ABSTRACT
The nutritional status of a patient has prognostic potency concerning short- and long-term outcomes, including survival, in many diseases. The controlling nutritional status (CONUT) score is a method for assessing nutritional status and predicting outcomes of several diseases. This study sought to systematically identify the prognostic role of preoperative CONUT score on posttreatment overall survival (OS), recurrence-free survival (RFS), and cancer-specific survival (CSS) in patients with cancer. The PubMed, SCOPUS, and Google Scholar databases and Google were searched for all dates until December 2019. Original articles investigating the association of preoperative CONUT score with survival in cancer patients who underwent surgery were included. Duplicate and irrelevant reports were screened out and the remaining articles assessed for quality and data extracted during critical analysis. Results of multivariate analysis were used to evaluate the prognostic competence of CONUT score in predicting survival. The search method identified an initial 181 articles, of which 32 were included in the final analysis. Lower OS, CSS, and RFS rates were reported by 100%, 100%, and 87.0% of the included studies, respectively, in cancer patients with high CONUT scores. A prognostic role of the CONUT score for prediction of OS, CSS, and RFS in cancer patients was shown by 91.7%, 90.9%, and 52.6% of the studies, respectively. The receiver operating characteristic curve area under the curve (AUC) value of the CONUT score for predicting OS, CSS, and RFS was at an acceptable level (>0.5) in all studies with available AUC values (n = 19). Sixty percent (12 of 20) of the studies reported that high CONUT score was significantly related to lower BMI. The findings promote confidence that a high preoperative CONUT score is associated with poor survival rate and is an independent prognostic factor of OS and CSS in patients with various types of cancer. Evaluation of the preoperative CONUT score might help clinicians in decision-making with respect to surgical implications.
Keywords: biomarker, cancer, cancer-specific survival, controlling nutritional status, overall survival, recurrence-free survival, prognosis
High preoperative CONUT score is associated with poor survival rate and is an independent prognostic factor for overall survival and cancer-specific survival in patients with various types of cancer.
Introduction
A biomarker is a biological feature that is measured to indicate the body's biological situation or condition, objectively, in relation to, e.g., normal or pathogenic processes, or pharmacologic reactions to a therapeutic approach (1). In clinical practice, biomarkers are often used for early diagnosis or monitoring of a disease; predicting the risk or outcome of a disease; measuring the effectiveness or harmfulness of a treatment regimen or other aspects of health; or screening of patients (2).
Prognostic biomarkers are baseline evaluations of disease features or patients’ characteristics that can predict the risk or outcome of the disease in patients regardless of therapy (3). In cancers, a prognostic biomarker could be an attribute of the disease (such as size of the tumor or stage or grade of the cancer) or an individual characteristic of the patient (such as age, sex, or weight loss) that may influence the outcome. Prognostic biomarkers are beneficial in predicting the outcomes, developing a treatment plan, and decision-making regarding surgical or chemotherapy implications (4).
Malnutrition is prevalent in cancer patients, which can affect short- and long-term outcomes. Nutritional status has a prognostic capacity for posttreatment long-term outcomes, including disease progression and survival, in patients (5). The controlling nutritional status (CONUT) score is an efficient nutritional screening tool for assessing the nutritional status of patients and is useful for early detection of undernutrition in all hospital inpatients. This tool is computed from the 3 clinical parameters of serum albumin, total cholesterol concentration, and total peripheral lymphocyte counts (6).
Multiple investigations have recently examined the prognostic capacity of the CONUT score on posttreatment complications, clinicopathological factors, and long-term outcomes in several types of diseases such as heart failure, liver disease, hypertension, and cancer (7–10). However, to our knowledge, the prognostic value of the CONUT score with regards to long-term outcomes has not yet been systematically reviewed in patients with cancer. The research question was “Is controlling nutritional status useful as a prognostic biomarker of survival in cancer patients who underwent surgery?” Therefore, this study aimed to systematically identify the prognostic role of preoperative CONUT score on posttreatment long-term outcomes including overall survival (OS), recurrence-free survival (RFS), and cancer-specific survival (CSS) in patients with cancer.
Methods
Search strategy and selection criteria
This review was conducted according to the guidelines indicated in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocols, 2015 statement. The PubMed, Google Scholar, and SCOPUS databases and Google were searched for all dates until December 2019. Journal articles and observational studies were searched. Original articles and congresses (if information of interest was available) in the English language that investigated the association of preoperative CONUT score with survival in cancer patients who underwent surgery were included. Inclusion criteria were as follows: articles that studied cancer patients who underwent surgery, and availability of postoperative survival information in the article. Research that studied cancer patients who underwent chemotherapy, radiotherapy, or immunotherapy without surgery, or with an unclear treatment method was excluded. Studies with postoperative CONUT score were also excluded. Studies that applied neoadjuvant therapy along with an operation were included in the present study if the effect of adjuvant therapy was considered in the multivariate analysis, otherwise they were excluded. Reviews, conferences, animal studies, editorials, and letters were also excluded. The following search terms were used: “controlling nutritional status” (in title) AND “survival” (in title/abstract) AND “cancer OR tumor OR carcinoma OR malignant” (in title/abstract). Reference lists of the articles were manually reviewed to identify further studies.
Screening of the articles
The extracted articles were saved in an EndNote file (Clarivate Analytics) and sorted to remove duplicate reports. The remaining titles and abstracts were reviewed to screen articles with the correct scope for the present review. The full texts of the screened articles were then critically analyzed separately for eligibility.
Quality assessment of the articles
The Newcastle-Ottawa scale for cohort studies was used for assessment of the quality of the included studies (11). Articles were categorized as good quality (if 3 or 4 stars in the selection domain AND 1 or 2 stars in the comparability domain AND 1 or 2 or 3 stars in the outcome/exposure domain were gained), fair quality (if 2 stars in the selection domain AND 1 or 2 stars in the comparability domain AND 2 or 3 stars in the outcome/exposure domain were achieved), or poor quality (if 0 or 1 star in the selection domain OR 0 stars in the comparability domain OR 0 or 1 star in the outcome/exposure domain were attained).
Data extraction
Full texts of the screened articles were carefully reviewed and data were extracted with regard to authors, year of publication, country, study design, type of cancer, type of treatment, number and age of patients, follow-up time, method and time of CONUT assessment, CONUT cutoffs, method of CONUT cutoff determination, efficacy of the CONUT score (sensitivity and specificity) for prognosis of survival, survival rates and their association with CONUT score, method of survival rate estimation, method of statistical analysis, confounding factors, and adjustments. Results of multivariate analysis were used to evaluate the prognostic effect of CONUT score on survival, in this review.
The time interval from surgery to radiological or histological detection of recurrence or metastasis or progression of disease was defined as RFS, disease-free-survival (DFS), or progression-free survival (PFS), which were all placed in 1 group (12, 13). The time interval from surgery to cancer-related death was defined as CSS (12, 14) and the time interval from surgery to overall death or last follow-up was defined as OS (12, 15). Studies that did not specify type of survival were considered as OS in this review.
Results
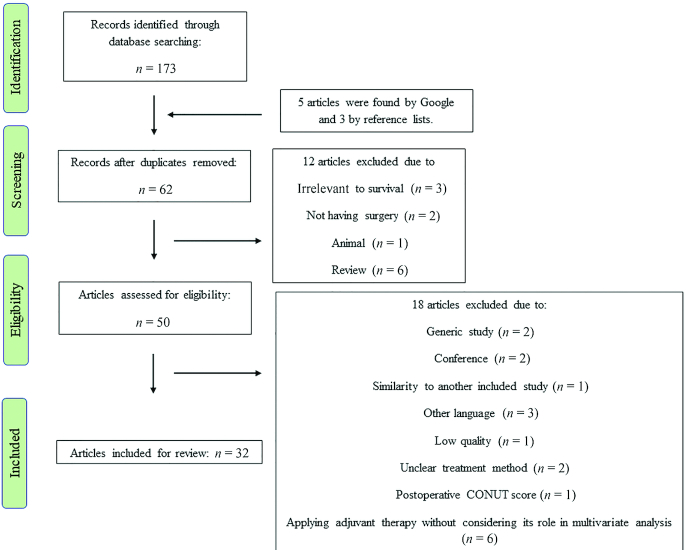
As Figure 1 shows, initially 181 titles and abstracts were found using the search method (173 by databases, 5 by Google, and 3 by reference list searching). After elimination of duplicate studies, 62 articles remained. During the screening stage, 50 studies were found to be relevant to the study subject. During critical analysis, 18 articles were excluded because of not being written in English (n = 3), being conference papers (n = 2), being generic studies (n = 2), similarity (n = 1), low quality (n = 1), unclear treatment method (n = 2), postoperative CONUT score (n = 1), and applying adjuvant therapy without considering it in the multivariate analysis (n = 6). Eventually, 32 original studies (10, 12, 16–45) (W Yang, C Shou, J Yu, Q Zhang, X Liu, H Yu, X Lin, unpublished results, 2019) matched the study scope and, thus, were comprised in the final review and analysis (Figure 1).
FIGURE 1.
Flow diagram of the study. CONUT, controlling nutritional status.
Characteristics of the studies
As Table 1 shows, 62.5% (n = 20) of the included studies were performed in Japan, 31.3% (n = 10) in China, and 6.3% (n = 2) in South Korea. All the studies included were retrospective cohort investigations except the report by Huang et al. (22), a prospective cohort study. Of the articles, 96.9% (31 of 32) were published between 2017 and 2019.
TABLE 1.
Characteristics of the studies included and findings on prognostic potential (according to multivariate analysis) and efficacy of the CONUT score in predicting survival1
| Reference | Country, study design | Type of cancer | Patients, n | Age, y | Follow-up time | CONUT score cut offs | Determination of optimal cutoff values for CONUT score/index | CONUT score efficacy for predicting survival | Calculation method of survival rate, HR, and CONUT score2 | Findings | Relation of CONUT score and BMI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahiko et al. (16) | Japan, retrospective cohort | Colorectal cancer (Stage I–IV), underwent surgery | 830 (470 males, 360 females) | Median (range): 78 (75–94) | ≥5 y | 0–1 (n = 508)2–3 (n = 249)≥4 (n = 73) | Not indicated. | Not indicated. | *¶◊ | Higher CONUT group had the lowest 5-y OS rate (P < 0.0001).CONUT score was a significant prognostic factor of OS (≥4 vs. 0–1: HR: 2.24; 95% CI: 1.48, 3.30; P < 0.001) after adjustment for confounders. | BMI did not differ between CONUT groups. |
| Akamine et al. (17) | Japan, retrospective | Lung adenocarcinoma, underwent surgery | 109 (76 males, 33 females) | Mean (range): 72 (45–85) | 4–13 y | High: ≥1 (n = 74)Low: 0 (n = 35) | ROC curve/— | AUC: 0.596 Sensitivity: 0.6711; specificity: 0.4375 | *¶◊ | High-CONUT group had lower 5-y OS (P = 0.04) and DFS (P = 0.01) rates.CONUT score was independently associated with DFS (HR: 2.63; 95% CI: 1.33, 5.68; P = 0.004) and OS (HR: 2.64; 95% CI: 1.06, 7.80; P = 0.04). | High-CONUT group had lower BMI (P = 0.025). |
| Elghiaty et al. (12) | South Korea, retrospective cohort | Nonmetastatic clear cell renal cell carcinoma, underwent radical or partial nephrectomy | 1046 (745 males and 301 females) | Median (range): 56 (46–64) | Median (range): 63 (43–87) mo | High: >2 (n = 115)Low: ≤2 (n = 931) | ROC curve/Youden Index | AUC (based on OS) = 0.633 Sensitivity = 46.4%; specificity = 73.7% (P = 0.001) | *¶◊ | High-CONUT group had lower 3- and 5-y RFS (P < 0.001), CSS (P = 0.006), and OS (P < 0.001) rates.High CONUT score was an independent predictor of RFS (HR: 3.09; 95% CI: 1.45, 6.59; P = 0.003), CSS (HR: 4.66; 95% CI: 1.62, 13.39; P = 0.004), and OS (HR: 2.81; 95% CI: (1.44, 5.50; P = 0.003). | High-CONUT group had lower BMI (P = 0.001). |
| Harimoto et al. (18) | Japan, retrospective cohort | Hepatocellular carcinoma, underwent hepatic resection | 2461 (1785 males, 676 females) | Mean ± SD: Low CONUT: 68.2 ± 10.1; High CONUT: 69.8 ± 9.2 | Not indicated | High: ≥4 (n = 540)Low: ≤3 (n = 1921) | ROC curve/Youden index | AUC (based on OS): 0.580 Sensitivity: 31.3%; specificity: 91.6% (P < 0.01) AUC (based on CSS): 0.563Sensitivity: 30.0%; specificity: 80.1% (P < 0.01) AUC (based on RFS): 0.536 Sensitivity: 63.5%; specificity: 41.9% (P < 0.01). | *¶◊ | High-CONUT group had lower OS and RFS rates (both, P < 0.01).Higher CONUT score was an independent predictor of poor OS (HR: 1.22; 95% CI: 1.06, 1.41; P = 0.006) and RFS (HR: 1.22; 95% CI: 1.06, 1.40; P = 0.006). | High-CONUT group had low BMI (P < 0.01). |
| Harimoto et al. (19) | Japan, retrospective | Hepatocellular carcinoma, underwent hepatic resection | 357 (270 males, 87 females) | Mean ± SD: Low CONUT: 67.3 ± 10.7; High CONUT: 69.8 ± 8.5 | ≥5 y | High: >3 (n = 69)Low: ≤3 (n = 288) | ROC curve/Youden index | AUC (based on OS): 0.621Sensitivity: 56.06%; specificity: 66.56% AUC (based on DSS): 0.651 Sensitivity: 65.7%; specificity: 59.5%. | *¶◊ | High-CONUT group had lower 5-y OS, RFS, and DSS rates (P < 0.01).Higher CONUT score was associated with poor OS (HR: 2.16; 95% CI: 1.25, 3.72; P = 0.03), but not with RFS. | BMI did not differ between CONUT groups. |
| Hirahara et al. (20) | Japan, retrospective | Esophageal cancer, underwent curative thoracoscopic esophagectomy | 148 (132 males, 16 females) | Mean ± SD: CONUT 0: 65.6 ± 8.2; CONUT 1: 67.5 ± 8.4; CONUT 2-3: 65.3 ± 8.9 | Not indicated | Normal nutrition: 0–1 (n = 70)Mild malnutrition: 2–4 (n = 62)Moderate–severe malnutrition: 5–12 (n = 16) | Not indicated. | Not indicated. | *¶◊ | CONUT was independently associated with worse prognosis for CSS (HR: 1.99; 95% CI: 1.07, 3.87), P = 0.03). | Not indicated. |
| Hirahara et al. (21) | Japan, retrospective cohort | Gastric cancer, underwent curative gastrectomy | 368 (254 males, 114 females) | Range: 36–91 | Median: 35.3 mo | High: ≥3 (n = 105)Low: ≤2 (n = 263) | ROC curve/— | AUC (based on 5-y OS): 0.625 Sensitivity: 65.0%; specificity: 57.9% | *¶◊ | High-CONUT group had lower 5-y OS rate (P < 0.001).Among all patients, OS was independently predicted by the CONUT score (HR: 2.25, P = 0.001).CONUT score was an independent prognostic factor for OS among the propensity score–matched subgroup (HR: 2.44; 95% CI: 1.46, 4.07; P < 0.001). | BMI did not differ between CONUT groups. |
| Huang et al. (22) | China, prospective cohort | Gastric cancer, underwent curative gastrectomy | 357 (275 males, 82 females) | Mean ± SD: 73.29 ± 5.24 | 1 y | Normal: 0–1 (n = 153)Light: 2–4 (n = 168)Moderate and severe: ≥5 (n = 36) | Not indicated. | Not indicated. | ¶◊ | CONUT score was an independent predictor of postoperative 1-y survival (OR: 2.91; 95% CI: 0.91, 9.31; P = 0.02). | Moderate–severe CONUT group had lower BMI (P < 0.001). |
| Iseki et al. (23) | Japan, retrospective | Colorectal cancer (Stage II/III), underwent curative surgery | 204 (112 males, 92 females) | Mean ± SD: High CONUT: 66.09 ± 9.23; Low CONUT: 71.13 ± 11.57 | 8 y or until their deaths | High: ≥3 (n = 54)Low: ≤2 (n = 150) | ROC curve/— | AUC (based on 5-y CSS): 0.624 (P = 0.076)Sensitivity: 0.5263; specificity: 0.7622 | *¶◊ | High-CONUT group had lower 5-y CSS (P = 0.002) and RFS (P = 0.002) rates.CONUT score was an independent risk factor for CSS (OR: 4.21; 95% CI: 1.21, 13.35; P = 0.02), but not for RFS. | Not indicated. |
| Ishihara et al. (24) | Japan, retrospective cohort | Localized urothelial carcinoma treated with radical nephroureterectomy | 107 (68 males, 39 females) | Mean ± SD: Low CONUT:72.7 ± 9.98; High CONUT: 76.1 ± 8.65 | Mean ± SD: 46.1 ± 32.8; 25.5 ± 18.4 mo | High: ≥3 (n = 24)Low: <3 (n = 83) | ROC curve/Youden index | AUC (based on RFS): 0.588 | *¶◊ | High-CONUT group had lower 5-y RFS (P = 0.04), CSS (P = 0.004), and OS (P = 0.01) rates. | Not indicated. |
| CONUT score was an independent predictor of CSS (HR: 5.44; 95% CI: 1.95, 14.8; P = 0.002), OS (HR: 2.90; 95% CI: 1.18, 6.75; P = 0.02), and RFS (HR: 2.26; 95% CI: 0.97, 4.94; P = 0.058). | |||||||||||
| Kang et al. (25) | Korea, retrospective cohort | Renal cell carcinoma, underwent surgery | 1881 (1361 males, 520 females) | Mean ± SD: Normal: 54.21 ± 12.17; Mild: 58.69 ± 12.80; Moderate to severe: 63.69 ± 12.83 | Median (range): 41 (6–178) mo | High: ≥2 (n = 508)Low: 0–1 (n = 1373) | ROC curve/— | ROC curve | *¶◊ | High-CONUT group had shorter RFS (P = 0.02) and CSM (P < 0.001).High CONUT score was an independent predictor of CSM (HR: 1.89; 95% CI: 1.12, 3.20; P = 0.02), but not for RFS. | High-CONUT group had lower BMI (P < 0.001). |
| Kato et al. (26) | Japan, retrospective | Pancreatic adenocarcinoma, underwent resection | 344 (207 males, 137 females) | Mean ± SD: 64.8 ± 9.9 | Median (range): 29.1 (0.6–178.5) mo | High: ≥4 (n = 79)Low: <4 (n = 265) | ROC curve/— | AUC (based on 2-y survival): 0.614 (95% CI: 0.56, 0.67).Sensitivity: 30.6%; specificity: 88.6% | *¶◊ | High-CONUT group showed lower OS (P = 0.002) but not RFS.High CONUT score was an independent prognostic risk factor for OS (HR: 1.64; 95% CI: 1.19, 2.26; P = 0.003). | Not indicated. |
| Kuroda et al. (27) | Japan, retrospective cohort | Gastric cancer, underwent curative resection | 416 (267 males, 149 females) | Median (range): 67.2 (25–94) | Median (range): 61.2 (1–134) mo | High: ≥4 (n = 62)Low: ≤3 (n = 354) | ROC curve/— | AUC (based on OS): 0.715 (95% CI: 0.68, 0.75)AUC (based on RFS): 0.658 (95% CI: 0.62, 0.70)AUC (based on CSS): 0.662 (95% CI: 0.61, 0.71) | *¶◊ | High-CONUT group had lower 5-y OS (P < 0.001), RFS (P = 0.02), and CSS (P = 0.02) rates.CONUT was an independent prognostic factor for OS (HR: 2.72; 95% CI: 1.74, 4.25; P < 0.001), but not for RFS and CSS. | High-CONUT group had lower BMI (P = 0.02). |
| Liang et al. (28) | China, retrospective cohort | Soft-tissue sarcomas, underwent surgical resection | 658 (393 males, 265 females) | Median (range): 43 (5–85) | Median (range): 103 (61–147) mo | High: ≥2 (n = 223)Low: 0–1 (n = 435) | ROC curve/Youden index | ROC curve | *¶◊ | High-CONUT group had lower 5-y OS (P < 0.001) and DFS (P < 0.001) rates.High CONUT was an independent predictor of OS (HR: 1.86; 95% CI: 1.47, 4.14; P < 0.001) and DFS (HR: 1.63; 95% CI: 1.26, 2.11; P < 0.001). | Not indicated. |
| Lin et al. (29) | China, retrospective cohort | Hepatocellular carcinoma, underwent curative hepatectomy | 380 (333 males, 47 females) | Median (range): 50 (19–80) | Median: 48.5 mo | High: ≥2 (n = 187)Low: <2 (n = 193) | ROC curve/— | AUC (based on OS): 0.618 (95% CI: 0.567, 0.667)Sensitivity: 66.3%; specificity: 56.5% | *¶◊ | High-CONUT group had lower 5-y OS (P < 0.001) and RFS (P = 0.02) rates. High CONUT was an independent prognostic indicator of decreased OS (HR: 2.40; 95% CI: 1.74, 4.25; P = 0.001), but not decreased RFS (HR: 1.36; 95% CI: 1.00, 1.85; P = 0.05). | Not indicated. |
| Miyata et al. (30) | Japan, retrospective | Intrahepatic cholangiocarcinoma, underwent curative hepatectomy | 71 (45 males, 26 females) | Mean ± SD: Low CONUT: 64.8 ± 1.7; High CONUT: 69.1 ± 1.9 | Mean: 36.9 mo | High: ≥2 (n = 31)Low: <2 (n = 40) | Not indicated. | Not indicated. | *¶◊ | High-CONUT group had lower 1-, 3-, and 5-y OS (P = 0.01), but not RFS.High CONUT was an independent prognostic factor for OS (HR: 3.02; 95% CI: 1.4, 6.8; P = 0.007), but not for RFS. | High-CONUT group had lower BMI (P = 0.009). |
| Ryo et al. (31) | Japan, retrospective cohort | Gastric cancer, underwent gastrectomy | 626 (435 males, 191 females) | Mean ± SD: 67.9 ± 10.9 | Median: 49.2 mo or until death | High: ≥2 (n = 289)Low: <2 (n = 337) | ROC curve/— | AUC (based on DFS): 0.656 Sensitivity: 0.66; specificity: 0.58 | *¶◊ | High-CONUT group had shorter OS (P < 0.0001) and DFS (P = 0.06) times. CONUT score was an independent prognostic factor for OS (HR: 1.74; 95% CI: 1.26, 2.41; P = 0.0007). | High-CONUT group had lower BMI (P < 0.0001). |
| Shoji et al. (32) | Japan, retrospective | Non–small cell lung cancer, underwent surgery | 138 (79 males, 59 females) | Mean (range): 68 (37–86) | Median (range): 58 (0–94) mo | High: ≥1 (n = 79)Low: 0 (n = 59) | ROC curve/— | AUC (based on CSS): 0.703Sensitivity = 91.67%; specificity = 46.07% | *¶◊ | High-CONUT group had lower 5-y RFS (P = 0.046), CSS, (P = 0.01), and OS (P = 0.01) rates. CONUT score was an independent prognostic factor for CSS (RR: 6.06; 95% CI: 1.07, 113.94; P = 0.04). | Not indicated. |
| Song et al. (33) | China, retrospective cohort | Nonmetastatic renal cell carcinoma, underwent surgery | 325 (231 males, 94 females) | Median (IQR): 57 (47–66) | Median (IQR): 64 (56.5–69) mo | High: ≥3 (n = 70)Low: <3 (n = 255) | ROC curve/Youden index | AUC (based on 5-y OS): 0.723, (P < 0.001)Sensitivity: 51.28%; specificity: 82.52% | *¶◊ | High-CONUT group had lower 5-y OS (P < 0.001), CSS (P < 0.001), and DFS (P < 0.001) rates. High CONUT was an independent risk factor for OS (HR: 3.36; 95% CI: 1.73, 6.56; P < 0.001), CSS (HR: 3.34; 95% CI: 1.59, 6.98; P = 0.001), and DFS (HR: 1.85; 95% CI: 1.07, 3.21; P = 0.03). | Not indicated. |
| Suzuki et al. (34) | Japan, retrospective | Gastric cancer, underwent curative resection | 211 (141 males, 70 females) | ≥75 | Median (range): 47 (5–185) mo | Normal nutrition: (n = 75) Light malnutrition: (n = 100) Moderate or severe malnutrition: (n = 36) | Not indicated. | Not indicated. | *¶◊ | Higher-CONUT group had shorter OS (P < 0.001) and CSS (P < 0.001).CONUT score was an independent prognostic factor for OS (HR: 2.12; 95% CI: 1.18, 3.69; P = 0.01) and CSS (HR: 3.75; 95% CI: 1.30, 10.43; P = 0.01). | Higher CONUT group had lower BMI (P = 0.008). |
| Takagi et al. (35) | Japan, retrospective cohort | Hepatocellular carcinoma, underwent hepatectomy | 295 (241 males, 54 females) | Mean ± SD: 65.8 ± 10.4 | Mean: 42.3 mo | High: ≥3 (n = 118)Low: ≤2 (n = 177) | ROC curve/— | AUC = 0.59 | *¶◊ | High-CONUT group had lower 5-y RFS (P = 0.01) and OS (P = 0.006) rates.The CONUT score was an independent predictor of RFS (HR: 1.64; 95% CI: 1.15, 2.30; P = 0.006) and OS (HR: 2.50; 95% CI: 1.47, 4.23; P = 0.001). | BMI did not significantly differ between CONUT groups. |
| Takagi et al. (36) | Japan, retrospective cohort | Hepatocellular carcinoma, underwent hepatectomy | 331 (269 males, 62 females) | Median (range): 67 (60–74) | 1 mo | High: ≥5 (n = 30)Low: ≤4 (n = 301) | Not indicated. | Not indicated. | ¶◊ | High-CONUT group had higher incidence of 30-d mortality (P < 0.001).High CONUT score was an independent predictor of in-hospital mortality after hepatectomy (HR: 9.41; 95% CI: 1.15, 77.4; P = 0.04). | BMI did not differ between CONUT groups (P > 0.05). |
| Takamori et al. (37) | Japan, retrospective | Malignant pleural mesothelioma | 83 (66 males, 17 females) | Median (range): 59 (31–81) | — | High: ≥3 (n = 31)Low: ≤2 (n = 52) | ROC curve/— | AUC (based on 1-y survival): 0.772Sensitivity: 73.1%; specificity: 64.0% | *¶◊ | High-CONUT group had lower OS and DFS rates (both, P < 0.001).High CONUT score was an independent predictive factor for OS (HR: 1.92; 95% CI: 1.17, 3.11; P = 0.01) and DFS (HR: 1.88; 95% CI: 1.14, 3.06; P = 0.01).High CONUT score was a prognostic factor of OS in patients who underwent surgery (HR: 4.86; 95% CI: 1.16, 19.14; P = 0.03). | Not indicated. |
| Tokunaga et al. (38) | Japan, retrospective | Colorectal cancer, underwent curative resection | 417 (247 males, 170 females) | Median (range): 68 (19–93) | Mean (range): 38.0 (1–115) mo | Normal: (n = 246)Light: (n = 127) Moderate: (n = 33) Severe: (n = 11) | Not indicated. | Not indicated. | *¶◊ | High-CONUT group (moderate/severe) had lower 5-y OS and RFS (both, P < 0.001).CONUT score was an independent prognostic factor for OS (moderate/severe vs. normal: HR: 5.92; 95% CI: 2.30, 14.92; P < 0.001; light vs. normal: HR: 2.74; 95% CI: 1.30, 5.87; P = 0.008), but not for RFS. | Moderate/severe CONUT group had lower BMI (P = 0.005). |
| Toyokawa et al. (39) | Japan, retrospective | Lung squamous cell carcinoma, underwent surgery | 108 (96 males, 12 females) | Median (range): 71 (45–89) | ≥5 y | High: ≥2 (n = 32)Low: 0–1 (n = 76) | ROC curve/— | AUC (based on OS): 0.590 Sensitivity: 0.786; specificity: 0.385 | *¶◊ | High-CONUT group had lower 5-y DFS (P = 0.02) and OS (P = 0.006) rates. High CONUT score was an independent prognostic factor for DFS (HR: 1.90; 95% CI: 1.04, 3.37; P = 0.04) and OS (HR: 1.91; 95% CI: 0.92, 3.86; P = 0.08). | BMI did not differ between CONUT groups (P > 0.05). |
| Wang et al. (40) | China, retrospective | Malignant peritoneal mesothelioma | 125 (46 males, 79 females) | Mean ± SD: 61.2 ± 9.3 | Median (range): 8 (0.6–53) mo | High: ≥3 (n = 81)Low: ≤2 (n = 44) | ROC curve/— | AUC (based on OS): 0.861 (P < 0.001)Sensitivity: 0.784; specificity: 0.821 | ¶◊ | CONUT score was an independent predictive factor for OS (RR: 1.26; 95% CI: 1.16, 1.38; P < 0.001). | BMI did not differ between CONUT groups (P > 0.05). |
| Yamamoto et al. (41) | Japan, retrospective | Colorectal cancer (stage I–IV) underwent surgery | 522 (291 males, 231 females) | — | ≥5 y | High: ≥3 (n = 158)Low: <3 (n = 364) | ROC curve/— | AUC (based on OS): 0.627 (P < 0.0001) | *¶◊ | High-CONUT group had a lower 5-y OS rate (P < 0.0001). | Not indicated. |
| Yang C et al. (42) | China, retrospective | Colorectal cancer, underwent curative resection | 160 (90 males, 70 females) | Mean ± SD: 58.4 ± 11.8 | Median (range): 30 (6–42) mo | High: ≥3 (n = 74)Low: <3 (n = 86) | ROC curve/Youden index | AUC (based on CSS): 0.759 (P < 0.001)Specificity: 0.821; sensitivity: 0.625 | *¶◊ | High CONUT score was correlated with poor RFS (P < 0.001) and CSS (P < 0.001).CONUT score was an independent prognostic factor for RFS (HR: 2.02; 95% CI: 1.19, 3.43; P = 0.01) and CSS (HR: 3.45; 95% CI: 1.68, 7.10; P = 0.001). | Not indicated. |
| W Yang, C Shou, J Yu, Q Zhang, X Liu, H Yu, X Lin, unpublished results, 2019 | —, retrospective | Gastrointestinal stromal tumors, underwent resection | 455 (222 males, 233 females) | Median (range): 57 (20–80) | Median (range): 110 (7–232) mo | Normal: 0–1 (n = 219)Light undernutrition: 2–4 (n = 196)Moderate–severe undernutrition: ≥5 (n = 40) | Not indicated. | Not indicated. | *¶◊ | Higher-CONUT group had a lower RFS rate (P = 0.001).CONUT score was an independent prognostic factor for RFS (COUNT ≥5 vs. CONUT = 0–1: HR: 2.83; 95% CI: 1.46, 5.50; P = 0.002). | Not indicated. |
| Zhang et al. (43) | China, retrospective cohort | Metastatic prostate cancer, underwent surgery | 94 males | Median (range): 71 (53–84) | Median (range): 16.31 (4.6–55.10) mo | High: ≥3 (n = 42)Low: 0–2 (n = 52) | X-tile program | Using the X-tile software | *◊ | High-CONUT group had shorter PFS before surgery (P < 0.05).High CONUT score was an independent prognostic factor for PFS (HR: 3.97; 95% CI: 1.05, 11.43; P = 0.004). | BMI did not differ between CONUT groups (P > 0.05). |
| Zheng Z-F et al. (44) | China, retrospective | Gastric cancer, underwent radical gastrectomy | 532 (403 males, 129 females) | Mean ± SD: 61.1 ± 11.5 | Median (range): 60 (2–76) mo | Normal nutrition: n = 291Light malnutrition: n = 183Moderate or severe malnutrition: n = 58 | Not indicated. | Not indicated. | *¶◊ | Higher-CONUT group had the lowest 5-y OS (P = 0.006) and RFS (P = 0.02) rates.CONUT score was not associated with 5-y OS and RFS. | Higher-CONUT group had low BMI (P = 0.01). |
| Zheng Y et al. (45) | China, retrospective | Renal cell carcinoma, underwent nephrectomy | 635 (400 males, 235 females) | Mean ± SD: 61.71 ± 12.51 | Median (range): 48.4 (29.3–80.1) mo | High: ≥2 (n = 349)Low: <2 (n = 286) | X-tile program | X-tile program | *¶◊ | High-CONUT group had shorter OS and CSS (both, P < 0.0001).CONUT score was an independent risk predictor of OS (HR: 3.01; 95% CI: 1.52, 5.95; P = 0.001) and CSS (HR: 3.00; 95% CI: 1.29, 6.98; P = 0.01). | High-CONUT group had low BMI (P < 0.001). |
CONUT, controlling nutritional status; CSS, cancer-specific survival; DFS, disease-free survival; OS, overall survival; PFS, progression-free survival; RFS, recurrence/relapse-free survival; ROC, receiver operating characteristic.
*Survival rate, ¶HR, ◊CONUT score. The Kaplan–Meier method and log-rank test were used to estimate survival rates. The Cox proportional hazards regression model was used to calculate HRs and 95% CIs. CONUT score was calculated from serum albumin and total cholesterol concentrations and total peripheral lymphocyte counts.
In all included studies the CONUT score was evaluated based on serum albumin concentration, total lymphocyte count, and total cholesterol concentration in each patient. Of the studies, 90.6% (29 of 32) used the Kaplan–Meier method and log-rank test to estimate survival rates and 96.9% (31 of 32) used Cox proportional hazards regression models to calculate HRs and 95% CIs.
The receiver operating characteristic (ROC) curve and the AUC were used to evaluate the efficacy of the CONUT score for predicting survival (65.6%, 21 of 32) and to determine the optimal cutoff for the CONUT score. The highest Youden index was used to attain the optimal cutoff of the CONUT score in 10 studies.
According to the Newcastle-Ottawa scale, all studies achieved good quality scores (Supplemental Table 1).
Relation between CONUT score and patients’ survival
Twenty-four articles studied the OS rate in different CONUT groups, for which all the studies (100%) showed that patients with high CONUT scores had significantly shorter OS than those with low CONUT scores (P < 0.05). Twenty-four of the studies investigated the prognostic effect of high CONUT score with regards to OS. Based on the multivariate analysis, 91.7% (22 of 24) of the studies demonstrated that CONUT score was an independent prognostic factor of OS (P < 0.05).
Eleven studies investigated CSS, cancer-specific mortality (CSM), or disease-specific survival (DSS) rate in different CONUT groups and all the studies (100%) indicated that patients with a high CONUT score had significantly shorter CSS than those with a low CONUT score (P < 0.05). According to multivariate analysis, 90.9% (10 of 11) of the studies reported that CONUT score was an independent prognostic factor of CSS (P < 0.05).
Twenty-three studies investigated RFS, DFS, or PFS rate in different CONUT groups and 87.0% (20 of 23) of the studies indicated that patients with a high CONUT score had significantly shorter RFS than those with a low CONUT score (P < 0.05). Nineteen studies evaluated the correlation between CONUT score and RFS. According to multivariate analysis, 52.6% (10 of 19) of the studies reported that the CONUT score was an independent prognostic factor for RFS (P < 0.05).
Efficacy of the CONUT score in prognosis of survival
Fourteen studies reported AUC values of ROC curves for OS, of which the minimum and maximum values were 0.58 and 0.86, respectively, among the studies. Eleven studies reported the sensitivity and specificity of CONUT score cutoffs for detection of OS. The minimum and maximum values of sensitivity were 30.6% and 82.0%, respectively, and for specificity were 38.5% and 91.6%, respectively, among the studies.
Four studies reported AUC values of ROC curves for RFS, ranging from 0.54 to 0.66 among the studies. Two studies reported CONUT score cutoffs’ sensitivity (ranging from 63.5% to 66.0% among the studies) and specificity (ranging from 41.9% to 58.0% across the studies) for detection of RFS.
Six studies reported AUC values of ROC curves for CSS with minimum and maximum values of 0.56 and 0.76, respectively, across the studies. Five studies reported CONUT score cutoffs’ sensitivity (ranging from 30.0% to 91.7% across the studies) and specificity (ranging from 46.1% to 80.1% across the studies) for detection of CSS.
Relation between BMI and CONUT score
Twenty studies measured the relation of BMI to CONUT score; 60.0% (n = 12) of the studies reported that the high-count group had significantly lower BMI (P < 0.05).
Discussion
Lower OS, CSS, and RFS rates in cancer patients with high CONUT scores were reported by 100%, 100%, and 86.7% of the studies, respectively. A prognostic role of the CONUT score for prediction of OS, CSS, and RFS in cancer patients was suggested by 91.7%, 90.9%, and 52.6% of the studies, respectively. The prognosis of outcomes (including tumor proliferation, patients’ survival, and posttreatment complications) in patients with cancer has been demonstrated to be strongly correlated with aspects of the host's nutritional situation such as BMI, visceral obesity, and sarcopenia (46–49). The CONUT score is a nutritional assessment tool that is easily, cheaply, and objectively estimated from serum albumin, total cholesterol, and total lymphocyte count (6). A prognostic impact of the CONUT score on survival has also been reported in hospitalized elderly people (50) and in patients with heart failure (7), end-stage liver disease (8), hypertension (9), and peritoneal dialysis (51).
The AUC value of the ROC curve for the CONUT score was at an acceptable level (>0.5) for predicting OS, CSS, and RFS in all of the studies where AUC values were presented (12, 18, 27). This indicates that the preoperative CONUT score is a reliable and independent prognostic marker of survival in cancer patients. Although sensitivity and specificity of the CONUT score for the prediction of survival were at an acceptable level (>50%) in most of the studies, the difference across the studies was possibly due to the adoption of various CONUT score cutoffs. It appears that a cutoff ≥3 has acceptable sensitivity and specificity in predicting survival.
Several studies have shown that the AUC of the CONUT score, according to the ROC curve, was significantly higher than the AUC of each component of CONUT including serum albumin and total cholesterol concentrations and total lymphocyte count (23, 27, 31, 38, 45). Furthermore, numerous studies have reported that individual components of the CONUT score, against the CONUT score itself, were not independent predictors of survival in patients with various types of cancers (18, 19, 23, 45, 52). Taken together, it may be interpreted that the CONUT score is a more valuable factor than its individual components for predicting survival.
In addition, several studies have compared prognostic accuracy of the CONUT score in predicting survival with other nutritional prognostic factors such as the Prognostic Nutritional Index (PNI) (29, 33, 53, 54). The PNI is calculated from the serum albumin concentration and total peripheral lymphocyte count. The prognostic value of the PNI for predicting survival in cancer patients has been confirmed in numerous studies (55–59). However, various studies have shown that the CONUT score had a higher ROC curve AUC for the prediction of survival than the PNI (29, 33, 53, 54). Moreover, contrary to the CONUT score, many studies have shown that the PNI was not an independent predictor of survival in patients with cancer (30, 36, 60). Iseki et al. (23) reported that the PNI was not an independent predictor of CSS in patients with colorectal cancer, whereas CONUT score was. Takagi et al. (35) also reported that the PNI, against CONUT score, was not an independent predictor of survival in patients with hepatocellular carcinoma. Y Zheng et al. (45) also demonstrated that the PNI was not an independent predictor of survival in patients with renal cell carcinoma, whereas CONUT score was. Collectively, considering the results of all the aforementioned studies, it is concluded that the CONUT score has higher prognostic accuracy than, and is preferable to, the PNI in predicting survival in different types of cancer.
The platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), and Glasgow Prognostic Score (GPS) are inflammatory factors that have recently been suggested as prognostic factors for predicting inflammation and survival in many diseases and/or cancers (61–65). Multiple studies have compared the prognostic accuracy of the CONUT score with the inflammatory markers in predicting survival. Li et al. (53) indicated that CONUT had a higher AUC (of ROC) for the prediction of survival than the NLR in patients with breast cancer. Leem et al. (54) reported that the CONUT had a higher ROC curve AUC than the GPS in patients with lung cancer. Lin et al. (29) showed that the CONUT score had a higher AUC value than the inflammatory parameters (NLR and PLR) in patients with hepatocellular carcinoma. Song et al. (33) reported that the CONUT AUC score for 5-y OS was higher than that of the NLR in patients with renal cell carcinoma. Toyokawa et al. (52) showed that the AUC of CONUT for predicting 3-y OS was higher than the AUCs of the PLR, NLR, and GPS in patients with esophageal cancer. Moreover, many studies have shown that the PLR, NLR, and GPS, against CONUT score, were not independent predictors of survival in patients with cancer (20, 29, 43, 45, 52). Putting together all the findings, it is suggested that the CONUT score, in comparison with the inflammatory markers, has higher prognostic accuracy and is superior in predicting survival in different types of cancer.
Carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) are 2 cancer markers with prognostic significance for prediction of survival in patients with cancer (66, 67). CEA is a protein commonly produced in very low concentrations in the blood of adults. The blood concentration of CEA may be raised in some types of cancer (68, 69). CA19-9 is a blood type antigen and it may be increased in patients with gastrointestinal cancers (69). None of the included studies compared the prognostic accuracy (AUC value of the ROC curve) of these markers with that of the CONUT score in predicting survival in cancer patients. However, several of the included studies showed that CEA and CA19-9, against CONUT score, were not independent predictors of survival in patients with cancer (10, 23, 26, 30, 32, 38, 42, 60). More investigations are needed to clarify the prognostic accuracy of the CONUT score in predicting survival compared with the CEA and CA19-9 markers in patients with cancer.
Conclusions
The findings of the present study promote confidence that a preoperative high CONUT score is associated with a poor survival rate and is an independent prognostic factor of OS and CSS after surgery in patients with cancer.
Application of the findings
This study suggests that the CONUT score is an easy and inexpensive indicator which not only could be used for evaluating nutritional status, but can also be beneficial as a prognostic marker of patients’ survival in various types of cancer. Evaluation of the CONUT score might help clinicians in decision-making with respect to surgical implications.
Strengths of the study
All the studies had good quality and, except 1, were published in the last 3 y. Nearly all of the studies used the same method to estimate survival rate and to analyze the correlation between CONUT score and survival. Further, in all studies included, the roles of main confounding factors including age, gender, BMI, stage of disease, tumor site, tumor number, tumor grade, tumor size, lymph node metastasis, presence of high-grade disease, adjuvant chemotherapy, immunohistochemistry, and operative procedures were considered in the analysis.
Limitations of the study
All included studies were from East Asia (Japan, China, and South Korea), which may restrict the utilization of the findings among other nations and ethnic groups. Most of the studies did not indicate the role of BMI as a nutritional factor in prediction of survival. Several of the studies did not indicate the value of the AUC of the CONUT score for prediction of survival. The optimal cutoff of the CONUT score varied across the different studies.
Suggestions for future research
The optimal cutoff of the CONUT score must be homogenized before its utilization in clinical practice. More investigations are needed to clarify the prognostic accuracy of the CONUT score in predicting survival compared with CEA and CA19-9 markers in patients with cancer.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—both of the authors: were involved in the searching process, selection of the articles, data extraction, manuscript writing, and read and approved the final manuscript.
Notes
Supported by Tabriz University of Medical Sciences.
Author disclosures: The authors report no conflicts of interest.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances.
Abbreviations used: CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; CONUT, controlling nutritional status; CSS, cancer-specific survival; DFS, disease-free survival; GPS, Glasgow Prognostic Score; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; PFS, progression-free survival; PLR, platelet-to-lymphocyte ratio; PNI, Prognostic Nutritional Index; RFS, recurrence/relapse-free survival; ROC, receiver operating characteristic.
Contributor Information
Sorayya Kheirouri, Department of Nutrition, Tabriz University of Medical Sciences, Tabriz, Iran.
Mohammad Alizadeh, Nutrition Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
References
- 1. Naylor S Biomarkers: current perspectives and future prospects. Expert Rev Mol Diagn. 2003;3(5):525–9. [DOI] [PubMed] [Google Scholar]
- 2. Carlomagno N, Incollingo P, Tammaro V, Peluso G, Rupealta N, Chiacchio G, Sandoval Sotelo ML, Minieri G, Pisani A, Riccio Eet al. Diagnostic, predictive, prognostic, and therapeutic molecular biomarkers in third millennium: a breakthrough in gastric cancer. Biomed Res Int. 2017:7869802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oldenhuis CN, Oosting SF, Gietema JA, de Vries EG. Prognostic versus predictive value of biomarkers in oncology. Eur J Cancer. 2008;44(7):946–53. [DOI] [PubMed] [Google Scholar]
- 4. Lu J, Chen Y, Liu Y, Ding J, Piao Z, Liu W. Clinical significance of prognostic score based on age, tumor size, and grade in gastric cancer after gastrectomy. Cancer Manag Res. 2018;10:4279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mantzorou M, Koutelidakis A, Theocharis S, Giaginis C. Clinical value of nutritional status in cancer: what is its impact and how it affects disease progression and prognosis?. Nutr Cancer. 2017;69(8):1151–76. [DOI] [PubMed] [Google Scholar]
- 6. Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, Rodríguez F, Fernández G. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20(1):38–45. [PubMed] [Google Scholar]
- 7. Formiga F, Chivite D, Corbella X. Utility of the Controlling Nutritional Status (CONUT) score in patients admitted due to acute heart failure. Int J Cardiol. 2017;235:203. [DOI] [PubMed] [Google Scholar]
- 8. Fukushima K, Ueno Y, Kawagishi N, Kondo Y, Inoue J, Kakazu E, Ninomiya M, Wakui Y, Saito N, Satomi Set al. The nutritional index ‘CONUT’ is useful for predicting long-term prognosis of patients with end-stage liver diseases. Tohoku J Exp Med. 2011;224(3):215–19. [DOI] [PubMed] [Google Scholar]
- 9. Sun X, Luo L, Zhao X, Ye P. Controlling Nutritional Status (CONUT) score as a predictor of all-cause mortality in elderly hypertensive patients: a prospective follow-up study. BMJ Open. 2017;7(9):e015649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Daitoku N, Miyamoto Y, Tokunaga R, Sakamoto Y, Hiyoshi Y, Iwatsuki M, Baba Y, Iwagami S, Yoshida N, Baba H. Controlling nutritional status (CONUT) score is a prognostic marker in metastatic colorectal cancer patients receiving first-line chemotherapy. Anticancer Res. 2018;38(8):4883–8. [DOI] [PubMed] [Google Scholar]
- 11. Penson DF, Krishnaswami S, Jules A, Seroogy JC, McPheeters ML. Newcastle-Ottawa quality assessment form for cohort studies. [Internet] In: Evaluation and treatment of cryptorchidism. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012. [Cited 2020 Aug 19] Available from: http://www.ncbi.nlm.nih.gov/books/NBK115843/bin/appe-fm3.pdf. [PubMed] [Google Scholar]
- 12. Elghiaty A, Kim J, Jang WS, Park JS, Heo JE, Rha KH, Choi YD, Ham WS. Preoperative controlling nutritional status (CONUT) score as a novel immune-nutritional predictor of survival in non-metastatic clear cell renal cell carcinoma of ≤ 7 cm on preoperative imaging. J Cancer Res Clin Oncol. 2019;145(4):957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. National Cancer Institute Definition of relapse-free survival – NCI Dictionary of Cancer Terms. [Internet] Bethesda (MD): National Cancer Institute; [cited 2020 Aug 19]. Available from: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/event-free-survival. [Google Scholar]
- 14. National Cancer Institute Definition of cause-specific survival – NCI Dictionary of Cancer Terms. [Internet] Bethesda (MD): National Cancer Institute; [cited 2020 Aug 19]. Available from: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/cause-specific-survival. [Google Scholar]
- 15. National Cancer Institute Definition of overall survival rate – NCI Dictionary of Cancer Terms. [Internet] Bethesda (MD): National Cancer Institute; [cited 2020 Aug 19]. Available from: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/overall-survival-rate. [Google Scholar]
- 16. Ahiko Y, Shida D, Horie T, Tanabe T, Takamizawa Y, Sakamoto R, Moritani K, Tsukamoto S, Kanemitsu Y. Controlling nutritional status (CONUT) score as a preoperative risk assessment index for older patients with colorectal cancer. BMC Cancer. 2019;19:946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akamine T, Toyokawa G, Matsubara T, Kozuma Y, Haratake N, Takamori S, Katsura M, Takada K, Shoji F, Okamoto Tet al. Significance of the preoperative CONUT score in predicting postoperative disease-free and overall survival in patients with lung adenocarcinoma with obstructive lung disease. Anticancer Res. 2017;37(5):2735–42. [DOI] [PubMed] [Google Scholar]
- 18. Harimoto N, Yoshizumi T, Inokuchi S, Itoh S, Adachi E, Ikeda Y, Uchiyama H, Utsunomiya T, Kajiyama K, Kimura Ket al. Prognostic significance of preoperative controlling nutritional status (CONUT) score in patients undergoing hepatic resection for hepatocellular carcinoma: a multi-institutional study. Ann Surg Oncol. 2018;25(11):3316–23. [DOI] [PubMed] [Google Scholar]
- 19. Harimoto N, Yoshizumi T, Sakata K, Nagatsu A, Motomura T, Itoh S, Harada N, Ikegami T, Uchiyama H, Soejima Yet al. Prognostic significance of preoperative controlling nutritional status (CONUT) score in patients undergoing hepatic resection for hepatocellular carcinoma. World J Surg. 2017;41:2805–12. [DOI] [PubMed] [Google Scholar]
- 20. Hirahara N, Matsubara T, Hayashi H, Takai K, Nakada S, Tajima Y. Prognostic importance of controlling nutritional status in patients undergoing curative thoracoscopic esophagectomy for esophageal cancer. Am J Ther. 2018;25(5):e524–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirahara N, Tajima Y, Fujii Y, Kaji S, Kawabata Y, Hyakudomi R, Yamamoto T, Taniura T. Controlling Nutritional Status (CONUT) as a prognostic immunonutritional biomarker for gastric cancer after curative gastrectomy: a propensity score-matched analysis. Surg Endosc. 2019;33(12):4143–52. [DOI] [PubMed] [Google Scholar]
- 22. Huang Y, Huang Y, Lu M, Sun W, Sun X, Chen X, Li L, Chandoo A, Li L. Controlling nutritional status (CONUT) score is a predictor of post-operative outcomes in elderly gastric cancer patients undergoing curative gastrectomy: a prospective study. Cancer Manag Res. 2019;11:9793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iseki Y, Shibutani M, Maeda K, Nagahara H, Ohtani H, Sugano K, Ikeya T, Muguruma K, Tanaka H, Toyokawa Tet al. Impact of the preoperative controlling nutritional status (CONUT) score on the survival after curative surgery for colorectal cancer. PLoS One. 2015;10(7):e0132488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ishihara H, Kondo T, Yoshida K, Omae K, Takagi T, Iizuka J, Tanabe K. Preoperative controlling nutritional status (CONUT) score as a novel predictive biomarker of survival in patients with localized urothelial carcinoma of the upper urinary tract treated with radical nephroureterectomy. Urol Oncol. 2017;35(9):539.e9–16. [DOI] [PubMed] [Google Scholar]
- 25. Kang HW, Seo SP, Kim WT, Yun SJ, Lee S-C, Kim W-J, Hwang EC, Kang SH, Hong S-H, Chung Jet al. Prognostic impact of nutritional status assessed by the controlling nutritional status (CONUT) score in patients with surgically treated renal cell carcinoma. Nutr Cancer. 2018;70(6):886–94. [DOI] [PubMed] [Google Scholar]
- 26. Kato Y, Yamada S, Suenaga M, Takami H, Niwa Y, Hayashi M, Iwata N, Kanda M, Tanaka C, Nakayama Get al. Impact of the controlling nutritional status score on the prognosis after curative resection of pancreatic ductal adenocarcinoma. Pancreas. 2018;47(7):823–9. [DOI] [PubMed] [Google Scholar]
- 27. Kuroda D, Sawayama H, Kurashige J, Iwatsuki M, Eto T, Tokunaga R, Kitano Y, Yamamura K, Ouchi M, Nakamura Ket al. Controlling nutritional status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. 2018;21(2):204–12. [DOI] [PubMed] [Google Scholar]
- 28. Liang Y, Hou T, Que Y, Zhao B, Xiao W, Zhang X, Zhou Z. Elevated controlling nutritional status (CONUT) score is associated with poor long-term survival in patients with low-grade soft-tissue sarcomas treated with surgical resection. Clin Orthop Relat Res. 2019;477(10):2287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin Z-X, Ruan D-Y, Jia C-C, Wang T-T, Cheng J-T, Huang H-Q, Wu X-Y. Controlling nutritional status (CONUT) score-based nomogram to predict overall survival of patients with HBV-associated hepatocellular carcinoma after curative hepatectomy. Clin Transl Oncol. 2020;22(3):370–80. [DOI] [PubMed] [Google Scholar]
- 30. Miyata T, Yamashita YI, Higashi T, Taki K, Izumi D, Kosumi K, Tokunaga R, Nakagawa S, Okabe H, Imai Ket al. The prognostic impact of controlling nutritional status (CONUT) in intrahepatic cholangiocarcinoma following curative hepatectomy: a retrospective single institution study. World J Surg. 2018;42(4):1085–91. [DOI] [PubMed] [Google Scholar]
- 31. Ryo S, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, Murai T, Asada T, Ishiyama A, Matsushita Het al. The controlling nutritional status score serves as a predictor of short- and long-term outcomes for patients with stage 2 or 3 gastric cancer: analysis of a multi-institutional data set. Ann Surg Oncol. 2019;26(2):456–64. [DOI] [PubMed] [Google Scholar]
- 32. Shoji F, Haratake N, Akamine T, Takamori S, Katsura M, Takada K, Toyokawa G, Okamoto T, Maehara Y. The preoperative controlling nutritional status score predicts survival after curative surgery in patients with pathological stage I non-small cell lung cancer. Anticancer Res. 2017;37(2):741–7. [DOI] [PubMed] [Google Scholar]
- 33. Song H, Xu B, Luo C, Zhang Z, Ma B, Jin J, Zhang Q. The prognostic value of preoperative controlling nutritional status score in non-metastatic renal cell carcinoma treated with surgery: a retrospective single-institution study. Cancer Manag Res. 2019;11:7567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suzuki S, Kanaji S, Yamamoto M, Oshikiri T, Nakamura T, Kakeji Y. Controlling nutritional status (CONUT) score predicts outcomes of curative resection for gastric cancer in the elderly. World J Surg. 2019;43(4):1076–84. [DOI] [PubMed] [Google Scholar]
- 35. Takagi K, Yagi T, Umeda Y, Shinoura S, Yoshida R, Nobuoka D, Kuise T, Araki H, Fujiwara T. Preoperative controlling nutritional status (CONUT) score for assessment of prognosis following hepatectomy for hepatocellular carcinoma. World J Surg. 2017;41(9):2353–60. [DOI] [PubMed] [Google Scholar]
- 36. Takagi K, Umeda Y, Yoshida R, Nobuoka D, Kuise T, Fushimi T, Fujiwara T, Yagi T. Preoperative controlling nutritional status score predicts mortality after hepatectomy for hepatocellular carcinoma. Dig Surg. 2019;36(3):226–32. [DOI] [PubMed] [Google Scholar]
- 37. Takamori S, Toyokawa G, Taguchi K, Edagawa M, Shimamatsu S, Toyozawa R, Nosaki K, Seto T, Hirai F, Yamaguchi Met al. The controlling nutritional status score is a significant independent predictor of poor prognosis in patients with malignant pleural mesothelioma. Clin Lung Cancer. 2017;18(4):e303–13. [DOI] [PubMed] [Google Scholar]
- 38. Tokunaga R, Sakamoto Y, Nakagawa S, Ohuchi M, Izumi D, Kosumi K, Taki K, Higashi T, Miyamoto Y, Yoshida Net al. CONUT: a novel independent predictive score for colorectal cancer patients undergoing potentially curative resection. Int J Colorectal Dis. 2017;32(1):99–106. [DOI] [PubMed] [Google Scholar]
- 39. Toyokawa G, Kozuma Y, Matsubara T, Haratake N, Takamori S, Akamine T, Takada K, Katsura M, Shimokawa M, Shoji Fet al. Prognostic impact of controlling nutritional status score in resected lung squamous cell carcinoma. J Thorac Dis. 2017;9(9):2942–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang Q-Q, Zheng G-Q, Yang D-L, Liang Y-F, Yin W-J, Su S-S. Pretreatment controlling nutritional status score and lactate dehydrogenase as predictive markers of survival in patients with malignant peritoneal mesothelioma. Nutr Cancer. 2018;70(8):1264–74. [DOI] [PubMed] [Google Scholar]
- 41. Yamamoto M, Saito H, Uejima C, Tanio A, Tada Y, Matsunaga T, Sakamoto T, Honjo S, Ashida K, Fujiwara Y. Prognostic value of combined tumor marker and controlling nutritional status (CONUT) score in colorectal cancer patients. Yonago Acta Med. 2019;62(1):124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang C, Wei C, Wang S, Han S, Shi D, Zhang C, Lin X, Dou R, Xiong B. Combined features based on preoperative controlling nutritional status score and circulating tumour cell status predict prognosis for colorectal cancer patients treated with curative resection. Int J Biol Sci. 2019;15(6):1325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang W, Wu Y, Zhang Z, Guo Y, Wang R, Wang L, Mao S, Zhang J, Yao X. Controlling nutritional status score: a new prognostic indicator for patients with oligometastatic prostate cancer. Curr Probl Cancer. 2019;43(5):461–70. [DOI] [PubMed] [Google Scholar]
- 44. Zheng Z-F, Lu J, Xie J-W, Wang J-B, Lin J-X, Chen Q-Y, Cao L-L, Lin M, Tu R-H, Zheng C-Het al. Preoperative skeletal muscle index vs the controlling nutritional status score: which is a better objective predictor of long-term survival for gastric cancer patients after radical gastrectomy?. Cancer Med. 2018;7(8):3537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zheng Y, Bao L, Wang W, Wang Q, Pan Y, Gao X. Prognostic impact of the controlling nutritional status score following curative nephrectomy for patients with renal cell carcinoma. Medicine (Baltimore). 2018;97(49):e13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Renfro LA, Loupakis F, Adams RA, Seymour MT, Heinemann V, Schmoll HJ, Douillard JY, Hurwitz H, Fuchs CS, Diaz-Rubio Eet al. Body mass index is prognostic in metastatic colorectal cancer: pooled analysis of patients from first-line clinical trials in the ARCAD database. J Clin Oncol. 2016;34(2):144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moon H-G, Ju Y-T, Jeong C-Y, Jung E-J, Lee Y-J, Hong S-C, Ha W-S, Park S-T, Choi S-K. Visceral obesity may affect oncologic outcome in patients with colorectal cancer. Ann Surg Oncol. 2008;15(7):1918–22. [DOI] [PubMed] [Google Scholar]
- 48. Akay S, Urkan M, Balyemez U, Erşen M, Taşar M. Is visceral obesity associated with colorectal cancer? The first volumetric study using all CT slices. Diagn Interv Radiol. 2019;25(5):338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, Hiyoshi Y, Iwagami S, Yoshida N, Watanabe Met al. Negative impact of skeletal muscle loss after systemic chemotherapy in patients with unresectable colorectal cancer. PLoS One. 2015;10(6):e0129742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cabré M, Ferreiro C, Arus M, Roca M, Palomera E, Serra-Prat M. Evaluation of CONUT for clinical malnutrition detection and short-term prognostic assessment in hospitalized elderly people. J Nutr Health Aging. 2015;19(7):729–33. [DOI] [PubMed] [Google Scholar]
- 51. Zhou H, Chao W, Cui L, Li M, Zou Y, Yang M. Controlling Nutritional Status (CONUT) score as immune-nutritional predictor of outcomes in patients undergoing peritoneal dialysis. Clin Nutr. 2020;39(8):2564–70. [DOI] [PubMed] [Google Scholar]
- 52. Toyokawa T, Kubo N, Tamura T, Sakurai K, Amano R, Tanaka H, Muguruma K, Yashiro M, Hirakawa K, Ohira M. The pretreatment controlling nutritional status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective study. BMC Cancer. 2016;16:722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li W, Deng Y, Ma G, Wu Q, Zhou Q. Controlling nutritional status (CONUT) score is a prognostic factor in patients with resected breast cancer. Ann Oncol. 2019;30(Supplement_3):mdz095.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leem AY, Jung JY, Lee SC, Kim EY, Lee SH, Chang J, Chung KY, Paik HC, Kim DJ, Lee JG. Prediction of postoperative pulmonary complications using preoperative controlling nutritional status (CONUT) score in patients with resectable non-small cell lung cancer. Curr Dev Nutr. 2019;3(Suppl 1):nzz035.P12–032-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ida N, Nakamura K, Saijo M, Kusumoto T, Masuyama H. Prognostic nutritional index as a predictor of survival in patients with recurrent cervical cancer. Mol Clin Oncol. 2018;8(2):257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hsieh M-C, Rau K-M, Chiang P-H, Sung M-T, Lan J, Luo H-L, Huang C-C, Huang C-H, Su HY-L. Impact of prognostic nutritional index on overall survival for patients with metastatic urothelial carcinoma. J Cancer. 2018;9(14):2466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sun J, Mei Y, Zhu Q, Shou C, Tjhoi WEH, Yang W, Yu H, Zhang Q, Liu X, Yu J. Relationship of prognostic nutritional index with prognosis of gastrointestinal stromal tumors. J Cancer. 2019;10(12):2679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hirahara N, Tajima Y, Fujii Y, Kaji S, Yamamoto T, Hyakudomi R, Taniura T, Kawabata Y. Prognostic nutritional index as a predictor of survival in resectable gastric cancer patients with normal preoperative serum carcinoembryonic antigen levels: a propensity score matching analysis. BMC Cancer. 2018;18(1):285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Luvián-Morales J, González-Trejo S, Carrillo JF, Herrera-Goepfert R, Aiello-Crocifoglio V, Gallardo-Rincón D, Ochoa-Carrillo FJ, Oñate-Ocaña LF. Association of the prognostic nutritional index and overall survival in patients with colorectal cancer: a STROBE compliant retrospective cohort study. Cancer Med. 2019;8(7):3379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu X, Zhang D, Lin E, Chen Y, Li W, Chen Y, Sun X, Zhou Z. Preoperative controlling nutritional status (CONUT) score as a predictor of long-term outcome after curative resection followed by adjuvant chemotherapy in stage II-III gastric cancer. BMC Cancer. 2018;18:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee YSG, Baradi A, Peverelle M, Sultani R, Adams H, Garlick J, Wilson AM. Usefulness of platelet-to-lymphocyte ratio to predict long-term all-cause mortality in patients at high risk of coronary artery disease who underwent coronary angiography. Am J Cardiol. 2018;121(9):1021–6. [DOI] [PubMed] [Google Scholar]
- 62. Wang J, Zhou X, He Y, Chen X, Liu N, Ding Z, Li J. Prognostic role of platelet to lymphocyte ratio in prostate cancer: a meta-analysis. Medicine (Baltimore). 2018;97(40):e12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bojaxhiu B, Templeton AJ, Elicin O, Shelan M, Zaugg K, Walser M, Giger R, Aebersold DM, Dal Pra A. Relation of baseline neutrophil-to-lymphocyte ratio to survival and toxicity in head and neck cancer patients treated with (chemo-) radiation. Radiat Oncol. 2018;13(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yang L, Huang Y, Zhou L, Dai Y, Hu G. High pretreatment neutrophil-to-lymphocyte ratio as a predictor of poor survival prognosis in head and neck squamous cell carcinoma: systematic review and meta-analysis. Head Neck. 2019;41(5):1525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jomrich G, Hollenstein M, John M, Baierl A, Paireder M, Kristo I, Ilhan-Mutlu A, Asari R, Preusser M, Schoppmann SF. The modified Glasgow prognostic score is an independent prognostic indicator in neoadjuvantly treated adenocarcinoma of the esophagogastric junction. Oncotarget. 2018;9(6):6968–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Auclin E, Taieb J, Lepage C, Aparicio T, Faroux R, Mini E, Folprecht G, Salazar R, Benetkiewicz M, Banzi Met al. Carcinoembryonic antigen levels and survival in stage III colon cancer: post hoc analysis of the MOSAIC and PETACC-8 trials. Cancer Epidemiol Biomarkers Prev. 2019;28(7):1153–61. [DOI] [PubMed] [Google Scholar]
- 67. Mirkin KA, Hollenbeak CS, Wong J. Prognostic impact of carbohydrate antigen 19-9 level at diagnosis in resected stage I–III pancreatic adenocarcinoma: a U.S. population study. J Gastrointest Oncol. 2017;8(5):778–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee SU, Jwa E, Kim DY, Kim TH, Baek JY, Cha Y, Chang HJ, Oh JH. Analysis of unexplained carcinoembryonic antigen elevation after curative treatment of locally advanced rectal cancer. Int J Clin Oncol. 2018;23(5):924–9. [DOI] [PubMed] [Google Scholar]
- 69. Alkady MM, Abdel-Messeih PL, Nosseir NM. Assessment of serum levels of the adipocytokine chemerin in colorectal cancer patients. J Med Biochem. 2018;37(3):313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.