Abstract
Breast cancer survivorship guidelines recommend at least annual follow-up visits, yet the degree to which this occurs in clinical practice is uncertain. Claims data from a US commercial insurance database (OptumLabs) were used to identify women treated with curative intent surgery for newly diagnosed breast cancer between 2006 and 2014. In 25 035 women, median follow-up was 3 years. In the second year after surgery, 9.6% of the patients did not visit a primary care provider, an oncologist, or a surgeon (guideline-nonadherent). The guideline-nonadherent proportion increased from 7.8% in women diagnosed in 2006 to 12.2% in those diagnosed in 2014 (two-sided Wald P < .001). During years 2–6, guideline-nonadherence was also associated with older age, nonwhite race, no radiation, no chemotherapy, no endocrine therapy, and increasing time after surgery. There is a substantial and increasing rate of inadequate follow-up among breast cancer survivors. This has the potential to impair outcomes.
More than three million breast cancer survivors live in the United States today (1). The American Cancer Society–American Society of Clinical Oncology and National Comprehensive Cancer Network guidelines recommend history and physical exams at least annually (2,3). In addition to evaluating for evidence of recurrence and new cancers, these visits, which may occur with primary care providers, oncologists, or surgeons, are intended to manage toxicities, encourage endocrine therapy adherence and healthy lifestyle, and provide psychosocial support. However, some survivors underuse follow-up care (4). We previously found that during year 1 of follow-up after chemotherapy for breast cancer, 2.0% of 6247 women had no oncologist or primary care provider visit (5). In addition, we found that mammography both in the first and fifth years of follow-up is less likely if a woman did not see oncology during that year (6).
Harnessing the data from OptumLabs Data Warehouse (Cambridge, MA), containing professional, facility, and outpatient medication claims for more than 100 million privately insured and Medicare Advantage enrollees throughout the United States (6), we conducted a retrospective analysis of office visits among a cohort of breast cancer survivors. This analysis was deemed exempt from institutional review board review.
Women older than 18 years who underwent breast surgery for invasive or noninvasive cancer between January 1, 2006, and December 31, 2014, were identified using claims-based algorithms (7,8). Continuous medical and pharmacy coverage was required for 12 months prior to the breast cancer diagnosis and at least 24 months after definitive breast surgery. We assessed care up to December 31, 2016, censoring for disenrollment from a covered plan, development of metastatic disease, or another cancer diagnosis.
Independent variables included age at diagnosis, race and/or ethnicity, region, surgery type, chemotherapy, endocrine therapy, radiation, and baseline comorbidities (captured by the International Classification of Diseases, 9th Revision, and International Classification of Diseases, 10th Revision, codes on claims during 12 months before the cancer diagnosis) (9). Comorbidity burden was evaluated using the Elixhauser score (none, 1, 2, 3, or ≥4). Use of endocrine therapy was based on pharmacy claims (yes vs no during each 12-month period).
Our primary endpoint was whether a patient had at least one primary care, oncology, or surgery office visit during each of the years 2–6 after surgery. Office visits were identified by restricting data to claims for which the place of service was reported as “office” and the Healthcare Common Procedure Coding System code was reported as 99201–99205 (new patients), 99211–99215 (established patients), or 99241–99245 (office consultations). If patients had multiple visits on the same day to a given specialty, only one was counted. Visits to hematology or oncology were classified as oncology, and visits to family medicine or internal medicine (not necessarily to a specific primary care provider) were classified as primary care (regardless of what diseases were billed for at that visit). Internal medicine subspecialists were not counted as primary care providers. Primary care visits, oncology visits, surgery visits, the sum of the three, and total visits (including other specialties) were counted for each 12-month period postsurgery.
We used mixed effects logistic regression, including an observation for each year of follow-up per patient, a random effect for patient, baseline characteristics, and an annual indicator for endocrine therapy to assess predictors of no primary care, oncology, or surgery visit that year. We reported odds ratios (ORs) and calculated overall two-sided Wald P values for the association between each baseline variable and no primary care provider, oncology, or surgery visit (considering P < .05 statistically significant) using Stata 15 (StataCorp, College Station, TX).
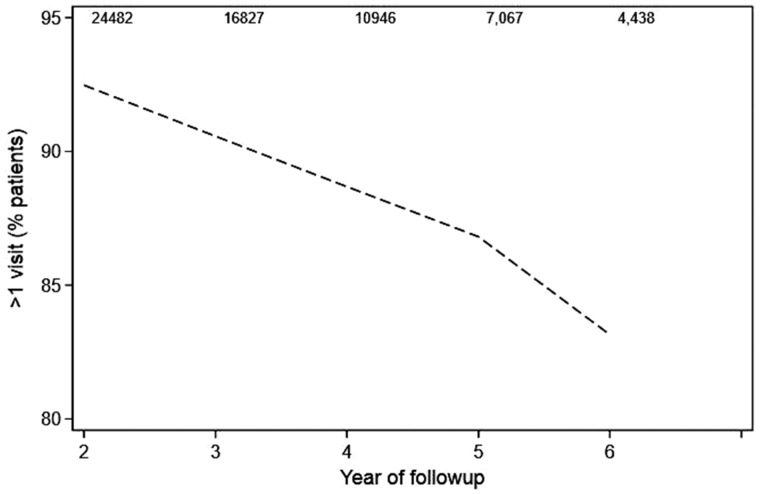
A total of 25 035 women were included, 69.6% of whom were younger than 65 years at the time of diagnosis, with 3 years′ median follow-up (interquartile range [IQR] = 2–6 years). Supplementary Table 1 (available online) provides demographic and clinical details. Visit frequencies over years 2–6 after surgery are illustrated in Supplementary Figure 1 (available online). In the second year, 9.6% did not visit a primary care provider, oncologist, or surgeon. The percentage of survivors without at least one primary care, oncology, or surgery visit increased over time (see Figure 1) to 12.2% (of 16 827 followed at least 3 years) in the third year, 14.5% (of 10 946 followed at least 4 years) in the fourth year, 16.9% (of 7067 followed at least 5 years) in the fifth year, and 20.7% (of 4438 followed at least 6 years) in the sixth year (P < .001). In addition to later years (3–6) of follow-up, our multivariable model revealed that the following were associated with no primary care, oncology, or surgery visit in a given year (shown in Table 1): older age, nonwhite race, no receipt of chemotherapy, no receipt of radiation, no endocrine therapy, receipt of lumpectomy, and more recent diagnosis (P < .001 for all except surgery type, for which P = .002). The proportion who had no primary care, oncology, or surgery visits in the second year after surgery increased from 7.8% for those diagnosed in 2006 to 12.2% for those diagnosed in 2014 (P < .001).
Figure 1.
Line graph depicting the proportion of patients who had at least one visit to primary care, oncology, or surgery during each year 2–6 after surgery. The number of patients available for assessment each year is shown at the top.
Table 1.
Multivariable model predicting no primary care, oncology, or surgery visit during a given year from 2–6
| Characteristic | OR (95% CI) | P * |
|---|---|---|
| Age, y | ||
| 18–34 | Referent | <.001 |
| 35–44 | 1.03 (0.42 to 1.65) | |
| 45–54 | 0.96 (0.40 to 1.52) | |
| 55–64 | 0.94 (0.39 to 1.49) | |
| 65–74 | 2.25 (0.91 to 3.60) | |
| ≥75 | 3.44 (1.36 to 5.53) | |
| Race/ethnicity | ||
| White | Referent | <.001 |
| Asian | 1.81 (1.24 to 2.38) | |
| Black | 1.37 (1.14 to 1.60) | |
| Hispanic | 1.14 (0.88 to 1.40) | |
| Other | 1.06 (0.77 to 1.35) | |
| Elixhauser (category) | ||
| None | Referent | .329 |
| 1 | 0.95 (0.81 to 1.09) | |
| 2 | 0.91 (0.76 to 1.06) | |
| 3 | 0.90 (0.72 to 1.08) | |
| ≥4 | 1.09 (0.88 to 1.31) | |
| Radiation | ||
| No | Referent | <.001 |
| Yes | 0.45 (0.38 to 0.52) | |
| Surgery | ||
| Lumpectomy | Referent | .002 |
| Mastectomy | 0.77 (0.64 to 0.90) | |
| Chemotherapy | ||
| No | Referent | <.001 |
| Yes | 0.33 (0.28 to 0.38) | |
| Reconstruction | ||
| No | Referent | .068 |
| Yes | 0.85 (0.70 to 1.00) | |
| Diagnosis year | ||
| 2006 | Referent | <.001 |
| 2007 | 0.99 (0.74 to 1.25) | |
| 2008 | 1.01 (0.76 to 1.27) | |
| 2009 | 1.24 (0.93 to 1.54) | |
| 2010 | 0.94 (0.70 to 1.18) | |
| 2011 | 1.06 (0.80 to 1.32) | |
| 2012 | 1.32 (0.99 to 1.65) | |
| 2013 | 1.29 (0.96 to 1.61) | |
| 2014 | 1.53 (1.12 to 1.95) | |
| Endocrine therapy† | ||
| 0 | Referent | <.001 |
| 1 | 0.20 (0.18 to 0.23) | |
| Year of follow-up | ||
| 2 | Referent | <.001 |
| 3 | 3.33 (2.79 to 3.87) | |
| 4 | 4.61 (3.88 to 5.35) | |
| 5 | 6.06 (5.07 to 7.05) | |
| 6 | 7.98 (6.60 to 9.36) | |
| Prior year visits | ||
| ≥1 visit to primary care, oncology, or surgery | Referent | <.001 |
| No visits | 2.84 (2.29 to 3.38) |
Two-sided Wald test. CI = confidence interval; OR = odds ratio.
Endocrine therapy defined by at least one pharmacy claim for tamoxifen, letrozole, anastrozole, or exemestane during the year of interest.
These results are similar to those from prior studies showing that many breast cancer survivors do not receive adequate long-term follow-up (4). With regard to the associations between lack of receipt of various therapies (including mastectomy) and less likelihood of follow-up, It is possible that a lower risk of recurrence or less need for management of toxicity drove the associations between lack of receipt of various therapies and less likelihood of follow-up visits. However, this should be investigated further using nonclaims-based methodology. This study is limited by the following: 1) its observational, claims-based design, which potentially allows unobserved confounders; 2) the fact that all included survivors had health insurance, which reduced generalizability, and the fact that type of plan and deductible data were not available for analysis; 3) the variable follow-up lengths, which produced missing data at later time points; and 4) its short median follow-up, which prevented an assessment of the relationship between visit frequency and disease-related outcomes. The rarity of primary care follow-up raises concerns regarding receipt of screening and preventive care, as well as management of nononcologic conditions (eg, cardiovascular disease). However, most national guidelines no longer recommend annual primary care visits for the general adult population. Our novel finding of an increased likelihood of no visits in survivors diagnosed more recently may be an unintended consequence of an increase in high-deductible health plans (with more cost sharing), as well as other obstacles to health-care access including a shortage of clinicians (particularly primary care providers). Indeed, we might find an even higher proportion without follow-up among uninsured survivors.
Funding
KJR was supported by KL2 TR002379 from the National Center for Advancing Translational Sciences of the National Institutes of Health. LS was supported by a 2016 National Comprehensive Cancer Network Foundation Young Investigator Award (principal investigator: Ruddy). RDH was supported by the Mayo Clinic Department of Medicine Catalyst for Advancing in Academics Award.
Notes
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health.
TCH reports consulting activities with TerSera Therapeutics (honoraria to institution) and research funding to institution from Takeda, all outside this submitted work; all other authors have no conflicts of interest to declare.
Supplementary Material
References
- 1. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol. 2016;34(6):611–635. [DOI] [PubMed] [Google Scholar]
- 3. Giordano SH, Elias AD, Gradishar WJ.. NCCN guidelines updates: breast cancer. J Natl Compr Canc Netw. 2018;16(5S):605–610. [DOI] [PubMed] [Google Scholar]
- 4. Ruddy KJ, Guo H, Baker EL, et al. Randomized phase 2 trial of a coordinated breast cancer follow-up care program. Cancer. 2016;122(22):3546. [DOI] [PubMed] [Google Scholar]
- 5. Leal AD, Van Houten H, Sangaralingham L, et al. Breast cancer survivorship care variations between adjuvant chemotherapy regimens. Clin Breast Cancer. 2018;18(4):e513–e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wallace PJ, Shah ND, Dennen T, et al. Optum Labs: building a novel node in the learning health care system. Health Aff (Millwood). 2014;33(7):1187–1194. [DOI] [PubMed] [Google Scholar]
- 7. Nattinger AB, Laud PW, Bajorunaite R, et al. An algorithm for the use of Medicare claims data to identify women with incident breast cancer. Health Serv Res. 2004;39(6 pt 1):1733–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gold HT, Do HT.. Evaluation of three algorithms to identify incident breast cancer in Medicare claims data. Health Serv Res. 2007;42(5):2056–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers of Disease Control and Prevention/National Center for Health Statistics. Classification of Diseases, Functioning, and Disability https://www.cdc.gov/nchs/icd/. Accessed October 8, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.