Abstract
Background
CD19-directed chimeric antigen receptor (CAR) T-cell therapy (CAR-T) has emerged as effective for relapsed/refractory large B-cell lymphoma (R/R LBCL). The neurologic toxicity seen with CAR-T, referred to as immune effector cell–associated neurotoxicity syndrome (ICANS), is poorly understood. To better elucidate the clinical characteristics, treatment outcomes, and correlative biomarkers of ICANS, we review here a single-center analysis of ICANS after CAR T-cell therapy in R/R LBCL.
Methods
Patients (n = 45) with R/R LBCL treated with axicabtagene ciloleucel (axi-cel) were identified. Data regarding treatment course, clinical outcomes, and correlative studies were collected. Patients were monitored and graded for ICANS via CARTOX-10 scoring and Common Terminology Criteria for Adverse Events (CTCAE) v4.03 criteria, respectively.
Results
Twenty-five (56%) patients developed ICANS, 18 (72%) of whom had severe (CTCAE grades 3–4) ICANS. Median time to development of ICANS was 5 days (range, 3–11). Elevated pre-infusion (day 0 [D0]) fibrinogen (517 vs 403 mg/dL, upper limit of normal [ULN] 438 mg/dL, P = 0.01) and D0 lactate dehydrogenase (618 vs 506 units/L, ULN 618 units/L, P = 0.04) were associated with ICANS. A larger drop in fibrinogen was associated with ICANS (393 vs 200, P < 0.01). Development of ICANS of any grade had no effect on complete remission (CR), progression-free survival (PFS), or overall survival (OS). Duration and total dose of steroid treatment administered for ICANS did not influence CR, PFS, or OS.
Conclusions
ICANS after CAR-T with axi-cel for R/R LBCL was seen in about half of patients, the majority of which were high grade. Contrary to previous reports, neither development of ICANS nor its treatment were associated with inferior CR, PFS, or OS. The novel finding of high D0 fibrinogen level can identify patients at higher risk for ICANS.
Keywords: chimeric antigen receptor T cells, fibrinogen, immunotherapy, lymphoma, neurotoxicity
Key Points.
1. ICANS is common after axi-cel CAR-T, occurring in about half of patients.
2. ICANS after axi-cel CAR-T is not associated with poor treatment outcomes.
3. Elevated baseline fibrinogen level at time of axi-cel infusion is predictive of ICANS.
Importance of the Study.
With increasing use of CAR-T in the treatment of malignancies, better understanding of the associated toxicities is critical. ICANS represents a particularly challenging CAR-T toxicity due to its unpredictability and wide range of clinical manifestations. This study analyzes and describes the clinical characteristics, outcomes, and correlative biomarkers of ICANS in a uniformly treated, single center population of large B-cell lymphoma patients receiving CD19-directed CAR-T with axi-cel. ICANS occurred in approximately 50% of the patients, the majority of which was severe (CTCAE grades 3–4). In contrast to the reported literature, development of ICANS was not associated with inferior treatment outcomes. A key novel finding reported here is the role of baseline fibrinogen as a predictive biomarker for ICANS, which can help identify patients at a higher risk for ICANS and contribute further understanding of the underlying pathophysiology of ICANS with the goal of improving CAR-T related morbidity.
Chimeric antigen receptor (CAR) T-cell therapy (CAR-T) has transformed the treatment of hematologic malignancies, with primary approvals of CD19-directed CAR-T in the United States for relapsed/refractory large B-cell lymphoma (R/R LBCL).1,2 With the emergence of CAR-T, clinicians have quickly adjusted to new challenges, including the monitoring and management of CAR-T related complications. These include cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS),3–5 which remain significant causes of CAR-T related morbidity that require further understanding and study.
While the exact mechanism leading to neurologic toxicity remains unclear, CAR-T mediated inflammation leading to endothelial activation and blood–brain barrier disruption may play a key role.6–8 Proposed predisposing factors for development of ICANS include concomitant neurologic conditions, prior CRS, higher CAR-T doses and peak expansion values, high tumor burden at the time of CAR-T infusion, low platelet count at CAR-T infusion, and an elevated inflammatory marker, such as C-reactive protein (CRP) or ferritin, or cytokines such as interleukin (IL)-1, IL-6, IL-10, and interferon-gamma.5–15
The incidence of ICANS across several CD19 CAR-T trials for lymphoma is reported to range 20–67%.1,2,16,17 In addition, a recent analysis reported an association of ICANS with inferior overall survival (OS).9 ICANS presents as a wide range of neurologic and cognitive clinical symptoms, from minor headaches, confusion, and handwriting changes to aphasia, seizures, cerebral edema, and even coma requiring mechanical ventilation.5–7,18 Most cases of ICANS are reversible; however, some reported cases were associated with prolonged morbidity and even mortality.1,6,19 While strategies to mitigate these risks are being explored, it is imperative for clinicians to monitor patients closely and recognize the initial signs of ICANS for early intervention. Preventative or preemptive treatment has been hindered by the lack of established predictive biomarkers for ICANS. Management of ICANS involves the use of corticosteroid therapy in addition to the IL-6 receptor antibody tocilizumab, if concurrent CRS is present.4,20 The optimal steroid dosing and duration has not been established, and it remains unclear if prolonged or high cumulative use of steroids blunts systemic CAR T-cell responses or affects clinical outcomes.
Further complicating the current landscape is that prior studies reporting on CAR-T related ICANS have been limited due to smaller sample size, heterogeneous group of disease histologies, or non-uniform CAR-T constructs.6,8,9 Herein we report a large single-center experience of 45 lymphoma patients uniformly treated with CD19-directed CAR-T with axicabtagene ciloleucel (axi-cel), which analyzes the clinical features, predictive biomarkers, and prognostic significance of ICANS.
Methods
Patient and Disease Characteristics
Patients with R/R LBCL treated with axi-cel at our center between April 2018 and May 2019 were identified. Data regarding patient and disease characteristics, treatment course, and clinical outcomes were collected (Table 1). Laboratory variables were collected at time of T-cell collection, day of initiation of lymphodepleting therapy, and day of CAR-T infusion (day 0 [D0]). For the acute-phase markers of CRP, ferritin, fibrinogen, and lactate dehydrogenase (LDH), we calculated both velocity, v, calculated as slope/rate of rise, and ΔX, defined as the difference between the D0 and maximum or minimum values of variable “X” within the first 28 days post CAR-T infusion. Patients were monitored and screened for ICANS via CAR-T associated toxicity 10-point (CARTOX-10) scoring.3 CRS was graded per the Lee criteria, and ICANS per the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.03 criteria.1,2,4,20 Severe ICANS was defined as grades 3–4. Initiation of steroid treatment followed axi-cel’s risk evaluation and mitigation strategy toxicity general guidelines, based on the presenting grade of ICANS. All patients had pretreatment fluorodeoxyglucose (FDG) PET/CT scans, which were used to calculate tumor metabolic volume (TMV), quantified by an independent radiologist review using Rover software (ABX Advanced Biochemical Compounds). Electroencephalographies (EEGs) were reviewed and classified by an independent neurologist. This study was approved by our center’s institutional review board and was conducted in accordance with the principles of the Declaration of Helsinki.
Table 1.
Baseline patient and disease characteristics
| Total (n = 45) | No ICANS (n = 20) | ICANS Grades 1–2 (n = 7) | ICANS Grades 3–4 (n = 18) | P-value | |
|---|---|---|---|---|---|
| Age, y, median (range) | |||||
| Sex, n (%) | 60 (26–75) | 58 (29–73) | 63 (54–68) | 58 (26–75) | 0.63 |
| Male | 22 (49) | 8 (40) | 3 (43) | 7 (39) | 0.43 |
| Female | 23 (51) | 12 (60) | 4 (57) | 11 (61) | |
| Ethnicity, n (%) | |||||
| Caucasian | 28 (62) | 11 (55) | 5 (71) | 12 (67) | |
| African American | 10 (22) | 5 (25) | 2 (29) | 3 (17) | 0.85 |
| Hispanic | 4 (9) | 3 (12) | 0 (0) | 1 (6) | |
| Asian | 3 (7) | 1 (4) | 0 (0) | 2 (11) | |
| Lymphoma type, n (%) | |||||
| DLBCL | 35 (78) | 14 (70) | 7 (100) | 14 (78) | |
| TFL | 7 (16) | 3 (15) | 0 (0) | 4 (22) | 0.41 |
| PMBCL | 3 (7) | 3 (15) | 0 (0) | 0 (0) | |
| Subtype, n (%) | |||||
| GCB | 26 (58) | 11 (55) | 3 (43) | 12 (67) | |
| non-GCB | 18 (40) | 9 (45) | 4 (57) | 5 (28) | 0.36 |
| N/A | 1 (2) | 0 (0) | 0 (0) | 1 (6) | |
| Double/triple, n (%) | |||||
| Double hit (FISH) | 10 (22) | 5 (25) | 1 (14) | 4 (22) | |
| Triple hit | 1 (2) | 0 (0) | 1 (14) | 0 (0) | 0.71 |
| Prior CNS disease, n (%) | 2 (4) | 2 (10) | 0 (0) | 0 (0) | 0.66 |
| Bridging therapy, n (%) | 30 (67) | 12 (60) | 4 (57) | 14 (78) | 0.33 |
| Tumor metabolic volume, cm3 median (range) | 35.01 (0.17–1425.2) | 16.46 (0.17–142.43) | 163.97 (19.58–1425.2) | 33.02 (1.67–407.65) | 0.93 |
| ALC at leukapheresis, K/µL | 0.8 (0.12–6.7) | 0.8 (0.4–6.7) | 0.9 (0.3–1.8) | 0.6 (0.12–2) | 0.09 |
| ANC on D0, K/µL | 1.3 (0–5.8) | 1.55 (0–4.8) | 0.92 (0–2.9) | 0.74 (0–5.8) | 0.30 |
| Platelets on D0, K/µL | 125 (10–331) | 132 (23–287) | 135 (19–166) | 109.5 (10–331) | 0.40 |
| Fibrinogen on D0, mg/dL | 461 (242–801) | 403 (242–620) | 437 (330–562) | 524.5 (337–801) | 0.003 |
| CRP on D0, mg/dL | 2 (0–33.7) | 1.9 (0–7.5) | 1.7 (0.9–7.6) | 2.1 (0–33.7) | 0.11 |
| Ferritin on D0, ng/mL | 342.4 (61.5–9508.2) | 299.6 (61.5–4007) | 486 (172.2–9508.2) | 638.2 (63.7–4172) | 0.35 |
| LDH on D0, units/L median | 540 (284–2343) | 506 (284–750) | 560 (377–1939) | 718.5 (356–2343) | 0.025 |
Statistical Analysis
Categorical variables were summarized as frequency counts and percentages and compared with Fisher’s exact test. Continuous variables were summarized as medians and interquartile ranges and compared with the Wilcoxon rank sum test. OS was calculated from time of treatment to death. PFS was calculated from time of treatment to either disease progression or death. Timetoevent data were summarized using the Kaplan–Meier method with surviving patients censored at the date of last followup or event (death or progression) and compared using logrank tests. The Cox proportional hazards model was used for multivariable analyses of potential prognostic factors. The results were summarized as hazard ratios, 95% confidence intervals (CIs), and P-values. Recursive partitioning analysis in a univariate fashion was used to identify the cutoff value with respect to survival (OS or PFS) for continuous variables. A logistic regression model was used to identify predictors of ICANS. The goodness of fit was summarized as receiver operating characteristic (ROC) curves and area under the curve (AUC). All statistical tests were 2sided. A P-value <0.05 was considered statistically significant. All statistical analyses were performed using R software package (R Development Core Team, 2012).
Results
Patient and Disease Characteristics
Forty-five patients with R/R LBCL treated with axi-cel CAR-T were identified (Table 1). Median patient age was 60 years (range, 26–75 y); 51% were female, 62% Caucasian, 22% African American, and 16% of other ethnicity. Lymphoma subtypes included 35 diffuse large B-cell lymphoma (DLBCL), 7 transformed follicular lymphoma (TFL), and 3 primary mediastinal B-cell lymphoma (PMBCL); these lymphomas were high grade, with median Ki-67 of 82.5%, 58% germinal center B-cell–like (GCB) subtype, and 24% were double- or triple-hit per fluorescence in situ hybridization (FISH) rearrangement studies. Two patients had prior central nervous system (CNS) involvement of their lymphoma that was cleared prior to axi-cel infusion. Ten patients had received prior hematopoietic stem cell transplantation (HSCT), 8 autologous and 2 allogeneic. Median absolute lymphocyte count (ALC) on day of CAR-T leukapheresis was 800K/μL. Most (67%) of the patients required bridging therapy during manufacturing of the CAR T cells, which included cytotoxic chemotherapy in 47%, radiation in 13%, and either steroids, biologics, or a combination thereof in the remaining patients. Median follow-up of this cohort was 7.1 months (interquartile range, 3.0–9.9).
CAR-T Infusion and ICANS Clinical Course
Twenty-five (56%) patients developed ICANS, 18 (72%) of whom developed grades 3–4, or severe, ICANS. Median time to development of ICANS was 5 days (range, 3–11 days). Most common initial ICANS symptoms were dysgraphia, word-finding difficulties, confusion, and somnolence. Less common ICANS symptoms included self-limited unilateral motor weakness, unilateral facial droop, unilateral anisocoria with ptosis, limb tremors, and gait ataxia. One patient developed an absence seizure followed by tonic-clonic activity, confirmed by EEG, which was controlled with escalation of anti-epileptic therapy. Two cases of autonomic dysfunction occurred. One patient developed acute left pontine infarct with associated tiny petechial hemorrhage, which led to neurogenic bladder, hydronephrosis, and overflow incontinence. Another patient developed severe dysautonomia with initial parasympathetic overactivity leading to asystole with successful resuscitation after administration of atropine and epinephrine, followed by transcutaneous and eventually transvenous pacing (TVP). After 2 days of TVP dependency, he developed symptoms consistent with sympathetic storm manifested by hypertensive emergency.8 All but one patient required broad-spectrum antibiotic therapy due to neutropenic fever during their treatment course, 8 of whom were found to have a concurrent infection. Twenty-eight (62%) patients received granulocyte-colony stimulating factor (G-CSF) support prior to D14 post CAR-T infusion. Median duration of hospitalization was 14 days for the whole cohort, longer for those who developed ICANS compared with those without, median 17 versus 11.5 days (P < 0.01), respectively.
The majority of patients developed CRS requiring treatment with tocilizumab (n = 36, median 2.5 doses). All ICANS patients had preceding CRS requiring treatment with tocilizumab (n = 25), and additional siltuximab (n = 2). Twenty-three (92%) patients with ICANS required steroid therapy, with a median total steroid dose equivalent to 221 mg of dexamethasone (range, 52–1630) for a median duration of 12.5 days (range, 4–27). Eight (18%) patients were treated initially with a high-dose pulse of i.v. methylprednisolone 1 g daily, and the remainder of patients with grade ≥2 ICANS were started on i.v. dexamethasone 10 mg every 6 hours (Supplementary Table 1). Five (11%) patients were discharged on prolonged steroid taper from hospital. Prophylactic anti-epileptic therapy with levetiracetam was used in all patients, with dose adjustments for those who exhibited seizure activity. ICANS symptoms recurred with tapering or discontinuation of steroids in 2 patients, requiring increase or re-initiation of steroids followed by a slow taper. Both patients eventually were able to discontinue steroid treatment with no recurrence of symptoms. Two patients exhibited persistent neurotoxicity manifested by short-term memory loss and profound weakness with immobility; both died without resolution of ICANS due to relapsed lymphoma.
Treatment Outcomes
Complete remission.—
Twenty-two (49%) patients achieved CR with axi-cel CAR-T. Five (11.1%) had progressive disease, and the remaining 16 (35.6%) had either partial response or stable disease. Sixteen patients (36.5%) achieved an early CR by D30 post CAR-T. Development of ICANS of any grade had no effect on achievement of CR (P = 0.66), nor did development of severe ICANS (P = 0.86).
PFS and OS.—
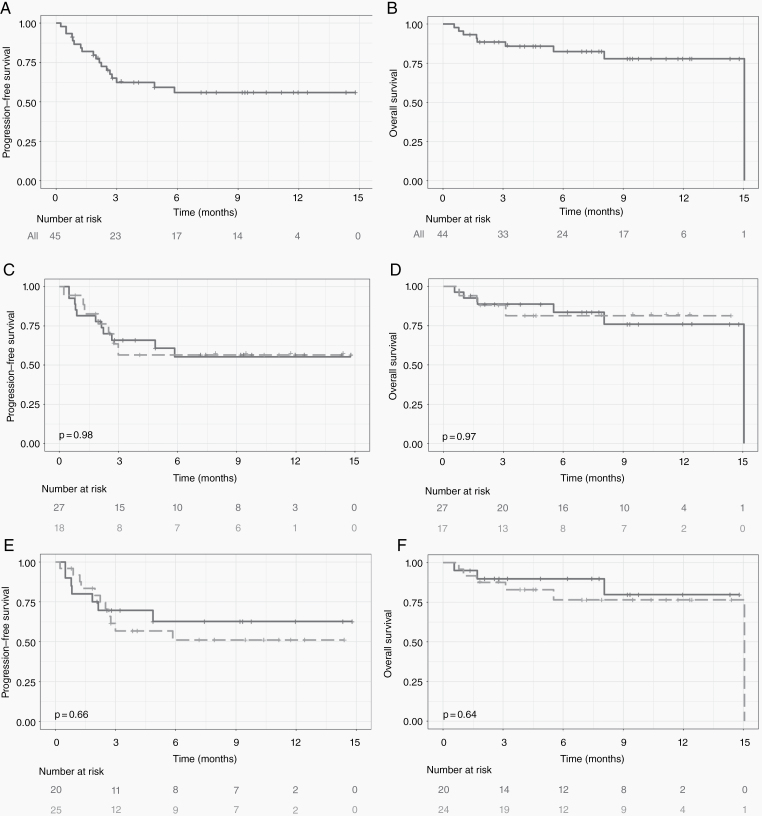
At the time of this analysis, the median follow-up for our cohort was 7 months. The median PFS was not reached, and median OS was 15.1 months (Fig. 1A, B). Development of any ICANS did not affect PFS (P = 0.98) or OS (P = 0.66) (Fig. 1E, F), and further, development of severe (grades 3–4) ICANS did not affect PFS (P = 0.97) or OS (P = 0.64) (Fig. 1C, D).
Fig. 1.
PFS (A) and OS (B) of whole patient cohort: not reached and 15 months, respectively. Kaplan–Meier estimate of PFS (C) and OS (D) for patients with (broken line/light gray) and without (solid line/dark gray) severe (grade 3–4) ICANS; PFS (E) and OS (F) with (broken line/light gray) and without (solid line/dark gray) any grade ICANS.
Effect of Toxicity Management on ICANS
All ICANS patients had preceding CRS, though no relationship between CRS grade—high (3–4) versus low (0–2)—and development of severe ICANS (P = 0.25) was found. Tocilizumab use was associated with development of ICANS (P < 0.01) and severe ICANS (P = 0.02).
In terms of ICANS management, duration and total dose of steroid treatment did not influence CR (P = 0.98, P = 0.86), PFS (P = 0.32, P = 0.59), or OS (P = 0.32, P = 0.58). G-CSF use prior to D14 also did not lead to higher rates of ICANS, nor did it influence CR (P = 0.91), PFS (P = 0.92), or OS (P = 0.95).
Correlative Studies—Biomarkers Predictive of ICANS
Elevated D0 fibrinogen (517 vs 403 mg/dL, ULN 438 mg/dL, P = 0.01) and D0 LDH (618 vs 506 units/L, ULN 618 units/L, P = 0.04) were associated with development of ICANS on univariate analysis. When focusing on severe ICANS, these same relationships were even more pronounced: higher D0 fibrinogen (525 vs 403 mg/dL, P < 0.01) and D0 LDH (719 vs 510 units/L, P = 0.03). Not predictive of ICANS were patient characteristics (including age, sex, and ethnicity), disease characteristics (including subtype or stage at diagnosis), and other D0 laboratory values (including platelet count, CRP, and ferritin) (Table 1).
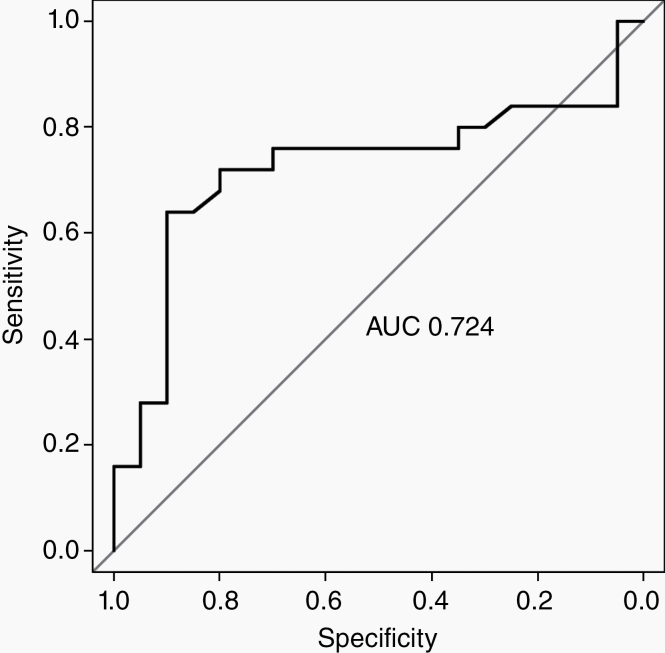
Elevated fibrinogen on day of axi-cel CAR-T infusion was predictive of ICANS (Fig. 2) with a ROC curve (AUC 0.724, 95% CI: 0.5642–0.88438). The high area under the ROC curve (AUROC) suggests fibrinogen’s discriminative ability in predicting ICANS (Fig. 2). Logistic regression showed that elevated D0 fibrinogen was predictive of any grade of ICANS (odds ratio [OR] 2.68 [1.36–6.33, P = 0.01], univariate analysis), and of severe ICANS (OR 3.27 [1.60–8.27, P < 0.01], univariate analysis) (Supplementary Table 2).
Fig. 2.
ROC curve (AUC 0.724, 95% CI: 0.5642–0.88438). The high area under the ROC curve (AUROC) suggests the discriminative ability of fibrinogen to predict ICANS.
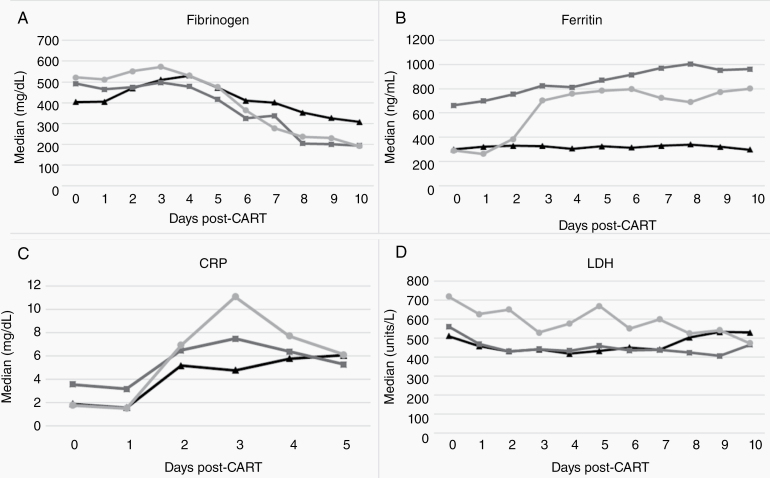
A larger drop in fibrinogen (Δfib) was associated with ICANS (393 vs 200, P < 0.01; multivariable OR 15.78 [2.23–356.06, P = 0.03]) and severe ICANS (408 vs 206, P < 0.01; univariate OR 3.43 [1.85–7.85, P < 0.01]); and a larger increase in ferritin levels (ΔFer) was also associated with ICANS (482.2 vs 173.9, P = 0.02), and severe ICANS (538.1 vs 217.4, P < 0.01) on univariate analysis (Fig. 3).
Fig. 3.
Median values for select correlative biomarker values and trends post CAR-T infusion: (A) fibrinogen (days 0–10), (B) ferritin (days 0–10), (C) CRP (days 0–5), (D) LDH (days 0–10).
Per legend: triangle markers (black line) represent patients with no ICANS; square markers (dark gray line) represent patients with grade 1–2 ICANS; circle markers (light gray line) represent patients with severe, or grade 3–4 ICANS.
Correlative Studies—Radiology and EEG Findings in ICANS
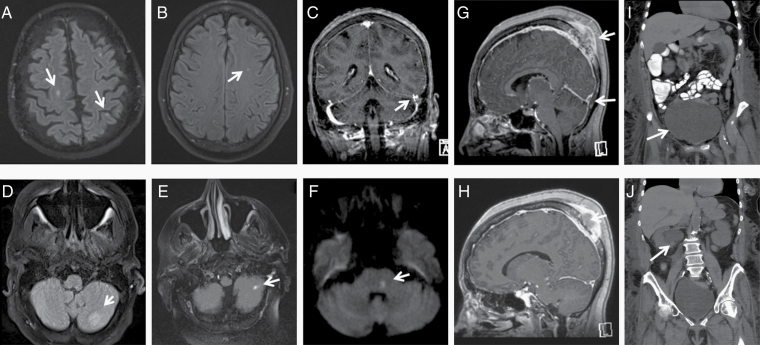
Brain MRI changes were found in 28% of ICANS patients, compared with pretreatment baseline brain MRIs. Acute abnormalities on brain MRI included tumor-associated nonocclusive thrombosis, petechial hemorrhages, pontine infarcts, and nonspecific white matter changes (Fig. 4). One striking finding in a patient with a transcalvarial tumor and a sagittal sinus tumor thrombus at time of CAR-T infusion was the development of pseudoprogression of the tumor thrombus by imaging at the time of onset of ICANS, resulting in effacement of the cerebral sulci (Fig. 4G, H). Another patient developed an acute pontine infarct which led to neurogenic bladder and subsequent obstructive hydronephrosis (Fig. 4F, I, J).
Fig. 4.
Radiologic findings in ICANS. Brain MRI showing: (A) cerebral late subacute infarcts on axial fluid attenuated inversion recovery (FLAIR); (B) nonspecific white matter lesion on axial FLAIR; (C) nonocclusive thrombosis of the left sigmoid and distal transverse sinuses on coronal T1 multiplanar reformation gadolinium (MPR gad); (D) a new focus of presumed petechial hemorrhage within the inferior left cerebellar hemisphere on axial FLAIR MR; (E) left cerebellar likely embolic acute/subacute infarct on axial FLAIR MR; (F) pretreatment transcalvarial mass, with thrombus within the superior sagittal sinus; (G) new subocclusive thrombus within left transverse sinus on sagittal T1 MPR gad; (H) with posttreatment pseudoprogression within the sagittal sinus with effacement of the sulci on sagittal T1 MPR gad; (F) an acute left pontine infarct with associated tiny petechial hemorrhage on axial diffusion weighted imaging (DWI) MR, which led to led to neurogenic bladder; neurogenic bladder seen on CT abdomen/pelvis demonstrating (I) urinary overflow incontinence (J) causing hydronephrosis.
EEG was done in 10 ICANS patients during ICANS symptoms; findings included: diffuse slowing, n = 7; generalized period discharges, n = 1; bilateral independent periodic discharges, n = 1; and stimulus-induced rhythmic, periodic, or ictal discharges, n = 1. Documented seizure activity on EEG was seen in 1 patient (4%), which required adjustment from prophylactic anti-epileptic dosing to therapeutic dosing and resolved thereafter with successful control.
By FDG-PET/CT, median pretreatment TMV was 34.6 cm3, with a trend toward higher pretreatment TMV values in those who developed ICANS compared with those who did not (38.2 vs 25.5 cm3, P = 0.10); baseline TMV also tended to be higher in patients with inferior OS (P = 0.09).
Discussion
ICANS is frequent among LBCL patients treated with axi-cel and is an important cause of treatment-associated morbidity and, rarely, mortality. While well recognized, the clinical features of ICANS are highly variable, and criteria for diagnosis, grading, and treatment response remain poorly defined. In our patient cohort, as seen in previous reports, ICANS occurred in more than half (56%) of LBCL patients treated with axi-cel, and was severe in the majority of cases (72%).1 Our report highlights the wide spectrum of presenting symptoms, including findings on EEG and MRI, and analyzes correlative biomarkers and disease characteristics which may predict ICANS onset, severity, and treatment outcomes.
In contrast to previous findings,9 we found no deleterious association between ICANS and OS, nor did we find an effect on CR rate or PFS. The mainstay of treatment for ICANS involves high doses of corticosteroids, raising obvious concern of reducing CAR-T efficacy. Our study found that therapy with steroids—regardless of total dose, average daily dose, initial high-dose pulsing, or duration—did not affect CAR-T treatment outcomes, including achievement of CR, PFS, or OS. Our findings contrast with early CAR-T reports suggesting that steroid use blunts the in vivo expansion of T cells requisite for efficacy, as well as interfering with other forms of immunotherapy, such as checkpoint inhibition.21–24 However, our findings are consistent with more recent CAR-T analyses that found steroids could be used to manage ICANS without impairing efficacy.1 In our cohort, we observed ICANS flares with rapid steroid taper, illustrating the importance of close monitoring for ICANS relapse during taper.
While early detection is important for timely initiation of therapy for ICANS, preventative and preemptive strategies have also been explored. Blockade of IL-1 or GM-CSF in vitro and in vivo in murine studies has been shown to effectively blunt the inflammatory cascade triggered by monocyte activation that has been associated with ICANS.14,15,25 Concern remains that a universally applied ICANS prevention strategy might hinder CAR-T efficacy. Thus, successful implementation of preventive therapy would be aided by identification of robust predictive biomarkers for ICANS. Early steroid use in CAR-T toxicity management is being assessed,26 and while additional prospective studies are needed, our findings may alleviate concerns about steroid usage in ICANS management under current guidelines and contribute to risk stratification efforts to identify patients at highest risk of ICANS who may benefit from preventative or preemptive therapies.
With respect to biomarkers, our results did not confirm reported associations between baseline high ferritin levels or low platelet counts and subsequent ICANS,9 but are in agreement with the predictive value of high LDH levels.9 LDH is likely a surrogate for tumor burden at time of treatment, which has been independently associated with development of ICANS.7,8,11,12 We report here novel associations between fibrinogen and ICANS: elevated fibrinogen prior to infusion on D0, as well as the decline in fibrinogen from baseline to the lowest measured level (Δfib) to be important predictors of ICANS. In addition to its well-described role in hemostasis, fibrinogen is an acute-phase reactant with pro-inflammatory functions. From a hemostatic perspective, high plasma levels of fibrinogen lead to activation of many cells, including platelets, endothelial cells, smooth muscle cells, and leukocytes. In the inflammatory setting, deposits of fibrinogen and its degradation products are found at sites of inflammation and tissue injury, exerting local effects which may lead to further vascular and tissue injury.27–29
Fibrinogen remains confined to the vasculature in healthy individuals, respecting the blood–brain barrier. However, in conditions associated with chronic blood–brain barrier disruption (eg, traumatic brain injury, encephalitis, bacterial meningitis, Alzheimer’s disease, multiple sclerosis), fibrinogen deposits are found within the CNS, where they trigger a pro-inflammatory cascade.28–34 Reported mechanisms of fibrinogen’s interaction with the immune system include effects on leukocyte function and migration, facilitation of leukocyte-endothelial interaction, and recruitment and activation of other cells to areas of damage, including platelets and monocytes, triggering their release of cytokines and chemokines.27,29 In vitro, CNS immune cells (ie, microglia) are directly activated by fibrinogen, which can trigger a pro-inflammatory cascade as well as lead to increased phagocytosis. Further, murine models of Alzheimer’s disease find that lower fibrinogen levels are associated with reduced microglial activation and, suggestively, with improved cognitive performance.29,30,32
In our analysis, the absolute decrement in fibrinogen level (Δfib) was associated with development of ICANS. IL-6, a well-described driver of inflammation, directly influences the transcription of fibrinogen at the mRNA level, consistent with the role of fibrinogen as an acute-phase reactant.29,34,36 Plausible explanations for the observed relationship between Δfib and ICANS include: (i) decreased fibrinogen levels reflective of an anti–IL-6 treatment response from tocilizumab and steroids, leading to reduced fibrinogen biosynthesis; that is, decreased fibrinogen resulted from treatment of ICANS rather than causing ICANS; and (ii) consumption and systemic deposition of fibrinogen and its degradation products at sites of vascular injury, with subsequent crossing of the blood–brain barrier into the CNS, resulting in increased clearance of circulating plasma fibrinogen. Interestingly, IL-1 promotes synthesis of fibrinogen and has been implicated in ICANS.14,29,35,36 IL-1 blockade is, therefore, a strategy of interest in mitigating ICANS.14,37 The baseline fibrinogen level and its decremental change during therapy are novel predictive markers for development of ICANS, particularly of severe ICANS. Further investigation into the role of fibrinogen in the development of ICANS may elucidate pathophysiologic mechanisms and identify avenues for targeted treatment and/or prevention.
One-third of our patients with ICANS exhibited changes on MRI imaging when compared with baseline pre-treatment Brain MRI, some prompting change in diagnosis and management. For example, for patients who developed new petechiae on imaging in the setting of CAR-T induced cytopenia, the threshold for platelet transfusions was raised to keep platelet levels above 50K/µL. On the other hand, our patient with evidence of tumor thrombus required initiation of anticoagulation, illustrating the importance of complete assessment of our patients during ICANS with brain MRI that can affect clinical practice. Findings support the practice of routine and serial imaging of ICANS patients to assess, manage, and monitor neurologic signs and symptoms until resolution. In our cohort, we assessed the TMV predictive value of baseline FDG-PET/CT, a promising investigational metric, on subsequent development of ICANS. Higher pretreatment TMV has been associated with more severe CRS in lymphoma patients undergoing CAR-T.38 However, TMV effect on treatment outcomes, including rate of CR and OS, has not been previously reported. While our analysis was underpowered, we did observe a non-significant trend toward higher ICANS severity and inferior survival among patients with higher baseline TMV. This finding warrants further study to confirm whether TMV can meaningfully predict the incidence and severity of ICANS as well as patient outcomes.
Limitations of this report include its retrospective and single-center design. Furthermore, potentially interesting and translational correlates such as cerebrospinal fluid analysis, CAR-T pharmacokinetics, and real-time peripheral blood cytokine analysis were not done routinely in our cohort, which would be important for further understanding of ICANS pathophysiology. Nevertheless, strengths of this analysis result from the relatively large cohort of patients with LBCL who uniformly received a single, CD19-directed CAR-T product (axi-cel) under “real-world” conditions following established institutional standard operating procedures. Other reports summarize ICANS findings from heterogeneous patient populations, CAR-T products and diseases, including acute lymphoblastic leukemia, small lymphocytic lymphoma/chronic lymphocytic leukemia, and/or hepatocellular carcinoma.9 The role of elevated baseline (D0) fibrinogen level and its decremental change following CAR T-cell therapy strongly predicts the development of ICANS. The potential contribution of this finding in elucidating pathophysiologic drivers of this condition warrants further prospective validation.
Conclusion
In our single-institution analysis, ICANS was seen in about half of patients after treatment with CD19-directed CAR-T with axi-cel for R/R LBCL. Nearly three-quarters of ICANS cases were severe (CTCAE grades 3–4). Contrary to other reports to date, development of ICANS was not associated with inferior rates of CR, PFS, or OS. Importantly, use of high-dose steroids, regardless of duration and total dose, had no discernible effect on patient treatment outcomes.
This is the first study to report that high D0 fibrinogen may identify patients at higher risk for ICANS, especially severe ICANS. Further, predictive biomarkers, such as fibrinogen and ferritin, could be monitored easily early in the CAR-T course to help evaluate those at higher risk for ICANS. The novel finding of fibrinogen as a predictive biomarker of ICANS may reflect its role in inflammation and vascular injury. Further investigation is warranted, hopefully leading to improved understanding of ICANS pathophysiology and to the development of preventative therapeutic strategies for ICANS.
Supplementary Material
Acknowledgments
The authors would like to thank our patients and the assisting cellular therapy team members, including our invaluable cell processing staff, research staff, and nurses.
Funding
None.
Conflict of interest statement. None to declare.
Authorship statement. NGH, AR, and SD designed the study and wrote the manuscript. NGH, AB, VK, HA, and EH assisted with data collection. NGH, HX, SB, and SD conducted data analysis. All authors provided critical feedback, edited, and approved the manuscript.
References
- 1. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schuster SJ, Bishop MR, Tam CS, et al. ; JULIET Investigators Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. [DOI] [PubMed] [Google Scholar]
- 3. Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. [DOI] [PubMed] [Google Scholar]
- 5. Garcia Borrega J, Gödel P, Rüger MA, et al. In the eye of the storm: immune-mediated toxicities associated with CAR-T cell therapy. Hemasphere. 2019;3(2):e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gust J, Hay KA, Hanafi LA, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Santomasso BD, Park JH, Salloum D, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8(8):958–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gauthier J, Turtle CJ. Insights into cytokine release syndrome and neurotoxicity after CD19-specific CAR-T cell therapy. Curr Res Transl Med. 2018;66(2):50–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karschnia P, Jordan JT, Forst DA, et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood. 2019;133(20):2212–2221. [DOI] [PubMed] [Google Scholar]
- 10. Kotani H, Faramand R, Lee SB, et al. Prediction of toxicity in R/R DLBCL treated with axicabtagene clioleucel (19-28z CAR T). Ann Oncol. 2019;30(Supplement 6):vi82. [Google Scholar]
- 11. Turtle CJ, Hay KA, Hanafi LA, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol. 2017;35(26):3010–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park JH, Rivière I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. J Clin Invest. 2016;126(6):2123–2138.27111235 [Google Scholar]
- 13. Taraseviciute A, Tkachev V, Ponce R, et al. Chimeric antigen receptor T cell-mediated neurotoxicity in nonhuman primates. Cancer Discov. 2018;8(6):750–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24(6):739–748. [DOI] [PubMed] [Google Scholar]
- 15. Sterner RM, Sakemura R, Cox MJ, et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood. 2019;133(7):697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abramson JS, Siddiqi T, Palomba ML, et al. High durable CR rates and preliminary safety profile for JCAR017 in R/R aggressive B-NHL (TRANSCEND NHL 001 study): a defined composition CD19-directed CAR T cell product with potential for outpatient administration. Biol Blood Marrow Transplant. 2018;24:S20–S24. [Google Scholar]
- 17. Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. El Chaer F, Siegel A, Holtzman NG, et al. Severe dysautonomia as a manifestation of neurotoxicity after CAR-T cell therapy for diffuse large B-cell lymphoma. Am J Hematol. 2020;95(6):E146–E148. [DOI] [PubMed] [Google Scholar]
- 19. Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36(28):2872–2878. [DOI] [PubMed] [Google Scholar]
- 24. Fucà G, Galli G, Poggi M, et al. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open. 2019;4(1):e000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sachdeva M, Duchateau P, Depil S, Poirot L, Valton J. Granulocyte-macrophage colony-stimulating factor inactivation in CAR T-cells prevents monocyte-dependent release of key cytokine release syndrome mediators. J Biol Chem. 2019;294(14):5430–5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Topp MS, van Meerten T, Houot R, et al. Earlier steroid use with axicabtagene ciloleucel (axi-cel) in patients with relapsed/refractory large B cell lymphoma (R/R LBCL). Biol Blood Marrow Transplant. 2020;26(3):S101. [Google Scholar]
- 27. Luyendyk JP, Schoenecker JG, Flick MJ. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. 2019;133(6):511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vilar R, Fish RJ, Casini A, Neerman-Arbez M. Fibrin(ogen) in human disease: both friend and foe. Haematologica. 2020;105(2):284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012;34(1):43–62. [DOI] [PubMed] [Google Scholar]
- 30. Cortes-Canteli M, Zamolodchikov D, Ahn HJ, Strickland S, Norris EH. Fibrinogen and altered hemostasis in Alzheimer’s disease. J Alzheimers Dis. 2012;32(3):599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Petersen MA, Ryu JK, Akassoglou K. Fibrinogen in neurological diseases: mechanisms, imaging and therapeutics. Nat Rev Neurosci. 2018;19(5):283–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paul J, Strickland S, Melchor JP. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer’s disease. J Exp Med. 2007;204(8):1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jenkins DR, Craner MJ, Esiri MM, DeLuca GC. Contribution of fibrinogen to inflammation and neuronal density in human traumatic brain injury. J Neurotrauma. 2018;35(19):2259–2271. [DOI] [PubMed] [Google Scholar]
- 34. Woods A, Brull DJ, Humphries SE, Montgomery HE. Genetics of inflammation and risk of coronary artery disease: the central role of interleukin-6. Eur Heart J. 2000;21(19):1574–1583. [DOI] [PubMed] [Google Scholar]
- 35. Perez RL, Roman J. Fibrin enhances the expression of IL-1 beta by human peripheral blood mononuclear cells. Implications in pulmonary inflammation. J Immunol. 1995;154(4):1879–1887. [PubMed] [Google Scholar]
- 36. Fish RJ, Neerman-Arbez M. Fibrinogen gene regulation. Thromb Haemost. 2012;108(3):419–426. [DOI] [PubMed] [Google Scholar]
- 37. Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24(6):731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang J, Hu Y, Yang S, et al. Role of fluorodeoxyglucose positron emission tomography/computed tomography in predicting the adverse effects of chimeric antigen receptor T cell therapy in patients with non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2019;25(6): 1092–1098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.