Abstract
We present a case of abdominal gastric band–associated Mycobacterium abscessus infection, manifesting after the onset of acute myeloid leukemia, complicated by immune reconstitution inflammatory syndrome (IRIS), and cured while receiving an allogeneic hematopoietic stem cell transplant. IRIS should be considered in less classical situations where there is unexplained clinical deterioration.
Keywords: rapid growing mycobacteria, Mycobacterium abscessus, gastric band, immune reconstitution inflammatory syndrome, hematopoietic stem cell transplantation
Mycobacterium abscessus complex is a group of rapidly growing mycobacteria (RGM) that cause predominantly cutaneous and pulmonary infection, and postsurgical infection including foreign body involvement is well described [1]. There is a variety of clinical manifestations of RGM in solid organ transplant recipients, with central venous catheter (CVC) infection being the predominant manifestation in hematopoietic stem cell transplant (HSCT) recipients [2]. Immune reconstitution inflammatory syndrome (IRIS) is an uncommon phenomenon in nontuberculous mycobacteria (NTM) infection in non-HIV patients. We present an unusual case of M. abscessus complex infection associated with gastric band infection, complicated by IRIS, and cured in the context of allogeneic HSCT for acute myeloid leukemia (AML).
CASE REPORT
A 54-year-old woman had undergone gastric band insertion in 2008 as a weight loss procedure without prior surgical complications and no port manipulation since insertion. She developed AML in July 2014 and was treated with idarubicin, cytarabine, etoposide induction, and 2 high-dose cytarabine consolidation chemotherapy cycles. In March 2015, she presented with relapse of AML and 1 month of epigastric pain. Her subsequent progress is detailed in Figure 1. Upper gastrointestinal endoscopy demonstrated erosion of the gastric band into the gastric lumen, which was laparoscopically removed but not sent for culture. Following surgical recovery, she had induction IDA-FLAG (idarubicin, high dose cytarabine, fludarabine, filgrastim) chemotherapy, complicated by wound breakdown and cellulitis at the right and left abdominal laparoscopic port sites. Computed tomography (CT) of the abdomen showed abdominal wall collections deep to the port sites (Figure 2), phlegmon adjacent to the lesser curvature of the stomach suggestive of persistent leak at the site of the previous gastric band, and moderate ascites. Abdominal wall tissue and ascite samples on cytology showed an acute inflammatory infiltrate of predominantly polymorphonuclear leucocytes and lymphocytes, by microscopy 3+ acid fast bacilli were seen, and on blood and chocolate agar grew an RGM, which on matrix-assisted laser desorption ionisation-time of flight mass spectrometry (MALDI-TOF MS) was Mycobacterium abscessus (score >2.0) and was identified by in-house polymerase chain reaction (PCR) as Mycobacterium abscessus complex (the PCR is validated for identification of M. abscessus complex but not to the species level). Phenotypic susceptibility testing by the Sensititre RAPMYCO microdilution panel (Thermo Fisher, Inc., Cleveland, OH, USA) showed a minimum inhibitory concentration for amikacin of 16 mg/L (susceptible), for cefoxitin of 32 mg/L (intermediate), for imipenem of 32 mg/L (intermediate), for tigecycline of 0.5 mg/L (no breakpoint), for linezolid of 16 mg/L (resistant), for trimethoprim/sulfamethoxazole of ≥8/152 mg/L (resistant), for ciprofloxacin of ≥4 mg/L (resistant), for moxifloxacin of ≥8 mg/L (resistant), and for minocycline of ≥8 mg/L (resistant). Clarithromycin showed inducible resistance (2 mg/L at 5 days and >16 mg/L at 14 days after incubation). Azithromycin phenotypic testing and erm gene determination were not performed. Notably, the patient had a history of rash with meropenem and was treated from April 21, 2015 (Day 0), with cefoxitin 12 g daily, tigecycline 100 mg daily, and azithromycin 500 mg daily. Azithromycin was continued in the regimen, as it is well tolerated and there is uncertainty regarding whether azithromycin induces erm gene expression to the same extent as clarithromycin [3, 4]. Neutrophil count recovered to >1.0 ×109/L by Day +20 (May 11, 2015), following which she developed worsening abdominal wall abscesses with new imaging findings of peritoneal nodularity (Figure 3). C-reactive protein (CRP) was mildly elevated (30 mg/L) and had not significantly increased. Repeat mycobacterial wound cultures were negative, and there was no response several weeks after intravenous amikacin 15 mg/kg/d was added to the regimen on June 4, 2015. We suspected IRIS, and there was prompt clinical, CRP (reduced to <1 mg/L), and radiological improvement to oral prednisolone 60 mg daily commenced Day +55 (June 15, 2015), which was reduced 10 mg per week. Amikacin was ceased. AML was in remission, and she proceeded to sibling allogeneic HSCT with fludarabrine/melphalan conditioning on Day +113 (August 12, 2015). With engraftment, there was a rapid rise of CRP to 200 mg/L with transient fever. The patient was given piperacillin-tazobactam for 5 days, and her CRP fell to 20–50 mg/L, at which point there was deterioration in the abdominal wounds, which were culture negative, and there was a rapid response in wounds and reduction in CRP after increasing prednisolone to 25 mg daily on Day +139 (September 7, 2015) with slow tapering. The dose was increased to 25 mg daily on Day +239 (December 15, 2015) for possible liver graft-vs-host-disease (GVHD) and was slowly weaned. Cefoxitin, tigecycline, and azithromycin were continued through until Day +281 (January 27, 2016; 6 months post-transplant). Following antibiotic cessation, there was no recurrence of infection. She subsequently developed chronic GVHD of skin, eyes, and mouth, but at last review in November 2020, she was alive and free of leukemia.
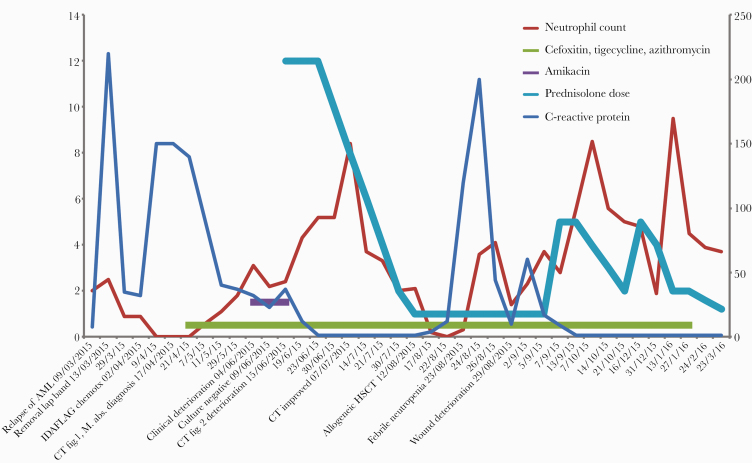
Figure 1.
Timeline of patient progress from the diagnosis of relapsed acute myeloid leukemia and laparoscopic band–associated Mycobacterium abscessus complex infection, March 2015, demonstrating periods of deterioration due to immune reconstitution inflammatory syndrome following neutrophil recovery after IDA-FLAG chemotherapy (June 4, 2015) and following engraftment after allogeneic hematopoietic stem cell transplant (August 29, 2015). The left y-axis shows units for neutrophil count (×109/L) and prednisolone dose (prescribed dose is 5 times the represented units in mg). The right y-axis shows units for C-reactive protein (mg/L). Abbreviations: AML, acute myeloid leukemia; CT, computed tomography; HSCT, hematopoietic stem cell transplant; IDA-FLAG, idarubicin, high dose cytarabine, fludarabine, filgrastim; IRIS, immune reconstitution inflammatory syndrome.
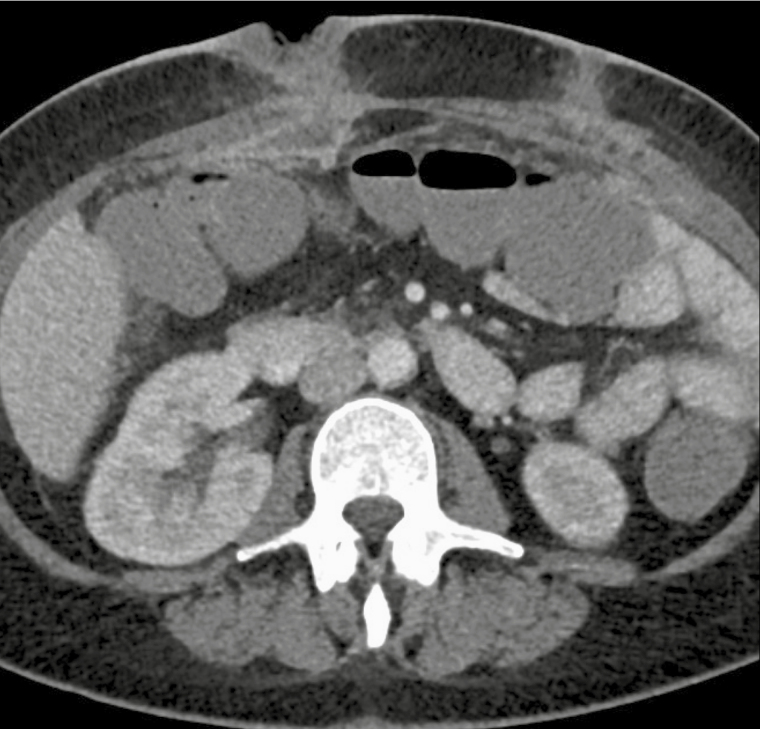
Figure 2.
Computed tomography of the abdomen at diagnosis, April 21, 2015, demonstrating abdominal wall collections deep to laparoscopic port sites.
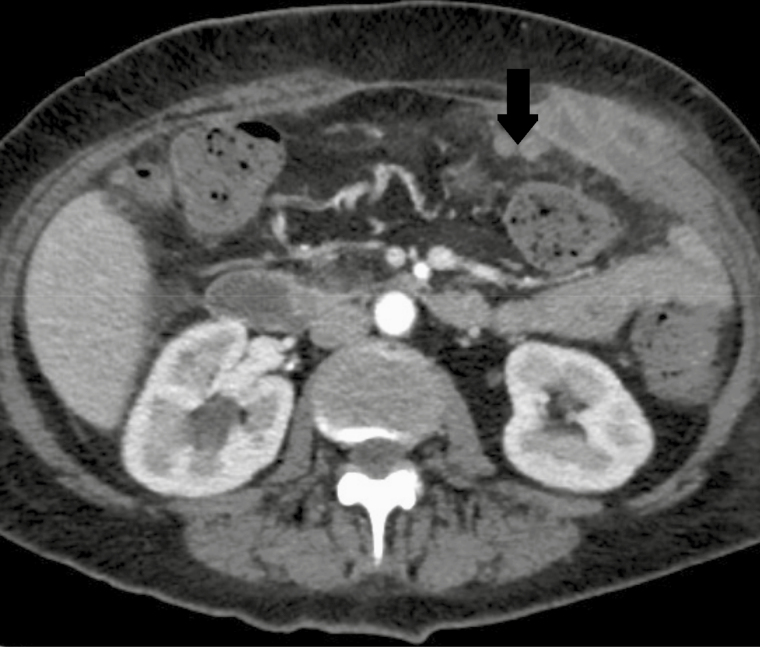
Figure 3.
Computed tomography of the abdomen, June 15, 2015, demonstrating peritoneal nodularity (arrow) at ~2 months after the commencement of mycobacterial treatment and following neutrophil recovery.
DISCUSSION
M. abscessus complex has caused infections of foreign bodies such as tympanostomy tubes, peritoneal dialysis catheters, breast implants, prosthetic joints, and prosthetic vascular grafts [1]. In our case, we infer that M. abscessus complex infection arose primarily from the gastric band because it initially manifested by erosion into the stomach, there was radiological evidence of infection at the lesser curvature of the stomach and peritoneum, and ascite culture was positive. Our conclusion is that abdominal wall and laparoscopic port site infection were secondary manifestations of deeper involvement. In the largest case series of RGM gastric band infections reported on the time period 2005–2011, 11 cases were due to Mycobacterium fortuitum and 7 were due to M. abscessus. Although the time from band placement to infection ranged from 21 days to 8 years, the majority presented within 3 months, manifested by peritonitis, band erosion, or chronic ulceration at the port site. The port was considered the primary location of infection in 10 patients (56%) [5]. We cannot be sure when infection of the gastric band occurred in our patient; however, it is possible that it occurred at insertion in 2008, remaining latent until the immunocompromise incurred 7 years later from AML and chemotherapy. We did not perform hospital environmental sampling for M. abscessus culture, but we believe that hospital-acquired infection is unlikely, as there were no other contemporaneous M. abscessus cases identified, nor any prior or subsequent M. abscessus cases related to the hospital environment or water supply.
Nontuberculous mycobacterial infections complicate 0.4% to 4.9% of HSCTs. Central venous catheter (CVC)–related infections with RGM and pulmonary infections with Mycobacterium avium complex (MAC) and other slow-growing mycobacteria are the predominant presentations, with cutaneous and disseminated infection being less common [2]. The largest series of RGM CVC infections (n = 23, M. abscessus = 10) after HSCT occurred at a median of 61 days post-transplant. The catheter was removed in all but 2 cases. Combination antibiotic therapy was given for a median of 6–7 weeks in the case of tunnel infection or bacteremia and 3 weeks for exit site infection. Cure was achieved in 21 patients, while 2 patients died of unrelated causes [6]. In the other major publication of RGM infection after HSCT, 6/7 were CVC-related, presenting 7–90 days post-transplant, and 6/7 infections resolved [7]. In our reported case, there was no suggestion of CVC infection, and blood cultures were negative. M. abscessus complex is notable for its antibiotic resistance, often only susceptible to amikacin and tigecycline, with intermediate susceptibility to cefoxitin and imipenem. Macrolide susceptibility is species dependent. We were highly concerned about proceeding to HSCT in the context of extensive active infection with this very resistant and virulent organism, but we had no other option. We have demonstrated that cure of this difficult infection can occur even through HSCT. Likewise, favorable results were obtained for 3 RGM CVC-related infections diagnosed shortly before HSCT [6].
A highly illustrative aspect of the case was the occurrence of IRIS. There are no agreed-upon diagnostic criteria for IRIS even where it has been more extensively studied in HIV patients. Recognized features are a new or worsening inflammatory condition after reconstitution of immunity that is not explained by another cause such as drug-resistant infection, superinfection, drug allergy, or noncompliance [8]. It is commonly responsive to corticosteroids, although this is not a component of proposed diagnostic criteria [8–10]. In our case, IRIS was diagnosed first following neutrophil recovery after chemotherapy and second after engraftment post-HSCT, when there was increased inflammation and deterioration in the wounds, radiological deterioration, sterile cultures, modestly raised CRP, no response to antibiotic augmentation, and rapid response to systemic corticosteroids. NTM-associated IRIS has been predominantly described with MAC infection in HIV patients [11], with few reports in neutropenic or transplant patients [9, 10, 12, 13]. The largest report by Manion and colleagues described 3 patients with primary immunodeficiency and disseminated MAC infection who underwent allogeneic HSCT. They had features of IRIS related to various stimuli such as neutrophil recovery, donor lymphocyte infusion, and when immunosuppression for GVHD was reduced. At times of IRIS, they demonstrated elevated CRP, interferon-γ, tumor necrosis factor–α, interleukin-6, interleukin-18, and acquisition of a CD4+ T lymphocyte MAC-specific cytokine response. Mycobacterial and IRIS treatment were not described [13]. RGM-associated IRIS has been reported once before in an AIDS patient who was diagnosed with disseminated M. abscessus complex infection 4 weeks after commencing antiretroviral therapy (ART), who improved with azithromycin, standard 4-drug tuberculosis (TB) therapy, and ART continuation, but without steroids. Mycobacterial species and antibiotic susceptibilities were not reported. This appears to have been a case of “unmasking” IRIS [14]. Prednisolone 20–40 daily for 4–8 weeks has been suggested for moderate to severe MAC-associated IRIS in HIV patients when there is no response to nonsteroidal anti-inflammatory drugs. Tumor necrosis factor inhibitors and thalidomide have been utilized in steroid-refractory TB-associated IRIS in HIV infection [15].
In summary, we present a highly unusual and illustrative case of M. abscessus complex infection associated with distant gastric band insertion, manifesting after the onset of AML and chemotherapy, complicated by IRIS, that was controlled and finally cured while receiving HSCT. Outcomes are not always poor in patients with difficult infection requiring HSCT, and we are reminded to consider IRIS in less classical situations where there is unexplained clinical deterioration.
Acknowledgments
Financial support. There was no funding for this manuscript.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. Written informed consent was obtained from the patient. The design of the work accords to the Australian National Statement on Ethical Conduct in Human Research [16], and was approved by Fiona Stanley Hospital.
References
- 1. Brown-Elliott BA, Wallace RJ. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapid growing mycobacteria. Clin Micro Rev 2002; 15: 716–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Doucette K, Fishman JA. Nontuberculous mycobacterial infection in hematopoietic stem cell and solid organ transplant recipients. Clin Infect Dis 2004; 38:1428–39. [DOI] [PubMed] [Google Scholar]
- 3. Choi GE, Shin SJ, Won CJ, et al. Macrolide treatment for Mycobacterium abscessus and Mycobacterium massiliense infection and inducible resistance. Am J Respir Crit Care Med 2012; 186:917–25. [DOI] [PubMed] [Google Scholar]
- 4. Maurer FP, Castelberg C, Quiblier C, et al. Erm(41)-dependent inducible resistance to azithromycin and clarithromycin in clinical isolates of Mycobacterium abscessus. J Antimicrob Chemother 2014; 69:1559–63. [DOI] [PubMed] [Google Scholar]
- 5. Wright HL, Thomson RM, Reid AB, et al. Rapidly growing mycobacteria associated with laparoscopic gastric banding, Australia, 2005–2011. Emerg Infect Dis 2014; 20:1612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gaviria JM, Garcia PJ, Garrido SM, et al. Nontuberculous mycobacterial infections in hematopoietic stem cell transplant recipients: characteristics of respiratory and catheter-related infections. Biol Blood Marrow Transplant 2000; 6:361–9. [DOI] [PubMed] [Google Scholar]
- 7. Roy V, Weisdorf D. Mycobacterial infections following bone marrow transplantation: a 20 year retrospective review. Bone Marrow Transplant 1997; 19:467–70. [DOI] [PubMed] [Google Scholar]
- 8. Haddow LJ, Easterbrook PJ, Mosam A, et al. Defining immune reconstitution inflammatory syndrome: evaluation of expert opinion versus 2 case definitions in a South African cohort. Clin Infect Dis 2009; 49:1424–32. [DOI] [PubMed] [Google Scholar]
- 9. Sun HY, Singh N. Immune reconstitution inflammatory syndrome in non-HIV immunocompromised patients. Curr Opin Infect Dis 2009; 22: 294–402. [DOI] [PubMed] [Google Scholar]
- 10. Sun HY, Singh N. Opportunistic infection-associated immune reconstitution syndrome in transplant recipients. Clin Infect Dis 2011; 53:168–76. [DOI] [PubMed] [Google Scholar]
- 11. Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis 2005; 5:361–73. [DOI] [PubMed] [Google Scholar]
- 12. Lemoine M, Laurent C, Hanoy M, et al. Immune reconstitution inflammatory syndrome secondary to Mycobacterium kansasii infection in a kidney transplant recipient. Am J Transplant 2015; 15:3255–8. [DOI] [PubMed] [Google Scholar]
- 13. Manion M, Dimitrova D, Pei L, et al. Immune reconstitution inflammatory syndrome as a posttransplantation complication in primary immunodeficiency with disseminated Mycobacterium avium. Clin Infect Dis 2020; 70:676–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abdulfattah O, Rahman EU, Shweta F, et al. Severe hypercalcemia in a patient with extrapulmonary Mycobacterium abscessus: granuloma or immune reconstitution inflammatory syndrome? First case of Mycobacterium abscessus presenting as retroperitoneal lymphadenopathy with severe hypercalcemia: a case report and literature review. J Community Hosp Intern Med Perspect 2018; 8:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America Available at: http://aidsinfo.nih.gov.smhslibresources.health.wa.gov.au/contentfiles/lvguidelines/adult_oi.pdf. Accessed 15 August 2020.
- 16. Australian Government: National Health and Medical Research Council. National statement on ethical conduct in human research (2007)—updated 2018 2018. Available at: https://www.nhmrc.gov.au/about-us/publications/national-statement-ethical-conduct-human-research-2007-updated-2018. Accessed 4 December 2020.