Abstract
Aims
The prognostic importance of cardiac procedural myocardial injury and myocardial infarction (MI) in chronic coronary syndrome (CCS) patients undergoing elective percutaneous coronary intervention (PCI) is still debated.
Methods and results
We analysed individual data of 9081 patients undergoing elective PCI with normal pre-PCI baseline cardiac troponin (cTn) levels. Multivariate models evaluated the association between post-PCI elevations in cTn and 1-year mortality, while an interval analysis evaluated the impact of the size of the myocardial injury on mortality. Our analysis was performed in the overall population and also according to the type of cTn used [52.0% had high-sensitivity cTn (hs-cTn)]. Procedural myocardial injury, as defined by the Fourth Universal Definition of MI (UDMI) [post-PCI cTn elevation ≥1 × 99th percentile upper reference limit (URL)], occurred in 52.8% of patients and was not associated with 1-year mortality [adj odds ratio (OR), 1.35, 95% confidence interval (CI) (0.84–1.77), P = 0.21]. The association between post-PCI cTn elevation and 1-year mortality was significant starting ≥3 × 99th percentile URL. Major myocardial injury defined by post-PCI ≥5 × 99th percentile URL occurred in 18.2% of patients and was associated with a two-fold increase in the adjusted odds of 1-year mortality [2.29, 95% CI (1.32–3.97), P = 0.004]. In the subset of patients for whom periprocedural evidence of ischaemia was collected (n = 2316), Type 4a MI defined by the Fourth UDMI occurred in 12.7% of patients and was strongly associated with 1-year mortality [adj OR 3.21, 95% CI (1.42–7.27), P = 0.005]. We also present our results according to the type of troponin used (hs-cTn or conventional troponin).
Conclusion
Our analysis has demonstrated that in CCS patients with normal baseline cTn levels, the post-PCI cTn elevation of ≥5 × 99th percentile URL used to define Type 4a MI is associated with 1-year mortality and could be used to detect ‘major’ procedural myocardial injury in the absence of procedural complications or evidence of new myocardial ischaemia.
Keywords: Elective PCI, Myocardial injury, Myocardial infarction, Procedural complication, Procedural myocardial injury, Procedural myocardial infarction
Graphical Abstract
Listen to the audio abstract of this contribution.
See page 335 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa983)
Introduction
Elective percutaneous coronary intervention (PCI) is considered a safe treatment for chronic coronary syndrome (CCS), with a very low rate of major procedural complications.1–3 Technical advances in PCI and new pharmacological therapies in coronary angiography procedures have resulted in a drastic reduction in PCI-related complications such as acute stent thrombosis, stroke, or vascular access bleeding. As a result, elective PCI can currently be performed in ambulatory systems of care with outpatients being treated and discharged on the same day. Although the rate of serious complications is low, post-PCI increases in cardiac biomarkers are frequent, especially in the era of high-sensitivity cardiac troponin (hs-cTn). However, the prognostic importance of post-PCI cTn elevations in CCS patients undergoing elective PCI in terms of recurrent cardiovascular events and long-term mortality remains debated.4–6
According to the definition and cardiac biomarker used, recent data have reported significant variability in the incidence of Type 4a myocardial infarction (MI) (ranging from 3% to 10%), and procedural myocardial injury (ranging from 25% to 70%),7–11 with some studies supporting an independent association with cardiovascular events and long-term mortality 6 , 7 and some not demonstrating a relationship with adverse outcomes.5 , 12 Similarly, minor troponin elevations may or may not be reflected in the loss of viable myocardium, and the association between the loss of small amounts of viable myocardium and further cardiovascular events remains unclear.13–15 In 2018, an expert consensus group comprising the European Society of Cardiology, the American Heart Association(AHA), the American College of Cardiology (ACC) and the World Heart Foundation published the Fourth Universal Definition of MI (UDMI) and defined procedural myocardial injury as a post-PCI increase of cTn >1 × 99th percentile upper reference limit (URL) in patients with normal pre-PCI baseline cTn (<1 × 99th percentile URL).10 The Academic Research Consortium 2 (ARC-2) consensus document advocates a much higher threshold of cTn increase (≥70 × 99th percentile URL) to identify significant procedural myocardial injury. Similarly, the Society for Cardiovascular Angiography and Interventions (SCAI) defined ‘clinically relevant MI’ as stand-alone troponin elevations ≥70× the upper limit of normal (ULN).14 , 16 The clinical validation of these definitions is challenging given the low rate of mortality in CCS patients undergoing elective PCI. Thus, using a pooled patient-level data analysis from large registries of CCS patients with normal pre-PCI baseline cTn levels undergoing elective PCI and the recent SASSICAIA randomized trial,17 we aimed to address the following important and unresolved questions for CCS patients undergoing elective PCI: (i) What is the optimal threshold of post-PCI cTn elevation for defining prognostically important procedural myocardial injury, in terms of its association with post-PCI all-cause 1-year mortality?; (ii) What is the relationship between the size of post-PCI cTn elevation and prognosis?; (iii) Do the results of the analyses differ according to whether conventional cTn or hs-cTn is used?; and (iv) Which patient, lesion, and procedure factors independently predict the risk of experiencing procedural myocardial injury and Type 4a MI post-PCI?
Methods
Study design and population
Principal investigators of articles investigating the impact of post-PCI cTn elevation on all-cause 1-year mortality were contacted and asked to share their patient-level characteristics and outcomes. We requested the following inclusion criteria to be present in the database: (i) CCS patients admitted for elective PCI for stable obstructive coronary lesions; (ii) baseline pre-PCI cTn <1 × 99th percentile URL (normal baseline cTn patients); (iii) at least one measurement of post-PCI cTn ≤48 h after the index procedure; and (iv) principal investigators agreeing to share all case-specific data. The requested individual patient data included baseline demographics, clinical characteristics, treatments, and baseline and post-PCI cTn levels. De-identified data were transferred in electronic format to the coordinating centre (Duke-NUS Medical School, Singapore) and analysed by the ACTION Study Group at the Pitié-Salpêtrière University Hospital, Paris, France. We contacted 48 investigators who have published or presented work on this topic and obtained 12 positive responses from those willing to share their data. The final list of studies and clinical trials used in the analysis is shown in Supplementary material online, Table S1A. 5 , 6 , 18–23 For each study, extensive consistency and completeness checks were carried out, followed by preliminary analyses to ensure agreement with the main published results. In addition, the values used for the 99th percentile URL were quality assured. Discrepancies were resolved by direct contact with the principal investigators.
Baseline and data collection
We collected all patient baseline characteristics and procedural aspects including medications in each database. Evidence of procedural complications and/or new myocardial ischaemia meeting the criteria for Type 4a MI in the Fourth UDMI was collected when available. The following high-risk features for PCI (both patients or procedure-related) were also collected: glomerular filtration rate <60 mL/min, diabetes mellitus, multiple stenting/stent (two or more stents), stent length, left main stem stenting, ACC/AHA lesion classification, lesion location (ostium), bifurcation, and chronic total occlusion.
Study objectives
The study objectives were the following: (i) to determine the incidences of procedural myocardial injury and Type 4a MI in CCS patients undergoing elective PCI; (ii) to identify the optimal threshold of post-PCI cTn elevation for defining prognostically important procedural myocardial injury in terms of a significant association with 1-year all-cause mortality after adjustment for potential confounders; (iii) to perform an interval analysis of post-PCI cTn elevations in order to evaluate the independent impact of different sizes of procedural myocardial injury on 1-year all-cause mortality; (iv) to determine whether the interval analyses vary according to whether hs-cTn or conventional cTn is used; (v) to evaluate the association between Type 4a MI and 1-year all-cause mortality in CCS patients undergoing elective PCI; and (vi) to evaluate the patient, lesion and PCI procedure factors which independently predict of 1-year all-cause mortality in CCS patients undergoing elective PCI.
Cardiac biomarkers
All types of cTn assays were considered, as long as they were used with consistency at baseline and post-PCI. The URL of the test was defined as the 99th percentile value obtained in a healthy population with cardiovascular risk <10% and provided by the manufacturer for each study. Special effort was made to ensure that all included studies had baseline values of cTn <1 × 99th percentile URL using published values rather than values used in the manuscripts per se. When it was unclear if the proper 99th percentile value had been employed, the principal investigator was consulted. When multiple measurements were performed ≤48 h after the PCI, peak values were considered for the analysis (Supplementary material online, Table S1B). Demographics of patients excluded because of missing troponin data are displayed in Supplementary material online, Table S1C.
Definitions of procedural myocardial injury and Type 4a myocardial infarction
In the overall population, procedural myocardial injury was defined as stated in the Fourth UDMI (post-PCI cTn elevation >1 × 99th percentile URL)9 , 10 in CCS patients with normal baseline pre-PCI cTn values. We also evaluated the rate of ‘major’ procedural myocardial injury defined by a post-PCI increase in cTn values greater than five-fold the 99th percentile URL regardless of the presence of procedural complication. In the ARC-2 consensus document, significant procedural myocardial injury was defined as post-PCI cTn elevation ≥70 × 99th percentile URL.24 The SCAI defined ‘clinically relevant MI’ as stand-alone post-PCI cTn elevation ≥70 × 99th percentile ULN in patients with normal baseline cTn and post-PCI cTn elevation ≥35 × 99th percentile ULN plus new pathological Q waves in ≥2 contiguous leads (or new persistent left bundle branch block).12
In the subset of patients for whom evidence of ischaemia was collected, Type 4a MI was defined as stated in the Fourth UDMI as post-PCI increases in cTn values greater than five-fold the 99th percentile URL associated with one of the following criteria: (i) signs of acute myocardial ischaemia (new ischaemic electrocardiogram (ECG) changes or new pathological Q wave); (ii) imaging evidence of loss of viable myocardium that is presumed to be new and in a pattern consistent with an ischaemic aetiology; and (iii) angiographic findings consistent with procedural flow-limiting complications such as coronary dissection, occlusion of a major epicardial artery or graft, side-branch occlusion-thrombus, disruption of collateral flow, or distal coronary embolization. Procedural myocardial injury and major procedural injury were defined by a post-PCI increases in cTn values >1 × 99th percentile and greater than five-fold the 99th percentile URL, respectively, without new evidence of ischaemia.
Statistical analysis
Baseline characteristics were reported as proportions for categorical variables and medians with interquartile ranges for continuous variables. A multivariate logistic regression was performed to evaluate the association between each unit of ratio used as a threshold and 1-year mortality including the following baseline clinical and angiographic characteristics—age, diabetes, active or prior smoking, hypertension, estimated glomerular filtration rate (eGFR) <60 mL/min, prior MI, prior coronary artery bypass graft surgery, multivessel stenting, number of stents,—associated with 1-year mortality and procedural myocardial injury or Type 4a MI (univariate P < 0.2). This analysis of thresholds was then stratified by Type of cTn (hs-cTn or conventional cTn). In the second set of analysis, a similar multivariate logistic regression models were used to perform the interval analysis estimating the association between pre-defined size categories of procedural myocardial injury (≥1–5 × URL; ≥5–35 × URL; ≥35–70 × URL; ≥70 × URL) and 1-year all-cause mortality as compared to the reference category of patients without post-PCI cTn elevation (<1 × URL). Survival curves were performed with the Kaplan–Meier method to display the risk of mortality according to these size categories and compared with the log-rank test. Following the statistical princeps of biomarker evaluation,25 , 26 the receiver operating characteristic (ROC) curve analysis was evaluated to assess the performance (sensitivity, specificity) of post-PCI cTn in predicting mortality. Finally, we performed a multivariate stepwise logistic regression using two models to determine the factors associated with procedural myocardial injury and Type 4a MI. In the first model, we included baseline clinical and angiographic variables with <10% of missingness associated with procedural myocardial injury and Type 4a MI (univariate P-value <0.2). In the second model, we included all available baseline characteristics, included angiographic complexity (lesion type ACC/AHA, ostial lesion or not, bifurcation or not), irrespective of the proportion of missing data. The statistical tests were performed using GraphPad Prism version 6.00 for Windows (GraphPad Software, San Diego CA, USA). Logistic regression was performed with the R foundation for statistical computing Vienna, Austria. All tests were two-sided with a statistical threshold for significance of 0.05.
Results
Patient, lesion, and percutaneous coronary intervention procedure characteristics
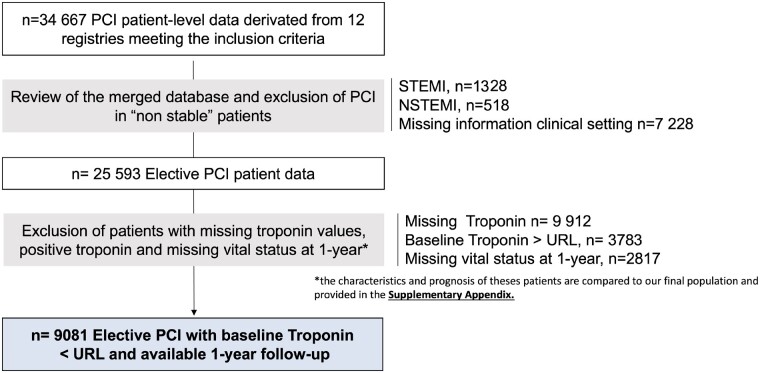
The consort diagram is presented in Figure 1. Among 34 667 patients with individual data, 25 593 patients underwent an elective PCI procedure, 9912 were excluded because they had a mildly elevated cTn, 3010 because of missing post-PCI cTn values, and 2817 because of missing 1-year status. The characteristics and outcomes of patients excluded are displayed in Supplementary material online, Table S1D and Supplementary material online, Figure S1. Finally, 9081 patients with a normal baseline cTn value, and a cTn measured within 48 h of PCI, and a 1-year follow-up were analysed. The biomarkers used in the overall population were high-sensitivity cTn in 52.0% of the patients and conventional cTn in 48.0%. cTnT was the most frequent biomarker used (59.3%) as compared with cTnI (40.7%). Among the 9081 patients with complete follow-up, 135 (1.49%) patients died within the first year of PCI. Baseline clinical and angiographic characteristics of our cohort according to the type of troponin are displayed in Tables 1 and 2.
Figure 1.
Consort diagram.
Table 1.
Baseline demographic characteristics
| Stable patients with elective PCI |
||
|---|---|---|
| Pre-PCI cTn levels | Hs-troponin | Conventional troponin |
| n (%) or median (Q1–Q3) | (n = 4719) | (n = 4362) |
| Demographics | ||
| Age (years) | 68.1 (60.3–74.1) | 66 (58.0–74.0) |
| Elderly patients (>75 years) | 997 (21.1) | 851 (19.5) |
| Men | 3635 (77.0) | 3315 (76.0) |
| Body mass index (kg/m²) | 26.8 (24.8–29.7) | 26.7 (24.5–29.4) |
| Diabetes | 1380 (29.2) | 1139 (26.1) |
| Hypertension | 3369 (71.4) | 1788 (41.0) |
| Dyslipidaemia | 3593 (76.1) | 1782 (40.9) |
| Current smoker | 831 (17.6) | 1501 (34.4) |
| Prior smoker | 1381 (29.3) | 1626 (37.3) |
| Prior stroke | 422 (8.9) | 226 (5.2) |
| Prior myocardial Infarction | 1152 (24.4) | 1665 (38.2) |
| Prior coronary artery bypass graft | 508 (10.8) | 577 (13.2) |
| Estimated glomerular filtration rate | 78.2 (60.7–99.1) | 74.6 (60.2–89.7) |
| Left ventricular ejection fraction | 60 (54–60) | 55.0 (50.0–60.0) |
| Treatment at baseline | ||
| Aspirin | 3362/4693 (71.6) | 3799/4362 (81.7) |
| Clopidogrel | 1510/4693 (32.2) | 1468/4362 (33.7) |
| Ticagrelor | 137/4693 (2.9) | 4/4362 (0.1) |
| Prasugrel | 244/4647 (5.3) | 0/4362 (0) |
| Beta-blocker | 2128/3458 (61.5) | 2995/4352 (68.8) |
| Renin angiotensin blockers | 2207/3458 (63.8) | 352/803 (43.8) |
| Statin | 2154/3458 (62.3) | 1986/2404 (81.9) |
Table 2.
Procedural characteristics
| n (%) or median (Q1–Q3) | Stable patients with elective PCI |
|
|---|---|---|
| Pre-PCI cTn levels | Hs-troponin | Conventional troponin |
| Lesions characteristics | n = 4719 | n = 4362 |
| Number of vessels treated | 1 (1–2) | 1 (1–2) |
| Multivessel stenting | 889 (18.8) | 1139 (26.1) |
| Drug eluting stent | 3249 (68.8) | 556 (12.7) |
| Number of stent total | 1 (1–2) | 1 (1–2) |
| >1 stent | 1689 (35.8) | 1431 (32.8) |
| >2 stents | 510 (10.8) | 223 (5.1) |
| Total stent length (mm) | 25 (18.0–40.0) | 31 (18–54) |
| ≥30 | 1884 (39.9) | 1632 (37.4) |
| ≥60 | 426 (9.0) | 123 (2.8) |
| Stent diameter (mm) | 3.0 (2.75–3.5) | 3.0 (3.0–3.5) |
| Left main stenting | 283 (6.0) | 430 (9.9 |
| Location | n = 3412 | n = 2048 |
| Distal | 592 (17.4) | 294 (14.4) |
| Mid-vessel | 1411 (41.4) | 451 (22.0) |
| Proximal | 581 (17.0) | 1022 (49.9) |
| Ostium | 828 (24.3) | 281 (13.7) |
| ACC/AHA lesion classification | n = 3484 | n = 2685 |
| A | 142 (4.1) | 251 (9.3) |
| B | 2653 (76.1) | 1830 (68.2) |
| C | 689 (19.8) | 604 (22.5) |
| Lesion type | n = 4719 | n = 4363 |
| Chronic total occlusion | 344 (7.3) | 213 (4.9) |
| Bifurcation | 492 (10.4) | 10 (0.2) |
| Atherectomy | 24 (0.5) | 6 (0.1) |
Incidence of procedural myocardial injury
Overall, the incidence of procedural myocardial injury as defined by the Fourth UDMI was 52.8%. This incidence varied with the type of cTn assay used, ranging from 23.8% in centres using conventional cTn assay to 79.8% in centres using the hs-cTn assay. When using the 5 × 99th percentile URL threshold, major procedural myocardial injury occurred in 18.2% of patients in our overall population (10.1% when evaluated by conventional cTn and 25.8% when evaluated by hs-cTn). The incidence of procedural myocardial injury as defined by ARC-2 was substantially lower and occurred in 1.05% of the patients with normal pre-PCI baseline cTn levels (1.08% when evaluated by conventional cTn and 1.02% when evaluated by hs-cTn).
Association between procedural myocardial injury on 1-year all-cause mortality
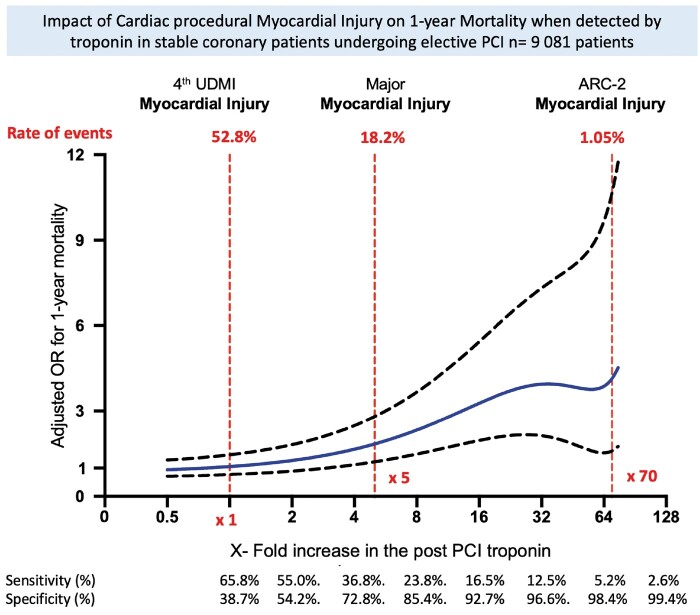
Associations between adjusted odds ratio (OR) for 1-year mortality and X-fold increase in the post-PCI cTn values in the population with baseline cTn levels are displayed in Take home figure. The association with 1-year mortality became significant beyond a three-fold elevation above the URL with a continuous increase in mortality until a 25-fold elevation with a significant and constant widening of the confidence interval (CI) as the number of events detected decreased accordingly. Procedural myocardial injury defined by the Fourth UDMI was not associated with 1-year mortality [adj OR, 1.35 95% CI (0.84–1.77), P = 0.21]. Major procedural myocardial injury defined as a post-PCI elevation in cTn ≥5 × 99th percentile URL was significantly associated with 1-year mortality with an adj OR of 2.29, 95% CI (1.32–3.97), P = 0.004. Importantly, the association of major procedural myocardial injury with 1-year mortality was similar when analysed separately for centre using conventional cTn [adj OR of 2.05, 95% CI (1.07–3.90), P = 0.02] or hs-cTn [adj OR of 2.31, 95% CI (1.27–4.20), P < 0.01]. On the other hand, the less frequent procedural myocardial injury as defined by ARC-2 was strongly associated with 1-year mortality [adj OR 4.15, 95% CI (1.62–10.64), P < 0.01]. The determination of threshold performance measured by the ROC analysis is displayed in Supplementary material online, Table S2 and the multivariate analysis of risk factors associated with 1-year mortality is presented in Table 3.
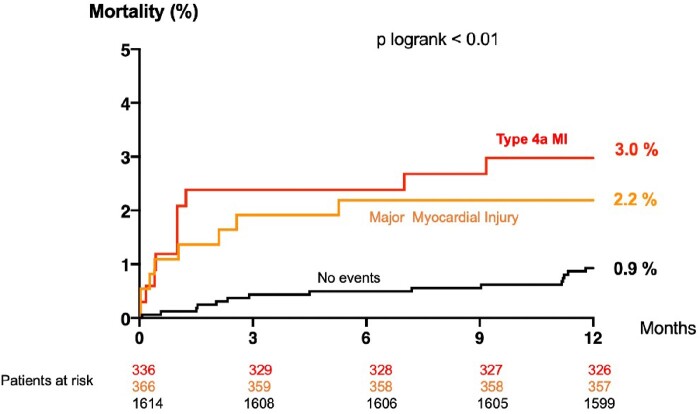
Figure 3.
All-cause mortality at 1 year according to the type of procedural event (type 4a MI according to Fourth UDMI in Red or prognostically important “Major” procedural myocardial injury in Orange) in patients with normal pre-PCI baseline cTn levels for whom evidence of new myocardial ischaemia were collected (n = 2316).
Table 3.
Multivariate analysis for the predictors of 1-year all-cause death in patients with normal pre-percutaneous coronary intervention baseline cardiac troponin levels
| Crude OR (95% CI) | Adj. OR (95% CI) | P-value | |
|---|---|---|---|
| Major procedural myocardial injury | 2.66 (1.55–4.56) | 2.29 (1.32–3.97) | 0.004* |
| Diabetes | 1.75 (1.02–3.00) | 1.80 (1.04–3.13) | 0.04* |
| Smoker | 1.03 (0.75–1.40) | 1.37 (0.97–1.94) | 0.074 |
| Age (risk per 5 years increase) | 1.44 (1.26–1.66) | 1.44 (1.23–1.68) | <0.001* |
| eGFR <60 mL/min | 2.08 (1.22–3.55) | 1.17 (0.64–2.12) | 0.61 |
| Left main or proximal left anterior descending artery | 1.02 (0.68–1.57) | 1.04 (0.69–1.62) | 0.79 |
| Prior myocardial infarction | 1.02 (0.65–1.60) | 1.15 (0.73–1.82) | 0.55 |
| Prior coronary artery bypass graft surgery | 1.36 (0.77–2.89) | 1.14 (0.53–2.46) | 0.73 |
| Women | 0.90 (0.49–1.84) | 1.11 (0.57–2.17) | 0.75 |
| Hypertension | 1.36 (0.77–2.41) | 1.14 (0.53–2.46) | 0.87 |
Variables with a univariate associated with 1-year mortality (P < 0.2) were included in the multivariate model: major procedural myocardial injury, age (per 5 years increase), diabetes, active or prior smoking, hypertension, prior MI, prior coronary artery bypass graft surgery, multivessel stenting, number of stent. Number of observations = 9004, AIC value = 576.
A significant P < 0.05.
Size categories of procedural myocardial injury and association with 1-year all-cause mortality
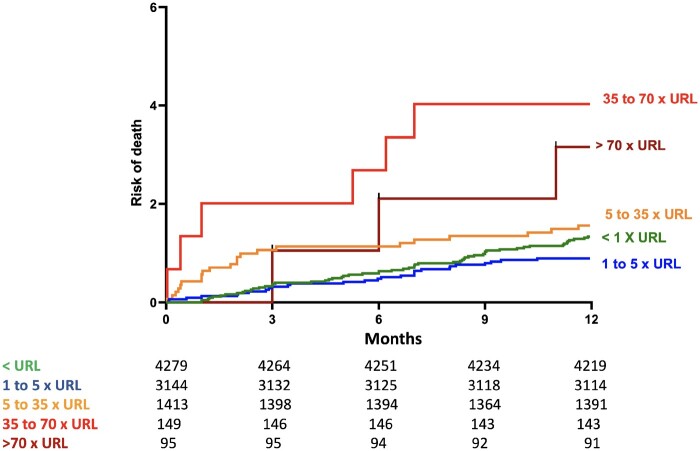
In the interval analysis, we evaluated the prognostic impact of different size categories of procedural myocardial injury according to the post-PCI cTn threshold stratified by type of cTn used (hs-cTn or conventional cTn) after adjustment for cofounders. The results of the unadjusted and adjusted OR for the association with 1-year mortality analysis are provided in Table 4, and the results of the Kaplan–Meier curves showing the survival of the population are displayed in Figure 2. We found that when using conventional cTn, only large procedural myocardial injury (≥70 × 99th percentile URL compared with <URL) was associated with increased risk of 1-year mortality [adj OR 5.97 95% CI (1.65–21.59), P = 0.002]. When using hs-cTn, a lower elevation of post-PCI cTn (≥5 to 35 × 99th percentile URL), which occurred in 22.78% of the patients, was associated with 1-year mortality [adj OR 3.99, 95% CI (1.12–14.25), P = 0.016]. The analysis survival stratified by the type of troponin and size categories of procedural myocardial injury is displayed in Supplementary material online, Figure S2.
Table 4.
Multivariate analysis for the association of categories of procedural myocardial injury as compared to the reference group separated by type of troponin
| Conventional troponin n = 4362 patients | Association with 1-year mortality |
|||||
|---|---|---|---|---|---|---|
| Size of periprocedural myocardial injury | % of population | No. death | Crude OR (95% CI) | P-value | Adj. OR (95% CI) | P-value |
| ≥70 URL | 1.08 | 3 | 6.07 (1.73–21.26) | 0.006 | 5.97 (1.65–21.59) | 0.023* |
| ≥35–70 × URL | 1.28 | 2 | 3.30 (0.75–14.51) | 0.13 | 3.14 (0.7–14.11) | 0.19 |
| ≥5–35 × URL | 7.84 | 6 | 1.62 (0.63–3.56) | 0.28 | 1.66 (0.65–4.25) | 0.31 |
| ≥1–5 × URL | 13.60 | 11 | 1.54 (0.71–3.32) | 0.26 | 1.57 (0.70–3.44) | 0.27 |
| Reference <1 × URL | 76.20 | 71 | — | — | — | — |
| Total | 100.00 | 93 | — | — | — | — |
| High-sensitivity troponin n = 4719 | Association with 1-year mortality | |||||
| Size of periprocedural myocardial injury | % of population | No. death | Crude OR | P-value | Adjusted OR | P-value |
| ≥70 URL | 1.02 | 1 | 6.75 (0.69–66.14) | 0.18 | 4.97 (0.46–53.18) | 0.28 |
| ≥35–70 × URL | 1.97 | 4 | 10.26 (3.14–54.73) | 0.005* | 9.38 (1.95–45.13) | 0.007* |
| ≥5–35 × URL | 22.78 | 16 | 4.79 (1.39–16.5) | 0.033* | 3.99 (1.12–14.25) | 0.016* |
| ≥1–5 × URL | 53.97 | 19 | 2.39 (0.70–8.08) | 0.25 | 2.06 (0.60–7.10) | 0.21 |
| Reference <1 × URL | 20.26 | 3 | — | — | — | — |
| Total | 100.00 | 43 | — | — | — | — |
Take home figure .
Adjusted odds ratio (OR) of mortality at 1 year according to post-PCI cTn level / URL ratio in patients withnormal pre-PCI baseline cTn levels. The solid blue line represents the adjusted odds ratio and the dotted lines represent the lower andupper 95% confidence intervals.
Incidence of Type 4a myocardial infarction and association with 1-year all-cause mortality
The number of patients having baseline normal cTn levels in whom evidence of procedural complication and/or new myocardial ischaemia was collected was limited (n = 2316). The incidence of Type 4a MI was 12.7% (n = 294) as determined by a post-PCI cTn elevation ≥5 × 99th percentile URL, and an additional criteria of procedural complication and/or evidence of myocardial ischaemia (Figure 3). Of these 294 patients with Type 4a MI, 250 (85.0%) had at least new ischaemic ECG changes or Q waves, 177 (60.0%) patients had angiographic evidence of coronary obstruction, and 12 (4.1%) had imaging evidence of loss of viable myocardium. Type 4a MI was independently associated with 1-year mortality in the multivariate analysis performed in this patient subset [adj OR 3.21, 95% CI (1.42–7.27), P = 0.005] (Table 5). Interestingly, we found that 70% of patients with a large procedural myocardial injury (defined as post-PCI cTn >35 × URL) were in fact a large Type 4aMI, with at least one reported angiographic complication or new ischaemic ECG changes.
Figure 2.
One-year mortality in chronic coronary syndrome patients with normal baseline cardiac troponin and post-percutaneous coronary intervention cardiac troponin elevations divided into five categories (unadjusted comparison).
Table 5.
Multivariate analysis for the predictors of 1-year all-cause death in the subgroup of patients where Type 4a myocardial infarction was classified
| Crude OR (95% CI) | Adj. OR (95% CI) | P-value | |
|---|---|---|---|
| Type 4a MI | 2.70 (1.22–5.98) | 3.21 (1.42–7.27) | 0.005* |
| Smoker | 1.29 (0.85–1.97) | 1.69 (1.09–2.63) | 0.019* |
| Diabetes | 1.31 (0.65–2.65) | 1.48 (0.72–3.04) | 0.006* |
| Age (risk per 5 years increase) | 1.34 (1.11–1.62) | 1.33 (1.07–1.65) | < 0.001* |
| eGFR <60 mL/min | 2.02 (1.4–2.07) | 1.46 (0.67–3.17) | 0.32 |
| Women | 1.08 (0.44–2.68) | 1.62 (0.63–4.18) | 0.63 |
| Prior myocardial infarction | 1.02 (0.65–1.60) | 1.15 (0.73–1.82) | 0.55 |
| Left main or proximal left anterior descending artery | 1.04 (0.52–2.12) | 1.02 (0.50–2.08) | 0.95 |
| Hypertension | 1.09 (0.52–2.3) | 0.93 (0.42–2.1) | 0.86 |
| Prior coronary artery bypass graft | 0.66 (0.16–2.82) | 0.54 (0.12–2.33) | 0.36 |
Variables with a univariate associated with 1-year mortality (P < 0.2) were included in the multivariate model: major procedural myocardial injury, age (per 5 years increase), diabetes, active or prior smoking, hypertension, prior MI, prior coronary artery bypass graft surgery, multivessel stenting, number of stent. Number of observations = 2221, AIC value = 305.
Independent predictors of procedural myocardial injury and Type 4a myocardial infarction
Risk factors for developing procedural myocardial injury (as defined by Fourth UDMI) and prognostically important procedural myocardial injury (as defined above) were identified in patients with normal cTn levels and are shown in Tables 6 and 7. Impaired kidney function, left main stem disease or left anterior descending artery PCI, and stent length and male sex were shown to be independent predictors of procedural myocardial injury. Interestingly, prior MI, patients treated for hypertension and women were less likely to develop such complications. Predictors of Type 4a MI could only be evaluated in the subgroup of patients with evidence of new myocardial ischaemia, and these were similar to the risk factors for procedural myocardial injury.
Table 6.
Multivariate analysis for predictors of procedural myocardial injury in patients with normal pre-PCI baseline cTn levels
| Normal baseline cTn population | Crude OR | Adjusted OR | P |
|---|---|---|---|
| Glomerular filtration rate <60 mL/min | 2.52 (2.09–3.03) | 2.5 (2.04–3.07) | <0.001* |
| Left main or proximal left anterior descending artery | 1.69 (1.43–1.99) | 1.48 (1.25–1.76) | <0.001* |
| Diabetes | 1.22 (1.02–1.45) | 1.29 (1.07–1.55) | 0.008* |
| Stent length (per 10 mm increase) | 1.21 (1.18–1.24) | 1.23 (1.21–1.25) | <0.001* |
| Age (risk per 5 years increase) | 1.03 (1.00–1.07) | 1.00 (0.99–1.02) | 0.701 |
| Stent diameter (risk per 0.25 mm increase) | 1.00 (1.00–1.03) | 1.00 (0.98–1.02) | 0.65 |
| Smoker | 1.00 (0.85–1.17) | 0.99 (0.83–1.18) | 0.89 |
| Prior coronary artery bypass graft | 0.77 (0.6–1) | 0.9 (0.68–1.18) | 0.43 |
| Women (vs. men) | 0.81 (0.67–0.99) | 0.72 (0.58–0.89) | 0.003* |
| Hypertension | 0.7 (0.59–0.84) | 0.64 (0.53–0.78) | <0.001* |
| Prior myocardial infarction | 0.48 (0.38–0.59) | 0.53 (0.42–0.65) | <0.001* |
Variables with a univariate associated with 1-year mortality (P < 0.2) were included in the multivariate model: major procedural myocardial injury, age (per 5 years increase), diabetes, active or prior smoking, hypertension, prior MI, prior coronary artery bypass graft surgery, multivessel stenting, number of stent. Number of observations = 8924, AIC value = 4641.
A significant P < 005.
Table 7.
Multivariate analysis for predictors of ‘Major’ procedural myocardial injury in patients with normal pre-PCI baseline cTn levels
| Normal baseline cTn population | Crude OR | Adjusted OR | P |
|---|---|---|---|
| Left main or proximal left anterior descending artery | 1.49 (1.12–1.17) | 1.19 (1.07–1.33) | 0.002* |
| Stent length (per 10 mm increase) | 1.14 (1.12–1.17) | 1.13 (1.10–1.16) | <0.001* |
| Age (risk per 5 years increase) | 1.08 (1.05–1.11) | 1.07 (1.04–1.1) | <0.001* |
| Stent diameter (risk per 0.25 mm increase) | 1.04 (1.03–1.05) | 1.02 (1.1–1.03) | 0.029* |
| Prior coronary artery bypass graft | 1.02 (0.86–1.21) | 1.00 (0.84–1.19) | 0.99 |
| Hypertension | 1.11 (0.99–1.20) | 1.03 (0.89–1.13) | 0.96 |
| Women (vs. men) | 1.02 (0.91–17) | 0.97 (0.85–1.11) | 0.65 |
| Smoker | 0.85 (0.76–0.94) | 0.94 (0.84–1.05) | 0.27 |
| Prior myocardial infarction | 0.81 (0.72–0.92) | 0.91 (0.8–1.03) | 0.12 |
| Diabetes | 0.89 (0.78–1.00) | 0.86 (0.76–0.98) | 0.02* |
Variables associated with 1-year mortality (univariate P-value <0.2) were included in the multivariate model: major procedural myocardial injury, age (per 5 years increase), diabetes, active or prior smoking, hypertension, prior MI, prior coronary artery bypass graft surgery, multivessel stenting, number of stent. Number of observations = 8924, AIC value = 6450.
A significant P < 005.
Discussion
The prognostic significance of procedural myocardial injury, as defined by the Fourth UDMI is unclear, with some studies showing an association between post-PCI cTn elevations (>1 × 99th percentile URL) and MACE or long-term mortality,4 , 6 , 7 whereas others do not.5 , 11 Some of this discordance may be due to many studies ignoring the need to only include patients with normal pre-PCI baseline cTn levels, others using inappropriately high values for the 99th percentile URL,27 and some discrepancies arising from using either conventional cTn or hs-cTn assays. The present study was designed to provide evidence regarding the optimal threshold of post-PCI cTn elevation for predicting 1-year all-cause mortality in CCS patients undergoing elective PCI and assess the association between the size of procedural myocardial injury and 1-year mortality. Both analyses were performed overall and separately using either conventional cTn or hs-cTn assays.
The results of this pooled analysis of patients with individual data can be summarized as follows: (i) the Fourth UDMI definition of procedural myocardial injury (>1 × 99th percentile URL) is very sensitive but not specific, as half of CCS patients met the diagnostic criteria after PCI, an incidence that was increased to almost 80% with hs-cTn and was not associated with 1-year all-cause mortality in our study; (ii) elevation of post-PCI cTn levels beyond three-fold the baseline level was independently associated with 1-year all-cause mortality with a continual increase in specificity, although this was also associated with a marked decrease in sensitivity and the proportion of the events detected; (iii) major myocardial injury defined with the same post-PCI cTn threshold elevation as for Type 4a MI (≥5 × 99th percentile URL), but without the additional evidence of new myocardial ischaemia, occurred in 18.2% of patients, and was independently associated with a two-fold increase in 1-year all-cause mortality, regardless of the type of troponin used (conventional cTn or hs-cTn); (iv) both larger and smaller extents of procedural myocardial injury were associated with an increased risk of mortality, although the former had a more pronounced prognostic impact as they were often underdiagnosed Type 4a MIs; (v) Type 4a MI, which is vastly under-reported in clinical studies as it combines cardiac biomarkers and ECG, clinical or angiographic criteria, occurred in 12.7% of patients in our study and was a strongly associated with 1-year all-cause mortality; (vi) the utilization of a hs-cTn assay increases by three-fold the incidence of procedural myocardial injury with definitions based on a lower threshold using conventional cTn (UDMI), whereas it remained unchanged with definitions using a higher threshold (ARC-2/SCAI) and our analysis suggests to use different thresholds of post-PCI cTn values according to the type of assay used (35-fold increase for conventional cTn and a five-fold increase for hs-cTn) instead of these definitions; and (vii) finally, we identified independent predictors of both procedural myocardial injury and Type 4a MI, as well as 1-year all-cause mortality, which can be considered to be risk factors in contemporary elective PCI.
The definition of procedural myocardial injury has been an ongoing subject of controversy in terms of its clinical relevance for the detection of PCI-related complications and their management, but also for trials evaluating revascularization strategies in CCS patients.28 CK-MB assays are no longer available in many centres and cTn assays, especially high-sensitivity assays, have progressively become the preferred cardiac biomarker given their superior sensitivity for the detection of procedural myocardial injury due to their higher range and discrimination properties. However, evidence linking post-PCI cTn elevations to patient prognosis using the Fourth UDMI is still lacking.
Our findings demonstrate that the 1 × 99th percentile URL threshold selected for defining procedural myocardial injury by the Fourth UDMI is too sensitive and is not associated with 1-year all-cause mortality after adjustment for confounding factors. On the contrary, a much higher threshold such as the one defined by the ARC-2 or SCAI initiative (≥70-fold)24 increased the specificity and selection of patients with the greatest extent of myocardium loss and risk of death, but this was associated with a significant reduction in sensitivity, thereby missing the vast majority of events in the population with normal baseline troponin.
Major procedural myocardial injury, defined by a post-PCI cTn elevations ≥5 × 99th percentile URL, regardless of the presence of angiographic complications and/or evidence of new myocardial ischaemia (ECG, angiographic, imaging) was associated with a significant two-fold increase in the risk of all-cause mortality. This was the case whether hs-cTn or conventional cTn was used. Crucially, the prognostic importance of the post-PCI cTn threshold elevation of ≥5 × 99th percentile URL was supported by the interval analysis demonstrating that even intermediate sized procedural myocardial injury (≥5× and <35 × 99th percentile URL) was associated with a 3.3× increase in 1-year all-cause mortality. However, for conventional cTn, a larger amount of procedural myocardial injury (≥35 × 99th percentile URL) was needed to show an independent association with an increase in 1-year all-cause mortality. The differences in thresholds between conventional cTn and hs-cTn likely relate to the differing risk profiles of the patient cohorts, with the group of patients with normal baseline hs-cTn likely to be of lower risk compared to the patient group with normal baseline conventional cTn due to the better prognostic performance of hs-cTn over conventional cTn at the lower values.
The present study also demonstrated that the additional angiographic or clinical criteria used to define Type 4a MI in the Fourth UDMI are unfortunately under-reported in PCI registries and that a threshold based solely on the post-PCI biomarker elevation appears to be an alternative to Type 4a MI in cases of the absence of adjudication or collection of evidence of new myocardial ischaemia on the ECG or the angiography. Of note, angiographic evidence of myocardial ischaemia was present in 90% of patients of our cohort with large periprocedural MIs (≥35 × 99th percentile URL).
Our study also demonstrates that this 5× threshold is meaningful when used in combination with a hs-cTn assay, which may serve as a warning flag to consider periprocedural angiographic defects not captured during the procedure. In addition, this threshold may be used to differentiate patients at low risk from higher-risk patients and enable to implement more intensive secondary prevention therapy or to guide early discharge in the context of ambulatory care. Of note, the incidence of Type 4a MI observed in our subgroup analysis (12.7%) was on the higher side when compared to previous studies including the SACCICAIA trial (3%), the cohort of Zeitouni et al.6 (7%), the study by Yang et al.11 (10.3%), and further large studies are needed to confirm the incidence of Type 4a MI in CCS patients undergoing PCI, especially with high-sensitivity assays.
It remains unclear whether mortality and hard clinical events following a procedural MI are a consequence of risk factors related to the complexity of the procedure and/or the patient’s vulnerability or a result from the extent of cardiac injury. Our results show higher adjusted ORs for 1-year mortality with a higher post-PCI cTn threshold, confirming that large extents of procedural myocardial injury have a stronger prognostic impact when compared to smaller extents of procedural myocardial injury. In 90% of patients with large procedural myocardial injury may, a loss of branch or distal embolization was reported by the investigators—displaying an actual MI rather than a biomarker reflecting an injury. Importantly, our analysis demonstrates for that smaller extents of procedural myocardial injury (post-PCI cTn elevations above the five-fold threshold and below the 35-fold threshold) are also prognostically significant as they are associated with a 2.5-fold increased risk of mortality when detected with hs-cTn. However, for conventional cTn, only a larger extent of cTn elevation (>35-fold threshold) was prognostically significant. Therefore, as expected, in CCS patients with normal baseline cTn levels, the threshold of post-PCI elevation that predicts clinical outcomes varies according to the type of cTn.
Although unmeasured risk factors such as atheroma burden, plaque composition, and blood vulnerability were not measured in our study, we found that both procedural- and patient-related high-risk features were risk factors for procedural cardiac injury and 1-year mortality. Recent ESC guidelines have proposed the use of more potent P2Y12 inhibitors such as ticagrelor or prasugrel in patients perceived to be at high ischaemic risk or requiring complex elective PCI procedures.29 The randomized control SASSICAIA trial comparing a pre-PCI loading dose of prasugrel to clopidogrel was prematurely stopped due to enrolment issues and found a non-significant 10% relative decrease in the rate of procedural events with prasugrel compared to clopidogrel (NCT0254861). Further studies on preventive strategies in patients with high-risk features are needed, and the ongoing randomized ALPHEUS trial (Assessment of Loading with the P2Y12 inhibitor Ticagrelor or clopidogrel to Halt ischaemic Events in patients Undergoing elective coronary Stenting, NCT02617290) should provide more data in this regard.30
Our analysis is not without its limitations. First, a large portion of patients were excluded from the database, because of missing PCI troponin measurements, potentially adding a degree of ascertainment bias although their baseline characteristics were similar (Supplementary material online, Table S1C and D). Missing data also involved characteristics associated with periprocedural MI, such as use of atherectomy, lesions calcifications, or left ventricular ejection fraction. Second, the mortality rate was low, but our large population carries enough power to identify the threshold associated with a two- to four-fold increased risk of mortality at 1 year after adjustment for confounders. Third, while we chose a homogenous set of low-risk patients admitted for elective PCI, there was some variability in the characteristics collected especially in the criteria of Type 4a MI, which were only reported in a minority of studies6 , 20 , 21 and the SASSICAIA trial—leading to potential variability in the proportion of Type 4a MI. Finally, CK-MB levels were not collected and serial measurement of cTn was not systematically performed, but this also reflects the current standard practice in elective PCI.
Conclusions
Procedural myocardial injury defined by the Fourth UDMI occurs in nearly 50% (using conventional cTn assays) and in nearly 80% (using hs-cTn assays) of CCS patients undergoing elective PCI with normal baseline cTn values and was not associated with an increase in 1-year all-cause mortality. Our participant-level pooled analysis has shown that ‘major’ procedural myocardial injury defined as a post-PCI elevation in cTn (either hs-cTn or conventional cTn) ≥5 × 99th percentile elevation was independently associated with a higher risk of 1-year all-cause mortality. This threshold was supported for hs-cTn, where the interval analysis demonstrated that a post-PCI elevation in cTn (≥5 and <35 × 99th percentile URL) was independently associated with an increased risk of 1-year all-cause mortality. However, conventional cTn was less discriminatory, as only a higher post-PCI cTn elevation (≥70 × 99th percentile URL) was significantly associated with 1-year all-cause mortality. Both major procedural myocardial injury and Type 4a MI are independent predictors of 1-year mortality after PCI and have the potential to serve as quality metrics in clinical practice and endpoints in clinical trials evaluating preventive pharmacological treatment or procedural strategies.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
ACTION Coeur research group (www.action-coeur.org) (to J.S.); British Heart Foundation (CS/14/3/31002 to D.J.H.); the National Institute for Health Research University College London Hospitals Biomedical Research Centre, Duke-National University Singapore Medical School, Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist-Senior Investigator scheme (NMRC/CSA-SI/0011/2017 to D.J.H.); and Collaborative Centre Grant scheme (NMRC/CGAug16C006 to D.J.H.). This article is based upon work from COST Action EU-CARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology). SASSICAIA trial was sponsored by Cardiology Department, Munich University Centre, Ludwig-Maximilians University, Munich, Germany
Conflict of interest: J.S. reports during the past 2 years the following disclosures: consulting fees or lecture fees or travel support from AstraZeneca, Bayer HealthCare SAS, Boehringer Ingelheim France, CSL Behring SA, Gilead Science, Sanofi-Aventis France, Terumo France SAS, Abbott Medical France SAS, and Stockholder of Pharmaseeds. G.M. reports consulting or speaker fees from Abbott, AIM group, Amgen, Actelion, American College of Cardiology Foundation, Astrazeneca, Axis-Santé, Bayer, Boston Scientific, Bristol-Myers Squibb, Beth Israel Deaconess Medical, Brigham Women’s Hospital, Fréquence Médicale, ICOM, Idorsia, Elsevier, Fédération Française de Cardiologie, Fréquence Médicale, ICAN, Lead-Up, Menarini, Medtronic, MSD, Novo-Nordisk, Pfizer, Quantum Genomics, Sanofi-Aventis, SCOR global life, Servier, and WebMD. J.M. receives institutional grants from Boston Scientific and lecture fees from Astra Zeneca, Bristol-Myers Squibb, Boston Scientific, and Edwards Lifescience. M.Z. receives research grants from Servier and Fédération Francaise de Cardiologie and BMS/Pfizer. Other authors have no disclosures to report.
Supplementary Material
Contributor Information
Johanne Silvain, Sorbonne Université, ACTION Study Group, Institut de Cardiologie, Hôpital Pitié-Salpêtrière (AP-HP), INSERM UMRS 1166, 47-83 bld de l’Hôpital, 75013 Paris, France.
Michel Zeitouni, Sorbonne Université, ACTION Study Group, Institut de Cardiologie, Hôpital Pitié-Salpêtrière (AP-HP), INSERM UMRS 1166, 47-83 bld de l’Hôpital, 75013 Paris, France.
Valeria Paradies, Cardiology Department, Maasstad Hospital, Rotterdam, Netherlands.
Huili L Zheng, Health Promotion Board, National Registry of Diseases Office, Singapore, Singapore.
Gjin Ndrepepa, Department of Adult Cardiology, Deutsches Herzzentrum München, Technische Universität, Munich, Germany.
Claudio Cavallini, Division of Cardiology, Ospedale S Maria della Misericordia, Piazzale Meneghini 1, Perugia 06100, Italy.
Dimitri N Feldman, Division of Cardiology, Weill Cornell Medical College, New York, NY, USA.
Samin K Sharma, Cardiac Catheterization Laboratory, Cardiovascular Institute, Mount Sinai Hospital, New York, NY, USA.
Julinda Mehilli, Munich University Clinic, Ludwig-Maximilians University, Munich, Germany; German Centre for Cardiovascular Research (DZHK), Partner Site Munich Heart Alliance, Munich, Germany.
Sebastiano Gili, Centro Cardiologico Monzino, IRCCS, Milan, Italy.
Emanuele Barbato, Department of Advanced Biomedical Sciences, University of Naples Federico II, Napoli, Italy.
Giuseppe Tarantini, Department of Cardiac, Thoracic and Vascular Sciences, University of Padua Medical School, Padua, Italy.
Sze Y Ooi, Eastern Heart Clinic, Prince of Wales Hospital, Sydney, NSW, Australia.
Clemens von Birgelen, Department of Cardiology, Thoraxcentrum Twente, Medisch Spectrum Twente, Enschede, Netherlands; Department of Health Technology and Services Research, Faculty of Behavioural, Management and Social Sciences, Technical Medical Centre, University of Twente, Enschede, Netherlands.
Allan S Jaffe, Department of Cardiology, Mayo Clinic, Rochester, MN, USA; Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN, USA.
Kristian Thygesen, Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark.
Gilles Montalescot, Sorbonne Université, ACTION Study Group, Institut de Cardiologie, Hôpital Pitié-Salpêtrière (AP-HP), INSERM UMRS 1166, 47-83 bld de l’Hôpital, 75013 Paris, France.
Heerajnarain Bulluck, Department of Cardiology, Norfolk and Norwich University Hospital, Norwich, UK.
Derek J Hausenloy, The Hatter Cardiovascular Institute, University College London, London, UK; Cardiovascular and Metabolic Disorders Program, Duke-National University of Singapore, Singapore, Singapore; National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore, Singapore; Cardiovascular Research Center, College of Medical and Health Sciences, Asia University, Taichung City, Taiwan; Yong Loo Lin School of Medicine, National University Singapore, Singapore, Singapore.
References
- 1. Giacoppo D, Colleran R, Cassese S, Frangieh AH, Wiebe J, Joner M, Schunkert H, Kastrati A, Byrne RA. Percutaneous coronary intervention vs coronary artery bypass grafting in patients with left main coronary artery stenosis: a systematic review and meta-analysis. JAMA Cardiol 2017;2:1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Valgimigli M, Frigoli E, Leonardi S, Vranckx P, Rothenbühler M, Tebaldi M, Varbella F, Calabrò P, Garducci S, Rubartelli P, Briguori C, Andó G, Ferrario M, Limbruno U, Garbo R, Sganzerla P, Russo F, Nazzaro M, Lupi A, Cortese B, Ausiello A, Ierna S, Esposito G, Ferrante G, Santarelli A, Sardella G, de Cesare N, Tosi P, van 't Hof A, Omerovic E, Brugaletta S, Windecker S, Heg D, Jüni P, Campo G, Uguccioni L, Tamburino C, Presbitero P, Zavalloni-Parenti D, Ferrari F, Ceravolo R, Tarantino F, Pasquetto G, Casu G, Mameli S, Stochino ML, Mazzarotto P, Cremonesi A, Saia F, Saccone G, Abate F, Picchi A, Violini R, Colangelo S, Boccuzzi G, Guiducci V, Vigna C, Zingarelli A, Gagnor A, Zaro T, Tresoldi S, Vandoni P, Contarini M, Liso A, Dellavalle A, Curello S, Mangiacapra F, Evola R, Palmieri C, Falcone C, Liistro F, Creaco M, Colombo A, Chieffo A, Perkan A, De Servi S, Fischetti D, Rigattieri S, Sciahbasi A, Pucci E, Romagnoli E, Moretti C, Moretti L, De Caterina R, Caputo M, Zimmarino M, Bramucci E, Di Lorenzo E, Turturo M, Bonmassari R, Penzo C, Loi B, Mauro C, Petronio AS, Gabrielli G, Micari A, Belloni F, Amico F, Comeglio M, Fresco C, Klinieken I, Van Mieghem N, Diletti R, Regar E, Sabaté M, Gómez Hospital JA, Díaz Fernández JF, Mainar V, de la Torre Hernandez JM. Radial versus femoral access and bivalirudin versus unfractionated heparin in invasively managed patients with acute coronary syndrome (MATRIX): final 1-year results of a multicentre, randomised controlled trial. Lancet 2018;392:835–848. [DOI] [PubMed] [Google Scholar]
- 3. Stone GW, Sabik JF, Serruys PW, Simonton CA, Généreux P, Puskas J, Kandzari DE, Morice M-C, Lembo N, Brown WM, Taggart DP, Banning A, Merkely B, Horkay F, Boonstra PW, Boven A. V, Ungi I, Bogáts G, Mansour S, Noiseux N, Sabaté M, Pomar J, Hickey M, Gershlick A, Buszman P, Bochenek A, Schampaert E, Pagé P, Dressler O, Kosmidou I, Mehran R, Pocock SJ, Kappetein P. Everolimus-eluting stents or bypass surgery for left main coronary artery disease. N Engl J Med 2016;375:2223–2235. [DOI] [PubMed] [Google Scholar]
- 4. Loeb HS, Liu JC. Frequency, risk factors, and effect on long-term survival of increased troponin I following uncomplicated elective percutaneous coronary intervention. Clin Cardiol 2010;33:E40–E44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ndrepepa G, Colleran R, Braun S, Cassese S, Hieber J, Fusaro M, Kufner S, Ott I, Byrne RA, Husser O, Hengstenberg C, Laugwitz K-L, Schunkert H, Kastrati A. High-sensitivity troponin t and mortality after elective percutaneous coronary intervention. J Am Coll Cardiol 2016;68:2259–2268. [DOI] [PubMed] [Google Scholar]
- 6. Zeitouni M, Silvain J, Guedeney P, Kerneis M, Yan Y, Overtchouk P, Barthelemy O, Hauguel-Moreau M, Choussat R, Helft G, Le Feuvre C, Collet J-P, Montalescot G. Periprocedural myocardial infarction and injury in elective coronary stenting. Eur Heart J 2018;39:1100–1109. [DOI] [PubMed] [Google Scholar]
- 7. Garcia-Garcia HM, McFadden EP, Birgelen C. V, Rademaker-Havinga T, Spitzer E, Kleiman NS, Cohen DJ, Kennedy KF, Camenzind E, Mauri L, Steg PG, Wijns W, Silber S, Es G-A. V, Serruys PW, Windecker S, Cutlip D, Vranckx P. Impact of periprocedural myocardial biomarker elevation on mortality following elective percutaneous coronary intervention. JACC Cardiovasc Interv 2019;12:1954–1962. [DOI] [PubMed] [Google Scholar]
- 8. Stone GW. Periprocedural myocardial infarction. JACC Cardiovasc Interv 2016;9:2229–2231. [DOI] [PubMed] [Google Scholar]
- 9. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Thygesen K, Alpert JS, White HD, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Chaitman BR, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Gabriel Steg P, Wijns W, Bassand J-P, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon J-L, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Ž, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Morais J, Aguiar C, Almahmeed W, Arnar DO, Barili F, Bloch KD, Bolger AF, Bøtker HE, Bozkurt B, Bugiardini R, Cannon C, de Lemos J, Eberli FR, Escobar E, Hlatky M, James S, Kern KB, Moliterno DJ, Mueller C, Neskovic AN, Pieske BM, Schulman SP, Storey RF, Taubert KA, Vranckx P, Wagner DR. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551–2567. [DOI] [PubMed] [Google Scholar]
- 10. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, Mickley H, Crea F, Van de Werf F, Bucciarelli-Ducci C, Katus HA, Pinto FJ, Antman EM, Hamm CW, De Caterina R, Januzzi JL, Apple FS, Alonso Garcia MA, Underwood SR, Canty JM, Lyon AR, Devereaux PJ, Zamorano JL, Lindahl B, Weintraub WS, Newby LK, Virmani R, Vranckx P, Cutlip D, Gibbons RJ, Smith SC, Atar D, Luepker RV, Robertson RM, Bonow RO, Steg PG, O’Gara PT, Fox KAA, Hasdai D, Aboyans V, Achenbach S, Agewall S, Alexander T, Avezum A, Barbato E, Bassand J-P, Bates E, Bittl JA, Breithardt G, Bueno H, Bugiardini R, Cohen MG, Dangas G, de Lemos JA, Delgado V, Filippatos G, Fry E, Granger CB, Halvorsen S, Hlatky MA, Ibanez B, James S, Kastrati A, Leclercq C, Mahaffey KW, Mehta L, Müller C, Patrono C, Piepoli MF, Piñeiro D, Roffi M, Rubboli A, Sharma S, Simpson IA, Tendera M, Valgimigli M, van der Wal AC, Windecker S, Chettibi M, Hayrapetyan H, Roithinger FX, Aliyev F, Sujayeva V, Claeys MJ, Smajić E, Kala P, Iversen KK, El Hefny E, Marandi T, Porela P, Antov S, Gilard M, Blankenberg S, Davlouros P, Gudnason T, Alcalai R, Colivicchi F, Elezi S, Baitova G, Zakke I, Gustiene O, Beissel J, Dingli P, Grosu A, Damman P, Juliebø V, Legutko J, Morais J, Tatu-Chitoiu G, Yakovlev A, Zavatta M, Nedeljkovic M, Radsel P, Sionis A, Jemberg T, Müller C, Abid L, Abaci A, Parkhomenko A, Corbett S; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2019;40:237–269.30165617 [Google Scholar]
- 11. Yang X, Tamez H, Lai C, Ho K, Cutlip D. Type 4a myocardial infarction: incidence, risk factors, and long-term outcomes. Catheter Cardiovasc Interv 2017;89:849–856. [DOI] [PubMed] [Google Scholar]
- 12. Bangalore S, Pencina MJ, Kleiman NS, Cohen DJ. Prognostic implications of procedural vs spontaneous myocardial infarction: results from the Evaluation of Drug Eluting Stents and Ischemic Events (EVENT) registry. Am Heart J 2013;166:1027–1034. [DOI] [PubMed] [Google Scholar]
- 13. Lim CCS, Gaal W. V, Testa L, Cuculi F, Arnold JR, Karamitsos T, Francis JM, Petersen SE, Digby JE, Westaby S, Antoniades C, Kharbanda RK, Burrell LM, Neubauer S, Banning AP. With the ‘universal definition,’ measurement of creatine kinase-myocardial band rather than troponin allows more accurate diagnosis of periprocedural necrosis and infarction after coronary intervention. J Am Coll Cardiol 2011;57:653–661. [DOI] [PubMed] [Google Scholar]
- 14. Selvanayagam JB, Porto I, Channon K, Petersen SE, Francis JM, Neubauer S, Banning AP. Troponin elevation after percutaneous coronary intervention directly represents the extent of irreversible myocardial injury: insights from cardiovascular magnetic resonance imaging. Circulation 2005;111:1027–1032. [DOI] [PubMed] [Google Scholar]
- 15. Rahimi K, Banning AP, Cheng ASH, Pegg TJ, Karamitsos TD, Channon KM, Darby S, Taggart DP, Neubauer S, Selvanayagam JB. Prognostic value of coronary revascularisation-related myocardial injury: a cardiac magnetic resonance imaging study. Heart 2009;95:1937–1943. [DOI] [PubMed] [Google Scholar]
- 16. Moussa ID, Klein LW, Shah B, Mehran R, Mack MJ, Brilakis ES, Reilly JP, Zoghbi G, Holper E, Stone GW. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol 2013;62:1563–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Julinda M, Moritz B, Willibald H, Katharina M, Christian T, Daniel A, Yujun X, Manuela T, Sarah G, Magda Z, David J, Dirk S, Stefanie S, Ulrich M, Ellen H, Adnan K, Franz-Josef N, Steffen M. Randomized comparison of intensified and standard P2Y12-receptor-inhibition before elective percutaneous coronary intervention. Circ Cardiovasc Interv 2020;13:e008649. [DOI] [PubMed] [Google Scholar]
- 18. Kini AS, Lee P, Marmur JD, Agarwal A, Duffy ME, Kim MC, Sharma SK. Correlation of postpercutaneous coronary intervention creatine kinase-MB and troponin I elevation in predicting mid-term mortality. Am J Cardiol 2004;93:18–23. [DOI] [PubMed] [Google Scholar]
- 19. Feldman DN, Kim L, Rene AG, Minutello RM, Bergman G, Wong SC. Prognostic value of cardiac troponin-I or troponin-T elevation following nonemergent percutaneous coronary intervention: a meta-analysis. Catheter Cardiovasc Interv 2011;77:1020–1030. [DOI] [PubMed] [Google Scholar]
- 20. Cavallini C, Verdecchia P, Savonitto S, Arraiz G, Violini R, Olivari Z, Rubartelli P, De Servi S, Plebani M, Steffenino G, Sbarzaglia P, Ardissino D; Italian Atherosclerosis, Thrombosis and Vascular Biology and Society for Invasive Cardiology–GISE Investigators. Prognostic value of isolated troponin I elevation after percutaneous coronary intervention. Circ Cardiovasc Interv 2010;3:431–435. [DOI] [PubMed] [Google Scholar]
- 21. Gili S, D'Ascenzo F, Moretti C, Omedè P, Vilardi I, Bertaina M, Biondi Zoccai G, Sheiban IMAD, Stone GW, Gaita F. Impact on prognosis of periprocedural myocardial infarction after percutaneous coronary intervention. J Intervent Cardiol 2014;27:482–490. [DOI] [PubMed] [Google Scholar]
- 22. Liou K, Jepson N, Kellar P, Ng B, Isbister J, Giles R, Friedman D, Allan R, Lau A, Pitney M, Ooi S-Y. Prognostic significance of peri-procedural myocardial infarction in the era of high sensitivity troponin: a validation of the joint ACCF/AHA/ESC/WHF universal definition of type 4a myocardial infarction with high sensitivity troponin T. Heart Lung Circ 2015;24:673–681. [DOI] [PubMed] [Google Scholar]
- 23. Di Serafino L, Borgia F, Maeremans J, Pyxaras SA, De Bruyne B, Wijns W, Heyndrickx GR, Dens J, Di Mario C, Barbato E. Periprocedural myocardial injury and long-term clinical outcome in patients undergoing percutaneous coronary interventions of coronary chronic total occlusion. J Invasive Cardiol 2016;28:410–414. [PubMed] [Google Scholar]
- 24. Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, Onuma Y, Morel M-A, Es G-A. V, Zuckerman B, Fearon WF, Taggart D, Kappetein A-P, Krucoff MW, Vranckx P, Windecker S, Cutlip D, Serruys PW; Academic Research Consortium. Standardized end point definitions for coronary intervention trials: the Academic Research Consortium-2 consensus document. Circulation 2018;137:2635–2650. [DOI] [PubMed] [Google Scholar]
- 25. Mj P, Rb D, Rs V. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med 2010;48:1703–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blangero Y, Rabilloud M, Laurent‐Puig P, Malicot KL, Lepage C, Ecochard R, Taieb J, Subtil F. The area between ROC curves, a non-parametric method to evaluate a biomarker for patient treatment selection. Biom J 2020;62:1476–1493. [DOI] [PubMed] [Google Scholar]
- 27. Jaffe AS, Apple FS, Lindahl B, Mueller C, Katus HA. Why all the struggle about CK-MB and PCI? Eur Heart J 2012;33:1046–1048. [DOI] [PubMed] [Google Scholar]
- 28. Ruel M, Falk V, Farkouh ME, Freemantle N, Gaudino MF, Glineur D, Cameron DE, Taggart DP. Myocardial revascularization trials. Circulation 2018;138:2943–2951. [DOI] [PubMed] [Google Scholar]
- 29. Neumann F-J, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J-P, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferović PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO, Wijns W, Glineur D, Aboyans V, Achenbach S, Agewall S, Andreotti F, Barbato E, Baumbach A, Brophy J, Bueno H, Calvert PA, Capodanno D, Davierwala PM, Delgado V, Dudek D, Freemantle N, Funck-Brentano C, Gaemperli O, Gielen S, Gilard M, Gorenek B, Haasenritter J, Haude M, Ibanez B, Iung B, Jeppsson A, Katritsis D, Knuuti J, Kolh P, Leite-Moreira A, Lund LH, Maisano F, Mehilli J, Metzler B, Montalescot G, Pagano D, Petronio AS, Piepoli MF, Popescu BA, Sádaba R, Shlyakhto E, Silber S, Simpson IA, Sparv D, Tavilla G, Thiele H, Tousek P, Van Belle E, Vranckx P, Witkowski A, Zamorano JL, Roffi M, Windecker S, Aboyans V, Agewall S, Barbato E, Bueno H, Coca A, Collet J-P, Coman IM, Dean V, Delgado V, Fitzsimons D, Gaemperli O, Hindricks G, Iung B, Jüni P, Katus HA, Knuuti J, Lancellotti P, Leclercq C, McDonagh TA, Piepoli MF, Ponikowski P, Richter DJ, Roffi M, Shlyakhto E, Sousa-Uva M, Simpson IA, Zamorano JL, Pagano D, Freemantle N, Sousa-Uva M, Chettibi M, Sisakian H, Metzler B, İbrahimov F, Stelmashok VI, Postadzhiyan A, Skoric B, Eftychiou C, Kala P, Terkelsen CJ, Magdy A, Eha J, Niemelä M, Kedev S, Motreff P, Aladashvili A, Mehilli J, Kanakakis I-G, Becker D, Gudnason T, Peace A, Romeo F, Bajraktari G, Kerimkulova A, Rudzītis A, Ghazzal Z, Kibarskis A, Pereira B, Xuereb RG, Hofma SH, Steigen TK, Witkowski A, de Oliveira EI, Mot S, Duplyakov D, Zavatta M, Beleslin B, Kovar F, Bunc M, Ojeda S, Witt N, Jeger R, Addad F, Akdemir R, Parkhomenko A, Henderson R; ESC Scientific Document Group. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 30. Silvain J, Cayla G, Beygui F, Range G, Lattuca B, Collet J-P, Dillinger J-G, Boueri Z, Brunel P, Pouillot C, Boccara F, Christiaens L, Labeque J-N, Lhermusier T, Georges J-L, Bellemain-Appaix A, Breton HL, Hauguel-Moreau M, Saint-Etienne C, Caussin C, Jourda F, Motovska Z, Guedeney P, El Kasty M, Laredo M, Dumaine R, Ducrocq G, Vicaut E, Montalescot G. Blunting periprocedural myocardial necrosis: rationale and design of the randomized ALPHEUS study. Am Heart J 2020;225:27–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.