Abstract
Gallbladder cancer (GBC) is a highly fatal cancer that can be cured through cholecystectomy if identified early. The presence of gallstones is the primary risk factor for GBC, but few people with gallstones develop GBC. A key question is what drives the development of GBC among persons with gallstones. We initiated the Chile Biliary Longitudinal Study (Chile BiLS) to address this question. From 2016 to 2019, Chile BiLS enrolled 4,726 women aged 50–74 years with ultrasound-detected gallstones from southern-central Chile, accounting for an estimated 36% of eligible women with gallstones in the study area. The median age was 59 years; 25% of the women were Amerindian (Mapuche), 60% were obese, 25% had diabetes, and 6% had cardiovascular disease. Participants will be followed for gallbladder dysplasia or cancer for 6 years. As of April 30, 2020, over 91% of those eligible completed the year 2 follow-up visit. Data being collected include epidemiologic and sociodemographic information, anthropometric measurements, blood pressure, and tooth counts. Biosamples being taken include baseline plasma, buffy coat, red blood cells, serum, blood clot from serum, and PAXgene whole blood (PreAnalytiX GmbH, Hombrechtikon, Switzerland). Complete gallbladder sampling is conducted for most participants undergoing cholecystectomy. The Chile BiLS cohort study will increase our understanding of GBC etiology and could identify potential risk stratification and early detection strategies in high-risk areas.
Keywords: Chile Biliary Longitudinal Study, etiology, gallbladder cancer, gallbladder dysplasia, gallstones, incidence
Abbreviations
- Chile BiLS
Chile Biliary Longitudinal Study
- FONASA
Fondo Nacional de Salud
- GBC
gallbladder cancer
Gallbladder cancer (GBC) is highly lethal, with 5-year survival rates less than 20% (1–4). While GBC is rare in much of the world, in some areas it is a major public health burden, such as south-central Chile (5). Because of its rarity at a global level, however, the etiology of GBC is poorly understood (6). Studying GBC in a high-risk area thus provides important opportunities to investigate its etiology and to identify potential strategies for prevention, early detection, and risk stratification.
Gallstones represent the main risk factor for GBC (6–8) and are present in up to 90% of cases in high-risk areas (9), but only a small proportion of people with gallstones develop GBC. The recommended treatment for symptomatic gallstones is cholecystectomy (i.e., surgical removal of the gallbladder); however, not all gallstone cases result in symptoms, and cholecystectomy may lead to medical complications and side effects, such as bile duct injury or increased long-term risk of other digestive diseases (10, 11). In addition, 10%–41% of patients experience persistent postcholecystectomy pain (12). Thus, a critical question is what drives the progression to gallbladder dysplasia, the immediate precursor of GBC (13), and eventually cancer among people with gallstones. We initiated the Chile Biliary Longitudinal Study (Chile BiLS) to address this question and identify potential risk stratification strategies. Because women are both twice as likely to have gallstones and twice as likely to develop GBC as men, we enrolled only women in the study (4, 14). Furthermore, GBC is among the top 5 causes of cancer death among women in Chile (15).
GBC is a particularly useful model for understanding inflammation-related carcinogenesis, since gallstones can lead to substantial inflammation in the gallbladder (16). Other proposed risk factors for GBC, such as obesity and infections like Salmonella enterica serovariant Typhi, also have an inflammatory component (17). Circulating inflammatory markers have been associated with both GBC and reduced survival after diagnosis (18–20). Conversely, statins, which reduce hyperlipidemia and have antiinflammatory effects, have been associated with lower incidence of biliary tract cancer, and aspirin has been associated with improved survival after GBC diagnosis (20, 21). Hormonal factors could also play a role, given the elevated risk of GBC among women and the increased GBC risk associated with increasing parity (8, 22). Novel associations have also been identified—for example, with aflatoxin (23, 24). Ultimately, prospective studies are needed to determine whether inflammatory markers and other factors contribute to the development of GBC among people with gallstones.
In this article, we describe the Chile BiLS cohort. The primary aim of Chile BiLS is to investigate epidemiologic and molecular risk factors for gallbladder dysplasia and cancer (e.g., inflammation markers, ultrasound characteristics such as a thickened gallbladder wall, and metabolic syndrome). A secondary aim is to examine the extent to which associations with factors like metabolic syndrome are mediated through inflammation. Chile BiLS will also facilitate evaluation of many novel associations with GBC in the context of gallstones.
METHODS
This cohort includes women aged 50–74 years with ultrasound-detected gallstones (Figure 1). The study was approved by the institutional review boards of the National Cancer Institute (US National Institutes of Health), the Pontificia Universidad Católica de Chile (Santiago, Chile), and the Chilean Ministry of Health. All participants provided written consent. Participants were recruited from 2 high-risk areas: the southern portion of the Araucanía region (Cautín Province) in southern Chile and Molina County in central Chile (Figure 2). In 2017, southern Araucanía had 752,100 inhabitants, including a large urban center (Temuco) with 282,415 inhabitants, and Molina had 45,976 inhabitants; 50% of the inhabitants in both areas were women (25). As of 2017, 17% of Araucanía residents and 13% of Maule residents were living in poverty and 29% and 23%, respectively, were in multidimensional poverty (based on measures of education, health, labor and social security, housing and local environment, and networks and social cohesion) (26).
Figure 1.
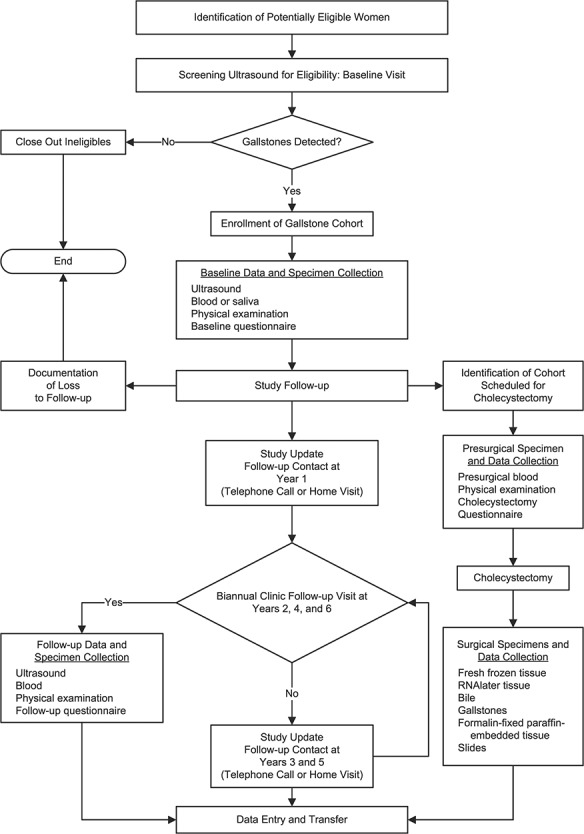
Design of the Chile Biliary Longitudinal Study.
Figure 2.
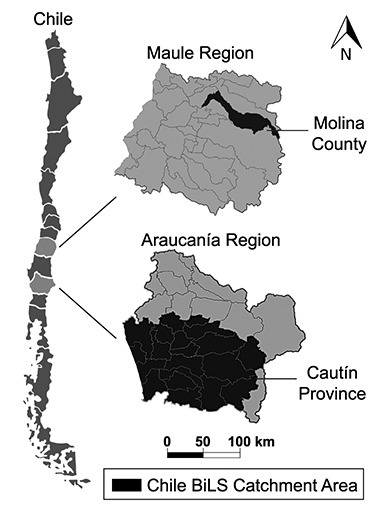
Catchment area of the Chile Biliary Longitudinal Study (Chile BiLS).
This study was initiated on January 18, 2016, and recruitment continued through January 16, 2019. Women were eligible if they were aged 50–74 years at the time of enrollment or turned 50 during the calendar year of their enrollment. Additionally, eligible women were required to reside within the catchment area, have no prior cholecystectomy, be mentally capable of answering study questionnaires, and be covered by Chile’s public health insurance system, the Fondo Nacional de Salud (FONASA), or another public insurance program provided to special groups in Chile (i.e., the Chilean armed forces or police service). FONASA covers approximately 80% of the Chilean population, with 4 hierarchical levels of coverage (FONASA groups A–D), determined by individual taxable income level.
Study recruitment mechanisms included door-to-door contact in selected urban areas (23%); recruitment efforts at local health centers, community centers, and hospitals and through media (63%); recruitment through ultrasonography, surgical consultation (to prescribe treatment, including cholecystectomy), and cholecystectomy waiting lists at health centers (12%); and invitation by local health center personnel (2%). In 3 sectors where we conducted a household census (out of 9 sectors in Temuco County)—Amanecer, Santa Rosa, and Pueblo Nuevo—we had sufficient information to calculate the response rate. Of 3,893 women contacted, 2,014 (51.7%) were eligible and 53 (1.4%) refused. Most ineligibility was due to previous cholecystectomy (n = 1,379/1,827; 75.5%). Of the eligible women contacted, 1,704 (84.6%) agreed to participate. Women from Molina were recruited through coenrollment with participants in the Maule Cohort, a prospective, population-based study of chronic diseases carried out in a high-risk area of Chile (27, 28).
We screened 19,239 women who met the entry criteria for gallstones using abdominal ultrasonography. Of these women, 4,726 (24.6%) had gallstones, completed the baseline visit, and were enrolled in Chile BiLS. All women identified as having gallstones agreed to be part of the study. As a benchmark for the representativeness of our population, we estimated the number of publicly insured women in our study regions who had gallstones. We used regional census data to obtain the number of women aged 50–74 years and assumed that 80% were enrolled in the public health system, 26% had cholecystectomy, and 25% had gallstones. We then compared the number of women expected with the number enrolled. On the basis of these calculations, we estimate that we enrolled 36% of eligible women with gallstones in the study area.
Participants will have follow-up study visits at years 2, 4, and 6. In addition, participants were contacted via telephone or through a home visit 1 year after their baseline visit to verify their contact information and maintain their interest in the study. Through April 30, 2020, compliance for the year 2 study visit was 91.4%. Participants who undergo cholecystectomy after enrollment are identified primarily through scheduled surgeries provided by all public hospitals and 1 private hospital in the study area, identification by one of the 3 pathology laboratories of these hospitals (Temuco Hospital, Clínica Mayor, or Curicó Hospital), or perisurgery notification by the study participant (see Web Table 1, available at https://academic.oup.com/aje). Participants are followed for death through periodic linkage with the death registry.
The primary outcomes of gallbladder dysplasia and cancer are identified through pathology or linkage with the death registry. On the basis of published data from Chile (29), we estimated that 50% of the cohort would undergo surgery over the course of the study. Preliminary data suggest that about 1% participants are proceeding to cholecystectomy each month, leading to half of the cohort undergoing surgery over the course of the study after accounting for loss to follow-up. The attrition rate to date is 25.5%. Censoring events included cholecystectomy (22.6%), death (1.1%), change of city of residence (0.1%), or refusal (1.7%). As of April 30, 2020, 3,506 participants are available for further follow-up. On the basis of cancer incidence data from the Valdivia Cancer Registry in the high-risk area of Chile (2) and published data from cholecystectomy patients from this region (13, 30), we estimate that we will identify approximately 100 GBC cases and at least as many gallbladder dysplasias. To account for potential prevalent disease, we will conduct sensitivity analyses excluding the first 2 years of follow-up. We will also correct estimates of association as previously described (31) using an estimated baseline prevalence of 5%–10% gallbladder dysplasia and cancer (13, 32).
At the baseline visit, participants underwent a detailed hepatobiliary ultrasound examination; completed an epidemiologic questionnaire with over 650 items, including questions on sociodemographic factors, medical history, gastrointestinal symptoms, medication use, family history, reproductive factors, behavioral factors, and pesticide exposure; underwent a physical examination, including anthropometric measurements, measurement of blood pressure, and a tooth count; and blood collection, including use of PAXgene (PreAnalytiX GmbH, Hombrechtikon, Switzerland) for gene expression (Table 1). At follow-up visits, participants undergo an ultrasonogram, a physical examination, blood collection, and a shortened form of the questionnaire for ascertainment of updated exposure information. If identified prior to surgery, participants have presurgery blood collected and are given a physical examination and a short questionnaire.
Table 1.
Data and Biospecimens Collected During Enrollment for the Chile Biliary Longitudinal Study, 2016–2019
| Data/Biospecimen | Description | Time Point(s) of Collection | Baseline Completion Rate | |||
|---|---|---|---|---|---|---|
| Baseline | Follow-up a | Perisurgery |
No. of Participants |
% | ||
| Hepatobiliary ultrasound | 20-minute ultrasonographic examination. Biliary and liver observations recorded and photographed by ultrasound technician. Ultrasound images stored for future digital analyses. | X | X | 4,726 | 100.0 | |
| Physical examination | Height, weight, waist circumference, hip circumference, blood pressure, and tooth count. | X | X | X | 4,695 | 99.3 |
| Blood sample | 20 mL of blood aliquoted into plasma, buffy coat, red blood cells, serum, and blood clot, plus 2.5 mL of PAXgene (PreAnalytiX GmbH, Hombrechtikon, Switzerland) whole blood (baseline only); if no blood available for DNA, 2 mL of Oragene (DNA Genotek, Ottawa, Ontario, Canada) saliva (baseline only). | X | X | X | 4,559 | 96.4 |
| Full questionnaire | 679 items, administered by a health technician. Includes questions on sociodemographic factors, medical history, gastrointestinal symptoms, medication use, family history, reproductive factors, behavioral factors, and pesticide exposure. | X | 4,673 | 98.9 | ||
| Abbreviated questionnaire | 382 items, administered by a health technician. Includes questions on medical history, gastrointestinal symptoms, medication use, reproductive factors, cigarette smoking, alcohol drinking, and diet. | X | ||||
| Perisurgery questionnaire | 83 items, administered by a health technician. Includes questions on gastrointestinal symptoms and medical history. | X | ||||
| Gallbladder tissue | Paraffin-embedded tissue blocks and slides created from the entire gallbladder (majority of cases) or routine blocks and slides created only. Fresh RNAlater (Fisher Scientific Company, Nazareth, Pennsylvania) and snap-frozen tissue also collected when possible. Formalin-fixed, paraffin-embedded tissue samples are histologically reviewed and scanned at high resolution for future digital pathology studies. | X | ||||
| Gallstones | Collected intraoperatively or at pathology laboratory. Frozen at −80°C. | X | ||||
| Bile | Collected intraoperatively (2.3–10 mL) or at pathology laboratory, when possible. Frozen at −80°C. | X | ||||
a Years 2, 4, and 6 of follow-up.
Additionally, snap-frozen and RNAlater-preserved (Fisher Scientific Company, Nazareth, Pennsylvania) tissue, bile, and gallstones are collected at cholecystectomy. Formalin-fixed, paraffin-embedded tissue is collected for the majority of cholecystectomized participants. Although it is common in clinical practice to collect only 1 formalin-fixed, paraffin-embedded block containing a section from the neck, body, and fundus of the gallbladder, in Chile BiLS the entire gallbladder is processed whenever cohort members in Araucanía are identified prior to pathology processing. To date, an average of 19 formalin-fixed, paraffin-embedded blocks (range, 2–70) are collected per participant among those with formalin-fixed, paraffin-embedded blocks collected. Our previous work has shown that diagnostic sampling techniques used in routine clinical practice may miss nearly 40% of low-grade gallbladder dysplasias (13). Thus, complete histological sampling and review in Chile BiLS offers us a unique opportunity to identify and comprehensively characterize histological changes throughout the gallbladder and to analyze associations with both cancer and low-grade dysplastic lesions. Data related to surgical procedures are obtained from the regional health-service databases. GBC and dysplasia are identified primarily through pathology review; however, some GBC cases are identified through death registry linkage.
The data that support the findings of this study are available upon request from the corresponding authors. The data are not publicly available because of privacy and/or ethical restrictions.
RESULTS
All participants completed the baseline hepatobiliary ultrasound examination (Table 1). In addition, 98.9% of them completed the baseline questionnaire, and 99.3% completed the physical examination. Furthermore, 96.4% provided blood, and of those who did not, 62.5% (115/184) provided saliva for DNA analysis.
The median age at enrollment was 59 years (interquartile range, 54–64), with the largest proportion (29.3%) being aged 50–54 years. Seventy-two percent of enrolled women identified as Chilean/Latino, while 25.1% identified as Mapuche, the major ethnic group indigenous to Chile’s southern region (Table 2). Over half of the cohort completed 8 or fewer years of education. The majority of women belonged to the lowest income groups (42.6% were in FONASA group A, which corresponds to no taxable income, and 33.3% were in FONASA group B, which corresponds to a taxable income at or below 1.6 times the poverty level, per the Chilean Ministry of Social Development (33, 34)).
Table 2.
Sociodemographic Characteristics of Women in the Chile Biliary Longitudinal Study (n = 4,726) at Enrollment, 2016–2019
| Characteristic | No. | % |
|---|---|---|
| Age, yearsa | 59 (54–64) | |
| 50–54 | 1,387 | 29.3 |
| 55–59 | 1,222 | 25.9 |
| 60–64 | 1,005 | 21.3 |
| 65–69 | 697 | 14.7 |
| 70–74 | 415 | 8.8 |
| Ethnicityb | ||
| Chilean/Latino | 3,407 | 72.1 |
| Mapuche | 1,184 | 25.1 |
| European | 25 | 0.5 |
| Other | 6 | 0.1 |
| Missing or unknownc | 104 | 2.2 |
| Education, no. of years completed | ||
| ≤8 | 2,517 | 53.3 |
| 9–12 | 1,741 | 36.8 |
| ≥13 | 388 | 8.2 |
| Missing or unknownc | 80 | 1.7 |
| Health coveraged | ||
| FONASA group | ||
| Group A | 2,014 | 42.6 |
| Group B | 1,573 | 33.3 |
| Group C | 294 | 6.2 |
| Group D | 274 | 5.8 |
| Unknown | 326 | 6.9 |
| Other health coverage | 102 | 2.2 |
| Missing or unknownc | 143 | 3.0 |
Abbreviation: FONASA, Fondo Nacional de Salud.
a Values are expressed as median (interquartile range).
b Ethnicity was self-reported. The Mapuche are an ethnic group indigenous to Chile’s southern region. “Other” includes Aymara/Quechua, Easter Islander, or another ethnicity.
c Includes missing data and the response “Participant doesn’t know/doesn’t respond.”
d FONASA is Chile’s public health insurance system. Groups A–D are hierarchical classifications used to determine the proportion of health-care costs covered by the government, based on taxable individual income. FONASA group A has the lowest income level and receives the highest level of governmental coverage of health-care costs. “Other health coverage” represents governmental health coverage provided specifically for the Chilean armed forces or police service, private insurance, or other insurance.
Table 3.
Continued
| Characteristic | No. | % |
|---|---|---|
| Weekly frequency of fried food consumptiona | ||
| None | 2,038 | 43.1 |
| Once | 1,961 | 41.5 |
| Twice or more | 639 | 13.5 |
| Missing or unknownb | 88 | 1.9 |
| Diabetes (ever diagnosed)a | ||
| No | 3,468 | 73.4 |
| Yes | 1,186 | 25.1 |
| Missing or unknownb | 72 | 1.5 |
| Cardiovascular disease (ever diagnosed)a,e | ||
| No | 4,357 | 92.2 |
| Yes | 284 | 6.0 |
| Missing or unknownb | 85 | 1.8 |
| Hepatitis (ever diagnosed)a | ||
| No | 4,471 | 86.6 |
| Yes | 167 | 3.5 |
| Missing or unknownb | 88 | 9.9 |
| Personal history of cancer (any)a | ||
| No | 4,397 | 93.0 |
| Yes | 251 | 5.3 |
| Missing or unknownb | 78 | 1.7 |
| Family history of gallstonesa | ||
| No | 2,366 | 50.1 |
| Yes | 2,360 | 49.9 |
| Family history of gallbladder cancera | ||
| No | 4,585 | 97.0 |
| Yes | 141 | 3.0 |
| Previous gallstone diagnosisa | ||
| No | 3,214 | 68.0 |
| Yes | 1,430 | 30.3 |
| Missing or unknownb | 82 | 1.7 |
| Biliary colic (ever experiencing intense epigastric pain lasting >30 minutes)a | ||
| No | 2,692 | 57.0 |
| Yes | 1,564 | 33.1 |
| Missing or unknownb | 470 | 9.9 |
| No. of gallstones detected on ultrasound | ||
| 1 | 1,902 | 40.2 |
| ≥2 | 2,179 | 46.1 |
| Not determinedf | 645 | 13.6 |
Table 3.
Continued
| Characteristic | No. | % |
|---|---|---|
| No. of lost teeth | ||
| 0 | 641 | 13.6 |
| 1–6 | 683 | 14.5 |
| 7–13 | 1,038 | 22.0 |
| 14–24 | 1,240 | 26.2 |
| >24 | 1,071 | 22.7 |
| Missing data | 53 | 1.1 |
| Body mass indexg | ||
| Underweight (<18.5) | 6 | 0.1 |
| Normal weight (18.5–24.9) | 420 | 8.9 |
| Overweight (25.0–29.9) | 1,427 | 30.2 |
| Obese (≥30.0) | ||
| Class I (30–34.9) | 1,503 | 31.8 |
| Class II (35–39.9) | 853 | 18.1 |
| Class III (≥40) | 463 | 9.8 |
| Missing or unknownb | 54 | 1.1 |
| Blood pressureh | ||
| Normal | 811 | 17.2 |
| Elevated | 526 | 11.1 |
| Hypertension (stage I/II) | 3,314 | 70.1 |
| Missing or unknownb | 75 | 1.6 |
a Self-reported via baseline risk factor questionnaire.
b Includes missing data and the response “Participant doesn’t know/doesn’t respond.”
c Never: <100 cigarettes smoked over a lifetime; former: smoked ≥100 cigarettes over a lifetime, not currently smoking; current: smoked ≥100 cigarettes over a lifetime, currently smoking.
d Never: has never consumed alcohol; former: does not currently consume alcohol but has consumed it in the past; current: currently consumes alcohol.
e Any self-reported diagnosis of heart attack, stroke, or any other cardiovascular disease.
f Not determined; unable to distinguish number of gallstones on ultrasound.
g Weight (kg)/height (m)2.
h Based on the average of 3 measurements taken an average of 5.8 minutes apart. Categorized per American College of Cardiology 2017 guidelines (45).
Most women (91.7%) had at least 1 live birth (Table 3). Among parous women, the median number of births was 3 (interquartile range, 2–4). About half of the women (52.5%) had ever used hormonal contraception, while only 4.9% had ever used hormone replacement therapy. The cohort includes 17.7% current cigarette smokers, 65.6% current alcohol drinkers, and 8.9% participants with exposure to pesticides. In addition, 20.9% reported regular use of aspirin, 2.7% regular use of nonsteroidal antiinflammatory drugs, and 25.9% regular use of statins. Fried food consumption was common, with 55.0% eating fried foods at least once per week. Twenty-five percent reported a previous diagnosis of diabetes, 6.0% cardiovascular disease, 3.5% hepatitis, and 5.3% cancer. Fifty percent had a family history of gallstones, and 3.0% had a family history of GBC. More than two-thirds of the cohort had lost 7 or more teeth. Sixty percent of the cohort was obese; 9.8% had severe (class III) obesity. The majority (70.1%) had stage I or stage II hypertension.
Table 3.
Baseline Epidemiologic and Clinical Characteristics of Women in the Chile Biliary Longitudinal Study (n = 4,726) at Enrollment, 2016–2019
| Characteristic | No. | % |
|---|---|---|
| No. of live birthsa | ||
| 0 | 319 | 6.7 |
| 1–2 | 1,612 | 34.1 |
| 3–4 | 1,938 | 41.0 |
| ≥5 | 783 | 16.6 |
| Missing or unknownb | 74 | 1.6 |
| Hormonal medication (ever use)a | ||
| Never | 2,068 | 43.8 |
| Contraceptives only | 2,338 | 49.5 |
| Hormone replacement therapy only | 92 | 1.9 |
| Both (contraceptives and hormone replacement) | 141 | 3.0 |
| Missing or unknownb | 87 | 1.8 |
| Cigarette smoking statusa,c | ||
| Never smoker | 2,845 | 60.2 |
| Former smoker | 971 | 20.6 |
| Current smoker | 837 | 17.7 |
| Missing or unknownb | 73 | 1.5 |
| Alcohol drinking statusa,d | ||
| Never drinker | 1,179 | 25.0 |
| Former drinker | 370 | 7.8 |
| Current drinker | 3,100 | 65.6 |
| Missing or unknownb | 77 | 1.6 |
| Pesticide exposure (ever exposed)a | ||
| No | 4,175 | 88.3 |
| Yes | 420 | 8.9 |
| Missing or unknownb | 131 | 2.8 |
| Regular use of aspirina | ||
| No | 3,266 | 69.1 |
| Yes | 989 | 20.9 |
| Missing or unknownb | 471 | 10.0 |
| Regular use of nonsteroidal antiinflammatory drugsa | ||
| No | 4,132 | 87.4 |
| Yes | 128 | 2.7 |
| Missing or unknownb | 466 | 9.9 |
| Regular use of statinsa | ||
| No | 3,030 | 64.1 |
| Yes | 1,223 | 25.9 |
| Missing or unknownb | 473 | 10.0 |
Most gallstones were asymptomatic. Only 30.3% reported a previous diagnosis of gallstones, and only a third of the cohort reported previously experiencing biliary colic (i.e., intense upper right quadrant or epigastric pain without diarrhea lasting more than 30 minutes) (Table 3). Of the 1,564 participants reporting biliary colic, 725 (46.4%) reported having had intense abdominal pain/cramps in the last 5 years, as did 453 participants without self-reported biliary colic. The number of gallstones could not be distinguished on ultrasound for 13.6% of the cohort (Table 3) (e.g., if the gallbladder was small and extensively fibrotic, it was not possible to determine whether there were many gallstones packed together or 1 large stone; or if many small stones were present, it may not have been possible to accurately determine the number of them). Among women for whom the number of gallstones could be determined, 46.1% had more than 1.
DISCUSSION
Chile has one of the highest GBC incidence and mortality rates in the world. The very high rates of GBC and gallstones in Chile provide a unique opportunity to test several emerging hypotheses that are difficult to examine in other populations. Although GBC is not common in developed countries like the United States, even in the United States there are populations that have a higher incidence of GBC, including Hispanics and Amerindians (35). Thus, Chile BiLS may help inform our understanding of risk in those populations as well (Figure 3).
Figure 3.
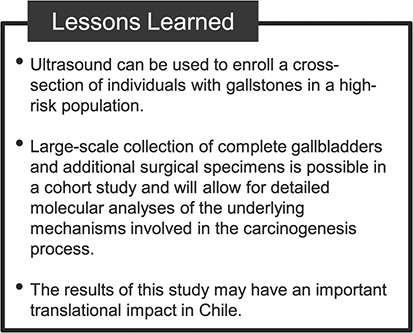
Lessons learned from the Chile Biliary Longitudinal Study.
Chile BiLS is unique in that it allows examination of risk factors for GBC and dysplasia in the context of gallstones in a high-risk population. Because Chile BiLS includes only people with ultrasound-confirmed gallstones, we will be able to assess whether potential risk factors contribute to GBC beyond their contribution to the risk of gallstones. Many prior studies have been unable to adequately ascertain risk factors that contribute to GBC independently of gallstones because of reliance on self-reported diagnoses of gallstones and cholecystectomies, which cannot capture undiagnosed stones.
The results of Chile BiLS may also have an important translational impact in Chile, where GBC is a leading cause of cancer death in women and the need for cholecystectomies exceeds the number of surgeons able to perform them. Current clinical guidelines in Chile dictate that persons aged 35–49 years with gallstones should be prioritized for prophylactic cholecystectomy through the public health system (36). This practice may lead to overtreatment among 35- to 49-year-olds and undertreatment of persons aged 50 years or more, who typically must wait longer for surgery. Currently, there is no approach for identifying and prioritizing patients at risk of developing GBC. Thus, in addition to informing etiology, the Chile BiLS cohort offers an important opportunity to identify potential risk stratification and early detection strategies.
Key risk factors we plan to evaluate in the future include inflammation (e.g., circulating immunological markers, immune infiltrates in tissue), ultrasound characteristics (e.g., a thickened gallbladder wall), diabetes, obesity, and metabolic syndrome. Based on case-control studies from Chile and Shanghai, China (18, 19), we hypothesize that persons who develop gallbladder dysplasia and cancer will have altered baseline levels of circulating immunological markers in comparison with those who do not develop gallbladder dysplasia and cancer. Further, we will explore whether gallbladder ultrasound images can identify and predict preneoplastic and neoplastic gallbladder lesions. We will also investigate the extent to which observed associations between metabolic syndrome, including diabetes and obesity, and GBC (37, 38) may be mediated through inflammation. Additionally, we plan to follow up on the recent observation that aflatoxin is associated with GBC (23, 24). Chile BiLS will provide an important opportunity to evaluate this association prospectively in a population where we have current data indicating elevated aflatoxin exposure. Other relevant exposures include infections like S. enterica serovariant Typhi (39) and metals and micronutrients (40).
In addition, collection of a range of biological specimens for participants undergoing cholecystectomy, including fresh frozen tissue, bile, gallstones, and formalin-fixed, paraffin-embedded tissue from the entire gallbladder, is a unique and important aspect of this study, as it is rarely possible to collect these specimens in routine clinical practice. We hope that these biospecimens will allow for detailed molecular analyses of the underlying mechanisms involved in the carcinogenic process—for example, through whole-genome sequencing, tumor transcriptomics, etc.
The Chile BiLS cohort is likely to be broadly representative of women with gallstones in this high-risk region, given that we estimate we have recruited 36% of all women aged 50–74 years with gallstones in the study area. The extensive questionnaire data cover a broad range of exposures. Biospecimens are available for nearly all participants. Collection of the complete gallbladder for the majority of women who undergo surgery is particularly important to facilitate studies of inflammatory infiltrates and other molecular characteristics. Mapping the heterogeneity of the molecular profile within the gallbladder and across the natural history of disease will greatly increase our understanding of the role of inflammation and other factors in carcinogenesis. Furthermore, given that the entire gallbladder will be available for the majority of participants who undergo surgery, it will be possible to examine molecular characteristics without bias from incomplete sampling.
Because all Chile BiLS participants have gallstones, findings may not apply to the general population. However, a gallstone cohort enables us to evaluate the factors that drive progression from gallstones to gallbladder dysplasia and cancer. Chile BiLS is a particularly valuable gallstone cohort, since most other gallstone cohorts are from the United States and Europe (41–44), where ethnicity, socioeconomic status, diet, and environmental exposures are quite different from those in this population with a high baseline risk of GBC. Many prior studies have only included patients with symptomatic gallstones, limiting the ability to understand drivers of progression among nonsymptomatic cases. In contrast, Chile BiLS includes not only symptomatic women who were recruited through clinics and hospitals but also women recruited through door-to-door contact and other community-based efforts. The fact that only a third of our participants reported having experienced biliary colic at baseline suggests we have succeeded in including a significant proportion of nonsymptomatic participants.
Although all participants have gallstones, there may be concerns that cholecystectomies could be driven by symptoms, and therefore observed associations with gallbladder dysplasia and cancer could potentially reflect associations with symptoms. To address this concern, we have collected detailed information on symptoms at baseline and follow-up and as participants undergo cholecystectomy, allowing us to take symptoms into account in analysis. We also expect that a majority of participants will undergo surgery over the course of follow-up, which will mitigate potential bias resulting from preferential selection of symptomatic cases for cholecystectomy. While Chile BiLS is a sizeable cohort in a high-risk population, the number of cancer outcomes may be limited. However, there will be many more cases of dysplasia and intestinal metaplasia, which will facilitate evaluation of associations across the natural history of disease.
Chile BiLS is a unique cohort aimed at assessing the factors that drive the development of gallbladder dysplasia and cancer among people with gallstones. Chile is an optimal setting given its high gallstone prevalence and GBC incidence, which facilitate studies of GBC risk among people with gallstones. This large, multidisciplinary, international collaboration will provide new insights into GBC etiology and could identify new approaches to cancer prevention and early detection.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, Maryland, United States (Jill Koshiol, Emma E. McGee, Ruth M. Pfeiffer, Sarah S. Jackson, Allan Hildesheim); Facultad de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile (Vanessa Van De Wyngard, Paz Cook, Fabio Paredes, Juan Carlos Roa, Catterina Ferreccio); Centro Avanzado de Enfermedades Crónicas, Fondo de Financiamiento de Centros de Investigación en Áreas Prioritarias, Santiago, Chile (Vanessa Van De Wyngard, Paz Cook, Fabio Paredes, Andrea Huidobro, Juan Carlos Araya, Catterina Ferreccio); Harvard T.H. Chan School of Public Health, Boston, Massachusetts, United States (Emma E. McGee); Hospital Dr. Hernan Henríquez Aravena, Temuco, Chile (Noldy Mardones, Karie Medina, Miguel Villaseca, Enrique Bellolio, Hector Losada, Juan Carlos Araya); Westat, Inc., Rockville, Maryland, United States (Vanessa Olivo, Karen Pettit); Facultad de Medicina, Universidad Católica del Maule, Talca, Chile (Andrea Huidobro); Facultad de Medicina, Universidad de La Frontera, Temuco, Chile (Raúl Sanchez, Enrique Bellolio, Hector Losada, Juan Carlos Araya).
This work was supported by general funds from the Intramural Research Program of the US National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics; the Office of Research on Women’s Health, National Institutes of Health; the Comisión Nacional de Investigación Científica y Tecnológica for the Fondo de Financiamiento de Centros de Investigación en Áreas Prioritarias (grant 15130011) and the Fondo Nacional de Desarrollo Científico y Tecnológico (grant 1170395) from the government of Chile; the Facultad de Medicina of the Universidad Católica del Maule; the Facultad de Medicina of the Pontificia Universidad Católica de Chile; and the Universidad de La Frontera.
The success of this investigation would not have been possible without exceptional teamwork and the diligence of the field staff who oversaw the recruitment, interviews, and collection of data from study subjects. Special thanks are due to the following individuals: Ricardo Erazo, Marjorie Barrera, Dr. Macarena Garrido, Cristián Herrera, and Philippe Delteil from the Santiago team; Pía Riquelme, Marta Mercado, Verónica Toledo, Samuel Arias, Magdalena Fernandez, and Constanza Pardo from the Temuco team; Fernando Herrera, Katherine Brito, and Pía Venegas from the Molina team; and Dr. Flery Fonseca from the Universidad de La Frontera. Dr. Volkan Adsay provided pathology expertise. Jane Demuth and Greg Rydzak at Information Management Services, Inc. (Rockville, Maryland), provided study management assistance. We also express appreciation to the many women who agreed to participate in the study and to provide information and biospecimens in hopes of improving GBC prevention and outcomes in Chile.
Members of the Chile BiLS Study Group: Flery Fonseca, Sergio Muñoz, Marta Mercado, Verónica Toledo, Magdalena Fernández, Samuel Arias, Constanza Pardo, Mariana Poblete, Fabiola Valenzuela, Maryorie Melo, Viviana Espinosa, Susana Tropa, Yoselin Cisternas, Marisela Campos, and Rosa Paillaman.
This research was presented at the 51st Annual Meeting of the Society for Epidemiologic Research, Baltimore, Maryland, June 19–22, 2018, and the 2019 Annual Meeting of the NCI Cohort Consortium, Rockville, Maryland, November 18–20, 2019.
Conflict of interest: none declared.
REFERENCES
- 1. Lazcano-Ponce EC, Miquel JF, Muñoz N, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51(6):349–364. [DOI] [PubMed] [Google Scholar]
- 2. Bertran E, Heise K, Andia ME, et al. Gallbladder cancer: incidence and survival in a high-risk area of Chile. Int J Cancer. 2010;127(10):2446–2454. [DOI] [PubMed] [Google Scholar]
- 3. Randi G, Malvezzi M, Levi F, et al. Epidemiology of biliary tract cancers: an update. Ann Oncol. 2009;20(1):146–159. [DOI] [PubMed] [Google Scholar]
- 4. Koshiol J, Ferreccio C, Devesa SS, et al. Biliary tract cancer In: Thun MJ, Linet MS, Cerhan JR, et al., eds. Cancer Epidemiology and Prevention. 4th ed. New York, NY: Oxford University Press; 2017:661–670. [Google Scholar]
- 5. Miranda-Filho A, Piñeros M, Ferreccio C, et al. Gallbladder and extrahepatic bile duct cancers in the Americas: incidence and mortality patterns and trends. Int J Cancer. 2020;147(4):978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wistuba II, Gazdar AF. Gallbladder cancer: lessons from a rare tumour. Nat Rev Cancer. 2004;4(9):695–706. [DOI] [PubMed] [Google Scholar]
- 7. Ishiguro S, Inoue M, Kurahashi N, et al. Risk factors of biliary tract cancer in a large-scale population-based cohort study in Japan (JPHC Study); with special focus on cholelithiasis, body mass index, and their effect modification. Cancer Causes Control. 2008;19(1):33–41. [DOI] [PubMed] [Google Scholar]
- 8. Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118(7):1591–1602. [DOI] [PubMed] [Google Scholar]
- 9. Lai CH, Lau WY. Gallbladder cancer—a comprehensive review. Surgeon. 2008;6(2):101–110. [DOI] [PubMed] [Google Scholar]
- 10. Behari A, Kapoor VK. Asymptomatic gallstones (AsGS)—to treat or not to? Indian J Surg. 2012;74(1):4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nogueira L, Freedman ND, Engels EA, et al. Gallstones, cholecystectomy, and risk of digestive system cancers. Am J Epidemiol. 2014;179(6):731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Dijk AH, Wennmacker SZ, de Reuver PR, et al. Restrictive strategy versus usual care for cholecystectomy in patients with gallstones and abdominal pain (SECURE): a multicentre, randomised, parallel-arm, non-inferiority trial. Lancet. 2019;393(10188):2322–2330. [DOI] [PubMed] [Google Scholar]
- 13. Koshiol J, Bellolio E, Vivallo C, et al. Distribution of dysplasia and cancer in the gallbladder: an analysis from a high cancer-risk population. Hum Pathol. 2018;82:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shaffer EA Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century? Curr Gastroenterol Rep. 2005;7(2):132–140. [DOI] [PubMed] [Google Scholar]
- 15. International Agency for Research on Cancer Global Cancer Observatory. gco.iarc.fr. Accessed October 31, 2019.
- 16. Espinoza JA, Bizama C, García P, et al. The inflammatory inception of gallbladder cancer. Biochim Biophys Acta. 2016;1865(2):245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Izarzugaza MI, Fernández L, Forman D, et al. Burden of gallbladder cancer in Central and South America. Cancer Epidemiol. 2016;44(suppl 1):S82–S89. [DOI] [PubMed] [Google Scholar]
- 18. Koshiol J, Castro F, Kemp TJ, et al. Association of inflammatory and other immune markers with gallbladder cancer: results from two independent case-control studies. Cytokine. 2016;83:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koshiol J, Gao YT, Corbel A, et al. Circulating inflammatory proteins and gallbladder cancer: potential for risk stratification to improve prioritization for cholecystectomy in high-risk regions. Cancer Epidemiol. 2018;54:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu Z, Kemp TJ, Gao YT, et al. Circulating levels of inflammatory proteins and survival in patients with gallbladder cancer. Sci Rep. 2018;8(1):5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jackson SS, Pfeiffer RM, Liu Z, et al. Association between aspirin use and biliary tract cancer survival. JAMA Oncol. 2019;5(12):1802–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jackson SS, Adami HO, Andreotti G, et al. Associations between reproductive factors and biliary tract cancers in women from the Biliary Tract Cancers Pooling Project. J Hepatol. 2020;73(4):863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koshiol J, Gao YT, Dean M, et al. Association of aflatoxin and gallbladder cancer. Gastroenterology. 2017;153(2):488–494.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nogueira L, Foerster C, Groopman J, et al. Association of aflatoxin with gallbladder cancer in Chile. JAMA. 2015;313(20):2075–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Instituto Nacional de Estadísticas. Entrega Final Censo 2017. Estimaciones y proyecciones de la población de Chile 1992–2050 (total país). http://www.censo2017.cl/. Accessed October 31, 2019.
- 26. Ministerio de Desarrollo Social y Familia Encuesta CASEN 2017. http://observatorio.ministeriodesarrollosocial.gob.cl/casen-multidimensional/casen/casen_2017.php. Accessed October 31, 2019.
- 27. Ferreccio C, Roa JC, Bambs C, et al. Study protocol for the Maule Cohort (MAUCO) of chronic diseases, Chile 2014–2024. BMC Public Health. 2016;16:Article 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferreccio C, Huidobro A, Cortes S, et al. Cohort profile: the Maule Cohort (MAUCO). Int J Epidemiol. 2020;49(3):760–761i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pérez-Ayuso RM, Hernández V, González B, et al. Natural history of cholelithiasis and incidence of cholecystectomy in an urban and a Mapuche rural area. Rev Med Chil. 2002;130(7):723–730. [PubMed] [Google Scholar]
- 30. Roa I, de Aretxabala X, Araya JC, et al. Preneoplastic lesions in gallbladder cancer. J Surg Oncol. 2006;93(8):615–623. [DOI] [PubMed] [Google Scholar]
- 31. Lee T Bias-adjusted exposure odds ratio for misclassified data. Internet J Epidemiol. 2008;6(2):Article 5252 http://ispub.com/IJE/6/2/5252#. Accessed May 22, 2020. [Google Scholar]
- 32. Csendes A, Alvarez F, Medina E, et al. Prevalencia de sintomas digestivos en mujeres adultas normales y su asociacion a la litiasis biliar. Rev Med Chil. 1995;122(5):531–536. [PubMed] [Google Scholar]
- 33. Ministerio de Desarrollo Social y Familia, Gobierno de Chile Informe Mensual Valor de la Canasta Básica de Alimentos y Líneas de Pobreza Julio 2016. http://observatorio.ministeriodesarrollosocial.gob.cl/layout/doc/ipc/Valor%20CBA%20y%20LP%C2%B4s%2016.07.pdf. Accessed November 29, 2020.
- 34. Superintendencia de Salud, Gobierno de Chile Materias FONASA. http://www.supersalud.gob.cl/consultas/667/w3-article-6304.html. Accessed November 29, 2020.
- 35. Van Dyke AL, Shiels MS, Jones GS, et al. Biliary tract cancer incidence and trends in the United States by demographic group, 1999–2013. Cancer. 2019;125(9):1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Salud M Guía Clínica Colecistectomía Preventiva en Adultos de 35 a 49 años. Santiago, Chile: Subsecretaría de Salud Pública, División de prevención y Control de Enfermedades, Departamento Manejo Integral del Cáncer y otros Tumores; 2014. [Google Scholar]
- 37. Campbell PT, Newton CC, Kitahara CM, et al. Body size indicators and risk of gallbladder cancer: pooled analysis of individual-level data from 19 prospective cohort studies. Cancer Epidemiol Biomarkers Prev. 2017;26(4):597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jackson SS, Van Dyke AL, Zhu B, et al. Anthropometric risk factors for cancers of the biliary tract in the Biliary Tract Cancers Pooling Project. Cancer Res. 2019;79(15):3973–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koshiol J, Wozniak A, Cook P, et al. Salmonella enterica serovar Typhi and gallbladder cancer: a case-control study and meta-analysis. Cancer Med. 2016;5(11):3310–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee MH, Gao YT, Huang YH, et al. A metallomic approach to assess associations of serum metal levels with gallstones and gallbladder cancer. Hepatology. 2019;71(3):917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Festi D, Reggiani ML, Attili AF, et al. Natural history of gallstone disease: expectant management or active treatment? Results from a population-based cohort study. J Gastroenterol Hepatol. 2010;25(4):719–724. [DOI] [PubMed] [Google Scholar]
- 42. Gracie WA, Ransohoff DF. The natural history of silent gallstones: the innocent gallstone is not a myth. N Engl J Med. 1982;307(13):798–800. [DOI] [PubMed] [Google Scholar]
- 43. Maringhini A, Moreau JA, Melton LJ 3rd, et al. Gallstones, gallbladder cancer, and other gastrointestinal malignancies. An epidemiologic study in Rochester, Minnesota. Ann Intern Med. 1987;107(1):30–35. [DOI] [PubMed] [Google Scholar]
- 44. Wenckert A, Robertson B. The natural course of gallstone disease: eleven-year review of 781 nonoperated cases. Gastroenterology. 1966;50(3):376–381. [PubMed] [Google Scholar]
- 45. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–e248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


