). However, in this study, quadratic responses were observed with increasing whey permeate supplementation on the IL-8 concentration (maximum at 10.9% whey permeate) as well as the relative abundance of Corynebacteriaceae (maximum at 10.5%), Lactobacillaceae (maximum at 12.9%), and Rhodobacteraceae (minimum at 8.9%) in the jejunum. These results could be related to the functions of milk oligosaccharides in whey permeate. Previous studies show that milk oligosaccharides in whey permeate may reduce the adverse effects of fermentation of undigested lactose in the intestinal lumen (Solomons, 2002; Mattar et al., 2012). Milk oligosaccharides in whey permeate are shown to modulate the intestinal microbiota with decreased the growth of potential pathogens (Marcobal et al., 2010; Marcobal and Sonnenburg, 2012; Savaiano et al., 2013; Azcarate-Peril et al., 2017). Previous in vitro studies showed that milk oligosaccharides could inhibit the binding of pathogens to the enterocytes and help to excrete pathogens (Ashkenazi et al., 1991; Ruiz-Palacios et al., 2003).
In conclusion, dietary inclusion of whey permeate increased growth of nursery pigs with a potential increase of water intake during the initial weeks after weaning. G:F of pigs from 7 to 11 kg BW reached the maximum at 13.6% whey permeate in diets or 60.2 g whey permeate intake per day and maintained at higher levels or intake, respectively. Improvement in growth performance partly attributed to stimulating intestinal immune responses and enterocyte proliferation with positive changes of jejunal mucosa-associated microbiota in nursery pigs. Whey permeate not only could be an important source of lactose for growth but also possesses important functions from milk oligosaccharides to enhance the intestinal health of nursery pigs.
Acknowledgments
This study was awarded and funded by American Dairy Products Institute (Elmhurst, IL). Financial support was also provided by North Carolina Agricultural Foundation (660101, Raleigh, NC, USA) for this study. In-kind contributions were provided from Agri-Mark, Inc. (Middlebury, Vermont, USA), Pipestone Grow Finish (Pipestone, MN, USA), and NG Purvis Farm (Robbins, NC, USA) for the animal experiments.
Glossary
Abbreviations
- ADFI
average daily feed intake
- ADG
average daily gain
- BW
body weight
- CD
crypt depth
- CP
crude protein
- DDGS
distillers dried grains with soluble
- DNA
deoxyribonucleic acid
- F:B
Firmicutes to Bacteroidetes ratio
- G:F
feed efficiency
- IL-8
interleukin-8
- ME
metabolizable energy
- MUC2
mucin-2 protein
- rRNA
ribosomal ribonucleic acid
- SID
standardized ileal digestible
- STTD
standardized total tract digestible
- TGF-β1
transforming growth factor beta-1
- TNF-α
tumor necrosis factor-alpha
- VH
villus height
- VH:CD
villus height to crypt depth ratio
Conflict of interest statement
J..M.P. was employed by NG Purvis Farm (Robbins, NC, USA). The remaining two authors declare that the research was conducted in the absence of any commercial and financial relationships that could be construed as a potential conflict of interest.
Literature Cited
- Adhikari, B., S. W. Kim, and Y. M. Kwon. . 2019. Characterization of microbiota associated with digesta and mucosa in different regions of gastrointestinal tract of nursery pigs. Int. J. Mol. Sci. 20:1630. doi: 10.3390/ijms20071630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann, K., A. Wutkowski, S. Kämpfer, M. Klempt, P. C. Lorenzen, and I. Clawin-Rädecker. . 2015. Comparison of the efficiency of different NF membranes for the enrichment of milk oligosaccharides from bovine milk. Eur. Food. Res. Technol. 241:803–815. doi: 10.1007/s00217-015-2505-z [DOI] [Google Scholar]
- Andrews, C., M. H. McLean, and S. K. Durum. . 2018. Cytokine tuning of intestinal epithelial function. Front. Immunol. 9:1270. doi: 10.3389/fimmu.2018.01270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi, S., D. S. Newburg, and T. G. Cleary. . 1991. The effect of human milk on the adherence of enterohemorrhagic E. coli to rabbit intestinal cells. Adv. Exp. Med. Biol. 310:173–177. doi: 10.1007/978-1-4615-3838-7_21 [DOI] [PubMed] [Google Scholar]
- Azcarate-Peril, M. A., A. J. Ritter, D. Savaiano, A. Monteagudo-Mera, C. Anderson, S. T. Magness, and T. R. Klaenhammer. . 2017. Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals. Proc. Natl. Acad. Sci. U. S. A. 114:E367–E375. doi: 10.1073/pnas.1606722113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barile, D., N. Tao, C. B. Lebrilla, J. D. Coisson, M. Arlorio, and J. B. German. . 2009. Permeate from cheese whey ultrafiltration is a source of milk oligosaccharides. Int. Dairy J. 19:524–530. doi: 10.1016/j.idairyj.2009.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bering, S. B 2018. Human milk oligosaccharides to prevent gut dysfunction and necrotizing enterocolitis in preterm neonates. Nutrients 10:1461. doi: 10.3390/nu10101461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff, S. C., G. Barbara, W. Buurman, T. Ockhuizen, J. D. Schulzke, M. Serino, H. Tilg, A. Watson, and J. M. Wells. . 2014. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol. 14:189. doi: 10.1186/s12876-014-0189-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode, L 2012. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 22:1147–1162. doi: 10.1093/glycob/cws074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo, M., S. M. Martín-Orúe, M. Roca, E. G. Manzanilla, I. Badiola, J. F. Perez, and J. Gasa. . 2006. The response of gastrointestinal microbiota to avilamycin, butyrate, and plant extracts in early-weaned pigs. J. Anim. Sci. 84:2725–2734. doi: 10.2527/jas.2004-556 [DOI] [PubMed] [Google Scholar]
- Chen, H., S. Zhang, I. Park, and S. W. Kim. . 2017. Impacts of energy feeds and supplemental protease on growth performance, nutrient digestibility, and gut health of pigs from 18 to 45 kg body weight. Anim. Nutr. 3:359–365. doi: 10.1016/j.aninu.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claud, E. C., T. Savidge, and W. A. Walker. . 2003. Modulation of human intestinal epithelial cell IL-8 secretion by human milk factors. Pediatr. Res. 53:419–425. doi: 10.1203/01.PDR.0000050141.73528.AD [DOI] [PubMed] [Google Scholar]
- Comstock, S. S., M. Li, M. Wang, M. H. Monaco, T. B. Kuhlenschmidt, M. S. Kuhlenschmidt, and S. M. Donovan. . 2017. Dietary human milk oligosaccharides but not prebiotic oligosaccharides increase circulating natural killer cell and mesenteric lymph node memory T cell populations in noninfected and rotavirus-infected neonatal piglets. J. Nutr. 147:1041–1047. doi: 10.3945/jn.116.243774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstock, S. S., M. Wang, S. N. Hester, M. Li, and S. M. Donovan. . 2014. Select human milk oligosaccharides directly modulate peripheral blood mononuclear cells isolated from 10-d-old pigs. Br. J. Nutr. 111:819–828. doi: 10.1017/S0007114513003267 [DOI] [PubMed] [Google Scholar]
- Cromwell, G. L., G. L. Allee, and D. C. Mahan. . 2008. Assessment of lactose level in the mid- to late-nursery phase on performance of weanling pigs. J. Anim. Sci. 86:127–133. doi: 10.2527/jas.2006-831 [DOI] [PubMed] [Google Scholar]
- Dallas, D. C., V. Weinborn, J. M. de Moura Bell, M. Wang, E. A. Parker, A. Guerrero, K. A. Hettinga, C. B. Lebrilla, J. B. German, and D. Barile. . 2014. Comprehensive peptidomic and glycomic evaluation reveals that sweet whey permeate from colostrum is a source of milk protein-derived peptides and oligosaccharides. Food Res. Int. 63(Pt B):203–209. doi: 10.1016/j.foodres.2014.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte, M. E., F. X. Zhou, W. M. Dutra Jr, and S. W. Kim. . 2019. Dietary supplementation of xylanase and protease on growth performance, digesta viscosity, nutrient digestibility, immune and oxidative stress status, and gut health of newly weaned pigs. Anim. Nutr. 5:351–358. doi: 10.1016/j.aninu.2019.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom, K. E., N. J. Benevenga, and R. H. Grummer. . 1975. Changes in the intestinal lactase activity in the small intestine of two breeds of swine from birth to 6 weeks of age. J. Nutr. 105:1032–1038. doi: 10.1093/jn/105.8.1032 [DOI] [PubMed] [Google Scholar]
- El-Hawiet, A., E. N. Kitova, and J. S. Klassen. . 2015. Recognition of human milk oligosaccharides by bacterial exotoxins. Glycobiology 25:845–854. doi: 10.1093/glycob/cwv025 [DOI] [PubMed] [Google Scholar]
- Flint, H. J., K. P. Scott, S. H. Duncan, P. Louis, and E. Forano. . 2012. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 3:289–306. doi: 10.4161/gmic.19897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrer, A., N. Sprenger, E. Kurakevich, L. Borsig, C. Chassard, and T. Hennet. . 2010. Milk sialyllactose influences colitis in mice through selective intestinal bacterial colonization. J. Exp. Med. 207:2843–2854. doi: 10.1084/jem.20101098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda, S., H. Toh, K. Hase, K. Oshima, Y. Nakanishi, K. Yoshimura, T. Tobe, J. M. Clarke, D. L. Topping, T. Suzuki, . et al. 2011. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469:543–547. doi: 10.1038/nature09646 [DOI] [PubMed] [Google Scholar]
- Gahan, D. A., M. B. Lynch, J. J. Callan, J. T. O’Sullivan, and J. V. O′Doherty. . 2009. Performance of weanling piglets offered low-, medium- or high-lactose diets supplemented with a seaweed extract from Laminaria spp. Animal 3:24–31. doi: 10.1017/S1751731108003017 [DOI] [PubMed] [Google Scholar]
- Garrido, D., D. C. Dallas, and D. A. Mills. . 2013. Consumption of human milk glycoconjugates by infant-associated bifidobacteria: mechanisms and implications. Microbiology (Reading). 159(Pt 4):649–664. doi: 10.1099/mic.0.064113-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, P. L., D. C. Mahan, and R. G. Shields Jr. 1981. Effect of starter diet and length of feeding regimen on performance and digestive enzyme activity of 2-week old weaned pigs. J. Anim. Sci. 53:299–307. doi: 10.2527/jas1981.532299x [DOI] [Google Scholar]
- Gray, G. M., N. A. Santiago, E. H. Colver, and M. Genel. . 1969. Intestinal beta-galactosidases. II. Biochemical alteration in human lactase deficiency. J. Clin. Invest. 48:729–735. doi: 10.1172/JCI106030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, J. Y., C. E. Phillips, M. T. Coffey, and S. W. Kim. . 2015. Efficacy of a supplemental candy coproduct as an alternative carbohydrate source to lactose on growth performance of newly weaned pigs in a commercial farm condition. J. Anim. Sci. 93:5304–5312. doi: 10.2527/jas.2015-9328 [DOI] [PubMed] [Google Scholar]
- Hester, S. N., X. Chen, M. Li, M. H. Monaco, S. S. Comstock, T. B. Kuhlenschmidt, M. S. Kuhlenschmidt, and S. M. Donovan. . 2013. Human milk oligosaccharides inhibit rotavirus infectivity in vitro and in acutely infected piglets. Br. J. Nutr. 110:1233–1242. doi: 10.1017/S0007114513000391 [DOI] [PubMed] [Google Scholar]
- Holanda, D. M., and S. W. Kim. . 2020. Efficacy of mycotoxin deactivators on health and growth of newly weaned pigs under chronic dietary challenges of deoxynivalenol. Toxins. 12:311. doi: 10.1093/jas/skz258.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn, N., F. Ruch, G. Miller, K. M. Ajuwon, and O. Adeola. . 2014. Impact of acute water and feed deprivation events on growth performance, intestinal characteristics, and serum stress markers in weaned pigs. J. Anim. Sci. 92:4407–4416. doi: 10.2527/jas.2014-7673 [DOI] [PubMed] [Google Scholar]
- Illanes, A 2016. Chapter 1 – Lactose: production and upgrading. In: Illanes, A., C. Guerrero, C. Vera, L. Wilson, R. Conejeros, and F. B. T.-L.-D. P. Scott, editors. Lactose-derived prebiotics. San Diego (CA): Academic Press; p. 1–33. [Google Scholar]
- Jang, K. B., and S. W. Kim. . 2019. Supplemental effects of dietary nucleotides on intestinal health and growth performance of newly weaned pigs. J. Anim. Sci. 97:4875–4882. doi: 10.1093/jas/skz334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, K., J. H. Kim, J. M. Purvis, J. X. Chen, P. Ren, M. Vazquez-Anon, and S. W. Kim. . 2020. Effects of mineral methionine hydroxy analog chelate in sow diets on epigenetic modification and growth of progeny. J. Anim. Sci. 98:1–12. doi: 10.1093/jas/skaa271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, D., T. P. King, M. McFadyen, and A. J. Travis. . 1991. Effect of lactation on the decline of brush border lactase activity in neonatal pigs. Gut 32:386–392. doi: 10.1136/gut.32.4.386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. W 2017. Meeting amino acid requirements in pig nutrition. 2018. In: Wiseman, J, editor. Achieving sustainable production of pig meat. Vol 2. Animal breeding and nutrition (ISBN: 978-1-78676-092-0; ISSN: 2059-6936). Boca Raton (FL): Burleigh Dodds Science Publishing; p. 145–165. [Google Scholar]
- Kim, C. H 2018. Immune regulation by microbiome metabolites. Immunology 154:220–229. doi: 10.1111/imm.12930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. W., E. Van Heugten, F. Ji, C. H. Lee, and R. D. Mateo. . 2006. Fermented soybean meal as a vegetable protein source for nursery pigs : I. Effects on growth performance of nursery pigs. J. Anim. Sci. 88:214–224. doi: 10.2527/jas.2009-1993 [DOI] [PubMed] [Google Scholar]
- Kucharzik, T., J. T. Hudson 3rd, A. Lügering, J. A. Abbas, M. Bettini, J. G. Lake, M. E. Evans, T. R. Ziegler, D. Merlin, J. L. Madara, . et al. 2005. Acute induction of human IL-8 production by intestinal epithelium triggers neutrophil infiltration without mucosal injury. Gut 54:1565–1572. doi: 10.1136/gut.2004.061168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurakevich, E., T. Hennet, M. Hausmann, G. Rogler, and L. Borsig. . 2013. Milk oligosaccharide sialyl(α2,3)lactose activates intestinal CD11c+ cells through TLR4. Proc. Natl. Acad. Sci. U. S. A. 110:17444–17449. doi: 10.1073/pnas.1306322110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H., V. L. de MeloSilva, Y. Liu, and D. Barile. . 2015. Short Communication: Quantification of carbohydrates in whey permeate products using high-performance anion-exchange chromatography with pulsed amperometric detection. J. Dairy Sci. 98:7644–7649. doi: 10.3168/jds.2015-9882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, T., J. Gao, M. Du, and X. Mao. . 2019. Bovine α-lactalbumin hydrolysates ameliorate obesity-associated endotoxemia and inflammation in high-fat diet-fed mice through modulation of gut microbiota. Food Funct. 10:3368–3378. doi: 10.1039/c8fo01967c [DOI] [PubMed] [Google Scholar]
- Li, M., M. H. Monaco, M. Wang, S. S. Comstock, T. B. Kuhlenschmidt, G. C. Fahey Jr, M. J. Miller, M. S. Kuhlenschmidt, and S. M. Donovan. . 2014. Human milk oligosaccharides shorten rotavirus-induced diarrhea and modulate piglet mucosal immunity and colonic microbiota. ISME J. 8:1609–1620. doi: 10.1038/ismej.2014.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., D. N. Nguyen, K. Obelitz-Ryom, A. D. Andersen, T. Thymann, D. E. W. Chatterton, S. Purup, A. B. Heckmann, S. B. Bering, and P. T. Sangild. . 2018. Bioactive whey protein concentrate and lactose stimulate gut function in formula-fed preterm pigs. J. Pediatr. Gastroenterol. Nutr. 66:128–134. doi: 10.1097/MPG.0000000000001699 [DOI] [PubMed] [Google Scholar]
- Liu, Y., J. Han, J. Huang, X. Wang, F. Wang, and J. Wang. . 2009. Dietary l-arginine supplementation improves intestinal function in weaned pigs after an Escherichia coli lipopolysaccharide challenge. Asian-Australas. J. Anim. Sci. 22:1667–1675. doi: 10.1038/ismej.2014.10 [DOI] [Google Scholar]
- Mahan, D. C 1992. Efficacy of dried whey and its lactalbumin and lactose components at two dietary lysine levels on postweaning pig performance and nitrogen balance. J. Anim. Sci. 70:2182–2187. doi: 10.2527/1992.7072182x [DOI] [PubMed] [Google Scholar]
- Mahan, D. C 1993. Evaluating two sources of dried whey and the effects of replacing the corn and dried whey component with corn gluten meal and lactose in the diets of weanling swine. J. Anim. Sci. 71:2860–2866. doi: 10.2527/1993.71112860x [DOI] [PubMed] [Google Scholar]
- Mahan, D. C., N. D. Fastinger, and J. C. Peters. . 2004. Effects of diet complexity and dietary lactose levels during three starter phases on postweaning pig performance. J. Anim. Sci. 82:2790–2797. doi: 10.2527/2004.8292790x [DOI] [PubMed] [Google Scholar]
- Marcobal, A., M. Barboza, J. W. Froehlich, D. E. Block, J. B. German, C. B. Lebrilla, and D. A. Mills. . 2010. Consumption of human milk oligosaccharides by gut-related microbes. J. Agric. Food Chem. 58:5334–5340. doi: 10.1021/jf9044205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcobal, A., and J. L. Sonnenburg. . 2012. Human milk oligosaccharide consumption by intestinal microbiota. Clin. Microbiol. Infect. 18(Suppl 4):12–15. doi: 10.1111/j.1469-0691.2012.03863.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattar, R., D. F. de Campos Mazo, and F. J. Carrilho. . 2012. Lactose intolerance: diagnosis, genetic, and clinical factors. Clin. Exp. Gastroenterol. 5:113–121. doi: 10.2147/CEG.S32368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra, R., D. Barile, M. Marotta, C. B. Lebrilla, C. Chu, and J. B. German. . 2014. Novel high-molecular weight fucosylated milk oligosaccharides identified in dairy streams. PLoS One. 9:e96040. doi: 10.1371/journal.pone.0096040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menchik, P., T. Zuber, A. Zuber, and C. I. Moraru. . 2019. Short Communication : Composition of coproduct streams from dairy processing: acid whey and milk permeate. J. Dairy Sci. 102:3978–3984. doi: 10.3168/jds.2018-15951 [DOI] [PubMed] [Google Scholar]
- Montalto, M., V. Curigliano, L. Santoro, M. Vastola, G. Cammarota, R. Manna, A. Gasbarrini, and G. Gasbarrini. . 2006. Management and treatment of lactose malabsorption. World J. Gastroenterol. 12:187–191. doi: 10.3748/wjg.v12.i2.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow, A. L., G. M. Ruiz-Palacios, X. Jiang, and D. S. Newburg. . 2005. Human-milk glycans that inhibit pathogen binding protect breast-feeding infants against infectious diarrhea. J. Nutr. 135:1304–1307. doi: 10.1093/jn/135.5.1304 [DOI] [PubMed] [Google Scholar]
- Moukarzel, S., and L. Bode. . 2017. Human milk oligosaccharides and the preterm infant: a journey in sickness and in health. Clin. Perinatol. 44:193–207. doi: 10.1016/j.clp.2016.11.014 [DOI] [PubMed] [Google Scholar]
- Naranjo, V. D., T. D. Bidner, and L. L. Southern. . 2010. Comparison of dried whey permeate and a carbohydrate product in diets for nursery pigs. J. Anim. Sci. 88:1868–1879. doi: 10.2527/jas.2009-2438 [DOI] [PubMed] [Google Scholar]
- Nessmith, W. B. Jr, J. L. Nelssen, M. D. Tokach, R. D. Goodband, J. R. Bergström, S. S. Dritz, and B. T. Richert. . 1997. Evaluation of the interrelationships among lactose and protein sources in diets for segregated early-weaned pigs. J. Anim. Sci. 75:3214–3221. doi: 10.2527/1997.75123214x [DOI] [PubMed] [Google Scholar]
- Nguyen, T. T., J. W. Kim, J. S. Park, K. H. Hwang, T. S. Jang, C. H. Kim, and D. Kim. . 2016. Identification of oligosaccharides in human milk bound onto the toxin a carbohydrate binding site of Clostridium difficile. J. Microbiol. Biotechnol. 26:659–665. doi: 10.4014/jmb.1509.09034 [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed. Washington (DC): The National Academies Press. [Google Scholar]
- O′Doherty, J. V., S. Dillon, S. Figat, J. J. Callan, and T. Sweeney. . 2010. The effects of lactose inclusion and seaweed extract derived from Laminaria spp. on performance, digestibility of diet components and microbial populations in newly weaned pigs. Anim. Feed Sci. Technol. 157:173–180. doi: 10.1016/j.anifeedsci.2010.03.004 [DOI] [Google Scholar]
- Pacheco, A. R., D. Barile, M. A. Underwood, and D. A. Mills. . 2015. The impact of the milk glycobiome on the neonate gut microbiota. Annu. Rev. Anim. Biosci. 3:419–445. doi: 10.1146/annurev-animal-022114-111112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pié, S., J. P. Lallès, F. Blazy, J. Laffitte, B. Sève, and I. P. Oswald. . 2004. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J. Nutr. 134:641–647. doi: 10.1093/jn/134.3.641 [DOI] [PubMed] [Google Scholar]
- Pierce, K. M., J. J. Callan, P. McCarthy, and J. V. O’Doherty. . 2007. The interaction between lactose level and crude protein concentration on piglet post-weaning performance, nitrogen metabolism, selected faecal microbial populations and faecal volatile fatty acid concentrations. Anim. Feed Sci. Technol. 132:267–282. doi: 10.1016/j.anifeedsci.2006.02.010 [DOI] [Google Scholar]
- Ramani, S., C. J. Stewart, D. R. Laucirica, N. J. Ajami, B. Robertson, C. A. Autran, D. Shinge, S. Rani, S. Anandan, L. Hu, . et al. 2018. Human milk oligosaccharides, milk microbiome and infant gut microbiome modulate neonatal rotavirus infection. Nat. Commun. 9:5010. doi: 10.1038/s41467-018-07476-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikov, E. A., S. S. Comstock, C. Yi, N. Contractor, and S. M. Donovan. . 2014. Dietary bovine lactoferrin increases intestinal cell proliferation in neonatal piglets. J. Nutr. 144:1401–1408. doi: 10.3945/jn.114.196568 [DOI] [PubMed] [Google Scholar]
- Rhouma, M., J. M. Fairbrother, F. Beaudry, and A. Letellier. . 2017. Post weaning diarrhea in pigs: risk factors and non-colistin-based control strategies. Acta Vet. Scand. 59:31. doi: 10.1186/s13028-017-0299-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinninella, E., P. Raoul, M. Cintoni, F. Franceschi, G. A. D. Miggiano, A. Gasbarrini, and M. C. Mele. . 2019. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 7:14. doi: 10.3390/microorganisms7010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins, K. R., A. M. Saxton, and L. L. Southern. . 2006. Estimation of nutrient requirements using broken-line regression analysis. J. Anim. Sci. 84(Suppl):E155–E165. doi: 10.2527/2006.8413_supple155x [DOI] [PubMed] [Google Scholar]
- Ruiz-Palacios G. M., L. E. Cervantes, P. Ramos, B. Chavez-Munguia, and D. S. Newburg. . 2003. Campylobacter jejuni binds intestinal H(O) antigen (Fuc α-1,2 Gal β-1,4 GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J. Biol. Chem. 278:14112–14120. [DOI] [PubMed] [Google Scholar]
- Rusu, D., R. Drouin, Y. Pouliot, S. Gauthier, and P. E. Poubelle. . 2010. A bovine whey protein extract stimulates human neutrophils to generate bioactive IL-1Ra through a NF-κB- and MAPK-dependent mechanism. J. Nutr. 140:382–391. doi: 10.3945/jn.109.109645 [DOI] [PubMed] [Google Scholar]
- Savaiano, D. A., A. J. Ritter, T. R. Klaenhammer, G. M. James, A. T. Longcore, J. R. Chandler, W. A. Walker, and H. L. Foyt. . 2013. Improving lactose digestion and symptoms of lactose intolerance with a novel galacto-oligosaccharide (RP-G28): a randomized, double-blind clinical trial. Nutr. J. 12:160. doi: 10.1186/1475-2891-12-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierack, P., N. Walk, K. Reiter, K. D. Weyrauch, and L. H. Wieler. . 2007. Composition of intestinal Enterobacteriaceae populations of healthy domestic pigs. Microbiology (Reading). 153(Pt 11):3830–3837. doi: 10.1099/mic.0.2007/010173-0 [DOI] [PubMed] [Google Scholar]
- Shen, Y. B., G. Voilqué, J. D. Kim, J. Odle, and S. W. Kim. . 2012. Effects of increasing tryptophan intake on growth and physiological changes in nursery pigs. J. Anim. Sci. 90:2264–2275. doi: 10.2527/jas.2011-4203 [DOI] [PubMed] [Google Scholar]
- Smilowitz, J. T., C. B. Lebrilla, D. A. Mills, J. B. German, and S. L. Freeman. . 2014. Breast milk oligosaccharides: structure-function relationships in the neonate. Annu. Rev. Nutr. 34:143–169. doi: 10.1146/annurev-nutr-071813-105721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M. W., S. Lloyd, and P. S. James. . 1988. T Testing the hypothesis that crypt cell hyperplasia inhibits lactase expression by mouse jejunal enterocytes. Q. J. Exp. Physiol. 73:777–780. doi: 10.1113/expphysiol.1988.sp003197 [DOI] [PubMed] [Google Scholar]
- Solaymani-Mohammadi, S., and S. M. Singer. . 2011. Host immunity and pathogen strain contribute to intestinal disaccharidase impairment following gut infection. J. Immunol. 187:3769–3775. doi: 10.4049/jimmunol.1100606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomons, N. W 2002. Fermentation, fermented foods and lactose intolerance. Eur. J. Clin. Nutr. 56(Suppl 4):S50–S55. doi: 10.1038/sj.ejcn.1601663 [DOI] [PubMed] [Google Scholar]
- Su, Y., W. Yao, O. N. Perez-Gutierrez, H. Smidt, and W. Y. Zhu. . 2008. Changes in abundance of Lactobacillus spp. and Streptococcus suis in the stomach, jejunum and ileum of piglets after weaning. FEMS Microbiol. Ecol. 66:546–555. doi: 10.1111/j.1574-6941.2008.00529.x [DOI] [PubMed] [Google Scholar]
- Szilagyi, A., and N. Ishayek. . 2018. Lactose intolerance, dairy avoidance, and treatment options. Nutrients 10:1994. doi: 10.3390/nu10121994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Bruggencate, S. J. M., I. M. J. Bovee-Oudenhoven, M. L. G. Lettink-Wissink, and R. Van der Meer. . 2005. Dietary fructooligosaccharides increase intestinal permeability in rats. J. Nutr. 135:837–842. doi: 10.1093/jn/135.4.837 [DOI] [PubMed] [Google Scholar]
- Tokach, M. D., J. L. Nelssen, and G. L. Allee. . 1989. Effect of protein and(or) carbohydrate fractions of dried whey on performance and nutrient digestibility of early weaned pigs. J. Anim. Sci. 67:1307–1312. doi: 10.2527/jas1989.6751307x [DOI] [PubMed] [Google Scholar]
- Tran, H., R. Moreno, E. E. Hinkle, J. W. Bundy, J. Walter, T. E. Burkey, and P. S. Miller. . 2012. Effects of lactose and yeast-dried milk on growth performance, fecal microbiota, and immune parameters of nursery pigs. J. Anim. Sci. 90:3049–3059. doi: 10.2527/jas.2011-4544 [DOI] [PubMed] [Google Scholar]
- Triantis, V., L. Bode, and R. J. J. van Neerven. . 2018. Immunological effects of human milk oligosaccharides. Front. Pediatr. 6:190. doi: 10.3389/fped.2018.00190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustunol, Z., and C. Wong. . 2010. Effect of nonfat dry milk and major whey components on interleukin-6 and interleukin-8 production in human intestinal epithelial-like Caco-2 cells. J. Dairy Sci. 93:2311–2314. doi: 10.3168/jds.2009-2607 [DOI] [PubMed] [Google Scholar]
- Vemuri, R., R. Gundamaraju, M. D. Shastri, S. D. Shukla, K. Kalpurath, M. Ball, S. Tristram, E. M. Shankar, K. Ahuja, and R. Eri. . 2018. Gut microbial changes, interactions, and their implications on human lifecycle: an ageing perspective. Biomed Res. Int. 2018:4178607. doi: 10.1155/2018/4178607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema, K 2012. Intestinal fermentation of lactose and prebiotic lactose derivatives, including human milk oligosaccharides. Int. Dairy J. 22:123–140. doi: 10.1016/j.idairyj.2011.10.011 [DOI] [Google Scholar]
- Wang, C., M. Zhang, H. Guo, J. Yan, L. Chen, W. Teng, F. Ren, Y. Li, X. Wang, J. Luo, . et al. 2020. Human milk oligosaccharides activate epidermal growth factor receptor and protect against hypoxia-induced injuries in the mouse intestinal epithelium and Caco2 Cells. J. Nutr. 150:756–762. doi: 10.1093/jn/nxz297 [DOI] [PubMed] [Google Scholar]
- Yu, Z. T., C. Chen, and D. S. Newburg. . 2013. Utilization of major fucosylated and sialylated human milk oligosaccharides by isolated human gut microbes. Glycobiology 23:1281–1292. doi: 10.1093/glycob/cwt065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivkovic, A. M., and D. Barile. . 2011. Bovine milk as a source of functional oligosaccharides for improving human health. Adv. Nutr. 2:284–289. doi: 10.3945/an.111.000455 [DOI] [PMC free article] [PubMed] [Google Scholar]