Abstract
Sensory systems are tuned by selection to maximize organismal fitness in particular environments. This tuning has implications for intraspecies communication, the maintenance of species boundaries, and speciation. Tuning of color vision largely depends on the sequence of the expressed opsin proteins. To improve tuning of visual sensitivities to shifts in habitat or foraging ecology over the course of development, many organisms change which opsins are expressed. Changes in this developmental sequence (heterochronic shifts) can create differences in visual sensitivity among closely related species. The genetic mechanisms by which these developmental shifts occur are poorly understood. Here, we use quantitative trait locus analyses, genome sequencing, and gene expression studies in African cichlid fishes to identify a role for the transcription factor Tbx2a in driving a switch between long wavelength sensitive (LWS) and Rhodopsin-like (RH2) opsin expression. We identify binding sites for Tbx2a in the LWS promoter and the highly conserved locus control region of RH2 which concurrently promote LWS expression while repressing RH2 expression. We also present evidence that a single change in Tbx2a regulatory sequence has led to a species difference in visual tuning, providing the first mechanistic model for the evolution of rapid switches in sensory tuning. This difference in visual tuning likely has important roles in evolution as it corresponds to differences in diet, microhabitat choice, and male nuptial coloration.
Keywords: color vision, photoreceptor development, transcription factor, cone opsin, cichlid
Introduction
Color vision enhances the ability of organisms to detect signals from food, conspecifics, predators, and the environment and can therefore assert a profound influence on fitness (Cronin et al. 2014). Changes to the spectral tuning of visual signals are predicted to have dramatic effects on the evolutionary trajectory of species and may even lead to speciation (Seehausen et al. 2008). It is thus of considerable interest to understand the genetic basis of evolutionary changes in visual sensitivity. The most variable component in tuning color vision are cone opsins (Sharpe and Gegenfurtner 1999), G-coupled protein receptors that are expressed in the cone cells of the retina. In most cases, the opsins are bound to the chromophore 11-cis retinal to form a specific visual pigment with a unique visual sensitivity. Visual tuning can either change through differences in the amino acid sequence of the opsin genes or the expression level of the opsin genes relative to one another. In many organisms, opsin expression changes through development to suit the differing visual needs of larvae, juveniles, and adults (Bowmaker 1995). These changes involve the regulation of multiple opsins that are switched on and off at particular developmental times (Carleton et al. 2008). Once this developmental progression is established, evolution can produce diverse adult phenotypes between species by altering the developmental pattern. In that way, larval or juvenile opsins from one species might be differentially expressed in other species producing large shifts in adult visual sensitivity (Carleton and Kocher 2001; Fuller et al. 2004; Shand et al. 2008; Sandkam, Young, and Breden 2015; Sandkam, Young, Breden, et al. 2015). Such visual tuning is therefore a prime example of altered developmental patterns or heterochronies contributing to phenotypic diversity (Gould 1977; Raff 1996; Smith 2003).
Modulation of visual sensitivity through shifts in expression requires the upregulation of one opsin coupled with concomitant downregulation of another (Shand et al. 2002; Carleton et al. 2008; Fuller and Claricoates 2011). If each opsin is independently regulated by different gene regulatory networks, simultaneous evolutionary changes in the expression of two opsins would be extremely unlikely. Differences in visual tuning among species would then require multiple substitutions that might be constrained by mutational dynamics. Shifts in opsin expression could occur more quickly if a single regulatory element could downregulate expression of one opsin while simultaneously upregulating expression of another.
To determine whether such a common regulatory framework exists, we have examined the mechanisms of opsin gene expression in the radiation of cichlid fishes in East Africa. More than 1,000 closely related species of African cichlid fishes represent an excellent model system for studying the genetic basis of opsin gene expression. The genome of each cichlid species encodes seven distinct opsin genes; short wavelength sensitive (SWS)1, SWS2A, SWS2B are expressed in single cones, whereas Rhodopsin-like (RH2)Aα, RH2Aβ, RH2B, and long wavelength sensitive (LWS) are expressed in double cones. Despite carrying all seven opsin genes in the genome, individual species typically express only three genes as adults. The particular set of expressed genes differs among even closely related species but tunes visual sensitivity to either short (SWS1, RH2A, RH2B), medium (SWS2B, RH2A, RH2B), or long (SWS2A, RH2A, LWS) wavelengths (reviewed in Carleton et al. 2016). These differences among species are likely the result of heterochronic changes because the ancestral species expressed each set of opsin genes at different stages of development (Carleton et al. 2008; O’Quin, Smith, Sharma et al. 2011). In the closely related species to many great lake cichlids, Oreochromis niloticus, individuals begin life with a visual system tuned to be more sensitive to green light by expressing high levels of RH2 and low levels of LWS opsin, then switch to a more red sensitive visual system later in life by expressing high levels of LWS and low levels of RH2 opsin (Carleton et al. 2008).
In order to take an unbiased genetic approach to identify factors important for opsin expression, we previously analyzed a genetic cross between Aulonocara baenschi, a Lake Malawi cichlid with high expression of RH2A opsin and no expression of LWS opsin (green shifted vision), and Tramitichromis intermedius, a species with low RH2A expression but high LWS expression (O’Quin et al. 2012; red shifted vision). Among the F2 from the cross, we detected a strong negative correlation between the expression of LWS and RH2A (R2 = −0.78) (O’Quin, Smith, Naseer, et al. 2011). Using quantitative trait locus analysis, we have compared gene expression phenotypes among all the F2 and tested for association with markers spaced across the genome (supplementary fig. 1, Supplementary Material online). This controls for genetic effects at other loci in the genome. We now report the identification of a trans-acting expression quantitative trait locus (eQTL) on linkage group 10 (LG10) that explains 48.6% of the variation in LWS expression and 57.1% of the variation in RH2A. The negative correlation and overlapping eQTLs suggest a common regulatory factor that can simultaneously promote expression of LWS while repressing expression of RH2A. Our combined fine mapping, transcriptomics, and functional analyses show that the transcription factor Tbx2a likely controls this switch between LWS and RH2A expression.
Results
The Transcription Factor Tbx2a Is Strongly Correlated with the Switch Between LWS and RH2A Opsin Expression
In order to identify the factor responsible for switching LWS/RH2A expression between A. baenschi and T. intermedius, we first used microsatellite markers and previously developed RAD-seq markers (O’Quin et al. 2012) to fine map the LWS/RH2A eQTL to a region that spans 1.15 Mb and contains 31 genes (fig. 1A and B; supplementary table 1, Supplementary Material online). We predicted that the expression of transcription factors which might regulate LWS should be correlated with LWS expression. In retinal transcriptomes from T. intermedius (N = 2) and A. baenschi (N = 4; an average of 183 million reads), only five genes were differentially expressed between A. baenschi and T. intermedius with fragments per kilobase of gene model per million (FPKM) ratios >2 (adora2b [3.244], gjb1 [2.458], msi2 [2.365], LOC101484605 [2.239], and Tbx2a [11.293]; supplementary table 1, Supplementary Material online).
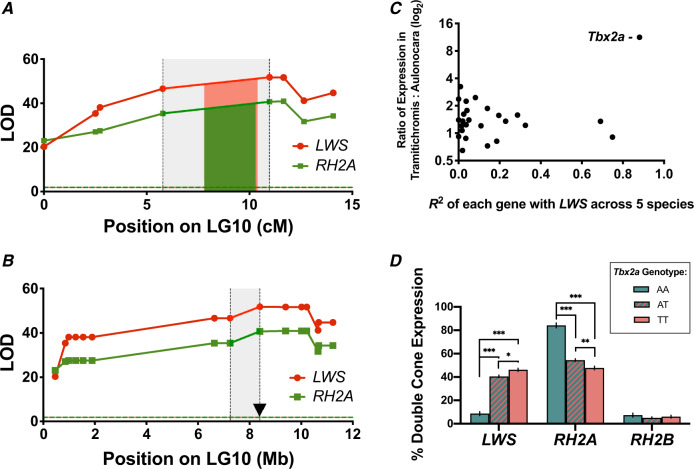
Fig. 1.
(A) Fine map of the LWS and RH2A eQTL from the cross between Aulonocara baenschi and Tramitichromis intermedius in mapping distance and (B) in genome space by mapping markers onto the Metriaclima zebra reference genome. Red and green regions denote the 95% Bayesian CI for LWS and RH2A, respectively. Gray shaded region indicates the space between the two markers surrounding the 95% CI. The candidate gene Tbx2a is located within this region, roughly 8 kb from the highest associated microsatellite marker and denoted as a triangle. (C) Expression of the genes in the region between QTL markers surrounding the 95% CI. Only the transcription factor Tbx2a is both highly correlated with LWS gene expression across species and strongly differentially expressed between T. intermedius and A. baenschi. X-axis: correlation between expression of each gene with LWS expression across five Malawi cichlid species not used in the cross. Y-axis: ratio of expression between T. intermedius (N = 2) and A. baenschi (N = 4) (log2). (D) Opsin expression by Tbx2a genotype across 285 F2. AA = homozygous A. baenschi Tbx2a, TT = homozygous T. intermedius Tbx2a, and AT = heterozygous. See Materials and Methods for how opsin expression was quantified. ***P < 0.0001, **P < 0.001, *P < 0.01.
Given that the basic structure of the opsin regulatory network is conserved across closely related cichlid taxa, we examined previously sequenced retinal transcriptomes of five additional cichlid species as a second means of identifying transcription factors regulating LWS. Of these five taxa, three had high LWS expression and two had low/no LWS expression in their transcriptomes (Brawand et al. 2014). Of the 31 eQTL genes, only three were strongly correlated (R2 > 0.7) with LWS expression (ubc [R2 = 0.92], cenpv [R2 = 0.74], and Tbx2a [R2 = 0.88]; supplementary table 1, Supplementary Material online).
Tbx2a was the only gene that was both differentially expressed between T. intermedius and A. baenschi and strongly correlated with LWS across the five additional cichlid species, suggesting that Tbx2a might explain the LWS/RH2A eQTL effect (fig. 1C). Further support for this hypothesis was provided by the F2 from the cross. LWS and RH2A expression differed significantly between individuals that were homozygous for the A. baenschi Tbx2a versus those with the T. intermedius Tbx2a genotype (fig. 1D).
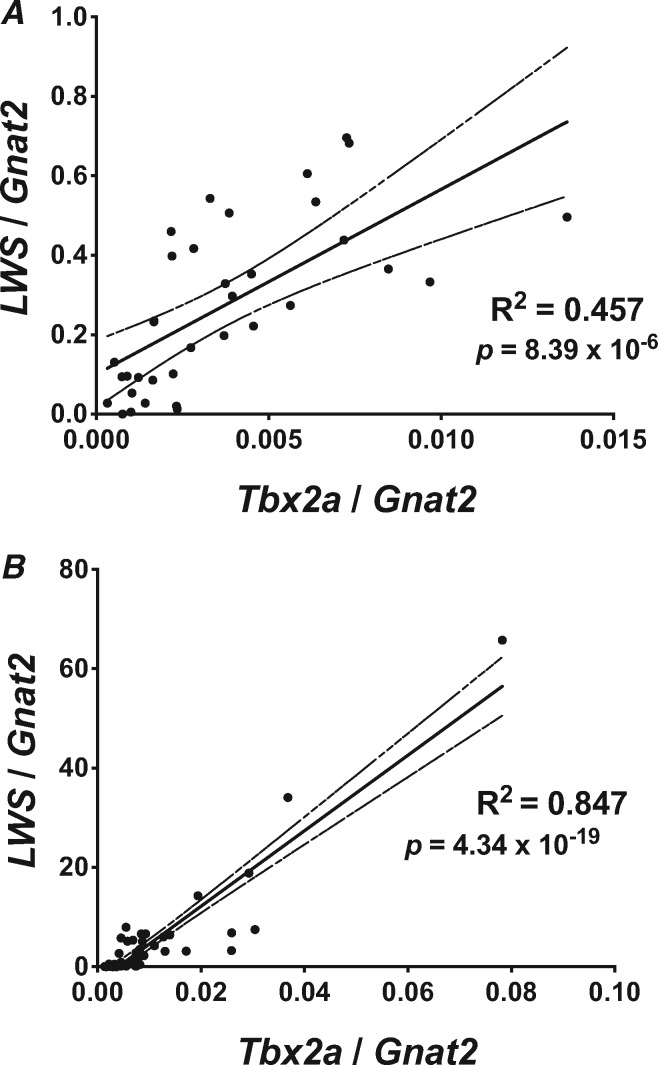
To confirm the correlation of Tbx2a with LWS expression, we first used qRT-PCR to measure retinal gene expression of Tbx2a and LWS relative to the cone-specific gene Gnat2. We detected a strong correlation between Tbx2a and LWS expression across F2 offspring from our cross between T. intermedius and A. baenschi (R2 = 0.457, P < 0.0001; fig. 2A). In addition, the expression of Tbx2a is strongly correlated with LWS expression across 58 additional species of Malawi cichlids that vary in LWS opsin gene expression (R2 = 0.847, P < 0.0001; fig. 2B). We were unable to correct for phylogeny due to the tenuous nature of the cichlid species relationships; however, a strong correlation was maintained even when the two most extreme datapoints were removed (R2 = 0.537, P < 0.0001).
Fig. 2.
Retinal expression of Tbx2a and LWS is strongly correlated (A) across 35 F2 individuals from the cross between Tramitichromis intermedius and Aulonocara baenschi in the lab, and (B) across 58 wild caught Malawi cichlid species. Solid line indicates the linear regression, dashed line denotes the 95% CI of the regression and P value denotes the probability that the slope is nonzero.
To test whether Tbx2a also corresponds to the switch from RH2 to LWS during the development of tilapia (a relative of both A. baenschi and T. intermedius), we generated whole-retina transcriptomes from tilapia at six stages of development. We found a strong positive correlation between the absolute expression of Tbx2a and LWS (R2 = 0.55) and a strong negative correlation between the absolute expression of Tbx2a and RH2 (R2 = −0.43).
The strong correlations of Tbx2a with LWS within a cross, among natural species variants, and across a developmental switch in a related species made Tbx2a a strong candidate to explain the LWS/RH2A eQTL. Furthermore, Tbx2a is located just 7.9 kb from the most highly associated eQTL microsatellite marker, which explained 54.8% of the variance in LWS expression and 46.6% of variance in RH2A expression across 285 F2. Members of the T-box 2 (Tbx2) transcription factor family can act as both activators and repressors of gene expression depending upon the promoter context and play a key role in retinal development (Takabatake et al. 2000; Behesti et al. 2009; Abrahams et al. 2010). Tbx2b plays a prominent role in specifying photoreceptor precursors to become short wavelength sensitive cone cells in zebrafish (Alvarez-Delfin et al. 2009).
How Does Tbx2a Regulate Opsin Expression?
In order to explore how Tbx2a modulates the switch in expression between LWS/RH2A, we identified transcription factor binding sites (TFBS) in regulatory regions of the LWS and RH2A opsins and tested the functionality of these sites experimentally. In cichlids, there are two RH2A loci (RH2Aα and RH2Aβ) that produce functionally identical proteins, but which have unique promoter sequences. To identify possible Tbx2a binding sites, we compared 800 bp of the proximal promoter from each of RH2Aα and RH2Aβ to the Homo sapiens TBX2 transcription factor binding matrix (JASPAR database of transcription factor binding profiles). We could identify only one TBX2 binding site upstream of the RH2Aβ transcriptional start site (TSS) (relative score = 0.921) and none upstream of the RH2Aα TSS.
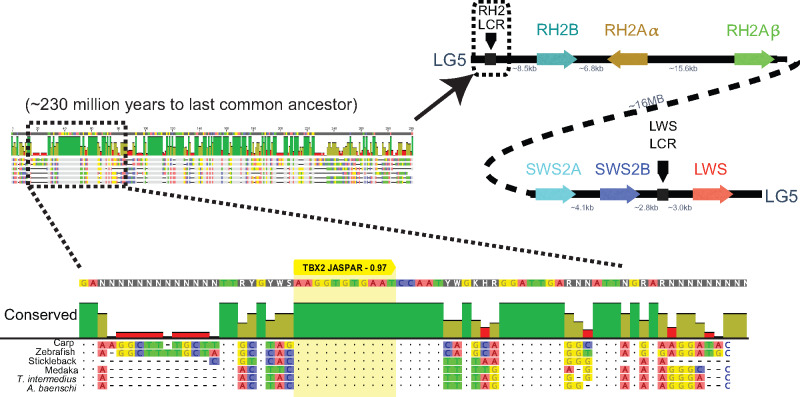
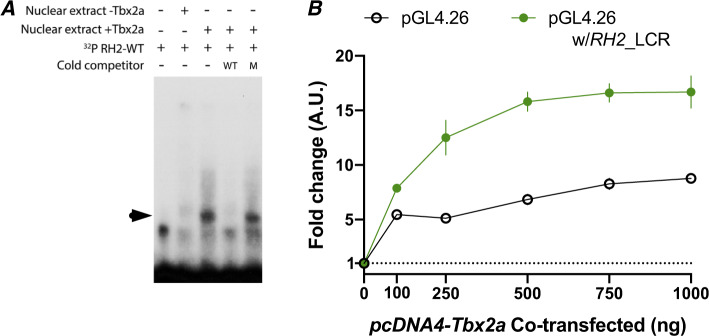
The RH2 gene array is downstream of a locus control region (LCR) that controls RH2 gene expression in zebrafish (Tsujimura et al. 2007, 2010). To identify whether Tbx2a could be interacting with the RH2 LCR, we aligned the RH2 LCR of A. baenschi, T. intermedius, carp (Cyprinus carpio), zebrafish (Danio rerio), stickleback (Gasterosteus aculeatus), and medaka (Oryzias latipes) (fig. 3). We detected a 16 bp region that was perfectly conserved across all six species (representing ∼230 Ma divergence) and contained a strongly predicted Tbx2 binding site based on the H. sapiens TBX2 matrix (relative profile score of 0.97). To test whether the cichlid Tbx2a transcription factor binds to the predicted sites in the RH2 LCR or RH2Aβ promoter, we cloned the coding region of Tbx2a into the pcDNA4-HisMax C vector and expressed the recombinant cichlid Tbx2a in the mammalian Madin-Darby canine kidney (MDCK) cell line. We used the Tbx2a enriched nuclear fraction to perform electrophoretic mobility shift assays (EMSA) with probes containing the predicted Tbx2a binding sites in the RH2 LCR and RH2Aβ promoter. The cichlid Tbx2a did not bind to the RH2Aβ promoter probe but shifted the RH2 LCR probe with high affinity. Furthermore, cichlid Tbx2a did not bind to a mutant version of the probe where the four bases corresponding to the key bases in the H. sapiens TBX2 binding motif were changed (fig. 4A;supplementary table 2, Supplementary Material online). These results show that the cichlid Tbx2a can bind to the highly conserved TBX2 TFBS in the RH2 LCR.
Fig. 3.
The RH2 LCR identified in zebrafish is highly conserved across teleosts. A Tbx2 binding site (yellow) is strongly predicted in the longest conserved region of the RH2 LCR when compared with the JASPAR database (score 0.97).
Fig. 4.
(A) Tbx2a directly binds to RH2 LCR in vitro. Radioactive-labeled RH2 LCR probes containing the putative Tbx2a binding site were incubated with nuclear extracts of MDCK cells transfected with empty vector (−Tbx2a) and vector encoding the cichlid Tbx2a transcription factor (+Tbx2a). The shift created by protein–DNA binding (indicated by arrowhead) was reduced by the addition of unlabeled probe in 400× molar excess (cold wild-type competitor, WT), but to a lesser degree by unlabeled probes with critical mutations in binding site residues (cold mutant competitor, M). (B) MDCK cells were cotransfected with constructs containing the RH2 LCR sequence upstream of a minimal promoter driving firefly luciferase reporter gene with increasing concentrations of Tbx2a expression plasmids. Constructs without the RH2 LCR sequence were used as a control. Fold change is relative to reporter gene activation in the absence of Tbx2a expression vectors.
To test whether Tbx2a binding to the RH2 LCR affects downstream gene expression, we cloned the RH2 LCR into a pGL4.26 vector upstream of luciferase driven by a minimal promoter. Luciferase expression increased substantially with increasing amounts of Tbx2a during cotransfection in MDCK cells but only in the presence of the pGL4.26 plasmid containing the RH2 LCR (fig. 4B). We detected a similar pattern of expression in both IMCD3 and HEK293 cell lines (supplementary fig. 2, Supplementary Material online). These results demonstrate that the interaction between Tbx2a and the RH2 LCR is capable of modulating downstream gene expression.
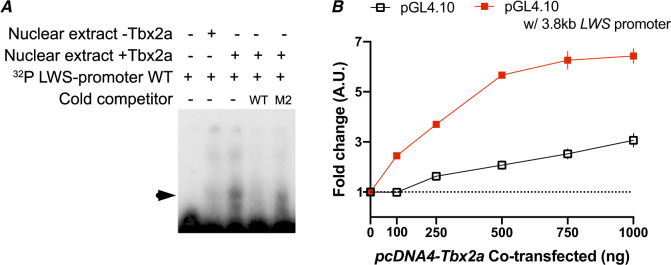
The LWS opsin is located on LG5, ∼16 Mb away from the RH2 array. However, the RH2 array is regulated by its own LCR and proximal promoter. This colocalization of RH2 and LWS on the same chromosome is shared across fishes, but it is generally assumed that the two arrays are so far apart that there is no effect of being on the same chromosome. The LWS LCR is located ∼3 kb upstream of the LWS coding sequence (Tam et al. 2011). JASPAR predicted two TBX2 TFBS in the LWS LCR but with lower confidence (relative scores of 0.85 and 0.81) than the site in the RH2 LCR (score of 0.97). Our EMSA results suggest that cichlid Tbx2a does not bind to either of these sites. JASPAR also predicted two TBX2 TFBS with a relative score of ≥0.90 (0.92 located 1,178 bp upstream and 0.96 located 471 bp upstream of the LWS TSS) within the 3 kb region between the LWS LCR and the beginning of the LWS coding sequence. EMSA demonstrated the binding of cichlid Tbx2a to the site 471 bp upstream of LWS TSS but not to the 1,178 bp upstream site (fig. 5A).
Fig. 5.
(A) Tbx2a directly binds to LWS promoter in vitro. Radioactive-labeled LWS promoter probes containing the putative Tbx2a binding site were incubated with nuclear extracts of MDCK cells transfected with empty vector (−Tbx2a) and vector encoding the cichlid Tbx2a transcription factor (+Tbx2a). The shift created by protein–DNA binding (indicated by arrowhead) was reduced by the addition of unlabeled probe in 400× molar excess (cold wild-type competitor, WT), but to a lesser degree by unlabeled probes with critical mutations in binding site residues (cold mutant competitor, M2). (B) MDCK cells were cotransfected with constructs containing the LWS promoter sequence upstream of a firefly luciferase reporter gene with increasing concentrations of Tbx2a expression plasmids. Constructs without the LWS promoter sequence were used as a control. Fold change is relative to reporter gene activation in the absence of Tbx2a expression vectors.
To test whether Tbx2a binding to the LWS promoter can modulate gene expression, we cloned the full 3.8 kb from upstream of the LWS LCR to the LWS TSS into a pGL4.10 vector upstream of a promoter-less copy of the luciferase gene. In the presence of Tbx2a protein, we observed a much larger increase in luciferase expression from the pGL4.10 vector containing the upstream LWS region compared with the control plasmids (fig. 5B). Thus, the interaction between Tbx2a and the LWS regulatory region is capable of modulating gene expression.
How Has Tbx2a Driven Differences Across Species?
The amino acid sequence of the Tbx2a binding domain is the same between A. baneschi and T. intermedius. To identify how Tbx2a might lead to the LWS/RH2A shift in visual tuning across species, we asked if differences in Tbx2a expression across species is due to changes in linked regulatory sequence between T. intermedius and A. baenschi. We used transcriptomes from T. intermedius, A. baenschi, and four F1 from the cross to examine allele-specific expression. We determined that 85.6% of the variance in Tbx2a expression is due to differences in cis-linked regulatory sequence. Our results strongly suggest that a change in regulatory sequence linked to the Tbx2a locus can explain the LWS/RH2A eQTL.
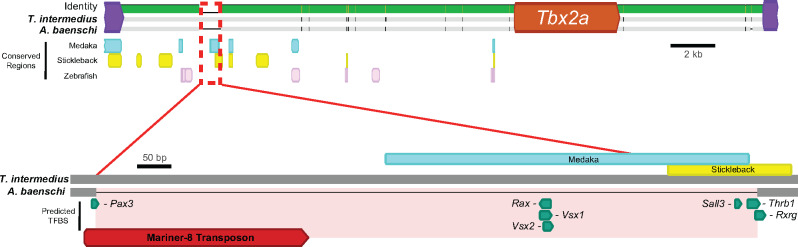
To identify the regulatory variant, we sequenced whole genomes from T. intermedius (N = 2) and A. baenschi (N = 2) and mapped the reads onto the Metriaclima zebra reference genome (coverage of 13.13 ± 9.19, 13.02 ± 8.99, 11.04 ± 7.71, and 9.13 ± 6.58, respectively). We identified only 10 fixed variants between T. intermedius and A. baenschi in the ∼19.5 kb region from the start of Tbx2a to the next gene upstream (protein phosphatase 1D), and only three fixed differences in the ∼5.5 kb region from the end of Tbx2a to the next gene downstream (acetyl-CoA carboxylase alpha; fig. 6, supplementary table 3, Supplementary Material online). The largest of these differences was a 938 bp deletion in A. baenschi located 13.4 kb upstream of the Tbx2a start site. A deletion downstream of Tbx2a in A. baenschi was only one base pair in length. The remaining 11 fixed differences were single-base pair changes. Interestingly, the 968 bp deletion contained a Mariner-8 transposon, a “cut and paste” transposable element (Hartl 2001).
Fig. 6.
Top: Alignment of the Tbx2a region between Tramitichromis intermedius and Aulonocara baenschi from the next gene upstream (protein phosphatase 1D) to the next gene downstream (acetyl-CoA carboxylase alpha). The 13 differences between species are denoted as vertical black lines (for single-base pair differences) or horizontal lines (for sequence missing in one species). Regions identified by MultiPipMaker to be conserved are shown as colored rectangles for medaka (blue), stickleback (yellow), and zebrafish (pink). Bottom: The 968 bp that is deleted in A. baenschi (shaded red). This region shows some conservation with both stickleback and medaka and contains several predicted TFBS for important retinal transcription factors as well as Pax3 (known to regulate Tbx2). The presence of a Mariner-8 transposon suggests this region was lost when this transposon excised from the area and took important regulatory sequence with it.
To delineate the regulatory implications of these sequence differences, we undertook a phylogenomic approach. We identified intergenic sequences that are conserved between T. intermedius, stickleback (Gasterosteus aculeatus, ∼128 Ma divergence), medaka (Oryzias latipes, ∼119 Ma divergence), and/or zebrafish (Danio rerio, ∼230 Ma divergence). Of the 18,818 bp between Tbx2a and the next gene upstream in T. intermedius, the MultiPipMaker (Schwartz et al. 2000) software identified only 4.6% of the sequence that was conserved between T. intermedius, stickleback and medaka, 1.8% conserved between T. intermedius, medaka and zebrafish, and only 0.3% was conserved among all four. The 938 bp deleted in A. baenschi contained the sequence that was partially conserved in both stickleback and medaka (fig. 6). In this region, we also identified predicted binding sites for six transcription factors that are known to play key roles in retinal development across vertebrates (Swaroop et al. 2010; Musser and Arendt 2017): Rxrg, Thrb1, Sall3, Vsx1, Rax, and Vsx2 (fig. 6). Moreover, the cichlid sequence contained a predicted binding site for Pax3, which is known to regulate Tbx2 expression (Liu et al. 2013). All of these potentially important regulatory sites have been lost in A. baenschi, which may explain the difference in Tbx2a expression between T. intermedius and A. baenschi.
Discussion
Spectral tuning of color vision is frequently accomplished by rapid evolutionary changes in opsin gene expression (Parry et al. 2005; Fuller and Claricoates 2011; Sandkam, Young, Breden, et al. 2015). However, evolution of gene expression through multiple independent mutations in distinct gene regulatory elements would be inherently slow and improbable (Halfon 2017). We propose that this could be overcome if regulatory factors could simultaneously downregulate one opsin while upregulating another opsin. Thus, a switch requiring only a single genetic change could allow rapid evolutionary changes in visual tuning among closely related taxa. Such a switch might have evolved to facilitate developmental shifts in opsin expression (Carleton et al. 2008; Shand et al. 2008). Here, we show that the transcription factor Tbx2a modulates an established switch between RH2A and LWS opsin gene expression. Using DNA-binding proteins and luciferase reporter assays, we show that Tbx2a can directly interact with the RH2 LCR and LWS promoter regions. We propose that a single change in the regulatory region upstream of Tbx2a is likely responsible for the rapid shift in expression-based visual tuning between T. intermedius (red shifted) and A. baenschi (green shifted). This shift in visual systems is associated with important ecological and evolutionary differences between these species including differences in male nuptial coloration (T. intermedius is red, whereas A. baenschi is yellow) and depth (A. baenschi forages at depths of 10–20 m, where red light has been attenuated). This work therefore identifies a regulatory element that controls the expression of multiple opsin genes and has thus played an important role in evolution and diversification.
Tbx2a Is a Major Regulatory Component of Switching Between RH2A and LWS Opsins
We have identified Tbx2a as a key component of the switch between RH2A and LWS opsin gene expression by fine mapping an eQTL on LG10 in a cross between A. baenschi (no LWS expression) and T. intermedius (high LWS expression). Support for the idea that Tbx2a explains the eQTL comes from its location only ∼8 kb from the highest associated microsatellite marker and the strong correlation of Tbx2a expression with LWS opsin expression in F2 hybrids. Tbx2a expression was also strongly correlated with LWS expression across 58 other species of Lake Malawi cichlids, as well as four cichlids in other African lakes and rivers, indicating that the role of Tbx2a is largely conserved across cichlids (fig. 2).
Many of the factors involved in photoreceptor gene regulation are highly conserved from fish to mammals (Viets et al. 2016). The Tbx2 class of transcription factors predates the vertebrate lineage. Musser and Arendt (2017) showed that the Tbx2 class has played a strong role in photoreceptor development since well before the emergence of the mammalian lineage. Although mammals have lost the RH2 class of opsins, they have maintained both LWS and TBX2. This raises the possibility that TBX2 may be acting as a conserved transcription factor modulating LWS expression. Nearly 20 years ago, Tbx2 was found to be expressed along the same dorsal–ventral pattern as M/LWS opsin during development in the mouse retina, which has high M/LWS expression in the dorsal, but not ventral, retina (Sowden et al. 2001). More recently, this possibility was further supported by a data set from single-cell transcriptomes of mouse retinal cells generated by Macosko et al. (2015). Reanalysis of this data set revealed that none of the “pure” SWS1 cones expressed Tbx2, yet some of the cones coexpressing Sws1 and Lws did show Tbx2 expression (Macosko et al. 2015; Musser and Arendt 2017). This pattern raises the possibility that the role of Tbx2a in promoting LWS expression could be conserved in even distantly related vertebrate lineages.
How Does Tbx2a Regulate Opsin Expression?
Unlike the other T-box transcription factors, members of the Tbx2/3 class are known to act as repressors as well as activators (Sebé-Pedrós and Ruiz-Trillo 2017). To determine how Tbx2a is modulating RH2A and LWS opsin expression, we examined predicted Tbx2 binding sites located in RH2A and LWS opsin regulatory sequence. Within the RH2 LCR, we found a predicted Tbx2a binding site that was completely conserved across six taxa (representing ∼230 Ma). Such strong conservation suggests that this location plays an important role in the mechanism by which the LCR controls downstream RH2 gene expression. Our EMSA results revealed that cichlid Tbx2a does indeed bind to the RH2 LCR at this location, and our luciferase results show that this Tbx2a–RH2 LCR complex can dramatically alter downstream gene expression. Since the interaction between Tbx2a and the RH2 LCR occurs at a highly conserved TFBS, it suggests an important role for Tbx2a in mediating how the RH2 LCR operates, which should be further explored.
Similar results were obtained with the LWS promoter. Our EMSA tests revealed that Tbx2a can indeed bind to a TFBS located 471 bp upstream of the LWS TSS and this Tbx2a-LWS promoter complex can dramatically alter downstream gene expression.
It was not expected that Tbx2a would increase expression of luciferase downstream of both the LWS promoter and the RH2 LCR. In cichlid retina, Tbx2a is directly correlated with LWS expression, but inversely correlated with expression of RH2A. However, LCRs are generally controlled by multiple transcription factors that interact with one another (Li et al. 2002). It seems plausible that Tbx2a interacts with additional cichlid cone cell-specific cofactors to produce the anticipated effect of decreasing RH2A expression, and that these cofactors were not present in the cell lines used in our luciferase experiments. Our recent QTL study in a new cichlid cross has identified additional genetic factors that contribute to variation in RH2A expression (Nandamuri et al. 2018) and these may help explain the cell-specific actions of the RH2 LCR on RH2A expression.
Single Changes to Opsin Regulatory Elements Can Switch Visual Tuning Between Species
Allele-specific expression in F1 hybrids between A. baenschi and T. intermedius confirmed that a cis-regulatory change in A. baenschi underlies the decrease in Tbx2a expression. Among the fixed differences between A. baenschi and T. intermedius, only the 968 bp deletion located 13 kb upstream of Tbx2a contained predicted TFBS for retinal transcription factors. Within this 968 bp deletion, 119 bp was conserved between T. intermedius, stickleback, and medaka. Conserved noncoding sequences can indicate important regulatory function (Levy et al. 2001). In this region, we found predicted TFBS for known retinal transcription factors including members of the Thrb1 and Rx families. In medaka, knocking out Rx3 resulted in the absence of Tbx2 expression in the retina, and plasmid injections of Rx3 rescued Tbx2 expression (Loosli et al. 2001), suggesting a feedback link between Rx and Tbx genes. Thus, this 968 bp deletion in A. baenschi might have resulted in the difference in Tbx2a expression between A. baenschi and T. intermedius.
Within the 968 bp deletion, we also detected a Mariner-8 transposable element (TE). The mariner class of TEs make a double strand break as they move throughout the genome. When the break is repaired through single-strand annealing, a deletion often occurs in the DNA flanking the break (Munoz-Lopez and Garcia-Perez 2010). African cichlid fishes are known to have high rates of TE movement (Brawand et al. 2014; Conte and Kocher 2015; Conte et al. 2017; Conte et al. 2019). We hypothesize that the deletion in A. baenschi was caused by a Mariner-8 transposable element leaving the area and taking the important Tbx2a regulatory sequence with it. However, stepwise variation in expression of single genes cannot be entirely ruled out.
Conclusion
We have identified an eQTL variant that causes the simultaneous upregulation of one opsin and downregulation of another. We identified the transcription factor Tbx2a as a major component of the regulatory framework, which drives this switch between RH2A and LWS opsin genes by binding to both the RH2 LCR and the LWS promoter. Our data demonstrate that single mutations within the components of this network are sufficient to change the expression of multiple opsin genes. Multiple, independent changes in the regulatory regions of each opsin gene are not required. This difference among species may have co-opted a pre-existing switch that controls normal developmental shifts in opsin expression. Thus, the single mutation in the regulation of Tbx2a observed here caused heterochronic shifts in a developmental pathway to create rapid evolutionary change in visual tuning. This difference in visual tuning corresponds to differences in diet, microhabitat choice, and male nuptial coloration.
Materials and Methods
Fine Mapping the LWS/RH2A Opsin eQTL on LG10
In our previous study of opsin eQTL, we established a cross between T. intermedius and A. baenschi and used restriction site-associated DNA sequences (RAD-seq) across 115 F2 to identify overlapping eQTL on LG10 that explained 48.6% and 57.05% of the expression of LWS and RH2A, respectively (O’Quin et al. 2012). Here, we developed eight fluorescently labeled microsatellite markers across these eQTL and genotyped 288 F2 adults. Primers flanking the microsatellites were designed with Primer3 (Untergasser et al. 2012) and obtained from Eurofins MWG Operon (Huntsville, AL). Forward primers were either fluorescently labeled or ordered with a CAG tag (GCAGTCGGGCGTCATCA) that matched a fluorescent CAG primer which was labeled with either 6’FAM or HEX dye. Polymerase chain reaction (PCR) products were run on an ABI 3730xl Genetic Analyzer and marker genotype was determined in GeneMapper (ABI). All individuals were sacrificed with an overdose of MS-222; DNA was extracted from fin clips using a DNeasy Kit (Qiagen) and retinas were dissected and stored in RNAlater (Invitrogen) at −20 °C prior to RNA extraction. Opsin expression phenotypes were determined by quantitative real-time polymerase chain reaction (qRT-PCR). Total RNA was extracted from whole retinas using an RNeasy kit (Qiagen) including a DNase step, and 0.5 µg was used to make cDNA with SuperScript III (Invitrogen) following manufacturer’s protocol. qRT-PCR was conducted for each of the cone opsins on a Roche 480 Lightcycler as described previously (Hofmann et al. 2009). Briefly, the seven cone opsins present in East African cichlids were measured individually using custom Taqman assays (Invitrogen) with the exception of RH2Aα and RH2Aβ, which were measured in combination because the sequence similarity between these genes is too high to tell them apart with qPCR-based methods (Spady et al. 2005). Results were corrected for relative assay efficiencies calculated by running a multiopsin construct containing all targets in a 1:1 ratio and the absolute efficiency of RH2A was determined from serial dilutions of cDNA (Spady et al. 2006).
The ratio of single to double cones in the cichlid retina is fixed in a highly structured retinal mosaic (Dalton et al. 2014). SWS genes are only expressed in single cones, whereas RH2 and LWS genes are only expressed in double cones (Dalton et al. 2014, 2017). Therefore, we follow the methods outlined in Yourick et al. (2019) and represent the expression of each of the double cone opsins as follows:
The eQTL was fine mapped by combining the previous RAD-seq markers with fluorescent microsatellite markers to provide 15 markers spanning the previously identified LWS/RH2A eQTL. Marker order was identified by BlastN (version 2.2.28+) (Altschul et al. 1997; Johnson et al. 2008) against the M. zebra reference genome (Conte et al. 2019). eQTLs and 95% Bayesian confidence intervals (CIs) were identified for LWS and RH2A using R/qtl (Broman et al. 2003) and the percent of variance in LWS and RH2A expression that was explained by the closest marker to Tbx2a was determined using the lm function in R v3.5.2 (R Core Team 2018). We conservatively identified putative transcription factors by finding all genes in the M. zebra UMD2a reference genome (Conte et al. 2019) between the two markers flanking the 95% CI (corresponding to a 1.15 Mb region).
Retinal Transcriptome Sequencing, Analyses, and Additional Species
To facilitate transcriptome comparisons across species, we first mapped the NCBI RefSeq genes for O. niloticus (RefSeq no. GCF_001858045) onto the M. zebra genome (Conte et al. 2019) using GMAP (version 2015-07-23; Wu and Watanabe 2005) to annotate the corresponding set of genes in M. zebra. We then generated retinal transcriptomes from four A. baenschi and two T. intermedius using a TruSeq RNA sample preparation kit version 2 (Illumina) and run on an Illumina HiSeq1500 with 100 bp single-end reads. The transcriptome reads were aligned to the gene models predicted in the M. zebra genome. Cuffdiff (version 2.2.1) was used to quantify transcript expression in terms of FPKM, which corrects for the size of the gene and the number of reads per sample (Trapnell et al. 2012).
We then compared differential expression between T. intermedius and A. baenschi to retinal transcriptomes of five other East African cichlids that were generated as part of the Broad Institute’s Cichlid Genome Project (Brawand et al. 2014). Two of the species express no LWS: M. zebra (Broad assembly v.0), and Neolamprologus brichardi (Broad assembly v.1). Three express high LWS: Pundamilia nyererei (Broad assembly v.1), Astatotilapia burtoni (Broad assembly v.1), and Oreochromis niloticus (Broad anchored assembly v1.1). To compare genes between species, we mapped the NCBI RefSeq transcripts for Oreochromis niloticus (RefSeq no. GCF_001858045) onto the genome for each of the five species using GMAP (Wu and Watanabe 2005). We then aligned retinal transcriptome reads and quantified relative gene expression in each species as FPKM using Tophat2 and Cuffdiff (Trapnell et al. 2012).
Of the 31 genes in the 95% CI, two were only expressed in one species and excluded from correlation analyses; the correlation of expression with LWS opsin expression (FPKM) was calculated using linear regressions across the five species. To test whether transcriptomes provided reliable indicators of gene expression, we compared the relative opsin expression levels within the transcriptomes to our previous qRT-PCR studies and found there to be good agreement (Schulte et al. 2014).
Identifying “Tbx2-Like” as Tbx2a
We found the gene annotated in the M. zebra genome as Tbx2-like to be both highly correlated with LWS across species and highly differentially expressed between T. intermedius and A. baenschi. To determine the true identity of this Tbx2-like locus, we built a phylogeny including Tbx2 sequences of eight additional vertebrate species (Danio rerio, Gasterosteus aculeatus, Oryzias latipes, Oreochromis niloticus, Gallus gallus, Homo sapiens, Bos taurus, and Mus musculus). Sequences were aligned using MAFFT (Katoh et al. 2009) and maximum likelihood trees were constructed using GARLI (Bazinet et al. 2014) with 100 bootstrap replicates to determine branch support (supplementary fig. 3, Supplementary Material online). The Tbx2-like fell within the Tbx2a clade, and identity was further confirmed by synteny in the UCSC genome browser across five species (M. zebra, O. niloticus, O. latipes, G. aculeatus, and D. rerio; supplementary fig. 4, Supplementary Material online).
Genome Sequencing of T. intermedius and A. baenschi
Genomes from two individuals of T. intermedius and two individuals of A. baenschi were sequenced using a TruSeq DNA sample preparation kit and run on an Illumina HiSeq1500 (100 bp paired-end reads with 550 bp inserts). Reads were filtered then quality checked and trimmed with Trimmomatic (Bolger et al. 2014) before being aligned to the M. zebra reference genome (Conte et al. 2019) using bwa-mem (version 0.7.12-r1039; Li and Durbin 2010). Alignments were converted to BAM format, duplicates were marked and whole genome metrics were determined using Picard (version 2.1.0). Alignments were viewed in IGV (Robinson et al. 2011) and regions of interest were edited manually in Geneious 11 before comparing the consensus sequence between species.
Correlating Expression of Tbx2a and LWS
The expression of Tbx2a, LWS, and Gnat2 (a cone-specific gene) was measured from retinal cDNA using custom probe-based qPCR assays (Taqman probes [Invitrogen]; see supplementary table 4, Supplementary Material online, for primer/probe sequences and efficiencies). Assay efficiencies were calculated from a 1,000-fold dilution series of a custom gene construct that contained the five target opsin genes in a 1:1 ratio. The expression of LWS and Tbx2a in each individual was normalized to expression of Gnat2 using the following equation:
such that E is the assay efficiency found from the dilution series and Ct is the critical threshold of that reaction in the sample. To determine if expression of Tbx2a is correlated with LWS expression across species, we conducted qPCR on 58 wild caught Malawi cichlid species (see supplementary table 5, Supplementary Material online, for species list and Hofmann et al. 2009 for description of collections). To determine the strength of the correlation of Tbx2a with LWS within the cross between A. baenschi and T. intermedius, we conducted qPCR on 35 F2 individuals. We ran a linear regression between the two genes in each data set in GraphPad Prism v.8.0.1.
Correlating Tbx2a, LWS, and RH2 Across Developmental Shift
We generated retinal transcriptomes from tilapia at six ages that span a developmental shift from RH2 to LWS opsin expression (Carleton et al. 2008) (14, 28, 36, 62, 114, and 196 days post hatch). Samples were prepared with a TruSeq RNA sample preparation kit v.2 (Illumina) and run on an Illumina HiSeq1500 with 100 bp single-end reads. Reads were aligned to the O. niloticus reference genome and Cuffdiff (version 2.2.1) was used to quantify transcript expression in terms of FPKM (Trapnell et al. 2012). The expression of Tbx2a, LWS, and RH2 genes were normalized by the expression of Gnat2 to account for differences in library size. To determine if Tbx2a corresponds with the developmental switch in opsin gene expression, we ran independent correlations of Tbx2a with LWS and RH2 opsins. If Tbx2a explains the developmental switch, then we expected to see a positive correlation with LWS and a negative correlation with RH2.
Cis Versus Trans Control of Tbx2a Expression Using Allele-Specific Expression
To determine if differences in Tbx2a expression were due to linked differences in regulatory sequence (thus supporting Tbx2a as a candidate for driving the LWS/RH2A eQTL), we identified single nucleotide polymorphisms in the expressed Tbx2a sequence that were differentially fixed between A. baenschi and T. intermedius using the transcriptomes described above. We then generated retinal transcriptomes from four F1 from the cross using a TruSeq RNA Sample preparation kit v.2 (Illumina) and sequenced on an Illumina HiSeq1000 with 100 bp single-end reads. Reads were mapped onto the M. zebra genome as before. We compared the number of reads of T. intermedius alleles to the number of reads of A. baenschi alleles. We then compared the log2 of this ratio to the log of the ratio of expression between the A. baenschi and T. intermedius parents (measured as FPKM described above).
Values closer to 1 reveal more of the variance in Tbx2a expression is explained by cis-linked regulatory differences (Wittkopp et al. 2004).
Identifying Fixed Variants Between Species Linked to Tbx2a
The genomes of T. intermedius (N = 2) and A. baenschi (N = 2) (described above) were compared to identify fixed differences around Tbx2a from the next gene upstream (Protein Phosphatase 1D (XM_004557358), located 19.5 kb upstream) and the next gene downstream (Acetyl-CoA carboxylase alpha [XM_014408615], located 5.5 kb downstream) in Geneious v.11. We found one large deletion in A. baenschi and verified this deletion with PCR and Sanger sequencing that spanned the deletion. To verify that this deletion was not specific to our lab population, we obtained Sanger sequence reads that spanned the deletion from two unrelated A. baenschi individuals collected in the wild in 2012 from Nkhoma Reef, Lake Malawi, Africa.
Transposable Element Within A. baenschi Deletion
To search for transposable elements in the vicinity of the A. baenschi deletion, we compared the intact sequence from T. intermedius, including 100 bp before and after the deletion, to the GIRI Repbase repository of transposable elements using CENSOR (Kohany et al. 2006).
Genomic Footprinting to Identify Sequence Conservation Upstream of Tbx2a
As Tbx2a is an evolutionarily conserved gene, important regulatory sequence is likely to be conserved across distantly related species. To find such conserved regions upstream of Tbx2a, we gathered the sequence between Tbx2a and the next gene upstream (protein phosphatase 1D [PPM1D]) from the medaka (23 kb), zebrafish (401 kb), and stickleback (18 kb) reference genomes on the UCSC genome browser. We then identified regions of conservation between these species and T. intermedius using MultiPipMaker (Schwartz et al. 2000).
Identifying Transcription Factor Binding Sites
The TRANSFAC repository of vertebrate TFBSs (Mathelier et al. 2016) was used to predict TFBS at P < 0.0001 from the T. intermedius sequence that was missing in A. baenschi. The list was then reviewed for transcription factors known to play key roles in vertebrate photoreceptor development (Musser and Arendt 2017).
Tbx2 Binding Sites in RH2 and LWS LCRs
The RH2 and LWS classes of opsins each have their own LCRs that have been highly conserved across a wide range of taxa (Tsujimura et al. 2010, 2015; Tam et al. 2011). These LCRs match between A. baenschi and T. intermedius. Using the JASPAR database, we scanned for Tbx2 binding sites (relative score >80%) in the RH2 and LWS LCRs. We also scanned for Tbx2 binding sites (relative score >90%) in the 3 kb region between LWS coding region and the LWS LCR, and 800 bp upstream of the RH2Aα and RH2Aβ genes.
Generating Cichlid Tbx2a Protein
A gBlock synthetic gene from Integrated DNA Technologies was designed to contain the full 1,839 bp of cichlid Tbx2a coding sequence flanked by restriction sites. To clone this gBlock into the pcDNA4-HisMax C mammalian expression vector (ThermoFisher Scientific), both were double digested (KpnI-HF and XhoI) and run on a PCR clean up column (Machery-Nagel).
The cut products were ligated together with a Quick Ligation kit (NEB) and used to transform DH5α Escherichia coli. Colonies were PCR screened for presence of the correct insert size. Correct reading frame and coding sequence of the construct was verified by Sanger sequencing. The pcDNA4-HisMax vector adds both poly-histidine and Xpress tags to the N terminal of expressed proteins. Because there is no cichlid-specific Tbx2a antibody, α-Xpress antibody was used for all western blots.
To produce recombinant Tbx2a protein, MDCK cells were transfected with the pcDNA4-Tbx2a construct using Lipofectamine 3000 (ThermoFisher Scientific) following manufacturer’s protocol. Cells were harvested 48 h after transfection and nuclear proteins were obtained with the classical method of nuclei isolation in hypotonic buffer. Protein extracts were quantified by BCA protein assay according to manufacturer’s protocol (Pierce) and solubilized in 4× Laemmli buffer + β-mercaptoethanol. After denaturation, 7 μg of nuclear protein extract was separated by SDS-PAGE and transferred to PVDF membrane (Trans-Blot Turbo System, Bio-Rad). We followed standard immunoblot procedures using primary antibodies raised against the Xpress tag (ThermoFisher R910-25, 1:5,000), and antimouse IgG HRP-conjugated secondary antibodies (Jackson ImmunoResearch 715-035-150, 1:10,000). Detection was performed by enhanced chemiluminescence using the SuperSignal West Pico Plus system (ThermoFisher). The expressed protein matched the predicted size for the cichlid Tbx2a (72 kDa) and was localized to the nucleus (supplementary fig. 5, Supplementary Material online).
Electrophoretic Mobility Shift Assay
The predicted binding motif of Tbx2 in H. sapiens is 11 bases long. We designed all EMSA probes to also include 15 bases upstream and downstream of the predicted Tbx2 binding site, making all probes 41 bp in length (supplementary table 2, Supplementary Material online). Mutant probes were designed such that the most influential sites in the Tbx2 binding motif were changed to intolerable bases. Probes were generated by annealing complementary oligos in duplex buffer (10 mM Tris–HCl pH 8.0, 1 mM EDTA, 50 mM NaCl), boiled in water and allowed to cool overnight at 4 °C. Annealing was verified by running on a 4% agarose gel next to single stranded oligos. We generated 32P-labeled probes with T4 polynucleotide kinase (PNK) and γ-32P ATP (30 pmol probe, 1X T4 PNK reaction buffer, 16.5 pmol γ-32P ATP, 20 units T4 PNK). Reactions were incubated at 37 °C for 30 min followed by heat inactivation at 65 °C for 20 min. Unincorporated γ-32P ATP was removed by running the product through Illustra MicroSpin G-25 Columns (GE Healthcare) and probe radioactivity was measured.
All EMSA reactions consisted of binding buffer (10 mM Tris, 50 mM KCl, 1 mM DTT; pH 7.5), 5 mM MgCl2, 0.05 μg/μl Poly dI-dC, and 100,000 dpm of 32P-labeled probe. Each probe containing the predicted Tbx2 binding site was run in the presence and absence of cichlid Tbx2 (15 μg of nuclear lysate). Unlabeled wild-type and mutant probes (≥400 molar excess) were used to test the specificity of the binding. Reactions were incubated for 60 min at room temperature and run on a 6% DNA Retardation gel (Invitrogen) in TBE buffer. Gels were then dried and exposed to radiographic film on an amplifying screen cassette overnight at −80 °C.
Luciferase Assays
To determine if Tbx2a directly affects expression of sequence downstream of the RH2 and LWS LCRs, we generated luciferase constructs. Because the RH2 LCR interacts with the promoter of all three downstream RH2 genes (Tsujimura et al. 2015), we chose to clone 495 bp of the RH2 LCR into pGL4.26, a luciferase plasmid that contains its own minimal promoter. For the LWS LCR, we cloned the entire 3.9 kb region from the LWS LCR to the LWS TSS into pGL4.10, a promoter-less luciferase plasmid. For both plasmids, PCR products were generated from genomic DNA with a Phusion High-Fidelity PCR kit (NEB) and primers that introduced KpnI and XhoI restriction sites. Cloning of PCR products was performed as described above. For luciferase assays, 15,000 MDCK cells were plated into each well of a 24-well plate in 500 μl of MEM alpha medium with 10% FBS. Two days after plating, all wells were transfected with 150 ng of the respective reporter vector (empty pGL4.26, pGL4.26-RH2_LCR, empty pGL4.10, or pGL4.10-LWS), and 5 ng of thymidine kinase promoter-Renilla luciferase reporter plasmid (pRL-TK) as a control for transfection efficiency. Wells were cotransfected with either 0, 100, 250, 500, 750, or 1,000 ng of the pcDNA4-Tbx2a plasmid described above. Three replicate wells were run in each experiment, and each experiment was repeated three times. An inert pUC19 plasmid was used to achieve transfections of equimolar amounts per well. Two days after transfection, cells were harvested, rinsed in PBS, lysed, and luciferase/Renilla signal was quantified on a GloMax-Multi+ dual injector luminometer using a Dual-Glo Luciferase Assay system (Promega). To determine the effect of increasing the amount of Tbx2a cotransfected on luciferase expression, we transformed the measure of luciferase/Renilla for each cotransfected reaction relative to the average measure of luciferase/Renilla of the same plasmid that was not cotransfected with Tbx2a. To determine if Tbx2a was interacting with the RH2 LCR or LWS promoter, we compared pGL4.26-RH2_LCR to empty pGL4.26 and pGL4.10-LWS to empty pGL4.10 at each treatment using t-tests in Prism v8.0.1.
Supplementary Material
Supplementary data are available at Molecular Biology and Evolution online.
Supplementary Material
Acknowledgments
We wish to thank Miranda Yourick, Daniel Escobar‐Camacho, Frances Clark, Thomas Kocher and the members of the Swaroop lab for valuable discussions and feedback. All animal procedures were approved by the University of Maryland College Park IACUC (Protocol # R-15-54). This work was supported by the National Eye Institute of the National Institutes of Health (R01EY024639 to K.L.C.; Intramural Research Program ZIAEY000450 and ZIAEY000546 to A.S.).
Author Contributions
B.A.S., K.L.C., L.C., and A.S. designed the research; C.O. and B.A.S. generated the eQTL map and qPCR data; B.A.S. and S.P.N. generated sequence data; B.A.S. and L.C. performed protein experiments; K.L.C., B.A.S., M.C., and W.J.G. performed genome and transcriptome analyses. B.A.S. and K.L.C. wrote the manuscript; all authors revised the manuscript.
DNA-seq and RNA-seq reads have been deposited at the NCBI Sequencing Read Archive (accession no. PRJNA551471). Additional data have been included as Supplementary Material.
References
- Abrahams A, Parker MI, Prince S.. 2010. The T-box transcription factor Tbx2: its role in development and possible implication in cancer. IUBMB Life. 62:92–102. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W, Lipman DJ.. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res. 25(17):3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Delfin K, Morris AC, Snelson CD, Gamse JT, Gupta T, Marlow FL, Mullins MC, Burgess HA, Granato M, Fadool JM.. 2009. Tbx2b is required for ultraviolet photoreceptor cell specification during zebrafish retinal development. Proc Natl Acad Sci U S A. 106(6):2023–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazinet AL, Zwickl DJ, Cummings MP.. 2014. A gateway for phylogenetic analysis powered by grid computing featuring GARLI 2.0. Syst Biol. 63(5):812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behesti H, Papaioannou VE, Sowden JC.. 2009. Loss of Tbx2 delays optic vesicle invagination leading to small optic cups. Dev Biol. 333(2):360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B.. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30(15):2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmaker JK. 1995. The visual pigments of fish. Prog Retinal Eye Res. 15(1):1–31. [Google Scholar]
- Brawand D, Wagner CE, Li YI, Malinsky M, Keller I, Fan SH, Simakov O, Ng AY, Lim ZW, Bezault E, et al. 2014. The genomic substrate for adaptive radiation in African cichlid fish. Nature. 513(7518):375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman KW, Wu H, Sen S, Churchill GA.. 2003. R/qtl: QTL mapping in experimental crosses. Bioinformatics. 19(7):889–890. [DOI] [PubMed] [Google Scholar]
- Carleton KL, Dalton BE, Escobar-Camacho D, Nandamuri SP.. 2016. Proximate and ultimate causes of variable visual sensitivities: insights from cichlid fish radiations. Genesis. 54(6):299–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton KL, Kocher TD.. 2001. Cone opsin genes of African cichlid fishes: tuning spectral sensitivity by differential gene expression. Mol Biol Evol. 18(8):1540–1550. [DOI] [PubMed] [Google Scholar]
- Carleton KL, Spady TC, Streelman JT, Kidd MR, McFarland WN, Loew ER.. 2008. Visual sensitivities tuned by heterochronic shifts in opsin gene expression. BMC Biol. 6(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte MA, Gammerdinger WJ, Bartie KL, Penman DJ, Kocher TD.. 2017. A high quality assembly of the Nile Tilapia (Oreochromis niloticus) genome reveals the structure of two sex determination regions. BMC Genomics. 18(1):341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte MA, Joshi R, Moore EC, Nandamuri SP, Gammerdinger WJ, Roberts RB, Carleton KL, Lien S, Kocher TD.. 2019. Chromosome-scale assemblies reveal the structural evolution of African cichlid genomes. Gigascience. 8(4): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte MA, Kocher TD.. 2015. An improved genome reference for the African cichlid, Metriaclima zebra. BMC Genomics. 16:724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin W, Johnsen S, Marshall J, Warrant J.. 2014. Visual ecology. Princeton (NJ: ): Princeton University Press. [Google Scholar]
- Dalton BE, de Busserolles F, Marshall NJ, Carleton KL.. 2017. Retinal specialization through spatially varying cell densities and opsin coexpression in cichlid fish. J Exp Biol. 220(2):266–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton BE, Loew ER, Cronin TW, Carleton KL.. 2014. Spectral tuning by opsin coexpression in retinal regions that view different parts of the visual field. Proc Biol Sci. 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RC, Carleton KL, Fadool JM, Spady TC, Travis J.. 2004. Population variation in opsin expression in the bluefin killifish, Lucania goodei: a real-time PCR study. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 190(2):147–154. [DOI] [PubMed] [Google Scholar]
- Fuller RC, Claricoates KM.. 2011. Rapid light-induced shifts in opsin expression: finding new opsins, discerning mechanisms of change, and implications for visual sensitivity. Mol Ecol. 20(16):3321–3335. [DOI] [PubMed] [Google Scholar]
- Gould SJ. 1977. Ontogeny and phylogeny. Cambridge (MA: ): Belknap Press of Harvard University Press. [Google Scholar]
- Halfon MS. 2017. Perspectives on gene regulatory network evolution. Trends Genet. 33(7):436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl DL. 2001. Discovery of the transposable element mariner. Genetics. 157(2):471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann CM, O’Quin KE, Marshall NJ, Cronin TW, Seehausen O, Carleton KL.. 2009. The eyes have it: regulatory and structural changes both underlie cichlid visual pigment diversity. PLoS Biol. 7(12):e1000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL.. 2008. NCBI BLAST: a better web interface. Nucleic Acids Res. 36(Web Server):W5–W9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Asimenos G, Toh H.. 2009. Multiple alignment of DNA sequences with MAFFT. Methods Mol Biol. 537:39–64. [DOI] [PubMed] [Google Scholar]
- Kohany O, Gentles AJ, Hankus L, Jurka J.. 2006. Annotation, submission and screening of repetitive elements in Repbase: Repbase Submitter and Censor. BMC Bioinformatics. 7:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S, Hannenhalli S, Workman C.. 2001. Enrichment of regulatory signals in conserved non-coding genomic sequence. Bioinformatics. 17(10):871–877. [DOI] [PubMed] [Google Scholar]
- Li H, Durbin R.. 2010. Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics. 26(5):589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Peterson KR, Fang X, Stamatoyannopoulos G.. 2002. Locus control regions. Blood. 100(9):3077–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Cao JX, Lv JH, Dong L, Pier E, Xu GX, Wang RA, Xu ZX, Goding C, Cui RT.. 2013. TBX2 expression is regulated by PAX3 in the melanocyte lineage. Pigment Cell Melanoma Res. 26:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loosli F, Winkler S, Burgtorf C, Wurmbach E, Ansorge W, Henrich T, Grabher C, Arendt D, Carl M, Krone A, et al. 2001. Medaka eyeless is the key factor linking retinal determination and eye growth. Development. 128(20):4035–4044. [DOI] [PubMed] [Google Scholar]
- Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM, et al. 2015. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 161(5):1202–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathelier A, Fornes O, Arenillas DJ, Chen C-Y, Denay G, Lee J, Shi W, Shyr C, Tan G, Worsley-Hunt R, et al. 2016. JASPAR 2016: a major expansion and update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 44(D1):D110–D115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Lopez M, Garcia-Perez JL.. 2010. DNA transposons: nature and applications in genomics. Curr Genomics. 11(2):115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser JM, Arendt D.. 2017. Loss and gain of cone types in vertebrate ciliary photoreceptor evolution. Dev Biol. 431(1):26–35. [DOI] [PubMed] [Google Scholar]
- Nandamuri SP, Conte MA, Carleton KL.. 2018. Multiple trans QTL and one cis-regulatory deletion are associated with the differential expression of cone opsins in African cichlids. BMC Genomics. 19(1):945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Quin KE, Schulte JE, Patel Z, Kahn N, Naseer Z, Wang H, Conte MA, Carleton KL.. 2012. Evolution of cichlid vision via trans-regulatory divergence. BMC Evol Biol. 12:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Quin KE, Smith AR, Sharma A, Carleton KL.. 2011. New evidence for the role of heterochrony in the repeated evolution of cichlid opsin expression. Evol Dev. 13:193–203. [DOI] [PubMed] [Google Scholar]
- O’Quin KE, Smith D, Naseer Z, Schulte J, Engel SD, Loh YH, Streelman JT, Boore JL, Carleton KL.. 2011. Divergence in cis-regulatory sequences surrounding the opsin gene arrays of African cichlid fishes. BMC Evol Biol. 11:120.21554730 [Google Scholar]
- Parry JW, Carleton KL, Spady T, Carboo A, Hunt DM, Bowmaker JK.. 2005. Mix and match color vision: tuning spectral sensitivity by differential opsin gene expression in Lake Malawi cichlids. Curr Biol. 15(19):1734–1739. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2018. R: a language and environment for statistical computing. Vienna (Austria: ): R Foundation for Statistical Computing. [Google Scholar]
- Raff RA. 1996. The shape of life: genes, development, and the evolution of animal form. Chicago (IL: ): University of Chicago Press. [Google Scholar]
- Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP.. 2011. Integrative genomics viewer. Nat Biotechnol. 29(1):24–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkam B, Young CM, Breden F.. 2015. Beauty in the eyes of the beholders: colour vision is tuned to mate preference in the Trinidadian guppy (Poecilia reticulata). Mol Ecol. 24(3):596–609. [DOI] [PubMed] [Google Scholar]
- Sandkam BA, Young CM, Breden FM, Bourne GR, Breden F.. 2015. Color vision varies more among populations than among species of live-bearing fish from South America. BMC Evol Biol. 15(1):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte JE, O’Brien CS, Conte MA, O’Quin KE, Carleton KL.. 2014. Interspecific variation in Rx1 expression controls opsin expression and causes visual system diversity in African cichlid fishes. Mol Biol Evol. 31(9):2297–2308. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Zhang Z, Frazer KA, Smit A, Riemer C, Bouck J, Gibbs R, Hardison R, Miller W.. 2000. PipMaker: a web server for aligning two genomic DNA sequences. Genome Res. 10(4):577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebé-Pedrós A, Ruiz-Trillo I.. 2017. Evolution and classification of the T-box transcription factor family. In: Frasch M, editor. T-box genes in development and disease Cambridge (MA: ): Academic Press; p. 1–26. [DOI] [PubMed] [Google Scholar]
- Seehausen O, Terai Y, Magalhaes IS, Carleton KL, Mrosso HD, Miyagi R, van der Sluijs I, Schneider MV, Maan ME, Tachida H, et al. 2008. Speciation through sensory drive in cichlid fish. Nature. 455(7213):620–626. [DOI] [PubMed] [Google Scholar]
- Shand J, Davies WL, Thomas N, Balmer L, Cowing JA, Pointer M, Carvalho LS, Trezise AE, Collin SP, Beazley LD, et al. 2008. The influence of ontogeny and light environment on the expression of visual pigment opsins in the retina of the black bream, Acanthopagrus butcheri. J Exp Biol. 211(9):1495–1503. [DOI] [PubMed] [Google Scholar]
- Shand J, Hart NS, Thomas N, Partridge JC.. 2002. Developmental changes in the cone visual pigments of black bream Acanthopagrus butcheri. J Exp Biol. 205(Pt 23):3661–3667. [DOI] [PubMed] [Google Scholar]
- Sharpe LT, Gegenfurtner KR.. 1999. Color vision: from genes to perception. Cambridge (UK: ): Cambridge University Press. [Google Scholar]
- Smith KK. 2003. Time’s arrow: heterochrony and the evolution of development. Int J Dev Biol. 47:613–621. [PubMed] [Google Scholar]
- Sowden JC, Holt JK, Meins M, Smith HK, Bhattacharya SS.. 2001. Expression of Drosophila omb-related T-box genes in the developing human and mouse neural retina. Invest Ophthalmol Vis Sci. 42(13):3095–3102. [PubMed] [Google Scholar]
- Spady TC, Parry JW, Robinson PR, Hunt DM, Bowmaker JK, Carleton KL.. 2006. Evolution of the cichlid visual palette through ontogenetic subfunctionalization of the opsin gene arrays. Mol Biol Evol. 23(8):1538–1547. [DOI] [PubMed] [Google Scholar]
- Spady TC, Seehausen O, Loew ER, Jordan RC, Kocher TD, Carleton KL.. 2005. Adaptive molecular evolution in the opsin genes of rapidly speciating cichlid species. Mol Biol Evol. 22(6):1412–1422. [DOI] [PubMed] [Google Scholar]
- Swaroop A, Kim D, Forrest D.. 2010. Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat Rev Neurosci. 11(8):563–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabatake Y, Takabatake T, Takeshima K.. 2000. Conserved and divergent expression of T-box genes Tbx2-Tbx5 in Xenopus. Mech Dev. 91(1–2):433–437. [DOI] [PubMed] [Google Scholar]
- Tam KJ, Watson CT, Massah S, Kolybaba AM, Breden F, Prefontaine GG, Beischlag TV.. 2011. Regulatory function of conserved sequences upstream of the long-wave sensitive opsin genes in teleost fishes. Vision Res. 51(21–22):2295–2303. [DOI] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L.. 2012. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 7(3):562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimura T, Chinen A, Kawamura S.. 2007. Identification of a locus control region for quadruplicated green-sensitive opsin genes in zebrafish. Proc Natl Acad Sci U S A. 104(31):12813–12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimura T, Hosoya T, Kawamura S.. 2010. A single enhancer regulating the differential expression of duplicated red-sensitive opsin genes in zebrafish. PLoS Genet. 6(12):e1001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimura T, Masuda R, Ashino R, Kawamura S.. 2015. Spatially differentiated expression of quadruplicated green-sensitive RH2 opsin genes in zebrafish is determined by proximal regulatory regions and gene order to the locus control region. BMC Genet. 16:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG.. 2012. Primer3: new capabilities and interfaces. Nucleic Acids Res. 40(15):e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viets K, Eldred K, Johnston RJ Jr.. 2016. Mechanisms of photoreceptor patterning in vertebrates and invertebrates. Trends Genet. 32(10):638–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittkopp PJ, Haerum BK, Clark AG.. 2004. Evolutionary changes in cis and trans gene regulation. Nature. 430(6995):85–88. [DOI] [PubMed] [Google Scholar]
- Wu TD, Watanabe CK.. 2005. GMAP: a genomic mapping and alignment program for mRNA and EST sequences. Bioinformatics. 21(9):1859–1875. [DOI] [PubMed] [Google Scholar]
- Yourick MR, Sandkam BA, Gammerdinger WJ, Escobar-Camacho D, Nandamuri SP, Clark FE, Joyce B, Conte MA, Kocher TD, Carleton KL.. 2019. Diurnal variation in opsin expression and common housekeeping genes necessitates comprehensive normalization methods for quantitative real-time PCR analyses. Mol Ecol Resour. 19(6):1447–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.