Abstract
Cigarette smoking during pregnancy is associated with numerous obstetrical, fetal, and developmental complications, as well as an increased risk of adverse health consequences in the adult offspring. Nicotine replacement therapy and electronic nicotine delivery systems (e-cigarettes) have been developed as a pharmacotherapy for smoking cessation and are considered safer alternatives for women to smoke during pregnancy. The safety of nicotine replacement therapy use during pregnancy has been evaluated in a limited number of short-term human trials, but there is currently no information on the long-term effects of developmental nicotine exposure in humans. However, animal studies suggest that nicotine alone may be a key chemical responsible for many of the long-term effects associated with maternal cigarette smoking on the offspring and increases the risk of adverse neurobehavioral outcomes, dysmetabolism, respiratory illness, and cancer. This review will examine the long-term effects of fetal and neonatal nicotine exposure on postnatal health.
Keywords: nicotine, pregnancy, fetal programming of adult disease, nicotine replacement therapy (NRT), electronic nicotine delivery system (e-cigarettes), dysmetabolism, lung development, neurotoxicity, childhood cancer
There is compelling evidence from multiple epidemiological studies and meta-analyses that cigarette smoking during pregnancy is associated with a number of adverse obstetrical outcomes including: spontaneous pregnancy loss (Pineles et al., 2014), placenta previa (Shobeiri and Jenabi, 2017), placental abruption (Shobeiri et al., 2017), preterm birth (Dahlin et al., 2016; Moore et al., 2016; Shah and Bracken, 2000), stillbirth (Bjørnholt et al., 2016; Marufu et al., 2015), impaired fetal growth (Abraham et al., 2017; Blatt et al., 2015; Pereira et al., 2017), and sudden infant death syndrome (Anderson et al., 2019; Zhang and Wang, 2013). Smoking cessation, or at least a reduction in cigarette smoking during pregnancy can improve many of these outcomes. Indeed, smoking cessation during pregnancy has been shown to reduce the incidence of impaired fetal growth (low birthweight or intrauterine growth restriction), prematurity and stillbirth (Hodyl et al., 2014; Räisänen et al., 2014; Soneji and Beltrán-Sánchez, 2019; Veisani et al., 2019).
Although cigarette smoking during pregnancy has been identified as a significant modifiable risk factor for adverse pregnancy outcomes, recent data suggest that many women still smoke during their pregnancies with prevalence rates ranging from 1.7% globally, to as high as 59% in some regions (Cui et al., 2014; Drake et al., 2018; Lange et al., 2018). Although approximately 75% of pregnant smokers want to quit, studies indicate that only half of women successfully abstain completely from smoking during pregnancy (Gilbert et al., 2015; Greaves et al., 2011; Orton et al., 2014; Tong et al., 2013). Nicotine dependence is a significant element of most women’s smoking behavior and a significant negative predictor of smoking abstinence during pregnancy (Baraona et al., 2017; Benowitz, 2010; Riaz et al., 2016). Consequently, nicotine replacement therapy (NRT; eg, nicotine containing gum, lozenges and transdermal patches) has been widely developed as a pharmacotherapy of smoking cessation (Moerke et al., 2020) and should be considered if the patient is unable to quit smoking by other means (Bar-Zeev et al., 2018; Behrakis et al., 2017; Ordean et al., 2017; Zwar et al., 2011). Recently, electronic nicotine delivery systems (e-cigarettes) have become available and marketed as effective smoking cessation tools (Collins et al., 2019; Farsalinos, 2018; Patel et al., 2016; Ramamurthi et al., 2016). Notably, in a recent study of pregnant smokers, the use of e-cigarettes to quit smoking was more common than any other FDA-approved smoking cessation pharmacotherapy (Oncken et al., 2017).
In pregnant women who use NRT or e-cigarettes for smoking cessation, nicotine crosses the placenta, concentrates in fetal blood and amniotic fluid, and is detectable in breast milk during lactation in some cases at higher concentrations than in maternal plasma (Jordanov, 1990; Lambers and Clark, 1996; Napierala et al., 2016; Rowe et al., 2015). NRT products were previously categorized as Pregnancy Category C (gum, nasal spray, and lozenges), or D (transdermal patches) drugs (Bruin et al., 2010). New FDA Pregnancy and Lactational Labeling Rules include additional details about the risks of drugs in pregnancy (Pernia and DeMaagd, 2016). In general, NRT product monographs suggest that NRT should only be considered for smoking cessation in pregnancy if the risk to the fetus or mother of continued smoking outweighs any potential adverse effects of NRT exposure (Drugs.com). Guidelines from a number of countries recommend that NRT be offered to pregnant women who are unable to quit smoking using nonpharmacologic means (Canadian Action Network for the Advancement, Dissemination and Adoption of Practice-informed Tobacco Treatment, 2011; National Institute for Health and Care Excellence, 2010; The Royal Australian and New Zealand College of Obstetricians and Gynaecologists, 2017; Zwar et al., 2011). Although NRT is highly effective for smoking cessation in nonpregnant smokers (Cahill et al., 2013), the evidence is much less clear during pregnancy. A recent Cochrane review of pharmacological interventions for promoting smoking cessation during pregnancy found evidence that in a pooled analysis of 9 studies and 2336 participants, NRT and behavioral support did increase cessation rates relative to control (relative risk [RR] 1.37, 95% CI 1.08–1.74), but when the analysis was limited to placebo-controlled randomized control trials there was no clear benefit of NRT use on cessation rates (RR 1.21, 95% CI 0.95–1.55) (Claire et al., 2020). The lack of a clear benefit of NRT for smoking cessation during pregnancy may be related to the low adherence to NRT among pregnant smokers; in studies where adherence was reported, complete adherence with the treatment protocol occurred in <40% of participants (Claire et al., 2020). Despite the lack of evidence to support the efficacy of NRT use during pregnancy, estimates suggest that approximately 10–20% of pregnant women are offered or prescribed NRT for smoking cessation (Dhalwani et al., 2014; Kapaya et al., 2015). Moreover, there has been an increase in e-cigarette usage during pregnancy; Cardenas et al. (2019) published a small cohort study using self-reports and noninvasive smoking biomarkers in 232 pregnant women and found that 6.8% of pregnant women were e-cigarette users, with most being concurrent smokers (75%).
NRT, Smokeless Tobacco and E-Cigarette Use in Pregnancy: Human Studies
As of 2019, there have only been 9 clinical trials which have investigated the efficacy and/or safety of NRT for smoking cessation during pregnancy (Claire et al., 2020); most of these have focused on obstetrical outcomes and the short term (<2 years) effects on the offspring. There are also a number of observational studies and retrospective cross-sectional studies which have looked at the effects of exposure to smoke-free nicotine containing products including NRT, snus/snuff, smokeless tobacco, Alaskan iq’mik, and e-cigarettes (Glover and Phillips, 2020). Although Claire et al., (2020) did not find evidence for increased rates of adverse pregnancy or infant outcomes (eg, miscarriage, stillbirth, low birthweight, preterm birth, neonatal intensive care unit admissions, neonatal death, and congenital anomalies) in randomized control trials of NRT use during pregnancy (Claire et al., 2020), there is some, albeit inconsistent, evidence from cohort studies that exposure to smoke-free nicotine products during pregnancy can increase the risk of congenital malformations, stillbirth, preterm birth, and low birthweight (Glover and Phillips, 2020). Using data from the Swedish Medical Birth Registry (1999–2009), Gunnerbeck et al. (2014) reported an increased risk of oral cleft malformations in infants whose mothers used snuff compared with nontobacco users (adjusted odds ratio [aOR] 1.48, 95% CI 1.00–2.21). Similarly, using data from the Danish National Birth Cohort (1997–2003), Morales-Suárez-Varela et al. (2006) showed an increased prevalence of congenital malformations in pregnant NRT users compared with nonsmokers. Conversely, analysis of primary care data from the UK (2001–2012) did not find an increased association between maternal NRT exposure and major congenital anomalies compared with the control group (aOR 1.12, 99% CI 0.84–1.48; Dhalwani et al., 2015). However, Although NRT use was not associated with an increased risk of stillbirth (Strandberg‐Larsen et al., 2008), the use of smokeless tobacco, which delivers doses of nicotine similar to cigarette smoking (Benowitz, 2011), has been reported to increase rates of stillbirth (Gupta and Subramoney, 2006; Hossain et al., 2018; Suliankatchi and Sinha, 2016). Moreover, while smoking is clearly associated with preterm birth and intrauterine growth restriction (Pereira et al., 2017; Shah and Bracken, 2000), the role of nicotine in causing this effect is unclear. Randomized control trials of NRT use in pregnancy have not shown increased rates of either low birthweight or preterm birth (Claire et al., 2020), yet retrospective studies have reported associations between nicotine exposure and these outcomes. For example, data from the 2004 Phase V Pregnancy Risk Assessment Monitoring System indicated that self-reported NRT use during pregnancy was associated with an approximately 2-fold increased risk of low birthweight and preterm birth compared with nonsmokers (Gaither et al., 2009). Similarly, maternal snuff and smokeless tobacco use have also been associated with preterm birth and low birthweight (Dahlin et al., 2016; Ratsch and Bogossian, 2014; Suliankatchi and Sinha, 2016). Finally, relative to pregnant women who did not use nor were exposed to tobacco or e-cigarettes, e-cigarette-only use during pregnancy increased the risk of having a small for gestational age infant (RR 5.1, 95% CI 1.2–22.2; Cardenas et al., 2019).
Taken together, evidence from human studies on the safety of NRT use (including e-cigarettes) during pregnancy remains uncertain (World Health Organization, 2013; Committee on Underserved Women and Committee on Obstetric Practice, 2017), yet there is some consensus that NRT may be a safer alternative to smoking during pregnancy because the mother and fetus are exposed to one chemical instead of the thousands of chemicals found in cigarette smoke (Benowitz et al., 2000). Indeed, it has been shown that conventional NRT use during pregnancy while abstinent from smoking results in lower maternal nicotine exposure than seen with smoking (Hickson et al., 2018), suggesting that the fetus will also be exposed to much less nicotine than children born to women who smoke during pregnancy. The same is not necessarily true for e-cigarette use. In fact, e-cigarette use has been reported to result in higher or comparable levels of nicotine exposure when compared with traditional tobacco cigarette use (Etter, 2014; Jarvis et al., 1984; St Helen et al., 2016). Although some studies have reported that amongst pregnant women there is a perception that e-cigarettes are less harmful than traditional cigarettes (Breland et al., 2019; Mark et al., 2015), e-cigarette use exposes the fetus to other chemicals including flavorants and thermal degradation products whose toxicity is largely unknown (Ward et al., 2020).
There is still considerable concern that there is no safe dose of nicotine during pregnancy (Bar-Zeev et al., 2018). Indeed, animal studies have demonstrated evidence of adverse behavioral, developmental, cardiovascular, respiratory, endocrine, and metabolic outcomes in the offspring following maternal nicotine exposure (Bruin et al., 2010; England et al., 2017; Holbrook, 2016). However, the evidence in human studies is less clear as most human studies of nicotine exposure use during pregnancy generally consider the acute risks of nicotine exposure on the developing fetus and in some cases, the long-term neurological effects. Moreover, nicotine exposure as a result of cigarette smoking or e-cigarette use also exposes the fetus to a multitude of other compounds. Nevertheless, considerable insight into the long-term effects of developmental nicotine exposure can be gained from animal models. Therefore, the goal of this review is to assess the current evidence regarding the long-term effects of fetal and neonatal nicotine exposure in animal and human studies. Searches were conducted on PubMed to collect relevant papers from 2010 to May 2020 based on the following keywords: “nicotine,” “nicotine replacement therapy,” “e-cigarette,” “smokeless tobacco,” “snus,” “snuff,” “iq’mik,” and “pregnancy” with, “fetal,” “dysmetabolism,” “obesity,” “diabetes,” “adiposity,” “cardiovascular,” “neurotoxicity,” “lung development,” and “childhood cancer”. Although most papers utilized animal and cell models, studies that involved human participants were prioritized where possible. These searches were not intended for the purpose of a systematic review but rather to evaluate recent findings on the effects of early life exposure to nicotine; as such, relevant studies prior to 2010 were included as appropriate. We will use these animal studies to consider the potential contribution of nicotine to the long-term toxicity associated with cigarette smoke exposure and e cigarette use during fetal and neonatal development in human populations.
LONG-TERM EFFECTS OF FETAL AND NEONATAL EXPOSURE TO NICOTINE
Neurodevelopmental and Behavioral Outcomes
Nicotine functions as a neurotransmitter, acting on nicotinic acetylcholine receptors (nAChR) which are distributed in specific brain regions throughout neurodevelopment (Dwyer et al., 2009). Nicotine has been clearly established as a neuroteratogen and numerous animal studies have shown that early life exposure to nicotine results in adverse neurodevelopmental outcomes including disrupted synaptic plasticity and neuronal maturation, deficits in the number of neurons, and changes in brain volume (Kazemi et al., 2020; Mahar et al., 2012; Muhammad et al., 2012; Sailer et al., 2019; Slotkin et al., 2007; Thomas et al., 2000; Zhu et al., 2012). These morphological and functional changes in brain development have been associated with altered behaviors including, increased risk of cognitive impairments, attention deficit/hyperactivity disorder (ADHD), depression, impaired behavioral flexibility, increased anxiety and somatosensory deficits in the nicotine-exposed offspring (reviewed in Abbott and Winzer-Serhan, 2012; Holbrook, 2016; Liao et al., 2012; Sailer et al., 2019). Interestingly, evidence from rodent studies suggests that many of these outcomes are sex dependent (Balsevich et al., 2014; Cross et al., 2017; Zhang et al., 2018). For example, maternal exposure to nicotine resulted in anxiety-like behaviors in male offspring but not female offspring, whereas ADHD-like behaviors were reported in both male and female offspring (Polli et al., 2020).
Adverse effects of nicotine exposure on neurodevelopment have also been reported following exposure to nicotine in e-cigarette vapors in animal and cell culture models (Church et al., 2020; Nguyen et al., 2018; Ruszkiewicz et al., 2020). Briefly, in vitro and in vivo rodent models investigating the influence of prenatal e-cigarette exposure on brain development suggest that exposure to e-cigarette aerosols increases neuronal death, reduces neuronal viability, worsens neonatal brain injury, and decreases learning and memory in adolescent offspring with a brain injury (Sifat et al., 2020). Similarly, maternal e-cigarette exposure in mice has resulted in increased short-term memory deficits, reduced anxiety, and hyperactivity in the offspring (Nguyen et al., 2018). Even though e-cigarette aerosols contain toxicants other than nicotine (Ward et al., 2020), the similarities in neurodevelopmental and behavioral outcomes following exposure to nicotine alone or nicotine as a component of e-cigarette aerosols suggest that exposure to nicotine, regardless of source, results in deficits in neurodevelopment and behavioral outcomes in rodent models, and supports the hypothesis that nicotine exposure may explain many of the adverse neurobehavioral outcomes in children born to women who smoke during pregnancy.
In humans, in utero exposure to cigarette smoke has been associated with adverse behavioral outcomes including greater irritability, hyperactivity, attention deficits, lower language comprehension and lower IQ (reviewed in Abbott and Winzer-Serhan, 2012; Liao et al., 2012; Smith et al., 2016). Of the numerous behavioral disorders that have been reported in the offspring of women who smoke, the strongest evidence is for an association between maternal smoking and ADHD in the offspring. A meta-analysis of prospective cohort studies reported an association between maternal smoking and an increased risk of ADHD in the offspring (pooled RR 1.58, 95% CI 1.33–1.88; He et al., 2017). Moreover, there is evidence that the severity of ADHD symptomology is positively correlated with the number of cigarettes smoked per day (Willoughby et al., 2009). Importantly, many rodent models of prenatal nicotine exposure have reported hyperactivity, attention deficit, working memory deficits, and impulsive-like behaviors in nicotine-exposed offspring (El Marroun et al., 2014; Polli et al., 2020; Zhu et al., 2012, 2017).
Although animal models have demonstrated that exposure to nicotine alone can affect neurodevelopment and offspring behavior, there is little information available in human populations (reviewed in Sailer et al., 2019). The single trial of NRT use in pregnancy, the Smoking, Nicotine And Pregnancy Trial, evaluated infant outcomes beyond the immediate perinatal period and reported that at 2 years of age, infants born to mothers who used NRT during pregnancy did not have an increased risk of impairments across 5 domains: communication, gross motor skills, fine motor skills, problem solving, and personal social development (Cooper et al., 2014). Although these results are reassuring, it is important to note that the compliance rate in the NRT study arm was below 10% at 1 month (Coleman et al., 2012). Therefore, the effect of early life exposure to nicotine alone, on neurodevelopment and postnatal behavior in children still remains largely unknown.
Metabolic Outcomes
Maternal smoking during pregnancy is consistently linked to an increased risk of obesity and dysmetabolism (ie, aberrant glucose and lipid homeostasis) in the offspring. Indeed, a recent meta-analysis, based on 39 studies of 236 687 children, reported an increased risk of obesity in children born to mothers who smoked during pregnancy (pooled aOR 1.55, 95% CI 1.40–1.73; Rayfield and Plugge, 2017). Pediatric obesity is associated with cardiovascular and metabolic comorbidities previously considered to be “adult” diseases (ie, type 2 diabetes mellitus, hypertension, nonalcoholic fatty liver disease [NAFLD], and dyslipidemia), lower health-related quality of life indicators and increased health care utilization and costs (Buttitta et al., 2014; Pelone et al., 2012; Lobstein and Jackson-Leach, 2016). Recent estimates suggest that the health care costs associated with childhood obesity attributable to maternal smoking are over $9 billion/year in the United States and $600 million/year in Canada (Chaiton and Holloway, 2016).
Since it is well-established that maternal cigarette smoking results in intrauterine growth restriction (Pereira et al., 2017) and that low birthweight is a significant risk factor for the development of cardiometabolic disease (Erkamp et al., 2019; Fall and Kumaran, 2019; Nakano, 2020), it has been suggested that the association of cigarette smoking with an increased risk of adverse postnatal health outcomes is simply a reflection of intrauterine growth restriction. However, maternal smoking increases the risk of adult-onset diseases in the offspring even after adjustment for a wide range of confounding factors including birthweight, infancy BMI, and socioeconomic status (Weng et al., 2012; Behl et al., 2013; Morgen et al., 2018; Rogers, 2019); suggesting that it may be a direct effect of intrauterine exposure to the chemicals in cigarette smoke that accounts for the increased risk of adverse health outcomes in the offspring of women who smoke during pregnancy. Of the thousands of chemicals in cigarette smoke, animal studies suggest that fetal exposure to nicotine alone may result in dysmetabolism (ie, increased weight gain/adiposity, aberrant glucose homeostasis and cardiovascular disease) in postnatal life.
Weight gain, adiposity, and dyslipidemia
Prenatal nicotine exposure has been shown to result in increased postnatal body weight and higher levels of body fat in animal models (Behl et al., 2013). The mechanisms by which early life exposure to nicotine can impact body weight homeostasis in animals are varied and include increased energy intake, reduced energy expenditure and effects on adipose tissue development and function. There is evidence that early life exposure to nicotine increases the hypothalamic expression of neuropeptide Y, an important orexigenic neuropeptide (Chen et al., 2012; Huang and Winzer-Serhan, 2007; Younes-Rapozo et al., 2013). The effects of nicotine on energy intake are complex as nicotine has been shown to increase food efficiency and preference for sugar but not overall food intake (Pinheiro et al., 2015; Somm et al., 2008; Younes-Rapozo et al., 2013). Interestingly, a rat model of early life exposure to cigarette smoke results in hyperphagia and an increased preference for a high-fat diet (Peixoto et al., 2019); these results are similar to what has been reported in the Saguenay Youth Study, a population-based cross sectional study investigating the long-term consequences of prenatal exposure to cigarette smoke (Haghighi et al., 2013; Lee et al., 2015a). Similarly, children exposed in utero to cigarette smoke had significantly higher ad libitum energy intake at a palatable lunch buffet but not over a 7-day period (Cameron et al., 2018). Taken together, these data suggest that early life exposure to nicotine in humans may influence body weight homeostasis via changes in energy intake and food preferences; although the effects of exposure to nicotine in the absence of the other components of cigarette smoke remains to be determined.
As obesity is a disorder of energy imbalance between energy intake and energy expenditure, it is also possible that nicotine exposure in early life increases obesity risk in the offspring via persistent changes in energy expenditure. Indeed, decreased spontaneous activity and perturbed thermogenesis was reported in rats exposed prenatally to nicotine (Somm et al., 2008). Recent work has shown that brown adipose tissue (BAT) is a thermogenic tissue which regulates energy expenditure (Villarroya et al., 2018). In rats, perinatal exposure to nicotine has been shown to increase the accumulation of unilocular lipid droplets in BAT (ie, whitening of BAT) along with decreased expression of thermogenesis-specific genes and sympathetic nervous system activity (ie, reduced BAT activity) (Fan et al., 2016a; Peixoto et al., 2019). Neonatal exposure to cigarette smoke had similar effects (Peixoto et al., 2019). The influence of nicotine exposure on energy expenditure and/or BAT activity in humans is not well known as there are few studies which have investigated these outcomes in the offspring of women who smoke or use nicotine during pregnancy. To our knowledge, the single study which assessed energy expenditure in children born to women who smoke did not report any differences in resting energy expenditure (Cameron et al., 2018); however, there was no assessment of any markers of BAT activity. Given that reduced energy expenditure is a key component of obesity that has been shown to be affected by nicotine exposure, studies investigating these outcomes in children with early life exposure to nicotine are warranted.
Prenatal nicotine exposure has also been shown to lead to increased storage of lipids in both white adipose tissue (WAT) and peripheral tissues, an effect which is central to the development of obesity and insulin resistance (Blüher, 2013). Rats exposed in utero to nicotine have elevated expression of adipogenic markers in WAT and increased fat pad weights (Behl et al., 2013; Fan et al., 2016b; Somm et al., 2008). These effects may be due to direct effects of nicotine on the developing adipocytes as in vitro studies have shown that nicotine exposure can increase lipid accumulation and adipocyte maturation in mouse 3T3-LI cells in a dose-dependent manner (Zhang et al., 2020). Similarly, increased percent body fat and fat mass have also been reported in children born to women who smoke (Cameron et al., 2018; Moschonis et al., 2017). Nicotine exposure in animal models has also been reported to increase hepatic fat accumulation (Ma et al., 2014) which is a key component of NAFLD (Tiniakos et al., 2010). The effect of nicotine on hepatic fat accumulation may be mediated via epigenetic changes in genes important for lipid homeostasis (Suter et al., 2010). For example, in utero exposure to nicotine causes elevated hepatic triglyceride levels in association with increased histone H3 [K9,14] acetylation in the promoter of hepatic fatty acid synthase, a key gene involved in de novo lipogenesis, in nicotine-exposed offspring at 6 months of age (Ma et al., 2014). Importantly, liver fat is often associated with obesity and insulin resistance (Bellentani, 2017). These changes in insulin sensitivity may explain increased diabetes risk in children born to women who smoke (reviewed in Rogers, 2019). Alternatively, the increased risk of diabetes following in utero exposure to cigarette smoke (Rogers, 2019) may be attributed, in part, to nicotine-induced reductions in beta cell mass as has been reported in animal studies (reviewed in Bruin et al., 2010).
Hypertension
Hypertension is another health consequence associated with in utero exposure to cigarette smoking in humans (Bruin et al., 2010; Cabral et al., 2018; Rogers, 2019) and animal studies suggest that this may be mediated via nicotine exposure. Indeed, fetal and neonatal nicotine exposure in rodents results in increased blood pressure in adult life (Gao et al., 2008; Pausová et al., 2003; Xiao et al., 2015). A recent study has reported that children exposed in utero to snus, a smokeless tobacco that delivers nicotine but not the combustion products found in cigarette smoke, had increased blood pressure at 5–6 years of age (Felicia et al., 2019). The mechanisms underlying increased blood pressure following early life exposure to nicotine are not fully known but may involve abnormalities in heart development and/or function as has been reported in animal studies (Barra et al., 2017; Wang et al., 2015). Alternatively, perinatal nicotine exposure may induce vasoconstriction and facilitate the hypertensive phenotype in adulthood via epigenetic mechanisms (Xiao et al., 2014). Indeed, one study conducted in rats found that perinatal exposure to nicotine altered epigenetic regulation of vascular angiotensin II receptors (ATRs), as evidenced by reduced DNA methylation at ATR-specific gene promoter regions (Xiao et al., 2014). Other studies suggest that increased blood pressure following nicotine exposure could be related to increased fat mass, glucose abnormalities, or elevated circulating triglyceride levels, all of which have been identified as determinants of childhood hypertension (Liang et al., 2020) and reported in nicotine-exposed offspring (Bruin et al., 2010; Ma et al., 2014).
There is clear evidence from animal studies that early life exposure to nicotine can cause profound cardiometabolic deficits. Although many of these metabolic perturbations are similar to what has been reported in children born to mothers who smoked during pregnancy, cigarette smoke contains numerous constituents making it difficult to attribute these outcomes to nicotine alone. As there are no clinical studies of NRT use in pregnancy which have reported metabolic outcomes in children, the consequences of early life exposure to nicotine in humans remains to be determined and is a significant research gap in the field.
Respiratory Outcomes
Lung function and development
The developing lung is sensitive to exogenous compounds such as those found in maternal tobacco smoke (Dasgupta et al., 2012; Gibbs et al., 2016; Stocks et al., 2013). There are several epidemiological studies which have reported that perinatal tobacco smoke exposure increases the risk of upper and lower respiratory tract infections, bronchitis, asthma, wheezing, pulmonary hypertension, and impaired lung function in the offspring (den Dekker et al., 2015; Kalliola et al., 2013; Spindel and McEvoy, 2016). In fact, 2 meta-analyses have reported that maternal smoking during the prenatal period was associated with an increased risk of wheezing (Burke et al., 2012) and asthma (Silvestri et al., 2015) in childhood. Importantly, there is compelling evidence from several animal studies that suggest that majority of adverse effects associated with perinatal smoke exposure on the developing lung are mediated by nicotine alone (reviewed in Kuniyoshi and Rehan, 2019; Maritz and Harding, 2011; McEvoy and Spindel, 2017; Spindel and McEvoy, 2016).
Perinatal nicotine exposure alters normal lung development leading to reduced lung size and volume capacity, thickening of the airway walls and pulmonary vessels and reduced alveolar surface area (England et al., 2017; Krebs et al., 2010; Lavezzi et al., 2014; Maritz, 2013; Petre et al., 2011; Sekhon et al., 1999, 2002). Collectively, these outcomes compromise the primary role of the respiratory system to mediate optimal blood-gas exchange (Spindel and McEvoy, 2016; Wongtrakool et al., 2012). In vitro and in vivo studies reveal that the effects of nicotine on airway remodeling can be attributed to its activity at the α7 nAChR (reviewed in Gibbs et al., 2016; Spindel and McEvoy, 2016). Maternal nicotine exposure upregulates α7 nAChR expression in fetal lung macrophages and epithelial cells and fibroblasts (England et al., 2017; Sekhon et al., 1999, 2002) and it has been suggested that nicotine activity at α7 nAChR may play a role in altering lung development, morphogenesis and airway plasticity (Maritz and Harding, 2011; Sekhon et al., 1999). In support of this hypothesis, perinatal nicotine exposure did not cause impaired lung function (as measured by forced expiratory flows) in α7 nAChR knockout mice (Wongtrakool et al., 2012).
Defects in lung development are often associated with impaired lung function and an increased incidence of respiratory diseases including asthma and wheeze (Stocks et al., 2013). There is compelling evidence demonstrating that prenatal exposure to nicotine adversely alters pulmonary function parameters, with consistent reports of decreased forced expiratory flows. These findings are indicative of alterations to airway diameter and compliance during critical stages of lung development (Landau, 2008; Spindel and McEvoy, 2016). Similarly, results from epidemiological studies have reported impaired lung function and increased risk for wheezing, asthma and hospitalization due to respiratory complications in children exposed to tobacco smoke (reviewed in McEvoy and Spindel, 2017; Wang et al., 2020). However, the impact of prenatal exposure to tobacco smoke on asthma risk is complex and cannot be solely attributed to nicotine exposure as evidenced by the fact that infants with prenatal exposure to paternal tobacco smoke also have an increased risk of asthma by age 6 (Wu et al., 2019). Despite this, there is some evidence in humans that early life exposure to nicotine alone can impact lung function in the offspring.
In a recent cohort study including 192 498 children, a small number of exposed cases showed that offspring born of women prescribed NRT patches during their first trimester of pregnancy demonstrated a significant association between maternal NRT use and respiratory anomalies (OR 4.65, 99% CI 1.76–12.25; Dhalwani et al., 2015). Although in humans the effects of nicotine exposure via e-cigarette use during pregnancy are unknown, a recent study that used a mouse whole-body environmental e-cigarette exposure model found that neonates exposed to aerosols containing nicotine had significant reductions in alveolar cell proliferation and impaired lung health (McGrath-Morrow et al., 2015). Although it is unclear whether nicotine-containing e-cigarettes will have the same effect in humans, these findings suggest that the use of e-cigarette during pregnancy may adversely affect respiratory health of offspring.
Fibrosis
To date, several epidemiologic studies have reported an association between adult cigarette smoking and idiopathic pulmonary fibrosis (Ebrahimpour et al., 2019; Jensen et al., 2012; Kärkkäinen et al., 2017; Taskar and Coultas, 2006). There is some evidence that nicotine may accelerate pathogenesis; in vitro and in vivo studies have shown that nicotine plays a role in key profibrotic processes including increased pulmonary fibroblast transdifferentiation (Krebs et al., 2010; Rehan et al., 2012), activation of collagen-producing cells (Sekhon et al., 1999; Vicary et al., 2017; Wawryk-Gawda et al., 2018) and recruitment of inflammatory molecules (Nowak et al., 1990; Roomans et al., 2002). There is limited evidence demonstrating an association between early life exposure to nicotine and risk for idiopathic pulmonary fibrosis; however, one epidemiological study has reported an association between maternal smoking and risk for idiopathic pulmonary fibrosis in the offspring (OR 1.41, 95% CI 1.19–1.68; Bellou et al., 2017). Based on animal and cell culture studies, it is plausible that this association is mediated via nicotine’s effects on the developing lung.
The substitution of alveolar architecture with collagen and aberrant remodeling of lung structures are hallmarks of pulmonary fibrosis (Jensen et al., 2012; Pardo and Selman, 2016). Emerging evidence in animal models suggests that fetal and neonatal nicotine exposure inhibits alveolar development and increases the risk for idiopathic pulmonary fibrosis in offspring via upregulation of transforming growth factor β1 signaling (Dasgupta et al., 2012). In fact, in multiple species (rat, mouse, and monkey) prenatal nicotine-induced overexpression of transforming growth factor β1, and its downstream effectors such as connective tissue growth factor, has been shown to increase the risk of fibrosis, inflammation, epithelial-to-mesenchymal transdifferentiation and collagen deposition in the airways and pulmonary vessels of the offspring (Dasgupta et al., 2012; Gauldie et al., 2003; Sekhon et al., 2002; Tarantal et al., 2010; Wongtrakool et al., 2012). The nAChRs expressed on pulmonary fibroblasts and epithelial cells are responsive to exogenous stimulation by nicotine (Ebrahimpour et al., 2019), suggesting that the observed incidences of idiopathic pulmonary fibrosis in nicotine-exposed offspring may be attributed to nAChR signaling. In support of this hypothesis, genetic knock-out models of α7-nAChR reversed the inflammatory response and changes to the local microenvironment of the fibrotic lung in nicotine-exposed mice (Wongtrakool et al., 2012).
Interestingly, some of these increases in inflammation-related genes appear to be mediated via epigenetic alterations as a result of early life nicotine exposure. Offspring exposed to nicotine in utero showed decreased histone deacetylase activity and increased overall histone H3 acetylation in the lung (Rehan et al., 2012), paralleling in vitro and in vivo observations of cigarette smoke-induced methylation changes in histones H3 [K9] and increased expression of inflammatory genes, including interleukin-8 and tumor necrosis factor α (Chen et al., 2015). Although further investigation is needed to identify disease-specific genes altered by nicotine exposure alone, these data suggest that nicotine treatment may alter the fetal lung epigenome and increase its susceptibility to adult lung diseases, such as asthma and idiopathic pulmonary fibrosis (Suter et al., 2015).
Childhood Cancers
Prenatal exposure to tobacco smoke has been associated with increased risk of childhood cancers, including childhood brain tumors, hepatoblastomas, and leukemias/lymphomas as well as genotoxic effects which may increase the risk of adult cancers (reviewed in Fucic et al., 2017; Pattemore, 2013). The most widely studied of these childhood cancers is acute lymphoblastic leukemia. Recent work suggests that prenatal maternal smoke exposure increased leukemogenic somatic deletions found in acute lymphoblastic leukemia (de Smith et al., 2017). In women who smoke, maternal and neonatal blood samples found increased/altered signaling pathways related to cancer, including: tumor suppressor p53, epidermal growth factor receptor, hedgehog, and wingless-related integration site signaling, all of which are essential signal transduction pathways related to cancer pathogenesis (Gu, 2014). Likewise, other studies have reported that the largest cluster of biological pathways in newborns impacted by maternal smoking were related to cancer (Rotroff et al., 2016). Importantly, altered DNA methylation patterns associated with these pathways have been found to persist in exposed offspring into adolescence (Lee et al., 2015b; Rotroff et al., 2016). Taken together, these data suggest that early life exposure to tobacco smoke mediates genetic and epigenetic changes resulting in increased risk of childhood cancers, with potentially long-term effects. However, tobacco smoke contains many known carcinogens (ie, polycyclic hydrocarbons and tobacco-smoke-specific nitrosamines derivatives) that may also cross the placenta, making it difficult to attribute the increased risk of cancer to nicotine alone (reviewed in Fucic et al., 2017). There are now studies that have shown that nicotine can affect several pathways mediating cancer as well as chromosomal aberrations and DNA double-strand breaks important in cancer development (reviewed in Sanner and Grimsrud, 2015). Although this evidence suggests that NRT use during pregnancy has the potential to increase cancer risk in children, this has not been demonstrated in human clinical trials or cohort studies.
The mechanism(s) by which nicotine may affect the development of cancer are not well known, but it may involve activation of the nAChR. Nicotine activation of nAChR has been shown to influence signaling pathways involved in survival (mitogenic and antiapoptotic), proliferation, epithelial to mesenchymal transition, angiogenesis and metastasis; promoting a cancer-supporting microenvironment that can aid in the initiation and progression of many adult tumors and cancers (reviewed in Grando, 2014; Haussmann and Fariss, 2016; Improgo et al., 2011; Mishra et al., 2015; Sanner and Grimsrud, 2015). Despite the fact that nicotine can influence many procancer pathways, nicotine is not presently considered a human carcinogen (International Agency for Research on Cancer, 2004). Although there are no long-term studies assessing the association between in utero exposure to NRT or e-cigarettes and cancer risk in children, in vitro studies and animal experiments suggest that the long-term effects of early life exposure to nicotine and cancer risk deserve further attention.
CONCLUSIONS
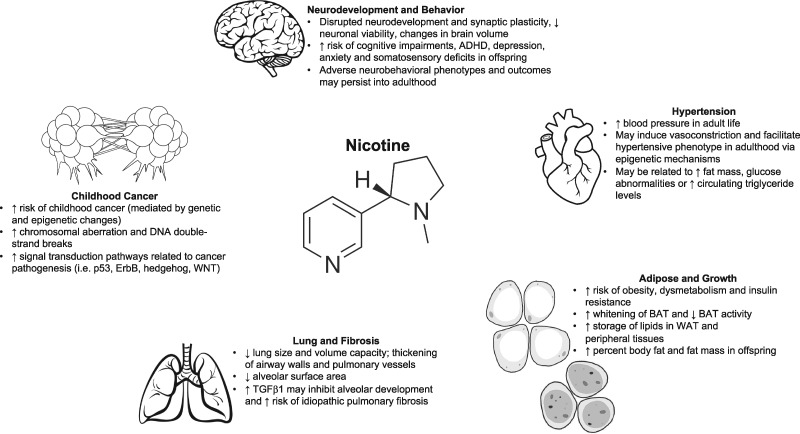
Cigarette smoking during pregnancy continues to be a significant modifiable risk factor for adverse pregnancy and fetal outcomes. Animal models have indicated that nicotine alone may be responsible for many of the long-term effects associated with maternal tobacco exposure including adverse metabolic (Buttitta et al., 2014; Liang et al., 2020; Lobstein and Jackson-Leach, 2016; Pelone et al., 2012), pulmonary (Gibbs et al., 2016; Kuniyoshi and Rehan, 2019; Maritz and Harding, 2011; McEvoy and Spindel, 2017) and neurobehavioral outcomes (Abbott and Winzer-Serhan, 2012; Clifford et al., 2012; Liao et al., 2012; Tiesler and Heinrich, 2014) and increased cancer risk in offspring (Bruin et al., 2010; England et al., 2017; Figure 1). However, the contribution of nicotine to these same outcomes in humans remains unclear.
Figure 1.
Summary, from animal studies, of the effects of prenatal nicotine exposure on the brain, heart, lungs, metabolic-regulating tissues and cancer.
Although NRT and e-cigarette usage are thought to be harm reduction strategies to reduce tobacco use during pregnancy, the postnatal health consequences of exposure to these products in humans are limited. There are few trials investigating NRT use during pregnancy most of which do not explore the postnatal health outcomes in the exposed children. Until there are carefully designed cohort studies to investigate long-term health outcomes in children exposed to NRT in fetal and neonatal life, there can be no definitive answer to whether or not there is a safe dose of nicotine during pregnancy (Bar-Zeev et al., 2018; Glover and Phillips, 2020). The long-term effects of maternal e-cigarette use on offspring health and the contribution of nicotine to any of these outcomes also remains largely unknown. There is evidence that pregnant women are using e-cigarettes for smoking cessation (Breland et al., 2019, Oncken et al., 2017) which can result in fetal exposure to nicotine alongside other toxicants. Studies have shown that other components of e-cigarette liquids (base components and flavoring compounds) can affect nicotine yield and may themselves have the ability to independently affect key pathways important for fetal development (DeVito and Krishnan-Sarin, 2018; Greene and Pisano, 2019). Moreover, e-cigarette liquids do not contain consistent nicotine concentrations, and the nicotine yield depends on puffing topography, the type of device used, and the other constituents present in the e-cigarette liquids. Therefore, there will be considerable challenges to understand the contribution of nicotine alone to any long-term health outcomes in offspring exposed to e-cigarette aerosols. Given that there is considerable potential for women to use nicotine alone during pregnancy through NRT or e-cigarette use, carefully designed human cohort studies and animal experiments to investigate long-term outcomes in the offspring are urgently needed.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This work was supported by the Canadian Institutes of Health Research (PJT-155981). L.J. was funded by a Canadian Graduate Scholarship—Master’s Award.
Contributor Information
Laiba Jamshed, Department of Obstetrics and Gynecology, McMaster University, Hamilton, Ontario L8N 3Z5, Canada.
Genevieve A Perono, Department of Obstetrics and Gynecology, McMaster University, Hamilton, Ontario L8N 3Z5, Canada.
Shanza Jamshed, Department of Obstetrics and Gynecology, McMaster University, Hamilton, Ontario L8N 3Z5, Canada.
Alison C Holloway, Department of Obstetrics and Gynecology, McMaster University, Hamilton, Ontario L8N 3Z5, Canada.
REFERENCES
- Abbott L. C., Winzer-Serhan U. H. (2012). Smoking during pregnancy: Lessons learned from epidemiological studies and experimental studies using animal models. Crit. Rev. Toxicol. 42, 279–303. [DOI] [PubMed] [Google Scholar]
- Abraham M., Alramadhan S., Iniguez C., Duijts L., Jaddoe V. W. V., Dekker H. T. D., Crozier S., Godfrey K. M., Hindmarsh P., Vik T., et al. (2017). A systematic review of maternal smoking during pregnancy and fetal measurements with meta-analysis. PLoS One 12, e0170946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T. M., Ferres J. M. L., Ren S. Y., Moon R. Y., Goldstein R. D., Ramirez J.-M., Mitchell E. A. (2019). Maternal smoking before and during pregnancy and the risk of sudden unexpected infant death. Pediatrics 143, e20183325 Available at: https://pediatrics.aappublications.org/content/143/4/e20183325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsevich G., Poon A., Goldowitz D., Wilking J. A. (2014). The effects of pre- and post-natal nicotine exposure and genetic background on the striatum and behavioral phenotypes in the mouse. Behav. Brain Res. 266, 7–18. [DOI] [PubMed] [Google Scholar]
- Baraona L. K., Lovelace D., Daniels J. L., McDaniel L. (2017). Tobacco harms, nicotine pharmacology, and pharmacologic tobacco cessation interventions for women. J. Midwifery Womens Health 62, 253–269. [DOI] [PubMed] [Google Scholar]
- Barra N. G., Lisyansky M., Vanduzer T. A., Raha S., Holloway A. C., Hardy D. B. (2017). Maternal nicotine exposure leads to decreased cardiac protein disulfide isomerase and impaired mitochondrial function in male rat offspring. J. Appl. Toxicol. 37, 1517–1526. [DOI] [PubMed] [Google Scholar]
- Bar-Zeev Y., Lim L. L., Bonevski B., Gruppetta M., Gould G. S. (2018). Nicotine replacement therapy for smoking cessation during pregnancy. Med. J. Aust. 208, 46–51. [DOI] [PubMed] [Google Scholar]
- Behl M., Rao D., Aagaard K., Davidson T. L., Levin E. D., Slotkin T. A., Srinivasan S., Wallinga D., White M. F., Walker V. R., et al. (2013). Evaluation of the association between maternal smoking, childhood obesity, and metabolic disorders: A National Toxicology Program Workshop Review. Environ. Health Perspect. 121, 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrakis P., Vardavas C, Papadakis S., eds. (2017). Tobacco Cessation guidelines for High-risk Populations (TOB.g). Hellenic Center for Disease Control & Prevention, Athens, Greece. [Google Scholar]
- Bellentani S. (2017). The epidemiology of non-alcoholic fatty liver disease. Liver Int. 37, 81–84. [DOI] [PubMed] [Google Scholar]
- Bellou V., Belbasis L., Konstantinidis A., Evangelou E. (2017). Tobacco smoking and risk for idiopathic pulmonary fibrosis: A prospective cohort study in UK Biobank. Eur. Respir. J. 50, PA4887 Available at: https://erj.ersjournals.com/content/50/suppl_61/PA4887. [DOI] [PubMed] [Google Scholar]
- Benowitz N. L. (2010). Nicotine addiction. N. Engl. J. Med. 362, 2295–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz N. L. (2011). Smokeless tobacco as a nicotine delivery device: Harm or harm reduction? Clin. Pharmacol. Ther. 90, 491–493. [DOI] [PubMed] [Google Scholar]
- Benowitz N. L., Dempsey D. A., Goldenberg R. L., Hughes J. R., Dolan-Mullen P., Ogburn P. L., Oncken C., Orleans C. T., Slotkin T. A., Whiteside H. P., et al. (2000). The use of pharmacotherapies for smoking cessation during pregnancy. Tob. Control 9, III91–III94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørnholt S. M., Leite M., Albieri V., Kjaer S. K., Jensen A. (2016). Maternal smoking during pregnancy and risk of stillbirth: Results from a nationwide Danish register-based cohort study. Acta Obstet. Gynecol. Scand. 95, 1305–1312. [DOI] [PubMed] [Google Scholar]
- Blatt K., Moore E., Chen A., Van Hook J., DeFranco E. A. (2015). Association of reported trimester-specific smoking cessation with fetal growth restriction. Obstetrics & Gynecology 125, 1452–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blüher M. (2013). Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract. Res. Clin. Endocrinol. Metab. 27, 163–177. [DOI] [PubMed] [Google Scholar]
- Breland A., McCubbin A., Ashford K. (2019). Electronic nicotine delivery systems and pregnancy: Recent research on perceptions, cessation, and toxicant delivery. Birth Defects Res. 111, 1284–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruin J. E., Gerstein H. C., Holloway A. C. (2010). Long-term consequences of fetal and neonatal nicotine exposure: A critical review. Toxicol. Sci. 116, 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke H., Leonardi-Bee J., Hashim A., Pine-Abata H., Chen Y., Cook D. G., Britton J. R., McKeever T. M. (2012). Prenatal and passive smoke exposure and incidence of asthma and wheeze: Systematic review and meta-analysis. Pediatrics 129, 735–744. [DOI] [PubMed] [Google Scholar]
- Buttitta M., Iliescu C., Rousseau A., Guerrien A. (2014). Quality of life in overweight and obese children and adolescents: A literature review. Qual. Life Res. 23, 1117–1139. [DOI] [PubMed] [Google Scholar]
- Cabral M., Fonseca M. J., González-Beiras C., Santos A. C., Correia-Costa L., Barros H. (2018). Maternal smoking: A life course blood pressure determinant? Nicotine Tob. Res. 20, 674–680. [DOI] [PubMed] [Google Scholar]
- Cahill K., Stevens S., Perera R., Lancaster T. (2013). Pharmacological interventions for smoking cessation: An overview and network meta-analysis. Cochrane Database Syst. Rev. (5), CD009329 Available at: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD009329.pub2/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron J. D., Doucet É., Adamo K. B., Walker M., Tirelli A., Barnes J. D., Hafizi K., Murray M., Goldfield G. S. (2018). Effects of prenatal exposure to cigarettes on anthropometrics, energy intake, energy expenditure, and screen time in children. Physiol. Behav. 194, 394–400. [DOI] [PubMed] [Google Scholar]
- Canadian Action Network for the Advancement, Dissemination and Adoption of Practice-informed Tobacco Treatment. (2011). Canadian Smoking Cessation Clinical Practice Guideline - Overview of Summary Statements, Vol. 8 Canadian Action Network for the Advancement, Dissemination and Adoption of Practice-informed Tobacco Treatment, Toronto, Canada. [Google Scholar]
- Cardenas V., Cen R., Clemens M., Moody H., Ekanem U., Policherla A., Fischbach L., Eswaran H., Magann E., Delongchamp R., et al. (2019). Use of Electronic Nicotine Delivery Systems (ENDS) by pregnant women I: Risk of small-for-gestational-age birth. Tob. Induc. Dis. 17, 44 Available at: http://www.journalssystem.com/tid/Use-of-Electronic-Nicotine-Delivery-Systems-by-Pregnant-Women-I-Risk-of-Small-for, 106089,0,2.html. Accessed May 13, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiton M., Holloway A. (2016). Population attributable risk of smoking during pregnancy on obesity in offspring. Can. J. Public Health 107, e336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Saad S., Sandow S., Bertrand P. (2012). Cigarette smoking and brain regulation of energy homeostasis. Front. Pharmacol. 3, 147. Available at: https://www.frontiersin.org/articles/10.3389/fphar.2012.00147/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Guan X., Peng X., Cui Z., Luan C., Guo X. (2015). Acetylation of lysine 9 on histone H3 is associated with increased pro-inflammatory cytokine release in a cigarette smoke-induced rat model through HDAC1 depression. Inflamm. Res. 64, 513–526. [DOI] [PubMed] [Google Scholar]
- Church J. S., Chace-Donahue F., Blum J. L., Ratner J. R., Zelikoff J. T., Schwartzer J. J. (2020). Neuroinflammatory and behavioral outcomes measured in adult offspring of mice exposed prenatally to e-cigarette aerosols. Environ. Health Perspect. 128, 047006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claire R., Chamberlain C., Davey M.-A., Cooper S. E., Berlin I., Leonardi‐Bee J., Coleman T. (2020). Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst. Rev. 3, CD010078 Available at: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD010078.pub3/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford A., Lang L., Chen R. (2012). Effects of maternal cigarette smoking during pregnancy on cognitive parameters of children and young adults: A literature review. Neurotoxicol. Teratol. 34, 560–570. [DOI] [PubMed] [Google Scholar]
- Coleman T., Cooper S., Thornton J. G., Grainge M. J., Watts K., Britton J., Lewis S. (2012). A randomized trial of nicotine-replacement therapy patches in pregnancy. N. Engl. J. Med. 366, 808–818. [DOI] [PubMed] [Google Scholar]
- Collins L., Glasser A. M., Abudayyeh H., Pearson J. L., Villanti A. C. (2019). E-cigarette marketing and communication: How e-cigarette companies market e-cigarettes and the public engages with e-cigarette information. Nicotine Tob. Res. 21, 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Underserved Women, Committee on Obstetric Practice. (2017). Committee Opinion No. 721: Smoking cessation during pregnancy. Obstet. Gynecol. 130, e200–e204. [DOI] [PubMed] [Google Scholar]
- Cooper S., Lewis S., Thornton J. G., Marlow N., Watts K., Britton J., Grainge M. J., Taggar J., Essex H., Parrott S., et al. ; for the Smoking, Nicotine And Pregnancy (SNAP) Trial Team. (2014). The SNAP trial: A randomised placebo-controlled trial of nicotine replacement therapy in pregnancy–clinical effectiveness and safety until 2 years after delivery, with economic evaluation. Health Technol. Assess. 18, 1–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S. J., Linker K. E., Leslie F. M. (2017). Sex-dependent effects of nicotine on the developing brain. J. Neurosci. Res. 95, 422–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Shooshtari S., Forget E. L., Clara I., Cheung K. F. (2014). Smoking during pregnancy: Findings from the 2009-2010 Canadian Community Health Survey. PLoS One 9, e84640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin S., Gunnerbeck A., Wikström A.-K., Cnattingius S., Bonamy A.-K. E. (2016). Maternal tobacco use and extremely premature birth – A population-based cohort study. BJOG 123, 1938–1946. [DOI] [PubMed] [Google Scholar]
- Dasgupta C., Xiao D., Xu Z., Yang S., Zhang L. (2012). Developmental nicotine exposure results in programming of alveolar simplification and interstitial pulmonary fibrosis in adult male rats. Reprod. Toxicol. 34, 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smith A. J., Kaur M., Gonseth S., Endicott A., Selvin S., Zhang L., Roy R., Shao X., Hansen H. M., Kang A. Y., et al. (2017). Correlates of prenatal and early-life tobacco smoke exposure and frequency of common gene deletions in childhood acute lymphoblastic leukemia. Cancer Res. 77, 1674–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Dekker H. T., Voort A. d., de Jongste J. C., Reiss I. K., Hofman A., Jaddoe V. W. V., Duijts L. (2015). Tobacco smoke exposure, airway resistance, and asthma in school-age children. Chest 148, 607–617. [DOI] [PubMed] [Google Scholar]
- DeVito E. E., Krishnan-Sarin S. (2018). E-cigarettes: Impact of e-liquid components and device characteristics on nicotine exposure. Curr. Neuropharmacol. 16, 438–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhalwani N. N., Szatkowski L., Coleman T., Fiaschi L., Tata L. J. (2014). Prescribing of nicotine replacement therapy in and around pregnancy: A population-based study using primary care data. Br. J. Gen. Pract. 64, e554–e560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhalwani N. N., Szatkowski L., Coleman T., Fiaschi L., Tata L. J. (2015). Nicotine replacement therapy in pregnancy and major congenital anomalies in offspring. Pediatrics 135, 859–867. [DOI] [PubMed] [Google Scholar]
- Drake P., Driscoll A. K., Mathews T. J. (2018). Cigarette smoking during pregnancy: United States, 2016. NCHS Data Brief No. 305, 1–8. [PubMed] [Google Scholar]
- Drugs.com. Nicotine Monograph for Professionals Drugs.com. Available at: https://www.drugs.com/monograph/nicotine.html. Accessed August 6, 2020.
- Dwyer J. B., McQuown S. C., Leslie F. M. (2009). The dynamic effects of nicotine on the developing brain. Pharmacol. Ther. 122, 125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimpour A., Shrestha S., Bonnen M. D., Eissa N. T., Raghu G., Ghebre Y. T. (2019). Nicotine modulates growth factors and microRNA to promote inflammatory and fibrotic processes. J. Pharmacol. Exp. Ther. 368, 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Marroun H., Schmidt M. N., Franken I. H. A., Jaddoe V. W. V., Hofman A., van der Lugt A., Verhulst F. C., Tiemeier H., White T. (2014). Prenatal tobacco exposure and brain morphology: A prospective study in young children. Neuropsychopharmacology 39, 792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England L. J., Aagaard K., Bloch M., Conway K., Cosgrove K., Grana R., Gould T. J., Hatsukami D., Jensen F., Kandel D., et al. (2017). Developmental toxicity of nicotine: A transdisciplinary synthesis and implications for emerging tobacco products. Neurosci. Biobehav. Rev. 72, 176–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkamp J. S., Jaddoe V. W. V., Mulders A., Steegers E. A. P., Reiss I. K. M., Duijts L., Gaillard R. (2019). Customized versus population birth weight charts for identification of newborns at risk of long-term adverse cardio-metabolic and respiratory outcomes: A population-based prospective cohort study. BMC Med. 17, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter J.-F. (2014). Levels of saliva cotinine in electronic cigarette users. Addiction 109, 825–829. [DOI] [PubMed] [Google Scholar]
- Fall C. H. D., Kumaran K. (2019). Metabolic programming in early life in humans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374, 20180123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Ping J., Zhang W., Rao Y., Liu H., Zhang J., Yan Y. (2016. a). Prenatal and lactation nicotine exposure affects morphology and function of brown adipose tissue in male rat offspring. Ultrastruct. Pathol. 40, 288–295. [DOI] [PubMed] [Google Scholar]
- Fan J., Zhang W., Rao Y., Xue J., Wang F., Zhang L., Yan Y. (2016. b). Perinatal nicotine exposure increases obesity susceptibility in adult male rat offspring by altering early adipogenesis. Endocrinology 157, 4276–4286. [DOI] [PubMed] [Google Scholar]
- Farsalinos K. (2018). Electronic cigarettes: An aid in smoking cessation, or a new health hazard? Ther. Adv. Respir. Dis. 12, 175346581774496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felicia N., Mikael N., Ronny W. (2019). Blood pressure and heart rate variability in preschool children exposed to smokeless tobacco in fetal life. J. Am. Heart Assoc. 8, e012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucic A., Guszak V., Mantovani A. (2017). Transplacental exposure to environmental carcinogens: Association with childhood cancer risks and the role of modulating factors. Reprod. Toxicol. 72, 182–190. [DOI] [PubMed] [Google Scholar]
- Gaither K. H., Brunner Huber L. R., Thompson M. E., Huet-Hudson Y. M. (2009). Does the use of nicotine replacement therapy during pregnancy affect pregnancy outcomes? Matern. Child Health J. 13, 497–504. [DOI] [PubMed] [Google Scholar]
- Gao Y.-J., Holloway A. C., Su L.-Y., Takemori K., Lu C., Lee R. M. K. W. (2008). Effects of fetal and neonatal exposure to nicotine on blood pressure and perivascular adipose tissue function in adult life. Eur. J. Pharmacol. 590, 264–268. [DOI] [PubMed] [Google Scholar]
- Gauldie J., Galt T., Bonniaud P., Robbins C., Kelly M., Warburton D. (2003). Transfer of the active form of transforming growth factor-beta 1 gene to newborn rat lung induces changes consistent with bronchopulmonary dysplasia. Am. J. Pathol. 163, 2575–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs K., Collaco J. M., McGrath-Morrow S. A. (2016). Impact of tobacco smoke and nicotine exposure on lung development. Chest 149, 552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert N. L., Nelson C. R. M., Greaves L. (2015). Smoking cessation during pregnancy and relapse after childbirth in Canada. J. Obstet. Gynaecol. Can. 37, 32–39. [DOI] [PubMed] [Google Scholar]
- Glover M., Phillips C. V. (2020). Potential effects of using non-combustible tobacco and nicotine products during pregnancy: A systematic review. Harm Reduct. J. 17, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grando S. A. (2014). Connections of nicotine to cancer. Nat. Rev. Cancer 14, 419–429. [DOI] [PubMed] [Google Scholar]
- Greaves L., Poole N., Okoli C. T. C., Hemsing N., Qu A., Bialystok L., O’Leary R. (2011). Expecting to Quit: A Best-Practices Review of Smoking Cessation Interventions for Pregnant and Postpartum Girls and Women, 2nd ed. British Columbia Centre of Excellence for Women’s Health, Vancouver. Available at: http://bccewh.bc.ca/2014/02/expecting-to-quit-a-best-practices-review-of-smoking-cessation-interventions-for-pregnant-and-postpartum-girls-and-women/. Accessed August 6, 2020.
- Greene R. M., Pisano M. M. (2019). Developmental toxicity of e-cigarette aerosols. Birth Defects Res. 111, 1294–1301. [DOI] [PubMed] [Google Scholar]
- Gu L. 2014. Integrative analysis of prostate cancer methylome and smoking-induced transgenerational epigenomic reprogramming. Available at: http://archiv.ub.uni-heidelberg.de/volltextserver/id/eprint/16011. Accessed May 11, 2020.
- Gunnerbeck A., Bonamy A.-K. E., Wikström A.-K., Granath F., Wickström R., Cnattingius S. (2014). Maternal snuff use and smoking and the risk of oral cleft malformations - A Population-Based Cohort Study. PLoS One 9, e84715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P., Subramoney S. (2006). Smokeless tobacco use and risk of stillbirth: A Cohort Study in Mumbai, India. Epidemiology 17, 47–51. [DOI] [PubMed] [Google Scholar]
- Haghighi A., Schwartz D. H., Abrahamowicz M., Leonard G. T., Perron M., Richer L., Veillette S., Gaudet D., Paus T., Pausova Z. (2013). Prenatal exposure to maternal cigarette smoking, amygdala volume, and fat intake in adolescence. JAMA Psychiatry 70, 98–105. [DOI] [PubMed] [Google Scholar]
- Haussmann H.-J., Fariss M. W. (2016). Comprehensive review of epidemiological and animal studies on the potential carcinogenic effects of nicotine per se. Crit. Rev. Toxicol. 46, 701–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Chen J., Zhu L.-H., Hua L.-L., Ke F.-F. (2017). Maternal smoking during pregnancy and ADHD: Results from a systematic review and meta-analysis of prospective cohort studies. J. Atten. Disord. 108705471769676. [DOI] [PubMed] [Google Scholar]
- Hickson C., Lewis S., Campbell K. A., Cooper S., Berlin I., Claire R., Oncken C., Coleman-Haynes T., Coleman T. (2018). Comparison of nicotine exposure during pregnancy when smoking and abstinent with nicotine replacement therapy: Systematic review and meta-analysis. Addiction 114, 406–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodyl N. A., Grzeskowiak L. E., Stark M. J., Scheil W., Clifton V. L. (2014). The impact of Aboriginal status, cigarette smoking and smoking cessation on perinatal outcomes in South Australia. Med. J. Aust. 201, 274–278. [DOI] [PubMed] [Google Scholar]
- Holbrook B. D. (2016). The effects of nicotine on human fetal development. Birth Defects Res. C Embryo Today 108, 181–192. [DOI] [PubMed] [Google Scholar]
- Hossain M. S., Kypri K., Rahman B., Milton A. H. (2018). Smokeless tobacco consumption and stillbirth: Population-based case–control study in rural Bangladesh. Drug Alcohol Rev. 37, 414–420. [DOI] [PubMed] [Google Scholar]
- Huang L. Z., Winzer-Serhan U. H. (2007). Nicotine regulates mRNA expression of feeding peptides in the arcuate nucleus in neonatal rat pups. Dev. Neurobiol. 67, 363–377. [DOI] [PubMed] [Google Scholar]
- Improgo M. R., Tapper A. R., Gardner P. D. (2011). Nicotinic acetylcholine receptor-mediated mechanisms in lung cancer. Biochem. Pharmacol. 82, 1015–1021. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. (2004). Smokeless Tobacco and Some Tobacco-specific N-Nitrosamines. International Agency for Research on Cancer. Available at: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Smokeless-Tobacco-And-Some-Tobacco-specific-Em-N-Em–Nitrosamines-2007.
- Jarvis M., Tunstall-Pedoe H., Feyerabend C., Vesey C., Salloojee Y. (1984). Biochemical markers of smoke absorption and self reported exposure to passive smoking. J. Epidemiol. Community Health 38, 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K., Nizamutdinov D., Guerrier M., Afroze S., Dostal D., Glaser S. (2012). General mechanisms of nicotine-induced fibrogenesis. FASEB J. 26, 4778–4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordanov J. S. (1990). Cotinine concentrations in amniotic fluid and urine of smoking, passive smoking and non-smoking pregnant women at term and in the urine of their neonates on 1st day of life. Eur. J. Pediatr. 149, 734–737. [DOI] [PubMed] [Google Scholar]
- Kalliola S., Pelkonen A. S., Malmberg L. P., Sarna S., Hämäläinen M., Mononen I., Mäkelä M. J. (2013). Maternal smoking affects lung function and airway inflammation in young children with multiple-trigger wheeze. J. Allergy Clin. Immunol.131, 730–735. [DOI] [PubMed] [Google Scholar]
- Kapaya M., Tong V., Ding H. (2015). Nicotine replacement therapy and other interventions for pregnant smokers: Pregnancy Risk Assessment Monitoring System, 2009–2010. Prev. Med. 78, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärkkäinen M., Kettunen H.-P., Nurmi H., Selander T., Purokivi M., Kaarteenaho R. (2017). Effect of smoking and comorbidities on survival in idiopathic pulmonary fibrosis. Respir. Res. 18, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi T., Avci N. G., Keller R. F., Akay Y. M., Akay M. (2020). Investigating the influence of perinatal nicotine exposure on genetic profiles of neurons in the sub-regions of the VTA. Sci. Rep. 10, 2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs M., Sakurai R., Torday J. S., Rehan V. K. (2010). Evidence for in vivo nicotine-induced alveolar interstitial fibroblast-to-myofibroblast transdifferentiation. Exp. Lung Res. 36, 390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuniyoshi K. M., Rehan V. K. (2019). The impact of perinatal nicotine exposure on fetal lung development and subsequent respiratory morbidity. Birth Defects Res. 111, 1270–1283. [DOI] [PubMed] [Google Scholar]
- Lambers D. S., Clark K. E. (1996). The maternal and fetal physiologic effects of nicotine. Semin. Perinatol. 20, 115–126. [DOI] [PubMed] [Google Scholar]
- Landau L. I. (2008). Tobacco smoke exposure and tracking of lung function into adult life. Paediatr. Respir. Rev. 9, 39–44. [DOI] [PubMed] [Google Scholar]
- Lange S., Probst C., Rehm J., Popova S. (2018). National, regional, and global prevalence of smoking during pregnancy in the general population: A systematic review and meta-analysis. Lancet Glob. Health 6, e769–e776. [DOI] [PubMed] [Google Scholar]
- Lavezzi A. M., Corna M. F., Alfonsi G., Matturri L. (2014). Possible role of the α7 nicotinic receptors in mediating nicotine’s effect on developing lung – Implications in unexplained human perinatal death. BMC Pulm. Med. 14, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. K., Abrahamowicz M., Leonard G. T., Richer L., Perron M., Veillette S., Reischl E., Bouchard L., Gaudet D., Paus T., et al. (2015. a). Prenatal exposure to cigarette smoke interacts with OPRM1 to modulate dietary preference for fat. J. Psychiatry Neurosci. 40, 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. K., Richmond R., Hu P., French L., Shin J., Bourdon C., Reischl E., Waldenberger M., Zeilinger S., Gaunt T., et al. (2015. b). Prenatal exposure to maternal cigarette smoking and DNA methylation: Epigenome-wide association in a discovery sample of adolescents and replication in an independent cohort at birth through 17 years of age. Environ. Health Perspect. 123, 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Xiao L., Luo Y., Xu J. (2020). Prevalence and risk factors of childhood hypertension from birth through childhood: A retrospective cohort study. J. Hum. Hypertens. 34, 151–164. [DOI] [PubMed] [Google Scholar]
- Liao C.-Y., Chen Y.-J., Lee J.-F., Lu C.-L., Chen C.-H. (2012). Cigarettes and the developing brain: Picturing nicotine as a neuroteratogen using clinical and preclinical studies. Tzu Chi Med. J. 24, 157–161. [Google Scholar]
- Lobstein T., Jackson-Leach R. (2016). Planning for the worst: Estimates of obesity and comorbidities in school-age children in 2025. Pediatr. Obes. 11, 321–325. [DOI] [PubMed] [Google Scholar]
- Ma N., Nicholson C. J., Wong M., Holloway A. C., Hardy D. B. (2014). Fetal and neonatal exposure to nicotine leads to augmented hepatic and circulating triglycerides in adult male offspring due to increased expression of fatty acid synthase. Toxicol. Appl. Pharmacol. 275, 1–11. [DOI] [PubMed] [Google Scholar]
- Mahar I., Bagot R. C., Davoli M. A., Miksys S., Tyndale R. F., Walker C.-D., Maheu M., Huang S.-H., Wong T. P., Mechawar N. (2012). Developmental hippocampal neuroplasticity in a model of nicotine replacement therapy during pregnancy and breastfeeding. PLoS One 7, e37219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maritz G. S. (2013). Perinatal exposure to nicotine and implications for subsequent obstructive lung disease. Paediatr. Respir. Rev. 14, 3–8. [DOI] [PubMed] [Google Scholar]
- Maritz G. S., Harding R. (2011). Life-long programming implications of exposure to tobacco smoking and nicotine before and soon after birth: Evidence for altered lung development. Int. J. Environ. Res. Public Health 8, 875–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark K. S., Farquhar B., Chisolm M. S., Coleman-Cowger V. H., Terplan M. (2015). Knowledge, attitudes, and practice of electronic cigarette use among pregnant women. J. Addict. Med. 9, 266–272. [DOI] [PubMed] [Google Scholar]
- Marufu T. C., Ahankari A., Coleman T., Lewis S. (2015). Maternal smoking and the risk of still birth: Systematic review and meta-analysis. BMC Public Health 15, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy C. T., Spindel E. R. (2017). Pulmonary effects of maternal smoking on the fetus and child: Effects on lung development, respiratory morbidities, and life long lung health. Paediatr. Respir. Rev. 21, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath-Morrow S. A., Hayashi M., Aherrera A., Lopez A., Malinina A., Collaco J. M., Neptune E., Klein J. D., Winickoff J. P., Breysse P., et al. (2015). The effects of electronic cigarette emissions on systemic cotinine levels, weight and postnatal lung growth in neonatal mice. PLoS One 10, e0118344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A., Chaturvedi P., Datta S., Sinukumar S., Joshi P., Garg A. (2015). Harmful effects of nicotine. Indian J. Med. Paediatr. Oncol. 36, 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerke M. J., McMahon L. R., Wilkerson J. L. (2020). More than smoke and patches: The quest for pharmacotherapies to treat tobacco use disorder. Pharmacol. Rev. 72, 527–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E., Blatt K., Chen A., Van Hook J., Defranco E. A. (2016). Relationship of trimester specific smoking patterns and risk of preterm birth. Am. J. Obstet. Gynecol. 215, 109.e1–109.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Suárez-Varela M. M., Bille C., Christensen K., Olsen J. (2006). Smoking habits, nicotine use, and congenital malformations. Obstet. Gynecol. 107, 51–57. [DOI] [PubMed] [Google Scholar]
- Morgen C. S., Ängquist L., Baker J. L., Andersen A. M. N., Michaelsen K. F., Sørensen T. I. A. (2018). Prenatal risk factors influencing childhood BMI and overweight independent of birth weight and infancy BMI: A path analysis within the Danish National Birth Cohort. Int. J. Obes. 42, 594–602. [DOI] [PubMed] [Google Scholar]
- Moschonis G., Kaliora A. C., Karatzi K., Michaletos A., Lambrinou C.-P., Karachaliou A. K., Chrousos G. P., Lionis C., Manios Y. (2017). Perinatal, sociodemographic and lifestyle correlates of increased total and visceral fat mass levels in schoolchildren in Greece: The Healthy Growth Study. Public Health Nutr. 20, 660–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad A., Mychasiuk R., Nakahashi A., Hossain S. R., Gibb R., Kolb B. (2012). Prenatal nicotine exposure alters neuroanatomical organization of the developing brain. Synapse 66, 950–954. [DOI] [PubMed] [Google Scholar]
- Nakano Y. (2020). Adult-onset diseases in low birth weight infants: Association with adipose tissue maldevelopment. J. Atheroscler. Thromb. 27, 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napierala M., Mazela J., Merritt T. A., Florek E. (2016). Tobacco smoking and breastfeeding: Effect on the lactation process, breast milk composition and infant development. Crit. Rev. Environ. Res. 151, 321–338. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. 2010. Smoking: Stopping in Pregnancy and After Childbirth | Public Health Guideline Available at: https://www.nice.org.uk/guidance/ph26. Accessed August 6, 2020.
- Nguyen T., Li G. E., Chen H., Cranfield C. G., McGrath K. C., Gorrie C. A. (2018). Maternal e-cigarette exposure results in cognitive and epigenetic alterations in offspring in a mouse model. Chem. Res. Toxicol. 31, 601–611. [DOI] [PubMed] [Google Scholar]
- Nowak D., Ruta U., Piasȩcka G. (1990). Nicotine increases human polymorphonuclear leukocytes chemotactic response—A possible additional mechanism of lung injury in cigarette smokers. Exp. Pathol. 39, 37–43. [DOI] [PubMed] [Google Scholar]
- Oncken C., Ricci K. A., Kuo C.-L., Dornelas E., Kranzler H. R., Sankey H. Z. (2017). Correlates of electronic cigarettes use before and during pregnancy. Nicotine Tob. Res. 19, 585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordean A., Wong S., Graves L. (2017). No. 349-substance use in pregnancy. J. Obstetr. Gynaecol. Canada 39, 922–937.e2. [DOI] [PubMed] [Google Scholar]
- Orton S., Bowker K., Cooper S., Naughton F., Ussher M., Pickett K. E., Leonardi-Bee J., Sutton S., Dhalwani N. N., Coleman T. (2014). Longitudinal cohort survey of women’s smoking behaviour and attitudes in pregnancy: Study methods and baseline data. BMJ Open 4, e004915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo A., Selman M. (2016). Lung fibroblasts, aging, and idiopathic pulmonary fibrosis. Ann. Am. Thorac. Soc. 13(Suppl. 5), S417–S421. [DOI] [PubMed] [Google Scholar]
- Patel D., Davis K. C., Cox S., Bradfield B., King B. A., Shafer P., Caraballo R., Bunnell R. (2016). Reasons for current E-cigarette use among U.S. adults. Prev. Med. 93, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattemore P. (2013). Tobacco or healthy children: The two cannot co-exist. Front. Pediatr. 1, 20Available at: http://journal.frontiersin.org/article/10.3389/fped.2013.00020/abstract. Accessed May 11, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pausová Z., Paus T., Šedová L., Bérubé J. (2003). Prenatal exposure to nicotine modifies kidney weight and blood pressure in genetically susceptible rats: A case of gene-environment interaction. Kidney Int. 64, 829–835. [DOI] [PubMed] [Google Scholar]
- Peixoto T. C., Moura E. G., Oliveira E., Younes-Rapozo V., Soares P. N., Rodrigues V. S. T., Torsoni M. A., Torsoni A. S., Manhães A. C., Lisboa P. C. (2019). Hypothalamic neuropeptides expression and hypothalamic inflammation in adult rats that were exposed to tobacco smoke during breastfeeding: Sex-related differences. Neuroscience 418, 69–81. [DOI] [PubMed] [Google Scholar]
- Pelone F., Specchia M. L., Veneziano M. A., Capizzi S., Bucci S., Mancuso A., Ricciardi W., de Belvis A. G. (2012). Economic impact of childhood obesity on health systems: A systematic review. Obes. Rev. 13, 431–440. [DOI] [PubMed] [Google Scholar]
- Pereira P. P. d S., Da Mata F. A. F., Figueiredo A. C. G., de Andrade K. R. C., Pereira M. G. (2017). Maternal active smoking during pregnancy and low birth weight in the Americas: A systematic review and meta-analysis. Nicotine Tob. Res. 19, 497–505. [DOI] [PubMed] [Google Scholar]
- Pernia S., DeMaagd G. (2016). The new pregnancy and lactation labeling rule. P T 41, 713–715. [PMC free article] [PubMed] [Google Scholar]
- Petre M. A., Petrik J., Ellis R., Inman M. D., Holloway A. C., Labiris N. R. (2011). Fetal and neonatal exposure to nicotine disrupts postnatal lung development in rats: Role of VEGF and its receptors. Int. J. Toxicol. 30, 244–252. Available at: https://journals.sagepub.com/doi/10.1177/1091581810395332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineles B. L., Park E., Samet J. M. (2014). Systematic review and meta-analysis of miscarriage and maternal exposure to tobacco smoke during pregnancy. Am. J. Epidemiol. 179, 807–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro C. R., Moura E. G., Manhães A. C., Fraga M. C., Claudio-Neto S., Younes-Rapozo V., Santos-Silva A. P., Lotufo B. M., Oliveira E., Lisboa P. C. (2015). Maternal nicotine exposure during lactation alters food preference, anxiety-like behavior and the brain dopaminergic reward system in the adult rat offspring. Physiol. Behav. 149, 131–141. [DOI] [PubMed] [Google Scholar]
- Polli F. S., Scharff M. B., Ipsen T. H., Aznar S., Kohlmeier K. A., Andreasen J. T. (2020). Prenatal nicotine exposure in mice induces sex-dependent anxiety-like behavior, cognitive deficits, hyperactivity, and changes in the expression of glutamate receptor associated-genes in the prefrontal cortex. Pharmacol. Biochem. Behav. 195, 172951. [DOI] [PubMed] [Google Scholar]
- Räisänen S., Sankilampi U., Gissler M., Kramer M. R., Hakulinen-Viitanen T., Saari J., Heinonen S. (2014). Smoking cessation in the first trimester reduces most obstetric risks, but not the risks of major congenital anomalies and admission to neonatal care: A population-based cohort study of 1 164 953 singleton pregnancies in Finland. J. Epidemiol. Community Health 68, 159–164. [DOI] [PubMed] [Google Scholar]
- Ramamurthi D., Gall P. A., Ayoub N., Jackler R. K. (2016). Leading-brand advertisement of quitting smoking benefits for e-cigarettes. Am. J. Public Health 106, 2057–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratsch A., Bogossian F. (2014). Smokeless tobacco use in pregnancy: An integrative review of the literature. Int. J. Public Health 59, 599–608. [DOI] [PubMed] [Google Scholar]
- Rayfield S., Plugge E. (2017). Systematic review and meta-analysis of the association between maternal smoking in pregnancy and childhood overweight and obesity. J. Epidemiol. Community Health 71, 162–173. [DOI] [PubMed] [Google Scholar]
- Rehan V. K., Liu J., Naeem E., Tian J., Sakurai R., Kwong K., Akbari O., Torday J. S. (2012). Perinatal nicotine exposure induces asthma in second generation offspring. BMC Med. 10, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz M., Lewis S., Coleman T., Aveyard P., West R., Naughton F., Ussher M. (2016). Which measures of cigarette dependence are predictors of smoking cessation during pregnancy? Analysis of data from a randomized controlled trial. Addiction 111, 1656–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. M. (2019). Smoking and pregnancy: Epigenetics and developmental origins of the metabolic syndrome. Birth Defects Res. 111, 1259–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roomans G. M., Vanthanouvong V., Dragomir A., Kozlova I., Wróblewski R. (2002). Effects of nicotine on intestinal and respiratory epithelium. J. Submicrosc. Cytol. Pathol. 34, 381–388. [PubMed] [Google Scholar]
- Rotroff D. M., Joubert B. R., Marvel S. W., Håberg S. E., Wu M. C., Nilsen R. M., Ueland P. M., Nystad W., London S. J., Motsinger-Reif A. (2016). Maternal smoking impacts key biological pathways in newborns through epigenetic modification in Utero. BMC Genomics 17, 976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe H., Baker T., Hale T. W. (2015). Maternal medication, drug use, and breastfeeding. Child Adolesc. Psychiatr. Clin. N. Am. 24, 1–20. [DOI] [PubMed] [Google Scholar]
- Ruszkiewicz J. A., Zhang Z., Gonçalves F. M., Tizabi Y., Zelikoff J. T., Aschner M. (2020). Neurotoxicity of e-cigarettes. Food Chem. Toxicol. 138, 111245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer S., Sebastiani G., Andreu-Férnández V., García-Algar O. (2019). Impact of nicotine replacement and electronic nicotine delivery systems on fetal brain development. Int. J. Environ. Res. Public Health 16, 5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanner T., Grimsrud T. K. (2015). Nicotine: Carcinogenicity and effects on response to cancer treatment – A review. Front. Oncol. 5, Available at: http://journal.frontiersin.org/Article/10.3389/fonc.2015.00196/abstract. Accessed May 11, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhon H. S., Jia Y., Raab R., Kuryatov A., Pankow J. F., Whitsett J. A., Lindstrom J., Spindel E. R. (1999). Prenatal nicotine increases pulmonary α7 nicotinic receptor expression and alters fetal lung development in monkeys. J. Clin. Invest. 103, 637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhon H. S., Keller J. A., Proskocil B. J., Martin E. L., Spindel E. R. (2002). Maternal nicotine exposure upregulates collagen gene expression in fetal monkey lung: Association with α 7 nicotinic acetylcholine receptors. Am. J. Respir. Cell Mol. Biol. 26, 31–41. [DOI] [PubMed] [Google Scholar]
- Shah N. R., Bracken M. B. (2000). A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. Am. J. Obstetr. Gynecol. 182, 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shobeiri F., Jenabi E. (2017). Smoking and placenta previa: A meta-analysis. J. Matern. Fetal Neonatal Med. 30, 2985–2990. [DOI] [PubMed] [Google Scholar]
- Shobeiri F., Masoumi S. Z., Jenabi E. (2017). The association between maternal smoking and placenta abruption: A meta-analysis. J. Matern. Fetal Neonatal Med. 30, 1963–1967. [DOI] [PubMed] [Google Scholar]
- Sifat A. E., Nozohouri S., Villalba H., Al Shoyaib A., Vaidya B., Karamyan V. T., Abbruscato T. (2020). Prenatal electronic cigarette exposure decreases brain glucose utilization and worsens outcome in offspring hypoxic-ischemic brain injury. J. Neurochem. 153, 63–79. [DOI] [PubMed] [Google Scholar]
- Silvestri M., Franchi S., Pistorio A., Petecchia L., Rusconi F. (2015). Smoke exposure, wheezing, and asthma development: A systematic review and meta-analysis in unselected birth cohorts. Pediatr. Pulmonol. 50, 353–362. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Ryde I. T., Tate C. A., Seidler F. J. (2007). Lasting effects of nicotine treatment and withdrawal on serotonergic systems and cell signaling in rat brain regions: Separate or sequential exposure during fetal development and adulthood. Brain Res. Bull. 73, 259–272. [DOI] [PubMed] [Google Scholar]
- Smith A., Dwoskin L., Pauly J. (2016). Early exposure to nicotine during critical periods of brain development: Mechanisms and consequences. J. Pediatr. Biochem. 1, 125–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somm E., Schwitzgebel V. M., Vauthay D. M., Camm E. J., Chen C. Y., Giacobino J.-P., Sizonenko S. V., Aubert M. L., Hüppi P. S. (2008). Prenatal nicotine exposure alters early pancreatic islet and adipose tissue development with consequences on the control of body weight and glucose metabolism later in life. Endocrinology 149, 6289–6299. [DOI] [PubMed] [Google Scholar]
- Soneji S., Beltrán-Sánchez H. (2019). Association of maternal cigarette smoking and smoking cessation with preterm birth. JAMA Netw. Open 2, e192514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindel E. R., McEvoy C. T. (2016). The role of nicotine in the effects of maternal smoking during pregnancy on lung development and childhood respiratory disease. Implications for dangers of e-cigarettes. Am. J. Respir. Crit. Care Med. 193, 486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Helen G., Havel C., Dempsey D. A., Jacob P., Benowitz N. L. (2016). Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction 111, 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocks J., Hislop A., Sonnappa S. (2013). Early lung development: Lifelong effect on respiratory health and disease. Lancet Respir. Med. 1, 728–742. [DOI] [PubMed] [Google Scholar]
- Strandberg-Larsen K., Tinggaard M., Nybo Andersen A.-M., Olsen J., Grønbaek M. (2008). Use of nicotine replacement therapy during pregnancy and stillbirth: A cohort study. BJOG 115, 1405–1410. [DOI] [PubMed] [Google Scholar]
- Suliankatchi R. A., Sinha D. N. (2016). The human cost of tobacco chewing among pregnant women in India: A systematic review and meta-analysis. J. Obstet. Gynecol. India 66, 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter M. A., Abramovici A. R., Griffin E., Branch D. W., Lane R. H., Mastrobattista J., Rehan V. K., Aagaard K. (2015). In utero nicotine exposure epigenetically alters fetal chromatin structure and differentially regulates transcription of the glucocorticoid receptor in a rat model. Birth Defects Res. A Clin. Mol. Teratol. 103, 583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter M., Abramovici A., Showalter L., Hu M., Shope C. D., Varner M., Aagaard-Tillery K. (2010). In utero tobacco exposure epigenetically modifies placental CYP1A1 expression. Metabolism 59, 1481–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantal A. F., Chen H., Shi T. T., Lu C.-H., Fang A. B., Buckley S., Kolb M., Gauldie J., Warburton D., Shi W. (2010). Overexpression of transforming growth factor- 1 in fetal monkey lung results in prenatal pulmonary fibrosis. Eur. Respir. J. 36, 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskar V. S., Coultas D. B. (2006). Is idiopathic pulmonary fibrosis an environmental disease? Proc. Am. Thorac. Soc. 3, 293–298. [DOI] [PubMed] [Google Scholar]
- The Royal Australian and New Zealand College of Obstetricians and Gynaecologists. 2017. Pre-pregnancy Counselling. Available at: https://ranzcog.edu.au/RANZCOG_SITE/media/RANZCOG-MEDIA/Women%27s%20Health/Statement%20and%20guidelines/Clinical-Obstetrics/Pre-pregnancy-Counselling-(C-Obs-3a)-review-July-2017_1.pdf? ext=.pdf. Accessed August 6, 2020.
- Thomas J. D., Garrison M. E., Slawecki C. J., Ehlers C. L., Riley E. P. (2000). Nicotine exposure during the neonatal brain growth spurt produces hyperactivity in preweanling rats. Neurotoxicol. Teratol. 22, 695–701. [DOI] [PubMed] [Google Scholar]
- Tiesler C. M. T., Heinrich J. (2014). Prenatal nicotine exposure and child behavioural problems. Eur. Child Adolesc. Psychiatry 23, 913–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiniakos D. G., Vos M. B., Brunt E. M. (2010). Nonalcoholic fatty liver disease: Pathology and pathogenesis. Annu. Rev. Pathol. Mech. Dis. 5, 145–171. [DOI] [PubMed] [Google Scholar]
- Tong V. T., Dietz P. M., Morrow B., D’Angelo D. V., Farr S. L., Rockhill K. M., England L. J.; Centers for Disease Control and Prevention (CDC). (2013). Trends in smoking before, during, and after pregnancy–Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000-2010. MMWR Surveill. Summ. 62, 1–19. [PubMed] [Google Scholar]
- Veisani Y., Jenabi E., Delpisheh A., Khazaei S. (2019). Effect of prenatal smoking cessation interventions on birth weight: Meta-analysis. J. Matern. Fetal Neonatal Med. 32, 332–338. [DOI] [PubMed] [Google Scholar]
- Vicary G. W., Ritzenthaler J. D., Panchabhai T. S., Torres-González E., Roman J. (2017). Nicotine stimulates collagen type I expression in lung via α7 nicotinic acetylcholine receptors. Respir. Res. 18, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya F., Cereijo R., Gavaldà-Navarro A., Villarroya J., Giralt M. (2018). Inflammation of brown/beige adipose tissues in obesity and metabolic disease. J. Intern. Med. 284, 492–504. [DOI] [PubMed] [Google Scholar]
- Wang B., Chen H., Chan Y. L., Wang G., Oliver B. G. (2020). Why do intrauterine exposure to air pollution and cigarette smoke increase the risk of asthma? Front. Cell Dev. Biol. 8, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Ouyang Q., Huang Z., Lin L., Yu E., Ferrari M. W. (2015). Prenatal nicotine exposure induces gender-associated left ventricular-arterial uncoupling in adult offspring. Mol. Med. Rep. 12, 410–418. [DOI] [PubMed] [Google Scholar]
- Ward A. M., Yaman R., Ebbert J. O. (2020). Electronic nicotine delivery system design and aerosol toxicants: A systematic review. PLoS One 15, e0234189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawryk-Gawda E., Chłapek K., Zarobkiewicz M. K., Lis-Sochocka M., Chylińska-Wrzos P., Boguszewska-Czubara A., Sławiński M. A., Franczak A., Jodłowska-Jędrych B., Biała G. (2018). CB2R agonist prevents nicotine induced lung fibrosis. Exp. Lung Res. 44, 344–351. [DOI] [PubMed] [Google Scholar]
- Weng S. F., Redsell S. A., Swift J. A., Yang M., Glazebrook C. P. (2012). Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch. Dis. Childhood 97, 1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby M. T., Kollins S. H., McClernon F. J.; The Family Life Investigative Group. (2009). Association between smoking and retrospectively reported attention-deficit/hyperactivity disorder symptoms in a large sample of new mothers. Nicotine Tob. Res. 11, 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongtrakool C., Wang N., Hyde D. M., Roman J., Spindel E. R. (2012). Prenatal nicotine exposure alters lung function and airway geometry through α7 nicotinic receptors. Am. J. Respir. Cell Mol. Biol. 46, 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2013). WHO | WHO Recommendations for the Prevention and Management of Tobacco Use and Second-Hand Smoke Exposure in Pregnancy. WHO, Geneva, Switzerland. Available at: http://www.who.int/tobacco/publications/pregnancy/guidelinestobaccosmokeexposure/en/. Accessed August 6, 2020. [PubMed] [Google Scholar]
- Wu C.-C., Hsu T.-Y., Chang J.-C., Ou C.-Y., Kuo H.-C., Liu C.-A., Wang C.-L., Chuang H., Chen C.-P., Yang K. D. (2019). Paternal tobacco smoke correlated to offspring asthma and prenatal epigenetic programming. Front. Genet. 10, 471 Available at: https://www.frontiersin.org/articles/10.3389/fgene.2019.00471/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D., Dasgupta C., Li Y., Huang X., Zhang L. (2014). Perinatal nicotine exposure increases angiotensin II receptor-mediated vascular contractility in adult offspring. PLoS One 9, e108161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D., Huang X., Li Y., Dasgupta C., Wang L., Zhang L. (2015). Antenatal antioxidant prevents nicotine-mediated hypertensive response in rat adult offspring. Biol. Reprod. 93, 66 Available at: https://academic.oup.com/biolreprod/article/93/3/66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes-Rapozo V., Moura E. G., Manhães A. C., Pinheiro C. R., Santos-Silva A. P., Oliveira E. D., Lisboa P. C. (2013). Maternal nicotine exposure during lactation alters hypothalamic neuropeptides expression in the adult rat progeny. Food Chem. Toxicol. 58, 158–168. [DOI] [PubMed] [Google Scholar]
- Zhang K., Wang X. (2013). Maternal smoking and increased risk of sudden infant death syndrome: A meta-analysis. Legal Med. 15, 115–121. [DOI] [PubMed] [Google Scholar]
- Zhang L., Spencer T. J., Biederman J., Bhide P. G. (2018). Attention and working memory deficits in a perinatal nicotine exposure mouse model. Rosenfeld CS, editor. PLoS One 13, e0198064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Chen H., Fan J., Li G., Sun A., Lan L., Zhang L., Yan Y. (2020). The association between maternal nicotine exposure and adipose angiogenesis in female rat offspring: A mechanism of adipose tissue function changes. Toxicol. Lett. 318, 12–21. [DOI] [PubMed] [Google Scholar]
- Zhu J., Fan F., McCarthy D. M., Zhang L., Cannon E. N., Spencer T. J., Biederman J., Bhide P. G. (2017). A prenatal nicotine exposure mouse model of methylphenidate responsive ADHD-associated cognitive phenotypes. Int. J. Dev. Neurosci. 58, 26–34. [DOI] [PubMed] [Google Scholar]
- Zhu J., Zhang X., Xu Y., Spencer T. J., Biederman J., Bhide P. G. (2012). Prenatal nicotine exposure mouse model showing hyperactivity, reduced cingulate cortex volume, reduced dopamine turnover, and responsiveness to oral methylphenidate treatment. J. Neurosci. 32, 9410–9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwar, N., Richmond, R., Borland, R., Peters, M., Litt, J., Bell, J., Caldwell, B., and Ferretter, I. (2011). Supporting Smoking Cessation: A Guide for Health Professionals Available at: https://www.racgp.org.au/clinical-resources/clinical-guidelines/key-racgp-guidelines/view-all-racgp-guidelines/supporting-smoking-cessation. Accessed August 6, 2020.