The use of scanning electron microscopy and transmission electron microscopy to elucidate the role of cholesterol crystals in atherosclerotic plaque rupture is discussed.
Atherosclerosis is a chronic inflammatory process accompanied by dyslipidaemia and other cellular derangements.1 As the quest for the development of innovative future therapies is ongoing, the ability to examine atherosclerotic vessels and the plaques within at an ultrastructural level has become requisite in order to further understand the underlying aetiological processes. In particular, electron microscopy techniques developed over the last several decades, together with classic histological imaging, have been of tremendous importance to understand plaque composition. Both scanning and transmission electron microscopy (SEM and TEM, respectively) have been used to illustrate the timeline of atherosclerosis.2–4 Advances made in these techniques have allowed for a deeper insight into the pathology of atherogenesis.
Often reported in the research community is the presence of empty spaces in advanced atherosclerotic lesions, which are featured prominently especially in paraffin sections and described as ‘clefts’ in electron microscopy. These ‘clefts’ were revealed to have been filled with crystals which were left vacuous after sample processing.5,6
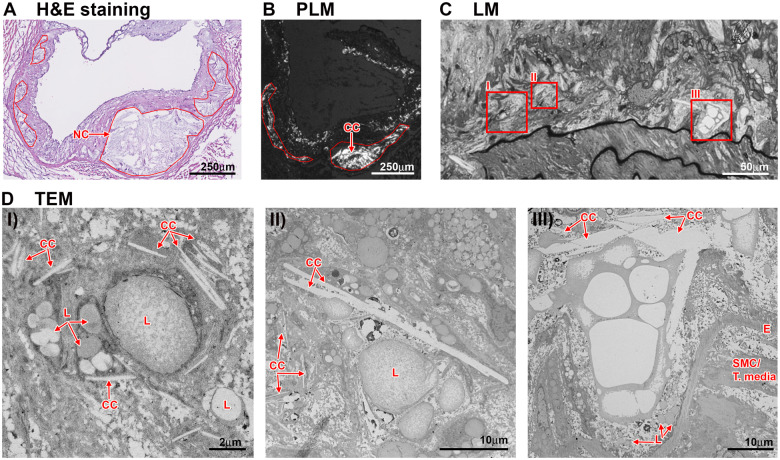
One of the first reports about crystalline cholesterol and cholesterol crystals (CC) in atherosclerosis was published in 19097 with several papers since demonstrating that it is indeed cholesterol monohydrate crystallizing in atherosclerotic lesions.8–10 The connection between CC and atherosclerosis is by now well-established11–14 as the presence of CC is a regular feature within the necrotic core of the atherosclerotic plaque (Figure 1 and Table 1). The necrotic core size and plaque composition are thought to play an important role in determining the propensity of plaques to rupture and cause major acute events such as myocardial infarction and stroke.15 In fact, two very recent studies utilized optical coherence tomography to report the possible detrimental impact of CC presence within the atherosclerotic lesion.16,17 Lesions found to have CC were associated with more major adverse cardiovascular events when compared to patients lacking CC within the lesion.17 Cholesterol crystals also play an important role CC embolism, a rare but detrimental outcome associated with invasive catheter-based manipulation,18–20 and an area to further investigate the origin and impact of CC.
Figure 1.
Cholesterol crystals in the necrotic core. Presence of cholesterol crystals as evaluated in the necrotic core region of 16-week HFD fed Ldlr−/− mice. (A) The outline (red) of the necrotic core area displays ‘clefts’ indicative for cholesterol crystal presences as shown in polarized light microscopy (B). (C/D) Thick sections (1 μm) of samples prepared for transmission electron microscopy show the necrotic core located right above the internal elastic lamina (C). Areas with cholesterol crystal are highlighted in red boxes. (D) Transmission electron microscopy imaging of these areas shows various component of the necrotic core, like lipid droplets (L) and cholesterol crystal of various shapes and sizes. CC, cholesterol crystal; E, internal elastic lamina; HFD, high fat diet; L, lipid; LM, light microscopy; NC, necrotic core; SMC, smooth muscle cell; T, media-tunica media.
Table 1.
CC presence, CC formation, and impact of CC on cell function and atherosclerosis in the past decade.
| Author, year, journal | Summary |
|---|---|
| Kobayashi, 2020, JACC Cardiovasc Interv42 | Case report suggesting CC involvement in atherosclerosis-like plaque formation in saphenous vein coronary bypass graft |
| Sugane, 2019, EHJ43 | Case report suggesting CC coronary atheroma as a precursor lesion to ACS |
| Lazareth, 2019, N Engl J Med44 | Case report of CC embolization after transcatheter aortic valve replacement |
| Fang, 2019, Atherosclerosis45 | CC are less frequently in culprit lesions of younger STEMI patients |
| Baumer, 2019, Atherosclerosis4 | Increasing presence of CC over time HFD is fed to LDLR−/− mice, ultrastructural analysis |
| Fujiyoshi, 2019, Atherosclerosis17 | Patients CC with culprit lesion had worse 1-year follow-up outcomes |
| Baumer, 2018, JCI Insight14 | Chronic inflammation accelerated macrophage CC formation and CC content in mouse atherosclerotic lesions |
| Lehti, 2018, Am J Pathol11 | CC presence shown by 3D electron microscopy in human lesions |
| Varsano, 2018, Proc Natl Acad Sci USA37 | Cholesterol monohydrate crystals from in two different structures with macrophages in vitro |
| Villinger, 2017, JACC: Card Imaging46 | Optical frequency-domain imaging allowed detection of CC within lesion |
| Baumer, 2017, Nat Commun13 | CC production by human aortic endothelial cells promotes atherogenesis |
| Abela, 2017, Am J Cardiol47 | Coronary artery aspirated after AMI had extensive deposits of CC and is associated with increased IL1b presence |
| Koide, 2017, PLoS One48 | Optical frequency-domain imaging detected increased CC presence in acute coronary syndrome when compared to stable angina pectoris |
| Nishimura, 2017, J Cardiol49 | Optical coherence tomography displays differences in CC location in human atherosclerotic lesions |
| Ho-Tin-Noe, 2017, J Pathol12 | CC detected in SMC during transition to fibroatheroma; CC-loaded SMC displayed CC formation |
| Kataoka, 2016, Circ Cardiovasc Imaging50 | Non-culprit plaques in woman less likely to harbour CC |
| Dai, 2016, Atherosclerosis51 | CC and vulnerable plaque features in humans identified by coherence tomography imaging |
| Janoudi, 2016, EHJ21 | CC induce arterial inflammation and are involved in plaque stability |
| Warnatsch, 2015, Science52 | CC-induced neutrophil NET formation priming macrophages and activating TH17 cells amplifying atherogenesis |
| Kataoka, 2015, JACC41 | CC within non-culprit lipid plaques associate with vulnerability of plaques |
| Sheedy, 2013, Nat Immunol38 | CD36 knockout in atherosclerotic mice resulted in lower serum IL-1β and decrease CC accumulation within plaques |
| Freigang, 2011, Eur J Immunol24 | CC-induced inflammasome activation is Nrf2 dependent impacting atherogenesis |
| Abela, 2010, J Clin Lipidol35 | CC-induced disruption of the atrial wall impacts inflammation |
| Rajamäki, 2010, PLoS One25 | CC activate the NLRP3 inflammasome in human macrophages |
| Duewell, 2010, Nature23 | NLRP3 inflammasome accelerates atherogenesis and is activated by CC |
While recent studies have contributed significantly to a broader understanding of the possible role of CC in plaque stability of human atherosclerosis utilizing SEM,21 most studies have focused on the ‘acute’ effects of CC on cellular derangement and subsequent disease pathogenesis. Treatment of macrophages with CC, as a simulation of CC presence within the atherosclerotic plaque, has been shown to:
impact macrophage cell membranes,22
activate the macrophage NLRP3 inflammasome,23–25 and
induce macrophage apoptosis.26
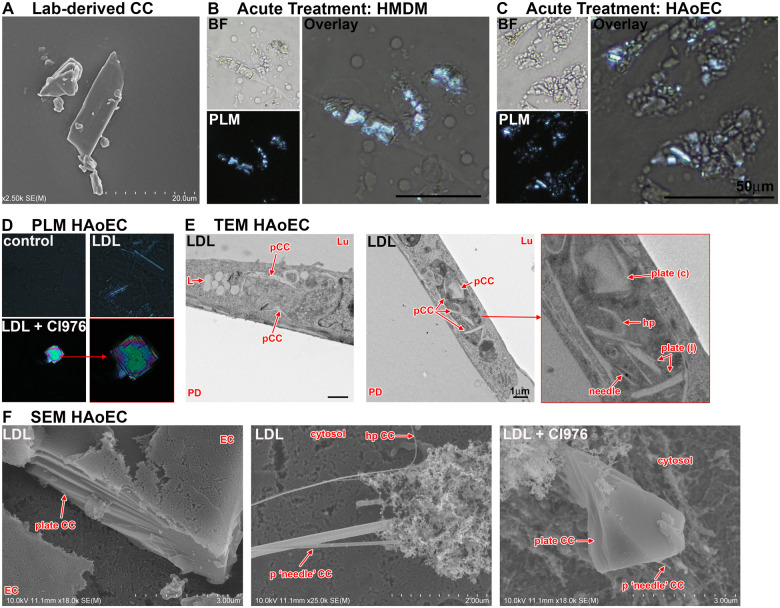
Cholesterol crystals are taken up by macrophages and other cell types (Figure 2A–C) which subsequently alter cellular function and survival. An involvement of cellular receptors has been described and discussed in the literature.27 The effect and impact of external CC on various cell types have also been studied in neutrophils28 and endothelial cells.29,30 Furthermore, the presence of CC has been implicated in the activation of the complement system,31 which by extension implicates CC’s involvement in coagulation events.32
Figure 2.
Cholesterol crystals in macrophages and endothelial cells. (A) Lab-derived cholesterol crystals imaged by scanning electron microscopy. (B/C) Human monocyte derived macrophages (HMDM) and human aortic endothelial cells (HAoEC) were incubated with 100 μg/mL CC for 24 h, fixed using 4% paraformaldehyde fixation buffer and imaged by polarized light microscopy. Cholesterol crystal can be seen to be taken up by human monocyte derived macrophages and human aortic endothelial cells. (D–F) Low-density lipoprotein treated HAoEC where imaged using polarized light microscopy (D), transmission electron microscopy (E), and scanning electron microscopy (F). Various shapes, sizes, and orientations of cholesterol crystal can be seen by either technique. Importantly, the impact of lipid-altering substances like CI-976 on cholesterol crystal shape and size highlights the importance of future research into signalling pathways regulating cholesterol crystal formation, shape, size, and origin. BF, bright field; CC, cholesterol crystal; EC, endothelial cell; hp, hairpin shaped CC; L, lipid; Lu, lumen/apical cell side; pCC, possible CC; PD, petri dish; plate (C), plate-shaped CC cross sectioned; plate (l), plate-shaped CC longitudinal sectioned; PLM, polarized light microscopy.
Despite the amount of research performed to understand the impact of CC within tissues and atherosclerotic plaques, relatively little is known about the underlying processes and mechanisms of cholesterol crystallization. Some suggest it to be an active process involving lipid bilayers33 and cell organelles,34 whereas some suggest it is a rather passive process due to saturation in the necrotic core.35 Crystallization of cholesterol has been observed in biliary diseases in which various forms and shapes of solid cholesterol have been described (needle-shaped, plate-shaped, filamentous, or helical) and have been implicated in their pathogenesis.36,37 In a major study of human carotid arteries, 3D electron microscopy was used to reveal both needle- and plate-shaped CC.11 Subsequent proteomic analysis showed apolipoprotein B to be the main component suggesting low-density lipoprotein (LDL) to be their origin.11 Furthermore, differences in the proteome of plasma lipoproteins and plaque-associated particles and crystals hint that lipolytic enzymes may be a contributing factor.11
Active CC formation within various cell types has been recently described. The most frequently studied cell type in the context of CC formation is the macrophage given its critical role in atherosclerotic plaque formation and progression.34 In addition to macrophages, active intracellular production of CC under hyperlipidaemic conditions has been shown in smooth muscle cells12 and aortic endothelial cells13 (Figure 2D–F), two additional important cell types important in atherogenesis. Although direct evidence of CC formation within a specific cell type is limited, in macrophages CC may form within lysosomes following treatment with oxidized LDL.34,38
Also, a recent study involving a monoclonal antibody that recognizes the narrow side of cholesterol monohydrate plates and cholesterol crystals indicates that cholesterol microdomains may participate in cellular CC formation and macrophage lipid homeostasis.39 Notably, the importance of lipid layers and membrane composition within the process of CC formation has been highlighted recently, indicating that the shape of CC produced by macrophages depends on the lipid composition of cellular membranes involved in the process of cholesterol crystallization.37 Amongst the physical and chemical factors impacting lab-derived CC formation, lower temperatures, increasing saturation and alkaline pH have been identified to influence CC formation.40
Electron microscopy evaluation of CC is of great interest given its resolution and strong foundation of existing literature on cell biology. However, an ongoing discussion amongst laboratories studying CC formation is the choice of techniques to determine the presence of CC. It is known, for example, that an ethanol dehydration step during sample preparation for SEM leads to lipid-based crystals dissolving in ethanol and thereby altering tissue morphology or underestimating quantity.5 An important factor in evaluating CC with TEM is necessarily equating the presence of CC with the ‘empty spaces/clefts’ left by the CC which has been dissolved during sample preparation with organic solvents (ethanol, resin). These indirect methods of demonstrating CC in the vasculature highlight the need for alternative imaging approaches or innovative ways of labelling CC for electron microscopy imaging. Additionally, it is difficult to differentiate between needle- and plate-shaped CC since the single ultra-thin (70 nm) TEM section does not permit 3D interpretation of the overall picture (Figure 2E/F). Polarized light microscopy does detect small, early CC but lacks the needed high-resolution to provide greater architecture. Thus, considering all of the challenges mentioned above in visualizing CC, we believe that focused ion beam scanning electron microscopy (FIB-SEM) with attached energy dispersive X-ray technology under cryo conditions, not requiring any organic solvents, could provide the highest resolution CC imaging in the most natural setting. However, because labs with the cryo-FIB-SEM setup are relatively rare and its operation is costly and time-consuming, analysing CC formation on a routine basis with this method in the vasculature or cell culture may be impractical for most labs.
Recent cardiovascular imaging advances have allowed scientists and medical professionals to have a much more detailed look into the morphology of atherosclerotic plaques, allowing a better understanding of the patients’ cardiovascular risk profile. Recent imaging advances using frequency-domain optical coherence tomography, for example, allows the visualization of CC in the patients coronary artery and formulation of a correlation to plaque vulnerability.41 Further development of innovative imaging techniques in the future could result in imaging of CC as an additional diagnostic tool to assess the extent, severity, and high-risk features of atherosclerotic plaques in patients. It will be important to determine the impact of lipid-lowering therapies like statins and anti-PCSK9 and/or anti-inflammatory therapies on CC formation in high-risk cardiovascular disease patients and its relationship with cardiovascular events. From the research perspective, it will be of critical importance to further understand and examine the underlying mechanisms behind cellular CC formation in atherogenesis. The deeper understanding of involved pathways as well as the actual cellular crystallization process itself will allow the development of targeted future therapies.
Data are available from the corresponding author upon reasonable request.
Supplementary Material
Acknowledgements
We would like to express our thanks and appreciation to Tina Weatherby from the Electron Microscopy Core at the University of Hawaii where all SEM and TEM images were taken presented in this review. The Hitachi HT7700 TEM was acquired with the help of NSF funding (DBI-1040548). We would also like to thank Dr J. Hammer and Dr E. Hellebrand at the School of Ocean and Earth Science and Technology (SOEST) at the University of Hawaii for the use of the polarized light microscope. The Social Determinants of Obesity and Cardiovascular Risk Laboratory is funded by the Division of Intramural Research at the National Heart, Lung, and Blood Institute, and the Intramural Research Program of the National Institute on Minority Health and Health Disparities. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; National Institute on Minority Health and Health Disparities; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Funding
The American Heart Association (#19TPA34850150 to W.B.).
Conflict of interest: The authors declare no conflicts of interest in relation to this work.
References
References are available as supplementary material at European Heart Journal online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.