Abstract
Background
Treatment for pediatric ependymoma includes surgical resection followed by local radiotherapy (RT). Proton RT (PRT) enables superior sparing of critical structures compared with photons, with potential to reduce late effects. We report mature outcomes, patterns of failure, and predictors of outcomes in patients treated with PRT.
Methods
One hundred fifty patients (<22 y) with World Health Organization grades II/III ependymoma were treated with PRT between January 2001 and January 2019 at Massachusetts General Hospital. Demographic, tumor, and treatment-related characteristics were analyzed. Event-free survival (EFS), overall survival (OS), and local control (LC) were assessed.
Results
Median follow-up was 6.5 years. EFS, OS, and LC for the intracranial cohort (n = 145) at 7 years were 63.4%, 82.6%, and 76.1%. Fifty-one patients recurred: 26 (51.0%) local failures, 19 (37.3%) distant failures, and 6 (11.8%) synchronous failures. One hundred sixteen patients (77.3%) underwent gross total resection (GTR), 5 (3.3%) underwent near total resection (NTR), and 29 (19.3%) underwent subtotal resection (STR). EFS for the intracranial cohort at 7 years for GTR/NTR and STR was 70.3% and 35.2%. With multivariate analysis, the effect of tumor excision persisted after controlling for tumor location. There was no adverse effect on disease control if surgery to RT interval was within 9 weeks of GTR/NTR.
Conclusion
PRT is effective and safe in pediatric ependymoma. Similar to previous studies, GTR/NTR was the most important prognostic factor. Intervals up to 9 weeks from surgery to PRT did not compromise disease outcomes. There was no LC benefit between patients treated with >54 Gray relative biological effectiveness (GyRBE) versus ≤54 GyRBE.
Keywords: pediatric ependymoma, proton, radiation
Key Points.
PRT for pediatric ependymoma is a safe and effective treatment modality.
The rate of RT necrosis and second malignancies is lower than has been reported.
There was no effect on disease control as long as radiation was started within 9 weeks from surgery in patients with a grade II tumor and GTR/NTR.
Importance of the Study.
Here we report a large cohort of pediatric patients with grades II/III brain and spinal ependymomas treated with PRT and a mature median follow-up time of 6.5 years. Gross total resection was the most significant prognostic predictor for EFS, OS, and LC. Notably, the median dose delivered to this cohort was 54 GyRBE and no benefit of dose escalation beyond 54 GyRBE was found. All spinal grades II/III ependymoma patients remained disease free, possibly indicating a favorable subset of patients. There was a low incidence of second tumors at 7 years (2.2%) and direct radiation brainstem injury (1.1%), but indirect brainstem injury did occur from inflamed irradiated residual tumor (1.4%). Intervals as long as 9 weeks from surgery to PRT do not compromise disease outcomes. Thus, providers should not rush treatment initiation if patients require additional time for postoperative recovery or referral to an appropriate treating facility.
Ependymoma accounts for 6.7% of all pediatric brain tumors, the majority of which occur in patients under 5 years of age.1 Though chemotherapeutic regimens have been employed with some benefit,2–6 the general frontline treatment strategy for localized disease includes maximal safe surgical resection followed by adjuvant focal radiotherapy (RT). Ependymomas can arise anywhere in the brain or spine, but the majority occur in the posterior fossa near the brainstem and other important cranial nerves and structures.7 Tumor location and the young age of patients pose technical challenges in achieving local control while minimizing late effects of treatment.
Proton radiotherapy (PRT) is a form of particle radiation that has unique properties which allow for conformal delivery of radiation dose to the target with reduced dose to surrounding normal structures. This reduced radiation exposure to healthy tissues should minimize the incidence and severity of late adverse side effects, which can negatively impact the quality of life of childhood cancer survivors.8,9 We have previously published early clinical outcomes of 70 pediatric patients with ependymoma who were treated with PRT at our institution.10 In this study, we report mature clinical outcomes in a large proton treated cohort—including disease control outcomes, patterns of failure, the incidence of neurologic injury and secondary malignancies, and the effects of clinical variables on outcomes.
Materials and Methods
Patient Selection
We reviewed outcomes of pediatric patients (<22 y of age at diagnosis) with ependymoma of the brain and spine (n = 169) who were treated at the Francis H. Burr Proton Beam Therapy Center or Harvard Cyclotron from January 15, 2001 to January 16, 2019. The study was approved by the institutional review board of Massachusetts General Hospital. Patients were excluded if they had less than 2 months of follow-up, gross metastatic disease, or World Health Organization grade I (myxopapillary) tumors (Fig. 1). Two patients were lost to follow-up and had no follow-up beyond 2 months after completion of RT. Patients treated with pre-irradiation chemotherapy were included in the analysis. One hundred fifty patients met eligibility criteria and were the overall cohort. The majority (55%) were followed prospectively on a clinical trial (NCT01288235) or through the Pediatric Proton/Photon Consortium Registry (NCT01696721). One hundred forty-five intracranial ependymoma patients were analyzed separately from the primary spinal patients to determine impact of tumor characteristics and treatment factors on outcomes.
Fig. 1.
CONSORT flow diagram.
Radiation Planning
Proton radiotherapy was delivered using either a passive scatter (n = 138; 92%) or pencil beam scanning technique (n = 12; 8%). A radiation planning CT scan with a custom immobilization device was obtained in the treatment position, and preoperative and postoperative MRI sequences were co-registered to the planning CT scan for target delineation. Gross tumor volume (GTV) was defined as the tumor bed, including any gross residual disease. The clinical target volume (CTV) was an 8–10 mm expansion on the GTV. However, CTV was drawn anatomically constrained at natural barriers, such as bone, and trimmed to include only 2–3 mm of the brainstem if it was not clearly involved. There was no planning target volume (PTV) volumetric expansion. The expansion for the aperture margin was 8–10 mm. We endeavored to follow the Children’s Oncology Group (COG) ACNS 0121 and ACNS 0831 guidelines for prescription volume coverage (100% of PTV coverage with 95% of the dose) but favored underdosing when critical structures such as the brainstem or spinal cord reached the tolerance dose. For patients who received >54 Gray relative biological effectiveness (GyRBE), portions of the CTV were underdosed to meet normal tissue constraints for the brainstem and spinal cord. The normal tissue goals were to keep mean dose to the cervical spinal cord 50.4 GyRBE or below, and point maximums to the brainstem below 55–58 GyRBE. Patients with gross residual disease near the brainstem received higher max point doses, but we endeavored to have a steep gradient of dose falloff above 55 GyRBE and tried to keep the median brainstem dose below 52–54 GyRBE. A representative dosimetric image of dose to residual tumor in a patient with gross residual disease near the brainstem is shown in Supplementary Figure 1.
The median prescribed radiation dose was 54.0 GyRBE (50.4–59.4 GyRBE).
• 53.5% of patients with intracranial disease who underwent a gross total resection (GTR) or near total resection (NTR) were given 54 GyRBE, and 41.4% received 55.8–59.4 GyRBE.
• 79.3% of patients with intracranial disease who underwent a subtotal resection (STR) were given 55.8–59.4 GyRBE.
Our dose guidelines respecting normal tissue constraints, along with the dose constraint recommendations of the University of Florida and MD Anderson, are summarized in the consensus paper by Haas Kogen et al.11
Clinical Data
Variables analyzed include the patient age at radiotherapy, sex, date of diagnosis, tumor location and grade, extent of surgical resection, dosimetric data, adjuvant treatment, patterns of failure, surgery-to-radiation time interval, time elapsed during radiation, and disease status at last follow-up. Data on brainstem dosimetry were available for 139 (95.9%) of patients with intracranial tumors. Extent of residual disease was delineated based on a review of operative reports and postoperative and radiation planning MR imaging. GTR was defined as a complete resection intraoperatively and radiographically with no evidence of residual tumor on postoperative imaging. NTR was defined as ≤5 mm residual disease (in any single dimension) on postoperative MRI or microscopic residual tumor noted by the neurosurgeon in the operative report. STR was defined as >5 mm residual disease on postoperative MRI in any dimension. Patients who received chemotherapy prior to RT and had no radiographic evidence of residual disease at the time of RT were analyzed with the GTR cohort. The follow-up duration was calculated for each patient from the start of radiation treatment to the date of last follow-up or date of death from any cause.
Statistical Analysis
All follow-up and disease control endpoints are measured from the start date of RT. An event was defined as a local, distant, or synchronous failure, development of second tumor, or death from any cause. Local control (LC) was defined by the date of the first local or synchronous failure.
We estimated rates of event-free survival (EFS) and overall survival (OS) using the Kaplan–Meier method and evaluated demographic, tumor, and treatment prognostic factors of EFS and OS using the log-rank test for univariate analyses. Cumulative incidence of local failure (LF) and rates of LC were calculated using death and distant failure (DF) as competing risks. Median follow-up time was estimated using the reverse Kaplan–Meier method. The cumulative incidence of both secondary tumor and brainstem injury/necrosis was calculated with death as a competing risk. A secondary tumor was defined as any benign or malignant skin, bone, soft tissue, or brain tumor identified in the radiation field. We estimated hazard ratios (HRs) using the Cox proportional hazards model and the Fine–Gray model for competing risks for both univariate and multivariate regression.12 The multivariate analysis for EFS, OS, and LC included those variables significantly associated with survival outcomes on univariate analysis and included tumor location and extent of surgical resection. Total dose and administration of pre-RT chemotherapy were excluded from the multivariate analysis as these variables were confounded with extent of surgical resection. We combined the GTR and NTR groups to improve the power of our models, as there were too few patients in the NTR group to have an adequate number of events for survival analysis. Brainstem dose characteristics were explored using Fisher’s exact test and the Wilcoxon rank sum test. We investigated the effect of RT timing on disease control by exploring patient groups dichotomized at various time points using clinical knowledge in combination with maximally selected rank statistics and effect sizes based on the hazard ratio. Fisher’s exact test was used to determine if tumor grade predicted type of failure. All P-values reported are based on two-sided tests. All analyses were performed using the R Statistical Package v3.5.
Results
Patient and Treatment Characteristics
Table 1 summarizes the baseline characteristics of all patients included in the cohort. The median time from surgery to PRT among patients who did not receive chemotherapy was 6.9 weeks. Of the 41 patients (27.3%) who received chemotherapy before radiation, 22 (57.3%) had residual disease after resection and 26 (63.4%) were <3 years of age at diagnosis. Nineteen (48.7%) of the patients who received chemotherapy before radiation had no evidence of residual disease at the start of RT—either because they underwent GTR/NTR after chemotherapy or because a complete response was achieved from chemotherapy. Median radiation treatment time was 43 days (range, 35–54). One patient required 83 days to complete radiation because of difficult social circumstances that impaired the family’s ability to bring the patient in for treatment. The most common chemotherapy regimen included vincristine, cisplatin (or carboplatin), cyclophosphamide, and etoposide. Three patients had microscopic disease in the cerebrospinal fluid (CSF) at diagnosis (M1). They underwent pre-irradiation chemotherapy, which included vincristine, cisplatin, cyclophosphamide, etoposide, and intrathecal or intravenous methotrexate and cleared their CSF prior to RT. One patient with M1 disease also received intrathecal cytarabine and hydrocortisone in addition to intrathecal methotrexate.
Table 1.
Patient characteristics
| Characteristics | N = 150 |
|---|---|
| Age at radiotherapy, y | |
| Median (range) | 3.6 (0.3–20.9) |
| <3 years | 67 (44.7%) |
| ≥3 years | 83 (55.3%) |
| Sex | |
| Male | 81 (54.0%) |
| Female | 69 (46.0%) |
| Extent of surgical resection | |
| GTR | 116 (77.3%) |
| NTR | 5 (3.3%) |
| STR | 29 (19.3%) |
| Tumor location | |
| Infratentorial | 102 (68.0%) |
| Supratentorial | 43 (28.7%) |
| Spine | 5 (3.3%) |
| Tumor histology | |
| WHO grade II | 58 (38.7%) |
| WHO grade III | 92 (61.3%) |
| Pre-irradiation chemotherapy | |
| Yes | 41 (27.3%) |
| No | 109 (72.7%) |
| Proton technique | |
| Pencil beam scanning | 12 (8.0%) |
| Passive scatter | 138 (92.0%) |
| Median surgery-to-RT interval, no. weeks (range) | 8.3 (3.3–266.3) |
| ≤9 wk | 81 (54.0%) |
| >9 wk | 69 (46.0%) |
| Median dose, GyRBE (range) | 54 (50.4–59.4) |
| ≤54 | 78 (52.0%) |
| >54 | 72 (48.0%) |
| Median follow-up, y (range) | 6.5 (0.2–18.7) |
| Median radiation treatment time, no. days (range) | 43 (35.0–83.0) |
Four of the 5 patients who received a dose of 50.4 GyRBE had WHO grades II/III ependymomas of the spinal cord. One patient with a grade II infratentorial ependymoma received 50.4 GyRBE due to concern for postoperative vascular injury. Otherwise, patients received a prescribed dose of 54–59.4 GyRBE at the discretion of the treating physician.
Treatment Outcomes
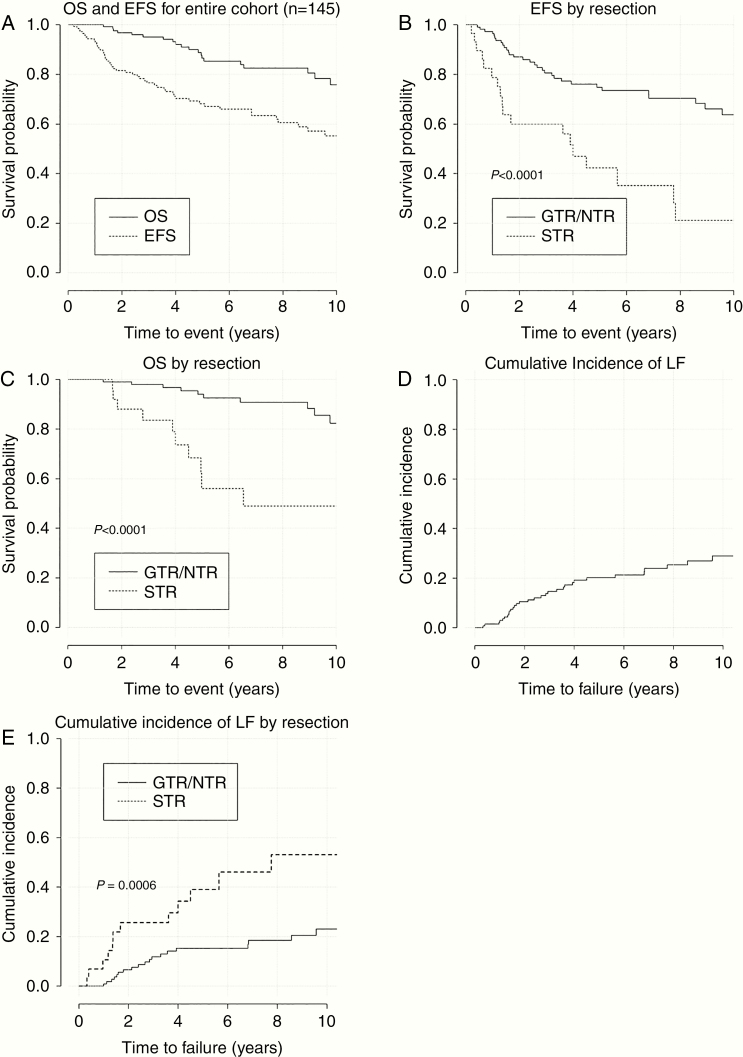
At a median follow-up of 6.5 years (range, 0.2–18.7), 22 patients died and an additional 29 patients had disease progression but were alive at last follow-up. The 7-year estimates of EFS and OS for the intracranial cohort were 63.4% and 82.6%, respectively (Fig. 2A). On univariate analysis (Table 2), STR was predictive of inferior EFS (HR [95% CI]: 3.5 [2.0–6.2], P < 0.0001), OS (HR [95% CI]: 4.9 [2.1–11.5], P < 0.0001), and LC (HR [95% CI]: 3.4 [1.7–6.7], P = 0.00006) (Table 2). The 7-year EFS and OS for GTR/NTR were 70.3% and 90.9% and 35.2% and 49.0% for STR, respectively (Fig. 2B, C). There was no significant difference in EFS or OS among patients with grades II and III tumors (EFS HR [95% CI]: 1.5 [0.8–2.6], P = 0.2 and OS HR [95% CI]: 1.1 [0.5–2.6], P = 0.8) (Supplementary Figure 2A, B). There was no radiation dose response relationship in the GTR/NTR cohort, and the 7-year LC was 84.2% versus 76.7% (P = 0.1) for patients who received ≤54 GyRBE and >54 GyRBE, respectively. On multivariate analysis, achievement of a GTR was the only predictor of EFS, OS, and LC in the intracranial cohort (P < 0.0001, P = 0.001, and P < 0.0001, respectively) (Supplementary Table 1).
Fig. 2.
Clinical outcomes for intracranial cohort and stratified by extent of resection. (A) OS and EFS for entire cohort (n = 145). (B) EFS by resection. (C) OS by resection. (D) Cumulative Incidence of LF. (E) Cumulative incidence of LF by resection.
Table 2.
Cox univariate analysis of EFS, OS, and LC at 5 and 7 years for intracranial tumors (n = 145)
| Variable | N | Events | Deaths | LF | Event-Free Survival (%) | Overall Survival (%) | Local Control (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Years | HR (95% CI) | P | Years | HR (95% CI) | P | Years | HR (95% CI) | P | ||||||||
| 5 | 7 | 5 | 7 | 5 | 7 | |||||||||||
| Entire Cohort | ||||||||||||||||
| 145 | 52 | 22 | 32 | 68.2 | 63.4 | - | - | 86.5 | 82.6 | - | - | 79.9 | 76.1 | - | - | |
| Tumor Grade | ||||||||||||||||
| Grade II | 57 | 20 | 11 | 10 | 71.2 | 71.2 | 1.0 | 85.5 | 85.5 | 1.0 | 84.6 | 84.6 | 1.0 | |||
| Grade III | 88 | 32 | 11 | 22 | 66.5 | 56.1 | 1.5 (0.8–2.6) | 0.2 | 88.0 | 79.7 | 1.1 (0.5–2.6) | 0.8 | 76.6 | 68.3 | 2.0 (0.9–4.1) | 0.06 |
| Tumor Location | ||||||||||||||||
| Supratentorial | 43 | 11 | 2 | 11 | 77.1 | 771.1 | 1.0 | 97.4 | 92.2 | 1.0 | 74.3 | 74.3 | 1.0 | |||
| Infratentorial | 102 | 41 | 20 | 21 | 64.7 | 58.0 | 1.7 (0.9–3.3) | 0.1 | 82.8 | 79.3 | 4.0 (0.9–17.2) | 0.04 | 82.1 | 76.9 | 0.7 (0.3–1.5) | 0.4 |
| Sex | ||||||||||||||||
| Female | 77 | 25 | 11 | 17 | 71.7 | 64.8 | 1.0 | 88.6 | 86.2 | 1.0 | 80.0 | 73.1 | 1.0 | |||
| Male | 68 | 27 | 11 | 15 | 64.8 | 62.4 | 1.2 (0.7–2.0) | 0.6 | 84.4 | 78.2 | 1.3 (0.6–3.1) | 0.5 | 79.8 | 79.8 | 0.9 (0.4–1.8) | 0.8 |
| Age at Diagnosis (years) | ||||||||||||||||
| ≥3 | 79 | 25 | 10 | 15 | 68.7 | 66.7 | 1.0 | 88.9 | 83.5 | 1.0 | 80.0 | 80.0 | 1.0 | |||
| <3 | 66 | 27 | 12 | 17 | 67.6 | 60.0 | 1.2 (0.7–2.1) | 0.4 | 84.1 | 81.6 | 1.4 (0.6–3.2) | 0.4 | 79.7 | 72.1 | 1.3 (0.7–2.6) | 0.5 |
| Total Dose (Gy RBE) | ||||||||||||||||
| ≤54 | 73 | 22 | 7 | 10 | 74.8 | 65.0 | 1.0 | 91.9 | 91.9 | 1.0 | 89.0 | 81.3 | 1.0 | |||
| >54 | 72 | 30 | 15 | 22 | 61.3 | 61.8 | 1.5 (0.9–2.6) | 0.1 | 80.9 | 73.2 | 2.3 (1.0–5.7) | 0.06 | 70.4 | 70.4 | 2.5 (1.2–5.2) | 0.01 |
| Surgical Extent* | ||||||||||||||||
| GTR and NTR | 116 | 33 | 12 | 19 | 74.8 | 70.3 | 1.0 | 94.0 | 90.9 | 1.0 | 84.7 | 81.5 | 1.0 | |||
| STR | 29 | 19 | 10 | 13 | 42.3 | 35.2 | 3.5 (2.0–6.2) | <0.0001 | 56.0 | 49.0 | 4.9 (2.1–11.5) | <0.0001 | 61.0 | 53.9 | 3.4 (1.7–6.7) | 0.0006 |
| Pre-RT Chemo | ||||||||||||||||
| No | 106 | 32 | 15 | 15 | 69.8 | 66.3 | 1.0 | 89.3 | 83.7 | 1.0 | 85.3 | 83.3 | 1.0 | |||
| Yes | 39 | 20 | 7 | 17 | 64.2 | 56.8 | 1.6 (0.9–2.7) | 0.1 | 79.5 | 79.5 | 1.2 (0.5–3.1) | 0.6 | 66.7 | 59.2 | 3.1 (1.6–6.1) | 0.001 |
| Surgery to RT Interval | ||||||||||||||||
| ≤9 Weeks | 81 | 27 | 13 | 12 | 68.0 | 63.7 | 1.0 | 87.9 | 83.3 | 1.0 | 85.3 | 82.9 | 1.0 | |||
| >9 Weeks | 64 | 25 | 9 | 20 | 68.4 | 62.8 | 1.2 (0.7–2.0) | 0.6 | 84.8 | 81.6 | 0.9 (0.4–2.1) | 0.8 | 73.2 | 67.7 | 2.2 (1.1–4.6) | 0.02 |
HR, hazard ratio; RT, radiation therapy; GTR, gross total resection; NTR, near total resection; STR, subtotal resection; LC, local control
*Patients who received chemotherapy prior to radiotherapy and had no radiographic evidence of residual disease at the time of radiotherapy were analyzed with the GTR cohort.
Of the 3 patients who initially presented with M1 disease but had no evidence of metastatic disease at the time of PRT, none exhibited disease recurrence at a median follow-up time of 3.2 years. There were 5 patients with spinal ependymoma, of which 1 was grade II and 4 were grade III (Supplementary Table 2). At a median follow-up of 8.0 years (range, 4.6–14.4), none of these patients developed disease recurrence after resection (STR n = 3, GTR n = 2) and focal RT. One patient was treated at the time of third recurrence after a repeat GTR and remained disease free at 8.0 years of follow-up. The patient with a grade II spinal ependymoma had a GTR but was treated with postoperative RT instead of observation due to aggressive histological features.
Patterns of Failure
A total of 51 patients in the intracranial cohort had recurrent disease. Twenty-six patients (51%) developed a local-only failure at a median time of 1.8 (range 0.9–9.5) years after completion of RT. The 7-year cumulative incidence of LF for the intracranial cohort was 23.4%. The 7-year LC was 76.1% (Fig. 2D). The 7-year LC for the GTR/NTR and STR cohorts were 81.5% and 53.9%, respectively, (HR [95% CI]: 3.4 [1.7–6.7], P = 0.0006; Fig. 2E). The difference in LC between grades II and III tumors trended toward significance (HR [95% CI]: 2.0 [0.9–4.1], P = 0.06; Supplementary Figure 2C). Nineteen patients (37.3%) developed a DF at a median time of 1.4 (0.2–8.9) years after completion of RT. Ten (52.6%) of these patients had grade III tumors. Notably, all 19 patients who developed a DF had primary infratentorial tumors. These failures were outside of the radiation field and were supratentorial, spinal, or leptomeningeal in nature. Six patients (11.8%) developed a synchronous LF + DF at a median time of 3.6 (0.3–11.9) years after completion of RT. Five (83.3%) of these patients had grade III tumors. Tumor grade was not predictive of the type of failure patients experienced (P = 0.43).
Time to Radiotherapy
The median time between surgical resection and PRT for the entire intracranial cohort was 8 weeks (range, 3.3–266.3). Patients who started RT >9 weeks from their last surgical resection had worse LC compared with those who did not (HR [95% CI]: 2.2 [1.1–4.6], P = 0.02) (Supplementary Figure 3A). However, this was confounded by extent of resection, and the effect of surgery-to-RT interval on LC did not persist among those who had a GTR/NTR (HR [95% CI]: 1.6 [0.7–3.9], P = 0.3) (Supplementary Figure 3B). Although the effect of surgery-to-RT interval on LC was nonsignificant among GTR/NTR patients, we detected a difference in magnitude of effect of surgery-to-RT interval among GTR/NTR when stratified by tumor grade. GTR/NTR patients with grade III tumors had similar rates of LC if treated with PRT regardless of the surgery-to-RT interval (HR [95% CI]: 1.0 [0.4–2.7]; P = 1.0) (Supplementary Figure 3C), while those with grade II tumors trended toward worse outcomes if PRT was delayed beyond 9 weeks (HR [95% CI]: 6.5 [0.7–58.9]; P = 0.07) (Supplementary Figure 3D). When patients who received pre-RT chemotherapy were excluded, the median time from surgery to PRT was 6.9 (3.3–183.7) weeks. The exclusion of these patients did not affect our analysis on the effect of surgery-to-RT interval on LC.
Brainstem Dosimetry
Brainstem dosimetry was available for 139 (95.9%) intracranial patients (Supplementary Table 3). The median dose to 50% of the brainstem (D50) and median maximum point dose for all patients with intracranial tumors was 52.4 GyRBE (range, 0–56.1) and 55 GyRBE (0–60.5). In patients with infratentorial tumors, the median brainstem D50 and the median maximum point dose was 53.2 GyRBE (16.5–56.1) and 55.1 GyRBE (49.6–60.5). Among patients with GTR/NTR, the median max point dose to the brainstem was 54.9 GyRBE, whereas the median max point dose to the brainstem among STR patients was 56.6 GyRBE (P < 0.001; Supplementary Table 3).
Table 3.
Literature review of children treated with radiotherapy for pediatric intracranial ependymoma
| Study | N | RT Technique | Median Follow-Up (y) | GTR/NTR vs STR | Grades I-II/III | OS | EFS | LC | Crude Incidence Brainstem Necrosis |
|---|---|---|---|---|---|---|---|---|---|
| Patteson et al, 2019** | 145 | Proton | 6.5 | 80.0/20.0 | 39.3/60.7 | 7 y: 82.6 | 7 y: 63.4 | 7 y: 76.1 | 0.7 |
| Merchant et al, 2019** (ACNS 0121) | 356 | Proton, IMRT, and 3D | 7.9 | 82.1/17.9 | 60.4/39.6 | 5 y: 83.8 | 5 y: 62.7 | N/A | N/A |
| Ducassou et al, 2018 | 202 | Proton, IMRT, and 3D | 4.5 | 85.6/14.4 | 36.1/63.9 | 5 y: 71.4 | 5 y: 50 | 5 y: 69.7 | 0 |
| Massimino et al, 2016 | 160 | Photons:3D | 5.6 | 69.0/31.0 | 47.5/52.5 | 5 y: 81.1 | 5 y: 65.4 | 5 y: 79.3 | N/A |
| Merchant et al, 2009* | 153 | Photons: 3D/IMRT | 5.3 | 81.7/19.3 | 44.4/55.6 | 7 y: 81 | 7 y: 69.1 | 7 y:81 | 1.6 |
| Mansur et al, 2005 | 60 | Photons: 3D | 12.5 | 23.0/72.0 (5 N/A) | 66.7/33.3 | 5 y:71.2 | 5 y: 58.4 | N/A | N/A |
| Shu et al, 2007 | 49 | Photons: 3D | 9.2 | 61.2/36.7 (~2 N/A) | 67.3/22.4 (10.2 N/A) | 5 y: 66.2 | 5 y: 40.7 | N/A | N/A |
| Indelicato et al, 2017** | 179 | Proton | 3.2 | 84.9/14.1 | 33.0/67.0 | 3 y: 90.4 | 3 y: 75.9 | 3 y: 85.4 | 5.6 |
| Sato et al, 2017 | 79 | IMRT or protons | 2.6 PRT, 4.9 IMRT | 84.8/15.2 | 19.0/81.0 | 3 y: 88 5 y: 76 | 3 y: 60 5 y: 60 | N/A | 7.6 |
IMRT = intensity modulated radiotherapy.
*NTR grouped with STR; **NTR grouped with GTR; N/A, not available
Late Toxicities
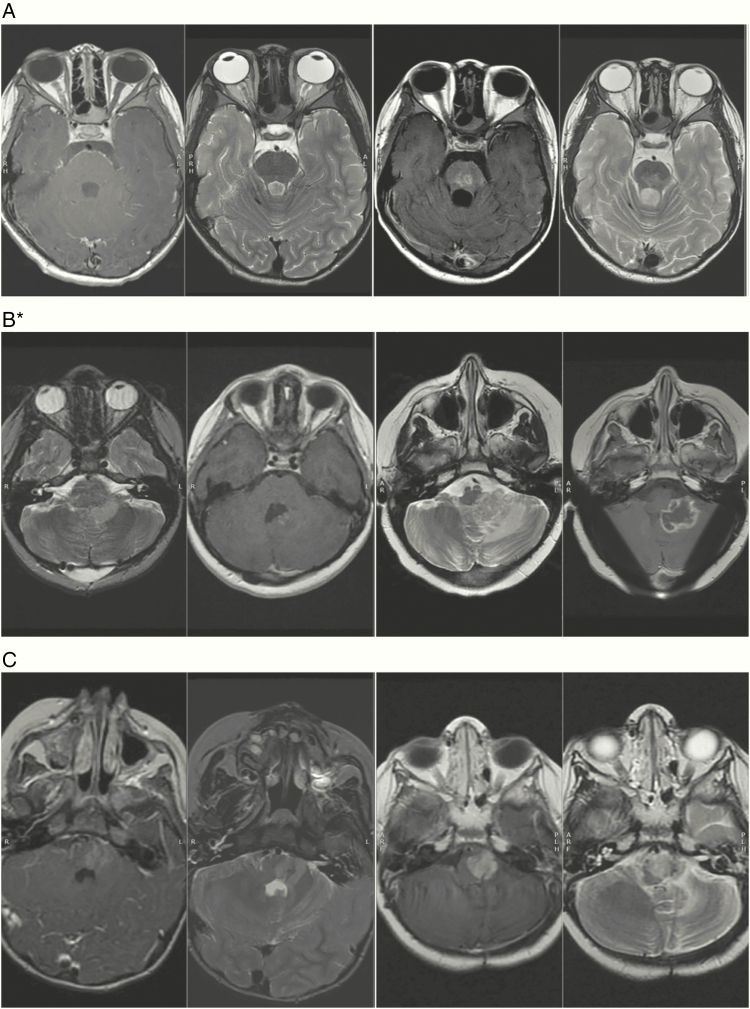
The 7-year cumulative incidence of grade II+ radiation injury/necrosis in the brainstem for the intracranial cohort was 1.1% (crude incidence: 0.7%) (n = 1). This patient had STR, sickle cell disease, and severe posterior fossa syndrome requiring daily anesthesia and multiple transfusions to keep hemoglobin S within a safe range (<30%) for anesthesia. He also required intensive inpatient rehabilitation postoperatively and during RT. He developed grade III brainstem injury 4 months after RT (Fig. 3A) and improved with steroid treatment. The prescription dose was 55.8 GyRBE with a mean, median (D50), and max dose to the brainstem in GyRBE of 51.2, 54.5, and 56.1, respectively. At last follow-up over 5 years after PRT, the patient is disease free with a Lansky score of 70 with persistent deficits in speech and cerebellar function. Two patients with residual disease adjacent to the brainstem developed tumor necrosis with edema and symptoms of brainstem compression and injury. One patient underwent biopsy of the mass and had pathology-confirmed viable tumor with tumor necrosis (Fig. 3B). The second patient exhibited partial resolution of these MRI changes but remained clinically symptomatic with balance and swallowing deficits (Fig. 3C). Both patients developed progressive disease and later died. There were no cases of grade II+ radiation necrosis outside of the brainstem.
Fig. 3.
Pretreatment and posttreatment images for 3 children. (A) Brainstem injury/necrosis with lesion intrinsic to the brainstem. (B–C) Tumor swelling with brainstem compression causing injury but no direct radiation injury or necrosis in the brainstem.
The 7-year cumulative incidence of secondary tumors was 2.2% (crude incidence: 0.7%) (n = 1). One patient developed a radiation-induced glioblastoma 9 years after RT and died 11 months after diagnosis. The 7-year cumulative incidence of grade II+ radiation-induced vasculopathies in the cohort was 1.5% (crude incidence: 1.4%) (n = 2). There were no cases of myelopathy in the cohort.
Discussion
This is one of the largest studies to report mature disease outcomes for a cohort of patients with pediatric ependymoma treated with PRT. We show that disease control is comparable to other photon treated cohorts (Table 3). Our data reinforce the well-known prognostic significance of achieving GTR, which has been most recently validated by COG ACNS 0121 and previously validated in several other studies.13–19 Our STR cohort had a 5-year EFS of 42.3%, which is comparable to, albeit slightly higher than, the 37.2% reported in COG ACNS 0121. The 7-year LC for the STR cohort was 53.9%, comparable to other studies when accounting for length of follow-up time,12,14,16 although significantly lower than the 7-year LC for the GTR cohort at 81.5%. These data suggest that over half of patients with gross residual disease will have long-term LC. The role of adjuvant chemotherapy is still being explored in ongoing COG and SIOP trials with the hope of improving outcomes in this high-risk subset of patients.
The importance of other prognostic factors is less understood. Some studies have demonstrated worse EFS and LC in patients with anaplastic (grade III) tumors.13–15,18 Our series shows a trend toward inferior EFS and LC but failed to reach statistical significance, possibly due to insufficient statistical power. Other studies show worse outcomes in male patients,13,15,17 but our cohort did not confirm that finding. The apparent decrement in OS and LC on univariate analysis in patients given a dose above 54 GyRBE can be explained by the fact that these patients were more likely to have gross residual disease (P < 0.0001) at the time of RT. The treating physicians were escalating RT dose based on the knowledge that residual disease puts the patient at a higher risk of disease progression. Overall, we did not find a dose response relationship in our cohort, which is in itself an important finding given that the last two COG ependymoma protocols call for a prescription dose of 59.4 GyRBE.
The most appropriate radiation dose for proton-treated pediatric ependymoma patients merits discussion. In our study, 41.4% of our GTR/NTR patients received a dose >54 GyRBE. However, our analysis did not demonstrate an improvement in LC or other disease outcomes. Taken together with our low radiation brainstem injury rate, these findings suggest that the majority of proton-treated patients with a GTR/NTR do not require doses above 54 GyRBE to achieve disease outcomes as comparable to what has been reported in the literature. Furthermore, dose escalation above 54 GyRBE may be harmful to surrounding critical structures, such as the brainstem, and may contribute to higher rates of injury in other studies.20 Merchant et al specifically analyzed the effect of dose in the photon-treated pediatric ependymoma cohort and noted no difference in LC, EFS, or OS between patients treated with 54 GyRBE or 59.4 GyRBE.13 Given our proton cohort’s outcome data with doses <59.4 GyRBE and previous photon data failing to show a benefit to dose escalation to 59.4 GyRBE and the higher risk of injury to surrounding critical structures, we should reconsider using doses above 54 GyRBE in patients with GTR/NTR. Given the increased risk of disease progression in patients with STR, doses higher than 54 GyRBE may still be warranted with sufficient care taken to protect the brainstem and spinal cord.
We also evaluated whether a prolonged interval from the last surgical resection until the start of RT affected disease control. We found that the interval between surgery and RT initiation does not seem to influence treatment outcomes in the whole cohort of patients and in those with a GTR/NTR, specifically. Subgroup analysis in the GTR/NTR cohort by histologic grade revealed a trend toward worse LC in grade II patients (but not in the grade III patients) if the interval stretched longer than 9 weeks. The reason for this finding is unclear and should be explored in other ependymoma cohorts. Gunther et al from The University of Texas MD Anderson Cancer Center found that early initiation of RT may lead to a higher incidence of radiographic evidence of radiation injury in pediatric ependymoma patients.21 In summary, it appears that there is no decrement in disease control if RT is started within 9 weeks, which may allow more time for patients to recover from surgical morbidity and for referral to an appropriate pediatric radiation facility.
Five patients in our cohort had ependymoma of the spine. Three patients were treated after STR and 2 patients were treated after GTR. All remained controlled of their disease despite one patient being treated after multiple recurrences and surgical resections. Though observation is typically recommended in patients with a GTR and grade II spinal ependymoma, one patient underwent adjuvant PRT due to a worrisome pathology report indicative of more aggressive behavior. With an actuarial EFS of 100% at a median follow-up time of 8.0 years, PRT is an attractive option for patients with primary spinal ependymoma who require adjuvant RT for residual disease or anaplastic histology, as it spares abdominal and thoracic organs unnecessary radiation dose.
A limitation of this study is that a minority of the cohort (45%) was followed retrospectively, which could have introduced selection bias. Another limitation is that molecular subtyping and genetic profiling of the tumors was unavailable for most patients, since they were accrued over an 18-year period and subtyping only recently became available in the clinical setting. However, the recently published COG ependymoma study ACNS 0121 showed no significant effect on EFS among fusion status for RELA (v-rel avian reticuloendotheliosis viral oncogene homolog A) or grouping for posterior fossa A/posterior fossa B. Notably, however, 1q gain status was significantly associated with worse EFS among patients with infratentorial tumors.14
Late recurrences are not uncommon and reinforce the need for long-term surveillance. They also highlight the importance of reading the literature critically for median follow-up when drawing comparisons between studies. Favorable estimates of OS emphasize the importance of considering morbidity and late effects when determining treatment options. The incidence of radiation-related brainstem injury (7-year cumulative: 1.1%, crude, 0.7%) and secondary tumors (7-year cumulative: 2.2%, crude: 0.7%) in our cohort is low compared with other photon and proton series.14,17,19 Of note, in ACNS 0121, a predominantly photon-treated cohort (94%), the cumulative incidence of secondary malignancies was 3.4% at 10 years.
Radiation-induced brainstem injury is an important toxicity that has been recently evaluated and described in proton-treated pediatric brain tumor cohorts, who may be at higher risk of toxicity due to variable RBE.11,20,22 Here, we draw an important distinction between direct brainstem injury from RT and indirect injury from inflammatory changes of treated residual tumor. While patients’ symptoms can be identical, the causes are very different, and thus prevention and treatment strategies are also unique. The risk of direct radiation injury to the brainstem can be minimized by limiting the brainstem dose in accordance with published guidelines.20,23 The only patient with direct radiation-related brainstem injury in our cohort had sickle cell disease. These patients are at risk of infarctions without additional risk factors, such as RT. During treatment which required daily anesthesia, transfusions were used to maintain the hemoglobin S level under 30%. Though there are no data to guide management of patients with sickle cell disease needing RT, it is possible that aggressive exchange transfusion in the immediate post-RT period could reduce the risk of brainstem injury in future patients.
Two patients suffered from compressive injuries on the brainstem from inflamed, irradiated residual tumor. This type of indirect injury, though no less consequential for the patient, should not be considered direct radiation injury or necrosis of the brainstem. Aggressive attempts at surgical resection near the brainstem should be pursued whenever feasible to limit the volume of residual tumor and thereby limit this risk. Additionally, we advocate for early intervention with steroids, bevacizumab, or surgical intervention if the inflammatory reaction causes symptomatic brainstem compression. Finally, if adjuvant chemotherapy or immunotherapy is being administered at the time of symptom onset, we advocate immediate cessation, as many agents can augment the inflammatory injury.
PRT is safe and efficacious when brainstem constraints are kept within recently published guidelines11,20,23 and when overall prescription doses to the tumor bed and residual disease range 50.4–59.4 GyRBE. We advocate for aggressive surgical resection whenever possible, not only because STR confers significantly worse outcomes, but also because radiation to residual disease abutting brainstem can cause unwanted toxicity through compressive injury from inflammation. Importantly, the escalation of RT dose should not be seen as a substitute for GTR, as patients with STR who received RT dose >54 GyRBE had poor outcomes. With acceptable toxicity and comparable treatment outcomes to conformal photon RT, PRT will continue to play an important role in the treatment of childhood ependymoma.
Funding
The project was supported by the federal share of program income earned by the Massachusetts General Hospital on C06 CA059267 Proton Therapy Research and Treatment Center. The Pediatric Proton/Photon Consortium Registry also receives funding from Ion Beam Applications (IBA), Protom, Elekta, and MIM Software, Inc.
Conflict of interest statement. None.
Authorship statement. The following authors contributed to data collection: Brooke E. Patteson, Shannon MacDonald, Sara L. Gallotto, Megan J. Giblin, and Elizabeth A. Weyman, and Torunn I. Yock. The following authors contributed to data analysis and interpretation: Brooke E. Patteson, Sujith Baliga, Shannon M. MacDonald, Benjamin V. Bajaj, Beow Y. Yeap, Torunn I. Yock, David H. Ebb, Mary S. Huang, Robin M. Jones, and Nancy J. Tarbell. All authors have seen and approved the manuscript.
Supplementary Material
References
- 1. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018;20(suppl_4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robison NJ, Campigotto F, Chi SN, et al. A phase II trial of a multi-agent oral antiangiogenic (metronomic) regimen in children with recurrent or progressive cancer. Pediatr Blood Cancer. 2014;61(4):636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duffner PK, Horowitz ME, Krischer JP, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993;328(24): 1725–1731. [DOI] [PubMed] [Google Scholar]
- 4. Zacharoulis S, Levy A, Chi SN, et al. Outcome for young children newly diagnosed with ependymoma, treated with intensive induction chemotherapy followed by myeloablative chemotherapy and autologous stem cell rescue. Pediatr Blood Cancer. 2007;49(1):34–40. [DOI] [PubMed] [Google Scholar]
- 5. Geyer JR, Zeltzer PM, Boyett JM, et al. Survival of infants with primitive neuroectodermal tumors or malignant ependymomas of the CNS treated with eight drugs in 1 day: a report from the Childrens Cancer Group. J Clin Oncol. 1994;12(8):1607–1615. [DOI] [PubMed] [Google Scholar]
- 6. Grill J, Le Deley MC, Gambarelli D, et al. ; French Society of Pediatric Oncology Postoperative chemotherapy without irradiation for ependymoma in children under 5 years of age: a multicenter trial of the French Society of Pediatric Oncology. J Clin Oncol. 2001;19(5):1288–1296. [DOI] [PubMed] [Google Scholar]
- 7. Smyth MD, Horn BN, Russo C, Berger MS. Intracranial ependymomas of childhood: current management strategies. Pediatr Neurosurg. 2000;33(3):138–150. [DOI] [PubMed] [Google Scholar]
- 8. MacDonald SM, Yock TI. Proton beam therapy following resection for childhood ependymoma. Childs Nerv Syst. 2010;26(3):285–291. [DOI] [PubMed] [Google Scholar]
- 9. Ness KK, Gurney JG, Zeltzer LK, et al. The impact of limitations in physical, executive, and emotional function on health-related quality of life among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Arch Phys Med Rehabil. 2008;89(1):128–136. [DOI] [PubMed] [Google Scholar]
- 10. Macdonald SM, Sethi R, Lavally B, et al. Proton radiotherapy for pediatric central nervous system ependymoma: clinical outcomes for 70 patients. Neuro Oncol. 2013;15(11):1552–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haas-Kogan D, Indelicato D, Paganetti H, et al. National Cancer Institute workshop on proton therapy for children: considerations regarding brainstem injury. Int J Radiat Oncol Biol Phys. 2018;101(1):152–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 13. Merchant TE, Li C, Xiong X, Kun LE, Boop FA, Sanford RA. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10(3):258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Merchant TE, Bendel AE, Sabin ND, et al. Conformal radiation therapy for pediatric ependymoma, chemotherapy for incompletely resected ependymoma, and observation for completely resected, supratentorial ependymoma. J Clin Oncol. 2019;37(12):974–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Massimino M, Miceli R, Giangaspero F, et al. Final results of the second prospective AIEOP protocol for pediatric intracranial ependymoma. Neuro Oncol. 2016;18(10):1451–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shu HK, Sall WF, Maity A, et al. Childhood intracranial ependymoma: twenty-year experience from a single institution. Cancer. 2007;110(2):432–441. [DOI] [PubMed] [Google Scholar]
- 17. Indelicato DJ, Bradley JA, Rotondo RL, et al. Outcomes following proton therapy for pediatric ependymoma. Acta Oncol. 2018;57(5):644–648. [DOI] [PubMed] [Google Scholar]
- 18. Ducassou A, Padovani L, Chaltiel L, et al. Pediatric localized intracranial ependymomas: a multicenter analysis of the Societe Francaise de Lutte Contre les Cancers de l’Enfant (SFCE) from 2000 to 2013. Int J Radiat Oncol Biol Phys. 2018;102(1):166–173. [DOI] [PubMed] [Google Scholar]
- 19. Sato M, Gunther JR, Mahajan A, et al. Progression-free survival of children with localized ependymoma treated with intensity-modulated radiation therapy or proton-beam radiation therapy. Cancer. 2017;123(13):2570–2578. [DOI] [PubMed] [Google Scholar]
- 20. Indelicato DJ, Flampouri S, Rotondo RL, et al. Incidence and dosimetric parameters of pediatric brainstem toxicity following proton therapy. Acta Oncol. 2014;53(10):1298–1304. [DOI] [PubMed] [Google Scholar]
- 21. Gunther JR, Sato M, Chintagumpala M, et al. Imaging changes in pediatric intracranial ependymoma patients treated with proton beam radiation therapy compared to intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93(1):54–63. [DOI] [PubMed] [Google Scholar]
- 22. Yock TI, Constine LS, Mahajan A. Protons, the brainstem, and toxicity: ingredients for an emerging dialectic. Acta Oncol. 2014;53(10): 1279–1282. [DOI] [PubMed] [Google Scholar]
- 23. Gentile MS, Yeap BY, Paganetti H, et al. Brainstem injury in pediatric patients with posterior fossa tumors treated with proton beam therapy and associated dosimetric factors. Int J Radiat Oncol Biol Phys. 2018;100(3):719–729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.