Abstract
Objectives
Patients with RA and SLE have an excess cardiovascular risk. We aimed to evaluate outcomes of acute cardiovascular events in these patients.
Methods
Using a nationwide database of Taiwan, we identified adult patients who experienced first-time acute myocardial infarction (n = 191 008), intracranial haemorrhage (n = 169 923) and ischaemic stroke (n = 486 890) over a 13-year period. Odds ratios (ORs) of in-hospital mortality and hazard ratios (HRs) of overall mortality and adverse outcomes during long-term follow-up in relation to RA and SLE were estimated with adjustment for potential confounders.
Results
In each cohort, 748, 410 and 1419 patients had established RA; 256, 292 and 622 patients had SLE. Among acute myocardial infarction patients, RA and SLE were associated with in-hospital mortality (RA: OR 1.61, 95% CI 1.33, 1.95; SLE: OR 2.31, 95% CI 1.62, 3.28) and overall mortality. Additionally, RA (HR 1.28, 95% CI 1.18, 1.38) and SLE (HR 1.46, 95% CI 1.27, 1.69) increased the risk of major adverse cardiac events. After intracranial haemorrhage, patients with RA and SLE had higher risks of in-hospital mortality (RA: OR 1.61, 95% CI 1.26, 2.06; SLE: OR 3.00, 95% CI 2.33, 3.86) and overall mortality. After ischaemic stroke, RA and SLE increased in-hospital mortality (RA: OR 1.45, 95% CI 1.15, 1.83; SLE: OR 2.18, 95% CI 1.57, 3.02), overall mortality and recurrent cerebrovascular events (RA: HR 1.10, 95% CI 1.002, 1.21; SLE: HR 1.31, 95% CI 1.14, 1.51), among which ischaemic stroke (HR 1.39, 95% CI 1.19, 1.62) was more likely to recur in SLE patients.
Conclusion
Both RA and SLE are consistently associated with adverse outcomes following acute cardiovascular events, highlighting the necessity of integrated care for affected patients.
Keywords: RA, SLE, acute myocardial infarction, intracranial haemorrhage, ischaemic stroke
Rheumatology key messages
RA and SLE are associated with in-hospital mortality, overall mortality and major adverse cardiac events after acute myocardial infarction.
RA and SLE are associated with in-hospital and overall mortality after intracranial haemorrhage and ischaemic stroke.
Among ischaemic stroke patients, SLE is associated with recurrent cerebrovascular events, particularly ischaemic stroke.
Introduction
Cardiovascular diseases (CVDs) continue to be the leading cause of death globally [1]. Traditional risk factors, such as age, male sex and hypertension, account for the development of atherosclerosis and associated CVDs. Notably, decades of work have gradually established the relationship between chronic inflammation and atherosclerosis [2–5]. Autoimmune diseases, a diverse group of disease entities originating from dysregulated autoimmunity, may present with extra-articular manifestations, including non-resolving systemic inflammation that contributes to accelerated atherosclerosis and CVDs. The increased incidence of acute myocardial infarction (AMI) and stroke in patients with autoimmune diseases, including RA, SLE, SSc, PM/DM and inflammatory bowel diseases [2–14], has been well documented.
The higher susceptibility to CVD and cardiovascular death in patients with autoimmune diseases raises the suspicion as to whether autoimmune diseases might worsen the outcomes of acute cardiovascular events. Studies have compared the outcomes of AMI/acute coronary syndrome (ACS) and stroke in patients with autoimmune diseases, such as RA and SLE, vs the general population. However, the short-term outcomes (i.e. 30-day or in-hospital mortality) of AMI/ACS are inconsistently reported in RA vs non-RA patients [15–20], and long-term outcomes (i.e. overall mortality and recurrent ischaemia) after AMI/ACS rely substantially on studies involving a small number of patients [15, 20, 21]. Given that aetiologies and clinical outcomes vary with different stroke subtypes [7, 12, 22], results categorized into two major stroke subtypes [i.e. intracranial haemorrhage (ICH) and ischaemic stroke] in RA patients are unavailable [23–26]. More importantly, considerably little is understood regarding the outcomes of acute cardiovascular events in patients with SLE, because relevant studies on this topic are very few in number with no or a limited duration of follow-up [24, 27, 28]. Although patients with autoimmune diseases have a significant burden of CVDs that pose excess risks of morbidity and mortality, outcomes after acute cardiovascular events in patients with autoimmune diseases remain largely underdetermined. Accordingly, the impact of autoimmune diseases on the prognosis following acute cardiovascular events requires further appraisal.
In this study, we examined the hypothesis that autoimmune diseases might be associated with adverse outcomes after acute cardiovascular events. The large sample size and high validity of diagnosis for catastrophic illnesses (e.g. autoimmune diseases) render the National Health Insurance Research Database (NHIRD) of Taiwan valuable as a real-world platform to assess different clinical aspects of autoimmune diseases [29–33]. We attempted, through this population-based study, to describe the clinical characteristics and outcomes of AMI, ICH and ischaemic stroke in patients with autoimmune diseases and to investigate whether, or which, autoimmune diseases might be associated with increased risks of in-hospital mortality and adverse outcomes during long-term follow-up.
Methods
Study design
The National Health Insurance (NHI) programme of Taiwan is a mandatory single-payer health insurance system [34]. All Taiwanese citizens are obliged to participate in the programme, which has been implemented since 1995. The NHI programme covers 99% of the population of Taiwan (∼23 million people). More than 90% of the clinics and hospitals in Taiwan, including >100 tertiary referral medical centres and regional hospitals, provide medical services reimbursed by the NHI administration. The data associated with medical care are routinely collected by the National Health Research Institutes to generate the NHIRD. In NHIRD, the International Classification of Diseases, Ninth Revision, Clinical Modification is used to define diseases and procedures. The International Classification of Diseases, Ninth Revision, Clinical Modification codes used in this study are listed in supplementary Table S1, available at Rheumatology online. Our access to this de-identified database was approved by the Review Committee of the National Health Research Institutes, and the study protocol was approved by the Institutional Review Board of National Cheng Kung University Hospital (A-EX-108-007).
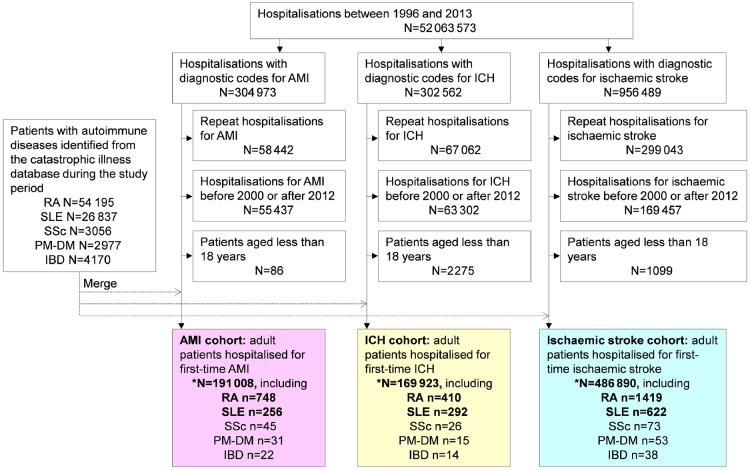
Using the in-patient hospital claims, we selected hospitalizations with diagnostic codes for AMI, ICH and ischaemic stroke from 1 January 2000 to 31 December 2012. The accuracy of the diagnoses in this database regarding AMI, ICH and ischaemic stroke has been previously validated [35–37]. The procedures used to establish the three study cohorts are summarized in Fig. 1. During the study period, we identified adult patients who had first-time AMI (n = 191 008), ICH (n = 169 923) and ischaemic stroke (n = 486 890) as the study cohorts. As shown in Fig. 1, the study flow chart shows that the three cohorts were assembled with a washout phase from 1996 to 1999, meaning that patients with hospitalizations for AMI, ICH or ischaemic stroke during those 4 years were eliminated from this study. The patients were collected from 2000 and thus were likely to have first-time AMI, ICH or ischaemic stroke. In Taiwan, patients with RA and SLE can apply for catastrophic illness certificates. Patients with a catastrophic illness certificate, which is issued after formal review by at least two speciality physicians at the time of application, are exempted from any co-payment for medical care in Taiwan. In this study, patients with SSc, PM/DM and inflammatory bowel diseases in these cohorts were few, and thus not further analysed.
Fig. 1.
Study flow chart: assembly of AMI, ICH and ischaemic stroke cohorts
AMI: acute myocardial infarction; ICH: intracranial haemorrhage; IBD: inflammatory bowel diseases.
Comorbidities and outcome measurements
Baseline medical comorbidities, including hypertension, diabetes mellitus, hyperlipidaemia, heart failure (HF), chronic kidney disease (CKD), peripheral artery disease (PAD), prior stroke (for the AMI cohort), ischaemic heart disease (IHD; for ICH and ischaemic stroke cohorts), chronic obstructive pulmonary disease (COPD), dementia, Parkinson’s disease, atrial fibrillation (AFib) and gout, were identified based on the prior hospitalizations and index hospitalization data of each patient [38]. Patients with end-stage renal disease (ESRD) were further distinguished from patients with CKD, if they were registered with dialysis catastrophic illness certificates. In the AMI cohort, whether the patient underwent reperfusion procedures (i.e. percutaneous coronary intervention or coronary artery bypass grafting) during the index hospitalization was recorded. Finally, the accreditation levels of the hospitals (i.e. tertiary referral centres, regional hospitals or other hospitals) were recognized.
All the outcomes were evaluated since the admission date of the index hospitalization for AMI, ICH and ischaemic stroke. In-hospital all-cause mortality was defined as death during the index hospitalization episode. In addition, overall all-cause mortality after AMI, ICH and ischaemic stroke was measured. For the AMI cohort, ischaemic events, revascularization and major adverse cardiac events (MACE) were evaluated. Ischaemic events were defined as rehospitalizations due to ACS after discharge from the index hospitalization. Revascularization was defined as rehospitalizations for either percutaneous coronary intervention or coronary artery bypass grafting after discharge. The composite endpoint MACE was defined as any occurrence of all-cause mortality, myocardial infarction and revascularization after AMI [39, 40]. For ICH and ischaemic stroke cohorts, recurrent cerebrovascular events, defined as rehospitalizations for ischaemic stroke, ICH, transient ischaemic attack, subarachnoid haemorrhage and other acute ill-defined cerebrovascular diseases after discharge, were evaluated. Selected adverse outcomes that occurred prior to censoring events, including mortality, withdrawal from the insurance programme or 31 December 2013, were recorded. The first event of each adverse outcome was analysed when the patients experienced more than one episode.
Statistical analysis
The patients without the abovementioned autoimmune diseases constituted the controls in each cohort. Patients with RA and SLE were compared with the controls separately to describe the differences in patient characteristics and outcomes. Categorical variables, expressed as numbers and percentages, were analysed using the χ2 test or Fisher’s exact test as needed. Continuous variables, expressed as median and interquartile range, were analysed using the Mann–Whitney U test. Odds ratios (ORs) of in-hospital mortality associated with RA and SLE were estimated using logistic regression analysis with adjustment for potential confounding variables, including age, sex, hypertension, diabetes mellitus, hyperlipidaemia, HF, CKD, PAD, COPD, dementia, Parkinson’s disease, AFib, gout and hospital levels, which have been considered in relevant cardiovascular studies [41, 42]. Prior stroke and reperfusion procedures in the AMI cohort and IHD in the ICH and ischaemic stroke cohorts were also added as covariates. Hazard ratios (HRs) of overall mortality, ischaemic events, revascularization, MACE and recurrent cerebrovascular events associated with RA and SLE were estimated using the Cox regression model after adjusting for potential confounding variables. Statistical analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC, USA). A P-value <0.05 was considered statistically significant.
Results
The AMI cohort
Patient characteristics of the controls, RA and SLE patients in the AMI cohort are summarized in Table 1. For the AMI cohort, there were 748 patients with RA, 256 patients with SLE and 189 922 controls. Of the 189 922 patients without autoimmune diseases, the median age was 70.0 years. The male-to-female ratio was 2.19. The median age of the 748 RA patients was 71.0 years. RA patients were predominantly female. Compared with the controls, RA patients had higher proportions of hypertension, HF, COPD and gout, lower proportions of diabetes mellitus, hyperlipidaemia and ESRD, and less frequently underwent reperfusion procedures after AMI during the index hospitalization (40.5 vs 45.1%, P = 0.01).
Table 1.
Characteristics of the controls, RA and SLE patients in the AMI cohort
| Characteristicsa | Controls (n = 189 922) | Patients with autoimmune diseases | |||
|---|---|---|---|---|---|
| RA patientsb (n = 748) | P-valuec | SLE patientsb (n = 256) | P-valuec | ||
| Age, median (IQR), years | 70.0 (58.0–79.0) | 71.0 (64.0–78.0) | <0.0001 | 53.5 (42.0–67.0) | <0.0001 |
| Sexd | <0.0001 | <0.0001 | |||
| Female | 59 514 (31.3) | 472 (63.1) | 190 (74.2) | ||
| Male | 130 286 (68.6) | 276 (36.9) | 66 (25.8) | ||
| Medical comorbidities | |||||
| Hypertension | 112 353 (59.2) | 484 (64.7) | 0.002 | 169 (66.0) | 0.03 |
| Diabetes mellitus | 74 606 (39.3) | 255 (34.1) | 0.004 | 43 (16.8) | <0.0001 |
| Hyperlipidaemia | 52 592 (27.7) | 135 (18.1) | <0.0001 | 51 (19.9) | 0.006 |
| HF | 47 745 (25.1) | 229 (30.6) | 0.0006 | 63 (24.6) | 0.85 |
| CKD | 14 138 (7.4) | 50 (6.7) | 0.43 | 63 (24.6) | <0.0001 |
| ESRD | 8120 (4.3) | 21 (2.8) | 0.048 | 47 (18.4) | <0.0001 |
| PAD | 6269 (3.3) | 28 (3.7) | 0.50 | 13 (5.1) | 0.11 |
| Prior stroke | 38 055 (20.4) | 145 (19.4) | 0.66 | 39 (15.2) | 0.06 |
| COPD | 26 283 (13.8) | 123 (16.4) | 0.04 | 20 (7.8) | 0.005 |
| Dementia | 6434 (3.4) | 25 (3.3) | 0.95 | 1 (0.4) | 0.008 |
| Parkinson’s disease | 3352 (1.8) | 18 (2.4) | 0.18 | 1 (0.4) | 0.10 |
| AFib | 17 086 (9.0) | 67 (9.0) | 0.97 | 10 (3.9) | 0.004 |
| Gout | 15 914 (8.4) | 97 (13.0) | <0.0001 | 18 (7.0) | 0.44 |
| Reperfusion procedures | 85 643 (45.1) | 303 (40.5) | 0.01 | 131 (51.2) | 0.051 |
| PCI | 79 365 (41.8) | 285 (38.1) | 123 (48.1) | ||
| CABG | 7349 (3.9) | 19 (2.5) | 10 (3.9) | ||
| Hospital level | 0.21 | <0.0001 | |||
| Tertiary referral centre | 63 441 (33.4) | 276 (36.9) | 163 (63.7) | ||
| Regional hospital | 77 260 (40.7) | 294 (39.3) | 56 (21.9) | ||
| Other hospitals | 49 221 (25.9) | 178 (23.8) | 37 (14.5) | ||
Data are expressed as patient number (percentage) unless otherwise indicated.
Nine patients with a combination of RA and SLE were counted in the groups of patients with RA and SLE.
The P-values were calculated by comparison with the controls.
Sex data were missing or undetermined in 122 control patients (0.06%). AMI: acute myocardial infarction; IQR: interquartile range; HF: heart failure; CKD: chronic kidney disease; ESRD: end-stage renal disease; PAD: peripheral artery disease; COPD: chronic obstructive pulmonary disease; AFib: atrial fibrillation; PCI: percutaneous coronary intervention; CABG: coronary artery bypass grafting.
Compared with the controls, the 256 SLE patients were significantly younger and predominantly female. SLE patients were much more likely to have hypertension, CKD and ESRD, and less likely to have diabetes mellitus, hyperlipidaemia, COPD, dementia and AFib. SLE patients appeared to undergo reperfusion procedures more frequently than the controls (51.2 vs 45.1%, P = 0.051) and were more likely to receive medical care in tertiary referral centres.
Outcomes of AMI in RA and SLE
The in-hospital mortality rates after AMI in the controls, RA patients and SLE patients were 12.3, 19.0 and 16.0%, respectively (Table 2). After adjusting for potential confounding variables, RA (adjusted OR 1.61, 95% CI 1.33, 1.95, P < 0.0001) and SLE (adjusted OR 2.31, 95% CI 1.62, 3.28, P < 0.0001) were independent predictors of in-hospital mortality after AMI.
Table 2.
Risks of adverse events after AMI associated with RA and SLE
| Adverse outcomes | Controls (n = 189 922) | Patients with autoimmune diseases | |
|---|---|---|---|
| RA patients (n = 748) | SLE patients (n = 256) | ||
| In-hospital mortality (%) | 23 439 (12.3) | 142 (19.0) | 41 (16.0) |
| Crude OR (95% CI) | 1.00 | 1.66 (1.39, 2.00) | 1.35 (0.97, 1.89) |
| Adjusted OR (95% CI)a | 1.00 | 1.61 (1.33, 1.95) | 2.31 (1.62, 3.28) |
| Overall mortality (%) | 100 045 (52.7) | 495 (66.2) | 134 (52.3) |
| Crude HR (95% CI) | 1.00 | 1.56 (1.43, 1.71) | 1.06 (0.89, 1.25) |
| Adjusted HR (95% CI)b | 1.00 | 1.51 (1.38, 1.65) | 1.94 (1.63, 2.29) |
| Ischaemic eventsc (%) | 55 693 (29.3) | 209 (27.9) | 74 (28.9) |
| Crude HR (95% CI) | 1.00 | 1.19 (1.04, 1.36) | 1.02 (0.81, 1.28) |
| Adjusted HR (95% CI)b | 1.00 | 1.23 (1.07, 1.41) | 1.25 (0.99, 1.57) |
| Re-AMI (%) | 35 191 (18.5) | 126 (16.8) | 48 (18.8) |
| Crude HR (95% CI) | 1.00 | 1.09 (0.92, 1.30) | 1.05 (0.79, 1.39) |
| Adjusted HR (95% CI)b | 1.00 | 1.13 (0.95, 1.35) | 1.42 (1.07, 1.89) |
| Revascularization (%) | 51 146 (26.9) | 146 (19.5) | 69 (27.0) |
| Crude HR (95% CI) | 1.00 | 0.85 (0.72, 1.00) | 1.02 (0.81, 1.29) |
| Adjusted HR (95% CI)b | 1.00 | 1.00 (0.85, 1.18) | 1.25 (0.99, 1.59) |
| Any MACEd (%) | 138 625 (73.0) | 595 (79.6) | 187 (73.1) |
| Crude HR (95% CI) | 1.00 | 1.28 (1.18, 1.39) | 1.01 (0.88, 1.17) |
| Adjusted HR (95% CI)b | 1.00 | 1.28 (1.18, 1.38) | 1.46 (1.27, 1.69) |
Based on the multiple logistic regression analysis with adjustment for age, sex, medical comorbidities (hypertension, diabetes mellitus, hyperlipidaemia, heart failure, chronic kidney disease, peripheral artery disease, chronic obstructive pulmonary disease, dementia, Parkinson’s disease, atrial fibrillation and gout), reperfusion procedures and hospital levels.
Based on the Cox proportional hazards regression model with adjustment for age, sex, medical comorbidities (hypertension, diabetes mellitus, hyperlipidaemia, heart failure, chronic kidney disease, peripheral artery disease, chronic obstructive pulmonary disease, dementia, Parkinson’s disease, atrial fibrillation and gout), reperfusion procedures and hospital levels.
Ischaemic events were defined as rehospitalizations due to acute coronary syndrome after discharge.
The composite endpoint MACE was defined as any occurrence of all-cause mortality, myocardial infarction and revascularization after AMI. AMI: acute myocardial infarction; OR: odds ratio; HR: hazard ratio; MACE: major adverse cardiac events.
The median follow-up time of the AMI cohort was 2.7 (interquartile range 0.3–6.2) years. After AMI, RA (adjusted HR 1.51, 95% CI 1.38, 1.65, P < 0.0001) and SLE (adjusted HR 1.94, 95% CI 1.63, 2.29, P < 0.0001) were independent predictors of overall mortality. RA was independently associated with ischaemic events (adjusted HR 1.23, 95% CI 1.07, 1.41, P = 0.003), whereas SLE was an independent predictor of re-AMI (adjusted HR 1.42, 95% CI 1.07, 1.89, P = 0.01). Finally, both RA (adjusted HR 1.28, 95% CI 1.18, 1.38, P < 0.0001) and SLE (adjusted HR 1.46, 95% CI 1.27, 1.69, P < 0.0001) were independent predictors of MACE.
The ICH and ischaemic stroke cohorts
Patient characteristics of the controls, RA and SLE patients in the ICH cohort are summarized in supplementary Table S2, available at Rheumatology online. For the ICH cohort, there were 410 patients with RA, 292 patients with SLE and 169 179 controls. The median age of the 169 179 patients without autoimmune diseases was 66.0 years, and the male-to-female ratio was 1.74. Compared with the control group, the 410 RA patients were significantly older and predominantly female. RA patients were more likely to have HF, CKD, ESRD, PAD and gout. Compared with the controls, the 292 SLE patients were significantly younger and predominantly female. SLE patients had significantly higher proportions of HF, CKD, ESRD and PAD, and lower proportions of hypertension, diabetes mellitus, COPD, dementia and gout. Both RA and SLE patients more frequently received care in tertiary referral centres.
Patient characteristics of the controls, RA and SLE patients in the ischaemic stroke cohort are summarized in supplementary Table S3, available at Rheumatology online. For the ischaemic stroke cohort, there were 1419 patients with RA, 622 patients with SLE and 484 713 controls. The median age of the 484 713 patients who did not have autoimmune diseases was 71.0 years and the male-to-female ratio was 1.40. Compared with the controls, the 1419 RA patients were predominantly female. RA patients had higher proportions of HF, CKD, PAD, IHD, COPD and gout, and lower proportions of diabetes mellitus, hyperlipidaemia and dementia. The 622 SLE patients were significantly younger than the controls and predominantly female. SLE patients had significantly higher proportions of CKD, ESRD and PAD and lower proportions of hypertension, diabetes mellitus, hyperlipidaemia, IHD, COPD, dementia, AFib and gout. Both RA and SLE patients were more likely to receive medical care in tertiary referral centres.
Outcomes of ICH and ischaemic stroke in RA and SLE
The in-hospital mortality rates after ICH in the controls, RA patients and SLE patients were 12.7, 20.2 and 32.9%, respectively (Table 3). After adjusting for potential confounding variables, RA (adjusted OR 1.61, 95% CI 1.26, 2.06, P < 0.0001) and SLE (adjusted OR 3.00, 95% CI 2.33, 3.86, P < 0.0001) were associated with in-hospital mortality. The median follow-up time of the ICH cohort was 2.7 (interquartile range 0.2–6.5) years. RA (adjusted HR 1.36, 95% CI 1.21, 1.53, P < 0.0001) and SLE (adjusted HR 2.27, 95% CI 1.98, 2.61, P < 0.0001) independently increased overall mortality after ICH. Neither RA nor SLE was associated with recurrent cerebrovascular events after ICH.
Table 3.
Risks of adverse events after ICH and ischaemic stroke associated with RA and SLE
| Adverse outcomes | Controls | Patients with autoimmune diseases | |
|---|---|---|---|
| RA patients | SLE patients | ||
| ICH | |||
| In-hospital mortality (%) | 21 460 (12.7) | 83 (20.2) | 96 (32.9) |
| Crude HR (95% CI) | 1.00 | 1.74 (1.37, 2.22) | 3.37 (2.64, 4.30) |
| Adjusted OR (95% CI)a | 1.00 | 1.61 (1.26, 2.06) | 3.00 (2.33, 3.86) |
| Overall mortality (%) | 96 850 (57.3) | 281 (68.5) | 207 (70.9) |
| Crude HR (95% CI) | 1.00 | 1.49 (1.32, 1.67) | 1.72 (1.50, 1.97) |
| Adjusted HR (95% CI)b | 1.00 | 1.36 (1.21, 1.53) | 2.27 (1.98, 2.61) |
| Recurrent cerebrovascular events (%) | 49 283 (29.2) | 100 (24.4) | 59 (20.2) |
| Crude HR (95% CI) | 1.00 | 0.97 (0.80, 1.18) | 0.96 (0.74, 1.23) |
| Adjusted HR (95% CI)b | 1.00 | 0.99 (0.82, 1.21) | 1.14 (0.88, 1.47) |
| Ischaemic stroke | |||
| In-hospital mortality (%) | 16 055 (3.3) | 78 (5.5) | 40 (6.4) |
| Crude HR (95% CI) | 1.00 | 1.70 (1.35, 2.13) | 2.00 (1.45, 2.76) |
| Adjusted OR (95% CI)a | 1.00 | 1.45 (1.15, 1.83) | 2.18 (1.57, 3.02) |
| Overall mortality (%) | 235 220 (48.5) | 781 (55.0) | 268 (43.1) |
| Crude HR (95% CI) | 1.00 | 1.38 (1.29, 1.48) | 0.95 (0.84, 1.07) |
| Adjusted HR (95% CI)b | 1.00 | 1.35 (1.26, 1.45) | 2.20 (1.95, 2.48) |
| Recurrent cerebrovascular events (%) | 164 049 (33.8) | 451 (31.8) | 196 (31.5) |
| Crude HR (95% CI) | 1.00 | 1.08 (0.98, 1.18) | 0.95 (0.82, 1.09) |
| Adjusted HR (95% CI)b | 1.00 | 1.10 (1.002, 1.21) | 1.31 (1.14, 1.51) |
Based on the multiple logistic regression analysis with adjustment for age, sex, medical comorbidities (hypertension, diabetes mellitus, hyperlipidaemia, heart failure, chronic kidney disease, peripheral artery disease, chronic obstructive pulmonary disease, dementia, Parkinson’s disease, atrial fibrillation and gout) and hospital levels.
Based on the Cox proportional hazards regression model with adjustment for age, sex, medical comorbidities (hypertension, diabetes mellitus, hyperlipidaemia, heart failure, chronic kidney disease, peripheral artery disease, chronic obstructive pulmonary disease, dementia, Parkinson’s disease, atrial fibrillation and gout) and hospital levels. ICH: intracranial haemorrhage; OR: odds ratio; HR: hazard ratio.
Regarding the ischaemic stroke cohort, the in-hospital mortality rates in the controls, RA patients and SLE patients were 3.3, 5.5 and 6.4%, respectively. After adjustment for potential confounding variables, RA (adjusted OR 1.45, 95% CI 1.15, 1.83, P < 0.0001) and SLE (adjusted OR 2.18, 95% CI 1.57, 3.02, P < 0.0001) were associated with in-hospital mortality. The median follow-up time of the ischaemic stroke cohort was 3.8 (interquartile range 1.6–7.1) years. RA (adjusted HR 1.35, 95% CI 1.26, 1.45, P < 0.0001) and SLE (adjusted HR 2.20, 95% CI 1.95, 2.48, P < 0.0001) were independently associated with overall mortality. In addition, RA (adjusted HR 1.10, 95% CI 1.002, 1.21, P = 0.045) and SLE (adjusted HR 1.31, 95% CI 1.14, 1.51, P = 0.0001) increased the risk of recurrent cerebrovascular events after ischaemic stroke.
Subtype of recurrent cerebrovascular events after ischaemic stroke
Finally, we explored which subtypes of recurrent cerebrovascular events were more likely to occur after ischaemic stroke in RA and SLE patients (supplementary Table S4, available at Rheumatology online). No particular subtype of recurrent cerebrovascular event was associated with RA. However, ischaemic stroke (HR 1.39, 95% CI 1.19, 1.62, P < 0.0001), but not ICH, transient ischaemic attack or subarachnoid haemorrhage, was more likely to occur in SLE patients.
Discussion
Although AMI, ICH and ischaemic stroke are considered different entities, these three major types of acute cardiovascular events are potentially life-threatening conditions. Our study provides epidemiologic evidence that patients with RA and SLE are at higher risk of in-hospital mortality and adverse outcomes during follow-up after acute cardiovascular events.
While investigators focusing on RA subjects in the cohorts of Sweden, US and Japan did not observe an increased risk of early mortality after AMI/ACS [15, 18, 20], others have reported different results [16, 17, 19]. An Australian cohort study demonstrated a two-fold increased risk in 30-day mortality after ACS in subjects with RA [19]. Another Swedish study using the national patient register showed increased 7- and 30-day mortalities for RA subjects by ∼50% [17]. In contrast, in a large cross-sectional study from the Nationwide Inpatient Sample in the US, patients with RA have a 34% better in-hospital mortality compared with other patients with AMI after adjusting for confounding variables [16]. Regarding the late outcomes, excess recurrent ischaemia and deaths in RA have been found after AMI/ACS [15, 20, 21]. In the present study, patients with RA and AMI received reperfusion procedures less frequently compared with controls, as reported previously [43]. More importantly, not only in-hospital mortality but also overall mortality, ischaemic events and MACE were substantially increased in RA patients than in non-RA patients after AMI. These results were maintained even after adjustment for potential confounding variables. Continuous exposure to systemic inflammation in RA may lead to accelerated atherosclerosis [2–5]. As demonstrated by coronary angiography, RA patients have more advanced coronary artery disease (CAD) than non-RA patients at diagnosis [44]. Among patients without established CAD, RA subjects have a higher prevalence, extent and severity of coronary plaque, as revealed by 64-slice CT angiography [45]. In one autopsy-based study, coronary artery inflammation and vulnerable coronary plaques are more commonly observed in RA patients than in controls [46]. Due to decreased pain perception and generalized hyposensitivity to myocardial ischaemia [19], RA patients have a higher severity of heart attack [4, 17, 43]. Therefore, pathological and clinical characteristics in RA patients may contribute to unfavourable outcomes following AMI.
While RA is associated with recurrence [26] and overall mortality [23] after stroke, similar early survival has been observed in RA vs non-RA patients [19, 25]. In the present study, we distinguished the outcomes of ICH from ischaemic stroke in RA subjects. After controlling for known risk factors, we provide the first evidence that RA exerted a negative impact on both early and overall survival among ICH and ischaemic stroke patients. Stroke prevention through CVD risk assessment for RA patients may help improve overall outcomes [4, 6]. As recommended by EULAR guidelines [6], rheumatologists, in collaboration with cardiologists and other disciplines, are responsible for the risk management of CVDs in RA. Patients with RA have more vulnerable carotid artery plaques than controls, but achieving remission in RA may stabilize this threat [47]. Also, insufficient treatment of CVDs may be a trigger of stroke in patients with RA and thus CVDs should be managed promptly to lower the subsequent stroke risks in these patients [12].
In addition to RA, the present study extended the scope to explore the impact of SLE on patients experiencing acute cardiovascular events. Consistently in all three cohorts, we found that patients with SLE received medical care for acute cardiovascular events more frequently in tertiary referral centres. Despite the younger age and lower frequency of traditional risk factors for CAD (e.g. diabetes mellitus and COPD), patients with SLE had a much higher prevalence of CKD and ESRD. Even after adjustment for known risk factors, we demonstrate for the first time that SLE increased the risks of early mortality after AMI, ICH and ischaemic stroke. Although long-term follow-up information after AMI in SLE is not available in the literature, a recent Swedish cohort study has shown that SLE is independently associated with 1-year death following ischaemic stroke but not ICH [27]. In this population-based study, SLE independently increased the risks of overall mortality following all three types of acute cardiovascular events. Notably, SLE patients tended to have re-AMI and MACE after AMI. Also, SLE carried a higher risk of recurrent cerebrovascular events after ischaemic stroke, and among stroke subtypes, ischaemic stroke was more likely to recur. The association between SLE and occurrences of AMI and ischaemic stroke has been established [3, 7, 11, 31]. Here, we demonstrate that the recurrent risks of AMI and ischaemic stroke were higher in patients with SLE, highlighting the importance of an integrated secondary-prevention programme for this high-risk population. Due to proatherogenic and prothrombotic states in SLE [4], intensive antiplatelet therapy might be considered after AMI and ischaemic stroke if not contraindicated.
Our study has some limitations. First, some variables of interest were unavailable from the database, such as the extent and distribution of CAD, the exact timing of reperfusion procedures after AMI, the status of smoking, details of immunomodulators, functional outcomes after stroke (e.g. the stroke severity index [37]) and causes of death; thus, we cannot include these variables in the analyses. Second, a sharp reimbursement reduction in Taiwan might have a detrimental effect on the quality of cardiac care [48]. This restricted medical expenditure could possibly bring more negative impacts on autoimmune disease patients who experience acute cardiovascular events as these patients inevitably need multi-disciplinary care. Further validation in other healthcare systems is thus required. Finally, a relatively small percentage of missing data or miscoded diagnoses is inherent in studies using large databases. However, such coding errors are likely random and non-differential misclassification biases the results towards the null.
In conclusion, our study may help rheumatologists, cardiologists and neurologists recognize the inherent risks associated with RA and SLE following AMI, ICH and ischaemic stroke. Previous investigators have demonstrated that patients with RA and SLE are at increased risk for CVDs. Our study further demonstrates that both RA and SLE are consistently associated with increased risks of adverse outcomes following three major types of acute cardiovascular events. Since CVDs are the major cause of death in patients with RA and SLE, integrated care is warranted to improve the outcomes of these high-risk patients.
Supplementary Material
Acknowledgements
The present study was based on data from the NHIRD provided by the National Health Insurance (NHI) administration, Ministry of Health and Welfare of Taiwan and the National Health Research Institutes (NHRI) of Taiwan. However, the interpretations and conclusions contained herein do not represent those of the NHI administration, Ministry of Health and Welfare of Taiwan or the NHRI of Taiwan. Study conception and design: C.-H.L., C.-Y.H., A.B., L.-M.T., Y.S. and C.-Y.L. Acquisition of data: C.-H.L., A.B., L.-C.H. and S.-C.C. Analysis and interpretation of data: C.-H.L., C.-Y.H., L.-C.H., S.-C.C., Y.S. and C.-Y.L. Manuscript drafting and critical review: all authors. Final approval of the manuscript: all authors.
Funding: This work was supported by a grant from the National Cheng Kung University Hospital, Tainan, Taiwan (NCKUH-10403012).
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1.GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1151–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Onat A, Direskeneli H.. Excess cardiovascular risk in inflammatory rheumatic diseases: pathophysiology and targeted therapy. Curr Pharm Des 2012;18:1465–77. [DOI] [PubMed] [Google Scholar]
- 3. Roifman I, Beck PL, Anderson TJ, Eisenberg MJ, Genest J.. Chronic inflammatory diseases and cardiovascular risk: a systematic review. Can J Cardiol 2011;27:174–82. [DOI] [PubMed] [Google Scholar]
- 4. Kaplan MJ. Management of cardiovascular disease risk in chronic inflammatory disorders. Nat Rev Rheumatol 2009;5:208–17. [DOI] [PubMed] [Google Scholar]
- 5. Shoenfeld Y, Gerli R, Doria A.. Accelerated atherosclerosis in autoimmune rheumatic diseases. Circulation 2005;112:3337–47. [DOI] [PubMed] [Google Scholar]
- 6. Agca R, Heslinga SC, Rollefstad S.. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2017;76:17–28. [DOI] [PubMed] [Google Scholar]
- 7. Wiseman SJ, Ralston SH, Wardlaw JM.. Cerebrovascular disease in rheumatic diseases: a systematic review and meta-analysis. Stroke 2016;47:943–50. [DOI] [PubMed] [Google Scholar]
- 8. Gandhi S, Narula N, Marshall JK, Farkouh M.. Are patients with inflammatory bowel disease at increased risk of coronary artery disease? Am J Med 2012;125:956–62. [DOI] [PubMed] [Google Scholar]
- 9. Ungprasert P, Charoenpong P, Ratanasrimetha P. et al. Risk of coronary artery disease in patients with systemic sclerosis: a systematic review and meta-analysis. Clin Rheumatol 2014;33:1099–104. [DOI] [PubMed] [Google Scholar]
- 10. Holmqvist M, Simard JF, Asplund K, Arkema EV.. Stroke in systemic lupus erythematosus: a meta-analysis of population-based cohort studies. RMD Open 2015;1:e000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arkema EV, Svenungsson E, Von Euler M, Sjöwall C, Simard JF.. Stroke in systemic lupus erythematosus: a Swedish population-based cohort study. Ann Rheum Dis 2017;76:1544–9. [DOI] [PubMed] [Google Scholar]
- 12. Meissner Y, Richter A, Manger B. et al. Serious adverse events and the risk of stroke in patients with rheumatoid arthritis: results from the German RABBIT cohort. Ann Rheum Dis 2017;76:1583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiang CH, Liu CJ, Huang CC. et al. Systemic sclerosis and risk of ischaemic stroke: a nationwide cohort study. Rheumatology (Oxford) 2013;52:161–5. [DOI] [PubMed] [Google Scholar]
- 14. Xiao Z, Pei Z, Yuan M. et al. Risk of stroke in patients with inflammatory bowel disease: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis 2015;24:2774–80. [DOI] [PubMed] [Google Scholar]
- 15. Sodergren A, Stegmayr B, Lundberg V, Ohman ML, Wallberg-Jonsson S.. Increased incidence of and impaired prognosis after acute myocardial infarction among patients with seropositive rheumatoid arthritis. Ann Rheum Dis 2006;66:263–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Francis ML, Varghese JJ, Mathew JM. et al. Outcomes in patients with rheumatoid arthritis and myocardial infarction. Am J Med 2010;123:922–8. [DOI] [PubMed] [Google Scholar]
- 17. Mantel Ä, Holmqvist M, Jernberg T, Wållberg-Jonsson S, Askling J.. Rheumatoid arthritis is associated with a more severe presentation of acute coronary syndrome and worse short-term outcome. Eur Heart J 2015;36:3413–22. [DOI] [PubMed] [Google Scholar]
- 18. Isogai T, Matsui H, Tanaka H. et al. Treatments and in-hospital mortality in acute myocardial infarction patients with rheumatoid arthritis: a nationwide retrospective cohort study in Japan. Clin Rheumatol 2017;36:995–1004. [DOI] [PubMed] [Google Scholar]
- 19. Van Doornum S, Brand C, King B, Sundararajan V.. Increased case fatality rates following a first acute cardiovascular event in patients with rheumatoid arthritis. Arthritis Rheum 2006;54:2061–8. [DOI] [PubMed] [Google Scholar]
- 20. McCoy SS, Crowson CS, Maradit-Kremers H. et al. Longterm outcomes and treatment after myocardial infarction in patients with rheumatoid arthritis. J Rheumatol 2013;40:605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Douglas KM, Pace AV, Treharne GJ. et al. Excess recurrent cardiac events in rheumatoid arthritis patients with acute coronary syndrome. Ann Rheum Dis 2006;65:348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hsieh FI, Lien LM, Chen ST. et al. Get With the Guidelines-Stroke performance indicators: surveillance of stroke care in the Taiwan Stroke Registry: Get With the Guidelines-Stroke in Taiwan. Circulation 2010;122:1116–23. [DOI] [PubMed] [Google Scholar]
- 23. Sodergren A, Stegmayr B, Ohman ML, Wallberg-Jonsson S.. Increased incidence of stroke and impaired prognosis after stroke among patients with seropositive rheumatoid arthritis. Clin Exp Rheumatol 2009;27:641–4. [PubMed] [Google Scholar]
- 24. Krishnan E. Stroke subtypes among young patients with systemic lupus erythematosus. Am J Med 2005;118:1415. [DOI] [PubMed] [Google Scholar]
- 25. Kang JH, Xirasagar S, Lin HC, Kao PF, Sung LC.. Risk of adverse outcomes in patients with rheumatoid arthritis hospitalized for stroke-a cross-sectional study. Clin Rheumatol 2018;37:2917. [DOI] [PubMed] [Google Scholar]
- 26. Chen YR, Hsieh FI, Lien LM. et al. Rheumatoid arthritis significantly increased recurrence risk after ischemic stroke/transient ischemic attack. J Neurol 2018;265:1810–8. [DOI] [PubMed] [Google Scholar]
- 27. Rossides M, Simard JF, Svenungsson E, von Euler M, Arkema EV.. Mortality and functionality after stroke in patients with systemic lupus erythematosus. J Rheumatol 2017;44:1590–6. [DOI] [PubMed] [Google Scholar]
- 28. Ward MM. Outcomes of hospitalizations for myocardial infarctions and cerebrovascular accidents in patients with systemic lupus erythematosus. Arthritis Rheum 2004;50:3170–6. [DOI] [PubMed] [Google Scholar]
- 29. Lai CH, Lai WW, Chiou MJ. et al. Outcomes of coronary artery bypass grafting in patients with inflammatory rheumatic diseases: an 11-year nationwide cohort study. J Thorac Cardiovasc Surg 2015;149:859–66.e1–2. [DOI] [PubMed] [Google Scholar]
- 30. Lai CH, Lai WW, Chiou MJ. et al. Outcomes of percutaneous coronary intervention in patients with rheumatoid arthritis and systemic lupus erythematosus: an 11-year nationwide cohort study. Ann Rheum Dis 2016;75:1350–6. [DOI] [PubMed] [Google Scholar]
- 31. Lin CY, Shih CC, Yeh CC. et al. Increased risk of acute myocardial infarction and mortality in patients with systemic lupus erythematosus: two nationwide retrospective cohort studies. Int J Cardiol 2014;176:847–51. [DOI] [PubMed] [Google Scholar]
- 32. Weng MY, Huang YT, Liu MF, Lu TH.. Incidence of cancer in a nationwide population cohort of 7852 patients with primary Sjögren’s syndrome in Taiwan. Ann Rheum Dis 2012;71:524–7. [DOI] [PubMed] [Google Scholar]
- 33. Lin JA, Liao CC, Lee YJ. et al. Adverse outcomes after major surgery in patients with systemic lupus erythematosus: a nationwide population-based study. Ann Rheum Dis 2014;73:1646–51. [DOI] [PubMed] [Google Scholar]
- 34. Hsieh CY, Su CC, Shao SC. et al. Taiwan’s National Health Insurance Research Database: past and future. Clin Epidemiol 2019;11:349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML.. Validation of the national health insurance research database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 2011;20:236–42. [DOI] [PubMed] [Google Scholar]
- 36. Cheng CL, Lee CH, Chen PS. et al. Validation of acute myocardial infarction cases in the national health insurance research database in Taiwan. J Epidemiol 2014;24:500–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hung LC, Sung SF, Hsieh CY. et al. Validation of a novel claims-based stroke severity index in patients with intracerebral hemorrhage. J Epidemiol 2017;27:24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sung SF, Hsieh CY, Lin HJ. et al. Validation of algorithms to identify stroke risk factors in patients with acute ischemic stroke, transient ischemic attack, or intracerebral hemorrhage in an administrative claims database. Int J Cardiol 2016;215:277–82. [DOI] [PubMed] [Google Scholar]
- 39. Kip KE, Hollabaugh K, Marroquin OC, Williams DO.. The problem with composite end points in cardiovascular studies: the story of major adverse cardiac events and percutaneous coronary intervention. J Am Coll Cardiol 2008;51:701–7. [DOI] [PubMed] [Google Scholar]
- 40. Hueb W, Lopes N, Gersh BJ. et al. Ten-year follow-up survival of the Medicine, Angioplasty, or Surgery Study (MASS II): a randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation 2010;122:949–57. [DOI] [PubMed] [Google Scholar]
- 41. Yeh RW, Sidney S, Chandra M. et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 2010;362:2155–65. [DOI] [PubMed] [Google Scholar]
- 42. Fang MC, Coca Perraillon M, Ghosh K, Cutler DM, Rosen AB.. Trends in stroke rates, risk, and outcomes in the United States, 1988 to 2008. Am J Med 2014;127:608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van Doornum S, Brand C, Sundararajan V, Ajani AE, Wicks IP.. Rheumatoid arthritis patients receive less frequent acute reperfusion and secondary prevention therapy after myocardial infarction compared with the general population. Arthritis Res Ther 2010;12:R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yalamanchili K, Aronow WS, Kilaru R. et al. Coronary artery disease is more severe in older persons with rheumatoid arthritis than in older persons without rheumatoid arthritis. Cardiol Rev 2006;14:55–6. [DOI] [PubMed] [Google Scholar]
- 45. Karpouzas GA, Malpeso J, Choi TY. et al. Prevalence, extent and composition of coronary plaque in patients with rheumatoid arthritis without symptoms or prior diagnosis of coronary artery disease. Ann Rheum Dis 2014;73:1797–804. [DOI] [PubMed] [Google Scholar]
- 46. Aubry MC, Maradit-Kremers H, Reinalda MS. et al. Differences in atherosclerotic coronary heart disease between subjects with and without rheumatoid arthritis. J Rheumatol 2007;34:937–42. [PubMed] [Google Scholar]
- 47. Semb AG, Rollefstad S, Provan SA. et al. Carotid plaque characteristics and disease activity in rheumatoid arthritis. J Rheumatol 2013;40:359–68. [DOI] [PubMed] [Google Scholar]
- 48. Chang GM, Cheng SH, Tung YC.. Impact of cuts in reimbursement on outcome of acute myocardial infarction and use of percutaneous coronary intervention: a nationwide population-based study over the period 1997 to 2008. Med Care 2011;49:1054–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.