Abstract
OBJECTIVES
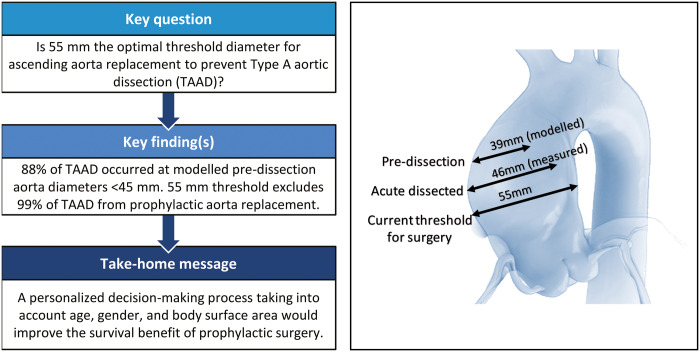
Current guidelines recommend prophylactic replacement of the ascending aorta at an aneurysmal diameter of >55 mm to prevent acute Type A aortic dissection (TAAD) in non-Marfan patients. Several publications have challenged this threshold, suggesting that surgery should be performed in smaller aneurysms to prevent this devastating disease. We reviewed our experience with measuring aortic size at the time of TAAD to validate the existing recommendation for prophylactic ascending aorta replacement.
METHODS
All patients who had been admitted for TAAD to our emergency department from 2014 to 2019 and underwent ascending aorta replacement were included. Marfan patients were excluded. The maximum diameter of the dissected aorta was measured preoperatively using CT scan. We estimated the aortic diameter at the time of dissection to be 7 mm smaller than the measured maximum diameter of the dissected aorta (modelled pre-dissection diameter).
RESULTS
Overall, 102 patients were included. Of these, 67 were male (65.6%) and 35 were female (34.4%), and the cohort’s mean age was 65 ± 12.1 years. In addition, 66% were treated for arterial hypertension. The mean maximum modelled pre-dissection diameter was 39.6 ± 4.8 mm: 39.1 ± 5.1 mm in men and 40.7 ± 2.8 mm in women (P = 0.1). The cumulative 30-day mortality rate was 19.6% (20/102).
CONCLUSIONS
TAAD occurred at a modelled aortic diameter below 45 mm in 87.7% of our patients. Therefore, the current aortic diameter threshold of 55 mm excludes ∼99% of patients with TAAD from prophylactic replacement of the ascending aorta. The maximum diameter of the ascending aorta warrants reappraisal and this parameter should be a distinct part of a personalized decision-making process that also takes into account age, gender and body surface area to establish the surgical indication for preventive aorta replacement aimed to improve the survival benefit of this procedure.
Keywords: Aortic aneurysm, Aortic dissection, Acute aortic syndrome, Aortic surgery
Acute aortic dissection of the ascending aorta (Stanford classification Type A) is a devastating disease that has an annual incidence of 2.9/100 000 [1].
INTRODUCTION
Acute aortic dissection of the ascending aorta (Stanford classification Type A) is a devastating disease that has an annual incidence of 2.9/100 000 [1]. Mortality rates of medically managed Type A acute aortic dissection (TAAD) are 20% at 24 h after presentation, 30% at 48 h, 40% at a week and 50% at a month after presentation [2, 3]. Therefore, surgical treatment is indicated immediately upon TAAD diagnosis. Despite major improvements in surgical techniques, cerebral perfusion protection strategies, and perioperative care, surgical mortality remains high, even in high-volume centres with the 30-day mortality of 7.6% and the operative mortality of 9.5% [2]. These rates have not significantly changed over time for patients receiving immediate and adequate surgical treatment.
Hospital-based databases only include patients who reach referral centres alive, and they exclude some 50% of TAAD patients who die at home or prior to hospital admission. This therefore leads to underestimating overall disease incidence and mortality [3].
Current guidelines recommend prophylactic ascending aorta replacement at an aneurysm diameter of >55 mm in an effort to prevent acute TAAD (class I, level C), in the absence of connective-tissue disorders [4]. These guidelines are essentially based on expert consensus and retrospective observational studies. The milestone paper, which was published in 1997, is a single-centre study of a heterogeneous 76-patient cohort [5].
In recent years, several publications have challenged the >55-mm threshold. Every single study that has looked at pre-dissection diameters has found that only a small percentage of TAAD patients actually had an aortic aneurysm above 55 mm. This comprises all major international registries, such as the International Registry for Aortic Dissections and German Registry for Acute Aortic Dissection type A [6, 7]. Based on a cohort of 343 patients, Rylski et al. modelled the pre-dissection aortic diameter from the dissected aorta by subtraction of the average diameter increase rate of 30% (according to the results of a study of human aortic geometry changes due to dissection). These authors concluded that >90% failed to meet the guidelines for elective ascending aorta replacement [8].
We have herein reviewed our experience of modelling pre-dissection aortic size to possibly validate existing recommendations for prophylactic ascending aorta replacement.
METHODS
We retrospectively evaluated clinical data, including demographics and cardiovascular risk factors, of 117 patients who consecutively underwent surgical treatment for TAAD in our institute and received at least a graft on the ascending aorta from January 2014 to October 2019. Patients with a clinical diagnosis of Marfan syndrome or connective-tissue disorders, iatrogenic or traumatic dissection, or bicuspid aortic valve were excluded. Within this cohort, we identified patients who had received adequate computed tomography angiography upon emergency department admission and we retrieved their clinical data. The following parameters were collected: date of birth, height, weight, gender and diagnosis of arterial hypertension. We defined the treatment of resistant systemic hypertension as requiring at least 3 antihypertensive drugs, such as beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, vasodilators or calcium channel blockers. Preoperative trans-oesophageal echocardiography was performed to exclude the presence of a bicuspid aortic valve.
Amidst our cohort, the maximum diameter and length of the dissected aorta were measured on pre-operatory contrast-medium CT scan. Centre line reconstructions were generated using the Carestream Health SA—Switzerland software package. Next, the maximum aortic diameter including true and false lumens, and outer to outer aortic wall diameter, was measured on axial transverse images perpendicular to the central line on the aortic segment between the sinotubular junction and origin of the brachiocephalic trunk. Based on our assumption, this is the most appropriate segment to study in patients without Marfan syndrome or connective-tissue disorders and a tricuspid aortic valve.
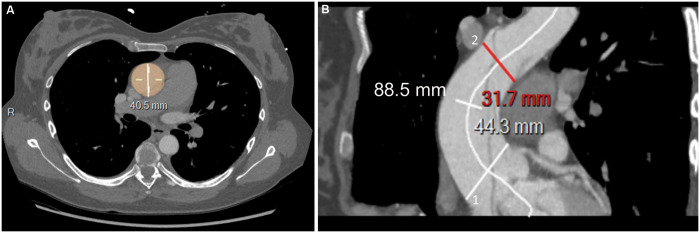
Finally, the aortic length was measured on centre line reconstructions starting from the plane corresponding to the sinotubular junction to the plane immediately proximal to the origin of the brachiocephalic artery (Figure 1). The manual identification of the centre line was required in case of either no or low contrast enhancement of the false channel.
Figure 1:
Centre line reconstructions of preoperative CT scan with contrast medium. (A) Patient 4: the maximum diameter of the dissected aorta was measured including true and false lumens, outer to outer aortic wall diameter, on axial transverse images perpendicular to the central line. (B) Patient 37: measure of the length of the ascending aorta. The ascending aorta begins at the plane corresponding to the sinotubular junction (white line 1) and extends to the plane immediately proximal to the origin of the brachiocephalic artery (red line 2): 88.5 mm corresponds to the length of the dissected ascending aorta.
To model the pre-dissection aortic dimension, we considered the 2 most recent publications on the effects of TAAD on aortic geometry changes and increases in aortic diameter: namely, the study by Rylski, in which the induced average increase in the mid-ascending aortic diameter was 13 ± 7mm (+32%) [8], and the study by Mansour, in which the pre-dissected aortic diameter was estimated to be 7 mm (−20%) smaller than the measured dissected aorta [9]. We decided to arbitrarily apply Mansour’s criteria to our cohort, so as to estimate pre-dissection aortic dimensions and identified it as a modelled pre-dissection diameter.
To compare our results with normal aortic size limits, the modelled pre-dissection ascending aorta diameters were compared between genders among 4 age categories (<45, 45–54, 55–64 and >65) and referred to gender-specific, age-specific and body surface area (BSA)-adjusted normal aortic diameters, as reported in a study involving 2952 patients by Wolak et al. [10].
The statistics have been presented as frequencies and percentages for categorical variables and as mean and standard deviation for continuous variables. For comparison of the continuous variables, the Mann–Whitney rank sum test was used. Categorical variables were compared using the Chi-squared test. In the event of small group sizes (n < 5), Fisher's exact t-test was used.
The study protocol was reviewed and accepted by our local ethics committee (authorization number 2020-01054), and we received written informed consent from each patient.
RESULTS
In total, 117 patients underwent surgical replacement of the ascending aorta, 28 (23.9%) of whom also received a prosthetic aortic valve and 13 (11.1%) of whom underwent a Bentall procedure. Fifteen patients (12.8%) were excluded from the study: 8 were Marfan patients, whereas a preoperative CT scan could not be found for the remaining 7. Of the 102 patients who were included, there were 67 men (65.6%) and 35 women (34.4%), and their mean age was 65 ± 12.1 years. Overall, 67 patients (66%) were treated for arterial hypertension and 54% suffered treatment-resistant hypertension. The cohort’s mean height was 173 ± 9 cm, and the mean weight was 80 ± 17.8 kg. The mean maximum modelled pre-dissection diameter was 39.6 ± 4.8 mm, which was 39.1 ± 5.1 mm in men and 40.7 ± 2.8 mm in women (P = 0.1) (Figure 2). The mean length of the ascending aorta was 87 ± 22 mm (Figure 3). The mean intensive care unit stay was 8 ± 0 days (range: 1–48 days). The operative and 30-day cumulative mortality rate was 19.6% (20/102). In total, 10 patients died due to neurological complications (10%), 9 died due to cardiovascular complications (haemorrhage, heart failure and intestinal ischaemia) and the remaining 1 died from multiple organ failure. None of the enrolled patients exhibited bicuspid aortic valves. Pathological examination of the resected dissected aorta revealed anomalies in all patients. Specifically, in 35 patients (34%) we found cystic media necrosis characterized by the local disappearance of elastic fibres in the arterial media, a reduction in smooth muscle cells and an increase in proteoglycans; 67 patients (64%) clearly presented only fragmentation of elastic fibres at the Elastica van Gieson stain.
Figure 2:
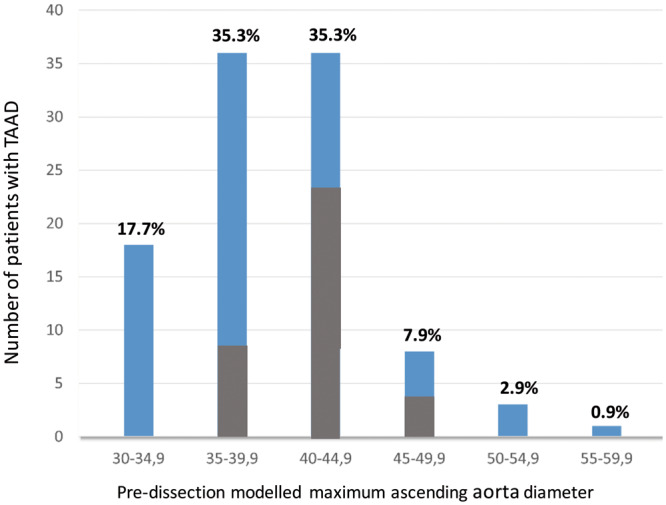
Distribution of pre-dissected maximum ascending aorta diameters, according to size and gender. The pre-dissected (modelled) diameter was obtained by measuring the maximum aortic diameter of the dissected ascending aorta of −7 mm, according to Mansour et al. results [9]. Grey columns correspond to female gender. TAAD: Type A aortic dissection.
Figure 3:
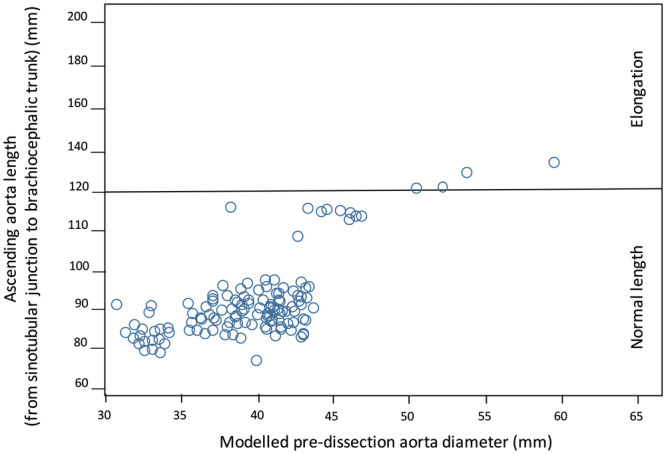
Scatter plot of dissected ascending aorta length. Aortic length was measured on centre line reconstructions starting from the plane corresponding to the sinotubular junction to the plane immediately proximal to the origin of the brachiocephalic artery. One hundred twenty millimetres is the cut-off value for calculating the Tübingen Aortic Pathoanatomy score [11].
DISCUSSION
Prophylactic replacement of the ascending aorta is the only effective strategy for preventing TAAD. As with all preventive treatments, a risk/benefit analysis appears crucial to determine the appropriate timing for the surgical procedure. Unlike coronary and cerebral vascular diseases, data on TAAD risk factors, incidence, and outcome are limited and no prospective population-based studies have been published as yet [3]. Several retrospective studies have highlighted the correlation between dissection and arterial hypertension. Indeed, hypertension that is resistant to medication has been shown to be the most significant modifiable risk factor for acute aortic dissection [3, 11]. The high rate of treatment-resistant hypertension in TAAD patients is most probably due to decreased aortic compliance, which is associated with fragility of the aortic wall. In our experience, 54% of patients suffered from treatment-resistant hypertension, and all these subgroup patients had an aortic diameter of smaller than 40 mm upon dissection, without any significant difference in the inter-gender variance of aortic diameters.
Although the exact sequence of events leading to TAAD remains poorly understood, several studies have demonstrated common pathways of medial degeneration. These comprise elastic fibre fragmentation and smooth muscle cell necrosis, which both lead to the progressive loss of aortic wall integrity and wall delamination [12, 13]. None of our patients displayed a histologically normal aortic wall. Histological examination of the resected dissected aorta demonstrated elastic fibre fragmentation in all patients and cystic media necrosis in 34% of them. This supports the hypothesis, which has been extensively described in the literature, that spontaneous aortic dissection does not occur in normal aortic walls [12]. Information on pathological aortic wall would play a key role in the decision-making process of preventive aortic replacement. Nevertheless, existing non-invasive investigational tools (ultrasounds, CT, and magnetic-resonance imaging) are unable to characterize aortic wall histology.
The ascending aortic transverse diameter is the only accepted morphological risk factor for TAAD and indication for preventive surgery. However, this is a poor predictor of the timing and location of dissection events. Historical studies using mathematical models to simulate aortic dissection underline how a dilatation phase of the ascending aorta (growing aorta) plays a key role in producing an intimal tear, which is followed by the acute dissection process [14]. However, size alone only seems to be a good predictor of ascending aortic dissection for diameters over 60 mm, which seems to be the hinge point for natural complications of aortic aneurysm [15, 16]. Approximately one-third of patients with aortic dissection exhibit a normal-sized or minimally enlarged aorta, and only 10% of patients display a true ascending aortic aneurysm [15]. In our cohort, only 9 (8.8%) patients had a pre-dissection aortic diameter larger than 55 mm.
An analysis of 3400 patients by the Yale Aortic Institute Database, which contains data on patients who had, by chance, an aortic CT scan just before aortic dissection, enabled Mansour et al. [9] to claim that the pre-dissection ascending aortic size is 7.65 mm smaller, just before dissection occurred. This is because rapid separation within the aortic media likely causes acute aortic wall weakness, which should theoretically trigger an immediate increase in aortic diameter. The reported threshold for the diameter’s increase due to dissection has a 21% variation coefficient upon measurements. This means that for a dissected aortic diameter of 60 mm, for example the pre-dissection diameter was between 48 and 53 mm. Other authors have reported more aggressive increases in aortic diameter due to acute dissection. In an elegant study, Rylski et al. reported that the maximum diameter of the ascending aorta increased by 12.8 mm (32%) from 40.1 mm (36.6 mm; 45.3 mm) to 52.9 mm (46.1 mm; 58.6 mm) (+12.8 mm; +32%; P < 0.001). A similar but less pronounced increase in dimensions was observed at the sinotubular junction level (+6.9 mm; p < 0.001). In contrast, the sinus of Valsalva diameter did not significantly increase [13].
If we apply the smallest correction of −7mm to our cohort, 51 (50%) TAAD patients probably displayed an aortic diameter that was smaller than 40 mm, whereas 89 (87.7%) exhibited an aortic diameter that was smaller than 45 mm at the time of dissection. This suggests that the size criterion for resection of ascending aorta aneurysm warrants reappraisal. In the personalized medicine era, for small aneurysms, aortic size cannot remain the only and absolute criterion for proposing the surgical replacement of the ascending aorta. One possibility to improve the parameter’s accuracy is to consider the aortic diameter with respect to age, gender and BSA. In Table 1, we have stratified our cohort according to gender, age and BSA at the time of dissection. We then compared the modelled pre-dissection diameter to the ‘normal’ ascending aorta diameter for the same class of age, gender and BSA, as reported by Wolak et al. [10]. Surprisingly, all patients exhibited a modelled pre-dissection diameter between 10% and 34% larger than it was supposed to be, according to Wolak’s data. This suggests that dissection occurs in dilated aorta with respect to the standard diameter even when the absolute value is below the accepted criteria for surgical resection. We have integrated these findings into a personalized decision-making process where an increase of at least 10% of patient aortic size with respect to ‘normal’ size for same class of age, gender and BSA represents an element in favour of early surgery. For example, in a 70-year-old male with a BSA of 2.2 m2, an ascending aorta of 38 mm could be considered normal, whereas in a 45-year-old woman with a BSA of 1.6 m2, this would signify an indication for surgical replacement because the aorta is 30% larger than what is considered as normal aortic diameter, this being 29.6 ± 2.8.
Table 1:
Gender-, age- and BSA-related ascending aorta diameter upper limits, according to Wolak et al. [10] compared to our cohort modelled pre-dissection data
| Age (years) | BSA (m2) | Ascending normal (mm)*, n = 2952 |
Ascending pre-dissection modelled (mm), n = 102 |
Diameter difference between normal and modelled (%) |
|||
|---|---|---|---|---|---|---|---|
| Female, n = 1147 | Male, n = 1805 | Female, n = 35 | Male, n = 67 | Female | Male | ||
| <45 | <1.70 | 28.4 ± 2.7 | 28.6 ± 2.2 | – | – | – | – |
| 1.70–1.89 | 30.0 ± 2.2 | 30.1 ± 3.1 | – | 34 ± 3.5 | – | +13.5 | |
| 1.90–2.09 | 29.8 ± 2.6 | 30.9 ± 2.7 | 38 | 38.5 ± 2 | +29.2 | +24.3 | |
| >2.1 | 31.3 | 32.3 ± 3.0 | 41 ± 3.5 | 37 ± 3.5 | +31.3 | +16.1 | |
| 45–54 | <1.70 | 29.6 ± 2.8 | 31.0 ± 3.8 | – | – | – | – |
| 1.70–1.89 | 31.4 ± 2.9 | 31.7 ± 3.2 | 42 | 40 ± 5.5 | +34.0 | +26.5 | |
| 1.90–2.09 | 32.5 ± 3.2 | 33.1 ± 3.3 | – | 37 | – | +12.1 | |
| >2.1 | 34.4 ± 3.1 | 34.4 ± 3.1 | – | 42.5 ± 3.5 | – | +23.2 | |
| 55–64 | <1.70 | 31.1 ± 2.9 | 31.5 ± 2.4 | 39 | – | +25.8 | – |
| 1.70–1.89 | 31.8 ± 2.6 | 33.5 ± 3.1 | 36 ± 2.5 | 39 ± 3.5 | +14.5 | +16.5 | |
| 1.90–2.09 | 33.0 ± 3.0 | 34.6 ± 3.3 | 39 | 40 | +18.2 | +15.9 | |
| >2.1 | 35.4 ± 3.3 | 36.1 ± 3.5 | – | 39.8 ± 4.5 | – | +10.5 | |
| >65 | <1.70 | 32.5 ± 2.5 | 33.9 ± 3.3 | 42.8 ± 3.5 | 39 | +31.1 | +15.0 |
| 1.70–1.89 | 33.4 ± 2.9 | 35.0 ± 3.0 | 43.5 ± 4.5 | 39.2 ± 2 | +30.0 | +12.0 | |
| 1.90–2.09 | 34.3 ± 4.2 | 35.8 ± 3.2 | 45.1 ± 4 | 41.5 ± 2.5 | +31.3 | +15.5 | |
| >2.1 | 32.8 | 36.8 ± 2.8 | – | 41.5 ± 4.5 | – | +13.0 | |
For our data, continuous variables were expressed as mean and standard deviation. An isolated value means that there was only 1 patient. Positive value in the diameter difference column indicates the diameter increase in the modelled pre-dissected aorta with respect to normal aorta.
*Data from Wolak et al.
BSA: body surface area.
Recently, the Aortic Institute at Yale New Haven Hospital published a risk stratification analysis involving their 780-patient cohort using regression models. Patients were stratified into 4 categories of yearly risk complications, based on their height-based aortic height index, which is defined as aortic size/height ratio. Using code colours and nomograms, the authors illustrated that a 17-cm-tall man with a 45-mm ascending aorta exhibits a 7% annual risk of aortic dissection, rupture or death. The group concluded that indexing absolute aortic diameter to anthropometric measurements allows for providing individualized risk classification with satisfactory results [17] and that surgical treatment should be recommended whenever patient-specific surgical mortality appears to be lower than the estimated risk of aortic dissection or death.
Other authors have underlined the role of aortic elongation in TAAD genesis. Unlike the circumferential aorta dilatation, its longitudinal dilatation, which is also defined as elongation, has been largely neglected in pathogenic TAAD models. Recently, Kruger et al. [11] reviewed their experience on 140 patients and proposed the Tübingen Aortic Pathoanatomy (TAIPAN) score to estimate the TAAD risk. They combined the ascending aorta ectasia and aorta elongation (defined as the central distance between the sinotubular junction and brachiocephalic trunk) to generate the following scoring system: ascending aorta diameter <45 mm = 0, 45–55 mm = 1, >55 mm = 2; ascending aorta length <120 mm = 0, >120 mm = 1. This scoring system was able to identify 23% of pre-TAAD patients and at least twice as many pre-TAAD patients as the diameter alone. Based on this retrospective morphological analysis, the authors recommended prophylactic ascending aorta replacement at ≥2 points [11]. When applying the TAIPAN score to our TAAD patient cohort, only 4 (3.4%) would have been an indication for elective ascending aorta replacement (Figure 3). However, our measurements corresponded to the length of the dissected aorta and not to that of the pre-dissected aorta, which should be used to calculate the TAIPAN score. There are no models to quantify the changes in aortic length due to acute aortic dissection as there are for aortic diameter changes.
As previously mentioned, surgical decision-making requires an accurate risk/benefit analysis. We must balance the reduced TAAD incidence, as a result of earlier surgery, against the operative risk of prophylactic surgery in an asymptomatic patient. Surgical mortality for elective ascending aorta replacement in otherwise healthy patients was estimated at 9% in 1997, when the threshold value of 55 mm was proposed. However, this estimated figure has meanwhile decreased to 1% [11, 18]. The mortality rate for emergency ascending aorta replacement in the most experienced hands was estimated at 22% in 1997. Notably, this has not wavered over the last 2 decades, and was 16.9% in the best series [1, 11, 19]. In our cohort, we observed a 19.6% cumulative mortality rate for 30-day stays. These data should tip the scales towards earlier surgery because the risk of TAAD has been proven to be greater than that of elective surgery. The number needed to treat could be calculated to match historical data from our experiences. To illustrate, a man with a 45–49-mm ascending aorta, without any connective-tissue disorders, and a tricuspid aortic valve exhibits an average annual death risk for aortic dissection of about 4.7% [20]. The mortality rate for elective ascending aorta replacement in this otherwise healthy patient is inferior to 1%. Therefore, preventive surgery reduces the mortality rate by 3.7%, which translates into an number needed to treat of 27 (the formal calculation is 100/3.7 = 27). Accordingly, we should treat 27 patients with 45–49 mm ascending aorta aneurysm to save 1 life.
However, about 7% of entry tears in TAAD are located in the aortic arch [19], and it is likely that the prophylactic surgical replacement of the ascending aorta is not able to prevent the dissection of the aortic arch and descending aorta.
We are aware that this study holds several methodological limitations, particularly its retrospective single-centre study design without any control group, the use of imprecise estimation of aortic diameter reduction (measurements were performed by a team of radiologists and surgeons without assessing intra-observer and interobserver variabilities) and the possibility of diameter increases due to dissection differing among patients. Moreover, we did not take into account TAAD-related morbidity, such as non-fatal neurological complications and renal function impairment, both of which affect the quality of life and could potentially be avoided with elective surgery. Although all these limitations could bias the conclusions, in our experience, the current aortic diameter threshold of 55 mm excludes ∼99% of TAAD patients from prophylactic replacement of the ascending aorta. Therefore, the absolute diameter of 55 mm deserves reappraisal. We should go beyond the guidelines and move towards a more personalized therapeutic approach that tailors treatment according to a multitude of parameters, in order to provide survival benefit by replacing the aneurysmal segment of the ascending aorta before the catastrophic event occurs. An international taskforce should adapt existing recommendations on surgical treatment of ascending aortic aneurysm to align with the most recent scientific evidence, including recommending prophylactic intervention at smaller aortic sizes in selected patients.
Conflict of interest: none declared.
Author contributions
Piergiorgio Tozzi: Conceptualization; methodology; writing—original draft preparation. Ziyad Gunga: Conceptualization, methodology, data curation. Lars Niclauss: Resources. Dominique Delay: Resources. Aurelian Roumy: Data curation. Raymond Pfister: Data curation. Sebastien Colombier: Data curation. Francesco Patella: Writing—review & editing. Salah Dine Qanadli: Methodology, review & editing. Matthias Kirsch: Methodology, review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Luca Di Marco, Tobias Krüger and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
ABBREVIATIONS
- BSA
Body surface area
- TAAD
Type A aortic dissection
REFERENCES
- 1. Mészáros I, Mórocz J, Szlávi J, Schmidt J, Tornóci L, Nagy L et al. Epidemiology and clinicopathology of aortic dissection. Chest 2000;117:1271–8. [DOI] [PubMed] [Google Scholar]
- 2. Abe T, Yamamoto H, Miyata H, Miyata H, Motomura N, Tokuda Y et al. Patient trends and outcomes of surgery for type A acute aortic dissection in Japan: an analysis of more than 10 000 patients from the Japan Cardiovascular Surgery Database. Eur J CardioThorac Surg 2020;57:660–7. [DOI] [PubMed] [Google Scholar]
- 3. Howard D, Banerjee A, Fairhead J, Perkins J, Silver LE, Rothwell PM. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control. 10-year results from the Oxford Vascular Study. Circulation 2013;127:2031–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Erbel R, Aboyans V, Boileau C, Bossone E, Di Bartolomeo R, Eggebrecht H et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). ESC Committee for Practice Guidelines. Eur Heart J 2014. 1;35:2873–926. [DOI] [PubMed] [Google Scholar]
- 5. Coady MA1, Rizzo JA, Hammond GL, Mandapati D, Darr U, Kopf GS et al. What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg 1997;113:476–91. [DOI] [PubMed] [Google Scholar]
- 6. Evangelista A, Isselbacher EM, Bossone E, Gleason TG, Eusanio MD, Sechtem U et al. Insights From the international registry of acute aortic dissection: a 20-year experience of collaborative clinical research. Circulation 2018;137:1846–60. [DOI] [PubMed] [Google Scholar]
- 7. Boening A, Karck M, Conzelmann LO, Easo J, Krüger T, Rylski B et al. German registry for acute aortic dissection type A: structure, results, and future perspectives. Thorac Cardiovasc Surg 2017;65:77–84. [DOI] [PubMed] [Google Scholar]
- 8. Rylski B, Branchetti E, Bavaria JE, Vallabhajosyula P, Szeto WY, Milewski R et al. Modeling of predissection aortic size in acute type A dissection: more than 90% fail to meet the guidelines for elective ascending replacement. J Thorac Cardiovasc Surg 2014;148:944–8. [DOI] [PubMed] [Google Scholar]
- 9. Mansour AM, Peterss S, Zafar MA, Rizzo JA, Fang H, Charilaou P et al. Prevention of aortic dissection suggests a diameter shift to a lower aortic size threshold for intervention. Cardiology 2018;319:139–46. [DOI] [PubMed] [Google Scholar]
- 10. Wolak A, Gransar H, Thomson LE, Friedman JD, Hachamovitch R, Gutstein A et al. Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age, gender, and body surface area. JACC Cardiovasc Imaging 2008;1:200–9. [DOI] [PubMed] [Google Scholar]
- 11. Kruger T, Boburg RS, Lescan M, Oikonomou A, Schneider W, Vöhringer L et al. Aortic elongation in aortic aneurysm and dissection: the Tübingen Aortic Pathoanatomy (TAIPAN) project. Eur J CardioThorac Surg 2018;54: 26–33. [DOI] [PubMed] [Google Scholar]
- 12. Nakashima Y. Pathogenesis of aortic dissection: elastic fiber abnormalities and aortic medial weakness. Ann Vasc Dis 2010;3:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rylski B, Blanke P, Beyersdorf F, Desai ND, Milewski RK, Siepe M et al. How does the ascending aorta geometry change when it dissects? J Am Coll Cardiol 2014;63:1311–9. [DOI] [PubMed] [Google Scholar]
- 14. Thubrikar MJ1, Agali P, Robicsek F. Wall stress as a possible mechanism for the development of transverse intimal tears in aortic dissections. J Med Eng Technol 1999;23:127–34. [DOI] [PubMed] [Google Scholar]
- 15. Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg 2002;74:S1877–80. [DOI] [PubMed] [Google Scholar]
- 16. Neri E, Barabesi L, Buklas D, Vricella LA, Benvenuti A, Tucci E et al. Limited role of aortic size in the genesis of acute type A aortic dissection. Eur J CardioThorac Surg 2005;28:857–63. [DOI] [PubMed] [Google Scholar]
- 17. Zafar MA, Li Y, Rizzo JA, Charilaou P, Charilaou P, Saeyeldin A et al. Height alone, rather than body surface area, suffices for risk estimation in ascending aortic aneurysm. J Thorac Cardiovasc Surg 2018;155:1938–50. [DOI] [PubMed] [Google Scholar]
- 18. Ziganshin BA, Zafar MA, Elefteriades JA. Descending threshold for ascending aortic aneurysmectomy: is it time for a “left-shift” in guidelines? J Thorac Cardiovasc Surg 2019;157:37–42. [DOI] [PubMed] [Google Scholar]
- 19. Sievers HH, Rylski B, Czerny M, Baier ALM, Kreibich M, Siepe M et al. Aortic dissection reconsidered: type, entry site, malperfusion classification adding clarity and enabling outcome prediction. Interact CardioVasc Thorac Surg 2020. 1;30:451–7. [DOI] [PubMed] [Google Scholar]
- 20. Pinard A, Jones GT, Milewicz DM. Genetics of thoracic and abdominal aortic diseases. Circ Res 2019;124:588–606. [DOI] [PMC free article] [PubMed] [Google Scholar]