Abstract
Background
Malignant astrocytic gliomas in children show a remarkable biological and clinical diversity. Small in-frame insertions or missense mutations in the epidermal growth factor receptor gene (EGFR) have recently been identified in a distinct subset of pediatric-type bithalamic gliomas with a unique DNA methylation pattern.
Methods
Here, we investigated an epigenetically homogeneous cohort of malignant gliomas (n = 58) distinct from other subtypes and enriched for pediatric cases and thalamic location, in comparison with this recently identified subtype of pediatric bithalamic gliomas.
Results
EGFR gene amplification was detected in 16/58 (27%) tumors, and missense mutations or small in-frame insertions in EGFR were found in 20/30 tumors with available sequencing data (67%; 5 of them co-occurring with EGFR amplification). Additionally, 8 of the 30 tumors (27%) harbored an H3.1 or H3.3 K27M mutation (6 of them with a concomitant EGFR alteration). All tumors tested showed loss of H3K27me3 staining, with evidence of overexpression of the EZH inhibitory protein (EZHIP) in the H3 wildtype cases. Although some tumors indeed showed a bithalamic growth pattern, a significant proportion of tumors occurred in the unilateral thalamus or in other (predominantly midline) locations.
Conclusions
Our findings present a distinct molecular class of pediatric-type malignant gliomas largely overlapping with the recently reported bithalamic gliomas characterized by EGFR alteration, but additionally showing a broader spectrum of EGFR alterations and tumor localization. Global H3K27me3 loss in this group appears to be mediated by either H3 K27 mutation or EZHIP overexpression. EGFR inhibition may represent a potential therapeutic strategy in these highly aggressive gliomas.
Keywords: (bi)thalamic, EGFR mutation, H3 K27M mutation, K27me3, pediatric-type high-grade glioma
Key Points.
This study confirms a distinct new subset of pediatric-type diffuse midline glioma with H3K27me3 loss, with or without H3 K27 mutation.
The poor patient outcome associated with these tumors is in line with the broader family of pediatric-type diffuse midline gliomas with H3 K27 mutation or EZHIP overexpression.
Frequent EGFR alterations in these tumors may represent a therapeutic target in this subset.
Importance of the Study
Malignant astrocytic gliomas in children show a remarkable biological and clinical diversity. Here, we highlight a distinct molecular class of pediatric-type malignant gliomas characterized by EGFR alteration and global H3K27me3 loss that appears to be mediated by either H3 K27 mutation or EZHIP overexpression. EGFR inhibition may represent a potential therapeutic strategy in these highly aggressive gliomas.
Malignant astrocytic gliomas of World Health Organization (WHO) grade III or IV are among the most common malignant central nervous system (CNS) tumors in childhood,1 and show remarkable biological and clinical heterogeneity. Despite common histopathological characteristics, pediatric malignant gliomas significantly differ from their adult counterparts in terms of molecular features, anatomical locations, and clinical outcomes.2–5 Over the past decade, (epi)genomic analyses have delivered broad insights into the molecular diversity of pediatric-type gliomas and enabled a subdivision into several biologically and clinically distinct entities defined by typical genetic alterations and DNA methylation profiles such as K27M or G34R/V mutations in histone 3, among others.6–8
In a recent study by Mondal et al,9 small in-frame insertions or missense mutations in the EGFR gene were observed in a subset of pediatric-type bithalamic diffuse gliomas, which also appeared to share a unique DNA methylation pattern and global loss of H3 K27me3. In order to further corroborate these findings, we used a screening approach based on unsupervised analysis of genome-wide DNA methylation profiling data of these initially reported cases alongside a large set of other CNS tumors (n > 30 000). This analysis revealed a molecularly distinct group of 58 tumors (including 5 of the cases initially described by Mondal et al), for which further molecular workup was performed using targeted next-generation DNA sequencing.
Materials and Methods
Sample Collection
Tumor samples and retrospective clinical data were provided by multiple international collaborating centers and collected at the Department of Neuropathology of the University Hospital Heidelberg. Case selection was based on genome-wide DNA methylation screening using DNA methylation data of the cases initially described by Mondal et al9 as a reference, which revealed a molecularly distinct tumor group comprising 5 of those tumors with an additional 53 cases. Additionally, DNA methylation data of numerous well-characterized reference samples representing CNS tumors of known histological and/or molecular subtype were used for comparative analyses.10–12 Detailed descriptions of the reference DNA methylation classes are outlined under https://www.molecularneuropathology.org/. Analysis of tissue and clinical data was performed in accordance with local ethics regulations. Clinical details of the patients are listed in Supplementary Table 1.
DNA Methylation Array Processing and Copy Number Profiling
Genomic DNA was extracted from fresh-frozen or formalin-fixed and paraffin-embedded (FFPE) tissue samples. DNA methylation profiling of all samples was performed using the Infinium MethylationEPIC (850k) BeadChip (Illumina) or Infinium HumanMethylation450 (450k) BeadChip array (Illumina) as previously described.10 Data of the samples of Mondal et al were retrieved from Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) accession GSE140124. All computational analyses were performed in R version 3.3.1 (R Development Core Team, 2016; https://www.R-project.org). Copy-number variation analysis from 450k and EPIC methylation array data was performed using the conumee Bioconductor package version 1.12.0. Raw signal intensities were obtained from IDAT files using the minfi Bioconductor package version 1.21.4.13 Illumina EPIC samples and 450k samples were merged to a combined dataset by selecting the intersection of probes present on both arrays (combineArrays function, minfi). Each sample was individually normalized by performing a background correction (shifting of the 5% percentile of negative control probe intensities to 0) and a dye-bias correction (scaling of the mean of normalization control probe intensities to 10 000) for both color channels. Subsequently, a correction for the type of material tissue (FFPE/frozen) and array type (450k/EPIC) was performed by fitting univariable, linear models to the log2-transformed intensity values (removeBatchEffect function, limma package v3.30.11). The methylated and unmethylated signals were corrected individually. Beta-values were calculated from the retransformed intensities using an offset of 100 (as recommended by Illumina). All samples were checked for duplicates by pairwise correlation of the genotyping probes on the 450k/850k array. To perform unsupervised nonlinear dimension reduction, the remaining probes after standard filtering10 were used to calculate the 1-variance weighted Pearson correlation between samples. The resulting distance matrix was used as input for analysis by t-distributed stochastic neighbor embedding (t-SNE) (Rtsne package v0.13). The following nondefault parameters were applied: theta = 0, pca = F, max_iter = 10 000 perplexity = 15.
Targeted Next-Generation DNA Sequencing and Mutational Analysis
Capture-based next-generation DNA sequencing for cases with sufficient material (n = 18) was performed on a NextSeq 500 instrument (Illumina), as previously described,14 using a custom brain tumor panel covering the entire coding and selected intronic and promoter regions of 130 or 160 genes of particular relevance in CNS tumors. Reads were aligned against the reference genome (GRch37). For 12 additional cases, sequencing data generated locally at the initial handling institute were integrated into the present study.
Gene Expression Profiling
Tumor samples with sufficient high-quality RNA (n = 5) were analyzed on the Affymetrix GeneChip Human Genome U133 Plus (v2.0) Array at the Microarray Department of the University of Amsterdam–the Netherlands or the Genomics and Proteomics Core Facility of the German Cancer Research Center, as previously described.15 Expression data were normalized using the MAS5.0 algorithm. Expression levels of EZH inhibitory protein (EZHIP) and epidermal growth factor receptor (EGFR) were compared with published reference cases of H3 K27M-mutant and wildtype diffuse midline glioma12,15 and with additional brain tumor reference samples (for EGFR). Analyses were performed using R2: Genomics Analysis and Visualization Platform (http://r2.amc.nl).
Immunohistochemistry
Immunohistochemistry was performed on a Ventana BenchMark ULTRA Immunostainer using the ultraView Universal DAB Detection Kit. Histone H3 lysine 27 trimethylated protein was detected using an anti-H3K27me3 antibody (rabbit polyclonal, diluted 1:500, Millipore).
Statistical Analysis
Data on overall survival (OS) could be retrospectively retrieved for 14 patients. OS was defined as time from initial diagnosis to patient death or last follow-up. Survival data were analyzed by the Kaplan–Meier method and compared by log-rank test using GraphPad Prism 8. P-values <0.05 were considered significant.
Results
DNA Methylation Profiling Reveals an Epigenetically Distinct Group of Pediatric-Type Malignant Gliomas
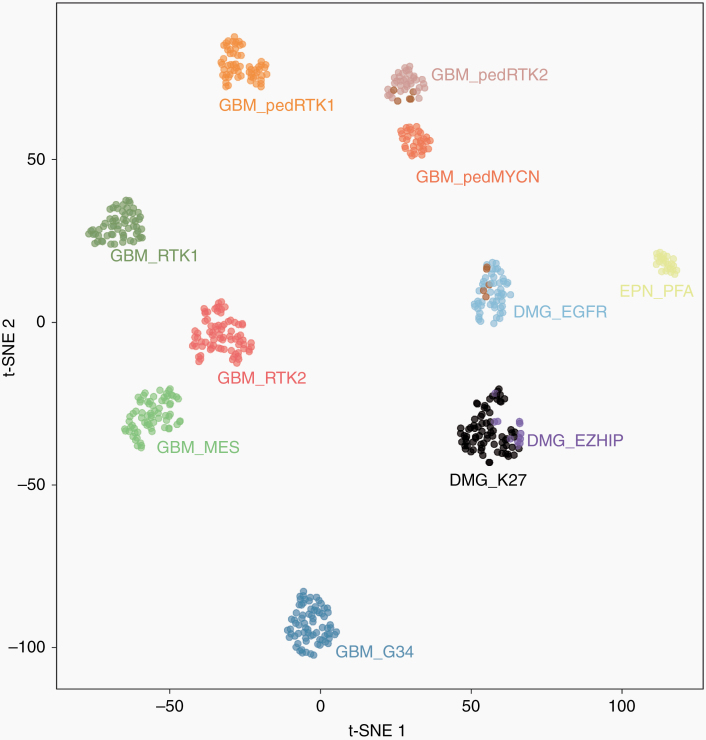
We first investigated the distribution of the samples from the study by Mondal et al based on unsupervised analysis of genome-wide DNA methylation profiling data alongside a large set of CNS tumors. Notably, this revealed a group of 53 tumor samples, clearly enriched for tumors of pediatric patients (see below), which formed a highly distinct DNA methylation class together with 5 of the recently published bithalamic glioma cases. A more focused t-SNE analysis of DNA methylation patterns of these 58 samples together with 516 well-characterized CNS neoplasms from the reference series encompassing various other types of pediatric-type and adult malignant astrocytic gliomas consistently confirmed the distinct nature of this novel group (Figure 1). The remaining 5 cases from the cohort described by Mondal et al showed a DNA methylation profile matching that of the pedRTK2 subgroup, which has also been shown to have a high frequency of EGFR alterations.11 Using the DNA methylation–based CNS tumor classification with the current public version v11b4, all tumors within the group reached the highest score for high-grade glioma classes.
Fig. 1.
t-SNE analysis of DNA methylation profiles of the 58 gliomas investigated (DMG_EGFR; 10 of the cases included from the Mondal et al cohort (UCSF) are highlighted in brown) alongside selected reference samples. Reference DNA methylation classes: glioblastoma IDH-wildtype, subclass RTK 1 (GBM_RTK1); glioblastoma IDH-wildtype, subclass RTK 2 (GBM_RTK2); glioblastoma IDH-wildtype, subclass mesenchymal (GBM_MES); glioblastoma IDH-wildtype, pediatric RTK 1 (GBM_pedRTK1); glioblastoma IDH-wildtype, pediatric RTK 2 (GBM_pedRTK2); glioblastoma IDH-wildtype, pediatric MYCN (GBM_pedMYCN); diffuse midline glioma H3 K27M-mutant (DMG_K27M); diffuse midline glioma H3-wildtype overexpressing EZHIP (DMG_EZHIP); glioblastoma IDH-wildtype, H3.3 G34-mutant (GBM_G34); ependymoma, posterior fossa group A (EPN_PFA).
EGFR Alteration and H3K27me3 Loss Are Frequent Features in This Group of Pediatric-Type Malignant Gliomas
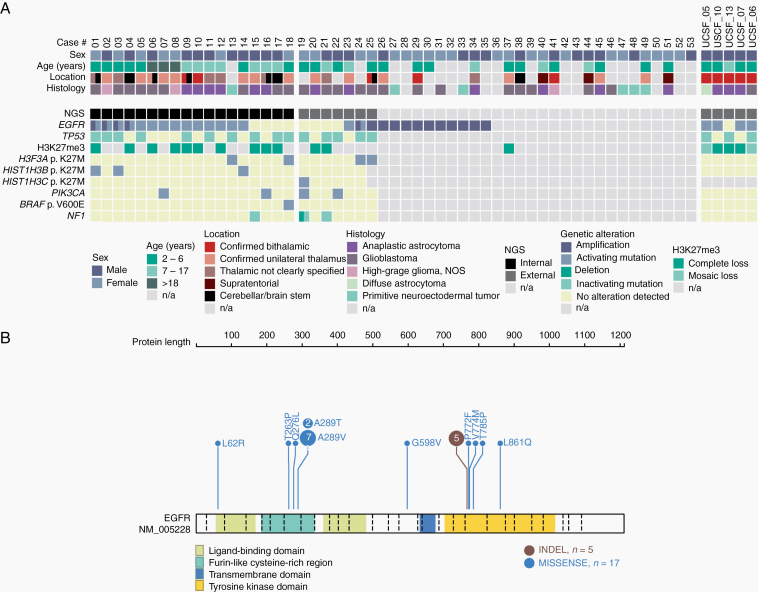
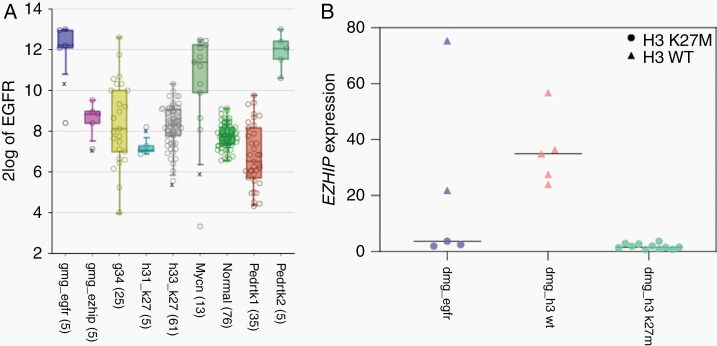
Analysis of copy number profiles (CNPs) derived from DNA methylation data revealed focal EGFR gene amplifications in 16 of the 58 tumor samples within the cohort (27%; Supplementary Figure 1a), as well as several additional chromosomal aberrations, including chromosome 7 gain, chromosome 1q gain, and chromosome 6q loss, each present in approximately half of all cases (Supplementary Figure 1b). In light of the high frequency of EGFR amplifications and previously reported mutations/indels in the bithalamic cases, we next performed DNA sequencing of tumor samples with sufficient material (n = 18). For an additional 12 cases, sequencing data provided by the original case submitter were integrated. In 15/30 analyzed tumors, a missense mutation in EGFR was detected, including 9 tumors with a hotspot p.A289V/T substitution and single cases showing p.L62R/p.V774M, p. L861Q/p.T263P, p.Q276L, p.P719F, p.G598V, and p.T785P substitutions, respectively (Figure 2). Five further tumors showed an in-frame insertion within exon 20 of EGFR, including 3 of the tumors initially described by Mondal et al.9 Five of the EGFR-mutant tumors harbored a concomitant amplification of EGFR. Analysis of EGFR expression in comparison to different other glioma showed the highest expression within the DMG_EGFR group (Figure 4). Additionally, 18 of 30 tumors (62%) harbored a mutation or deletion of the tumor suppressor gene TP53. Subsets of tumors harbored additional hotspot mutations in genes encoding members of the mitogen-activated protein kinase (MAPK) and/or phosphoinositide 3-kinase (PI3K) signaling pathways, including 4 tumors with a PIK3CA mutation and 4 tumors with either a BRAF V600E or a NF1 mutation. Details are given in Figure 2 and Supplementary Table 1.
Fig. 2.
(A) Clinicopathological characteristics and recurrent genetic alterations of the 58 gliomas. (B).Visualization of the EGFR mutation profile in the investigated glioma cohort created using the online tool ProteinPaint available at https://proteinpaint.stjude.org/
Fig. 4.
(A) EGFR expression in the DMG_EGFR group in comparison to different other glioma showing that tumors within the DMG_EGFR group exhibit the highest expression of EGFR. (B) Distribution of normalized EZHIP gene expression level in the investigated glioma cohort in comparison to reference cases of H3 K27M-mutant and wildtype diffuse midline gliomas showing that H3 K27M-wildtype tumors exhibit higher expression of EZHIP.
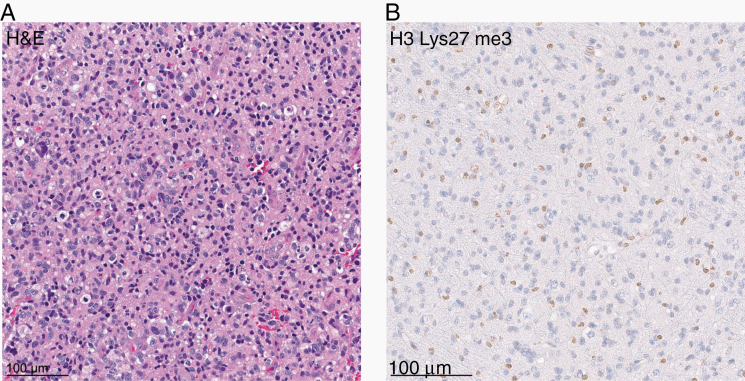
Notably, 8 of the 30 sequenced cases (27%) showed a K27M mutation in H3.1 or H3.3. Immunohistochemical staining for histone H3 lysine 27 trimethylation (H3K27me3) showed global loss of nuclear H3 K27me3 expression in tumor cells of all cases that could be evaluated (n = 18; one of them showing an H3.3 K27M mutation; Figure 3). Interestingly, analysis of EZHIP expression in 5 of the samples with available gene expression data indicated an overexpression in both of the H3-wildtype tumors compared with tumors with H3 K27M mutation (Figure 4), in line with an alternative mechanism of polycomb repressive complex 2 inhibition in glioma by EZHIP overexpression.12,16–19 Despite these findings, no striking similarity was observed with the DNA methylation profile of other H3 K27M-mutant tumors, including diffuse midline gliomas/diffuse intrinsic pontine gliomas as well as rare posterior fossa type A ependymomas. The DNA methylation profile was also distinct from that of the recently described H3 wildtype subset of diffuse midline glioma with EZHIP overexpression, which clustered close to their “typical” H3 K27-mutant counterparts12 (Figure 1).
Fig. 3.
(A) Hematoxylin and eosin (H&E) staining of one of the gliomas included in the investigated cohort (case #1) revealing a pleomorphic astrocytic neoplasm with mitotic figures. (B) Immunohistochemical staining for histone H3 lysine 27 trimethylation (H3K27me3) showing a loss of nuclear expression in the tumor cells.
Clinical Characteristics and Outcome
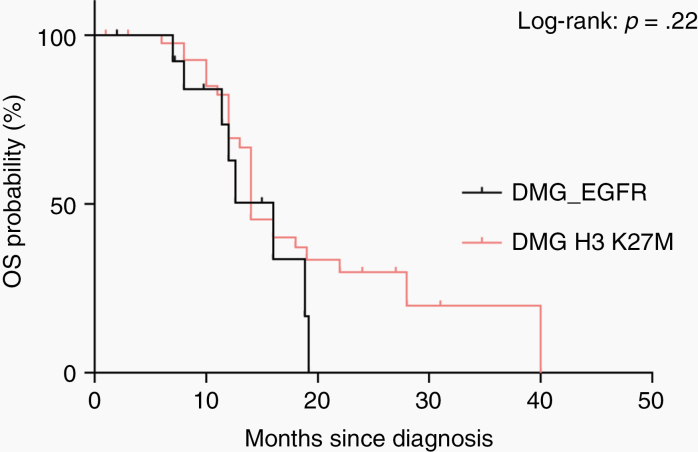
Limited clinical data were available for 36 of the patients. Tumors in most patients were histopathologically diagnosed as malignant astrocytic gliomas corresponding to glioblastoma or anaplastic astrocytoma (Figure 2). Median patient age at the time of diagnosis was 8.2 years (range: 1–43 y) and the sex distribution was equal (male:female ratio 1:1.1). The majority of the tumors (n = 31) were located in the thalamic region (Figure 5), with 5 of the new tumors showing a clear bithalamic growth pattern (in addition to the 5 tumors reported by Mondal et al). A small subset of tumors arose in the cerebral hemisphere (n = 3), brainstem (n = 2), and cerebellum (n = 1). Retrospective outcome data concerning OS were available for 14 patients. Analysis of OS of these patients in comparison to children with diffuse midline glioma, H3 K27M-mutant (n = 46) revealed a similarly poor outcome, and would support a putative assignment of WHO grade IV to these tumors (Figure 6).
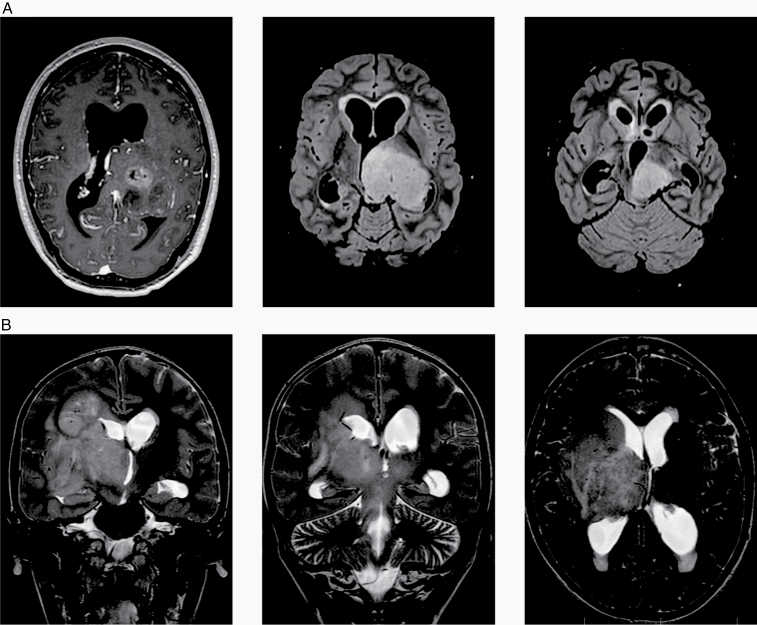
Fig. 5.
MR images from 2 representative patients of the investigated cohort showing (A) a 21-year-old male patient (case #6) with a mass in the left thalamus, extending into mesencephalon and left cerebellar peduncle and (B) a 12-year-old boy (case #21) with a mass in the right thalamus with diffuse infiltration of the right hemisphere and T2 changes also in the contralateral hemisphere.
Fig. 6.
Kaplan–Meier curves for OS of 14 patients from the investigated cohort (DMG_EGFR) for whom OS data were available. OS of 14 patients is compared with OS of 46 patients with diffuse midline glioma, H3 K27M-mutant, WHO grade IV (DMG H3 K27M) diagnosed at the University Hospital Heidelberg.
Discussion
Here, we provide further evidence for the remarkable biological diversity of malignant astrocytic gliomas in children by confirming the existence of an epigenetically distinct subset of diffuse gliomas with predominantly thalamic location that shows frequent gene amplification and/or mutation of the EGFR gene and loss of H3K27me3. Overall, this group shows significant similarities with the class of “pediatric bithalamic gliomas” that has recently been reported by Mondal et al.9 Although most of the tumors in our series were located in the thalamic region, a small subset occurred in other, mostly midline CNS locations.
In addition, we expand on the spectrum of EGFR alterations observed in this group, with a high number of cases showing EGFR amplification and/or other missense mutations in EGFR besides the previously described variants.9 Interestingly, only 2 additional tumors in our series (further to the 3/5 that were included from the Mondal et al9 cohort) harbored the small in-frame insertions/duplications within exon 20 of EGFR, which was a high-frequency event in the initial bithalamic glioma cohort. However, it should also be noted that sequencing data were available for only half of the novel cases in our larger cohort. The lack of evidence for an EGFR alteration in a few cases with available sequencing data might be explained by uncommon and/or not covered aberrations in the affected pathway. Alterations of EGFR have been reported rarely in other types of pediatric malignant gliomas but seem to be enriched particularly within the DNA methylation class glioblastoma isocitrate dehydrogenase (IDH)-wildtype, receptor tyrosine kinase III (RTK III) in the current version of the Heidelberg DNA methylation classifier (broadly equivalent to “pedRTK2” according to Korshunov et al11). The frequency of EGFR alterations in the present subset of pediatric-type malignant gliomas, however, is much higher than that reported for any other type of glioma except the RTK II subgroup of IDH-wildtype glioblastoma in adults, which also carries frequent EGFR alterations, in particular EGFR amplification.8 The A289 mutation hotspot was shown to be linked with a more diffuse, infiltrative growth pattern,20 which fits well with being a common mutation in this group of malignant gliomas, especially those with unusual bithalamic growth. These findings further identify a subset of pediatric-type malignant glioma with a potential therapeutic target, since potent EGFR inhibitors are available and previous work in a patient-derived orthotopic xenograft model has shown that pediatric gliomas with EGFR amplifications may respond to targeted inhibitors.21 However, this hypothesis needs clinical validation as several clinical trials of EGFR-targeting approaches have previously failed in clinical trials for adult glioblastoma.22–24
In line with the previous report,9 a high percentage of tumors in the present cohort harbored additional TP53 mutations and a smaller subset showed H3 K27M mutations. These observations interestingly reveal that not all diffuse gliomas involving midline structures of the CNS with histone H3 K27M mutation align by DNA methylation profile with the reference DNA methylation class “diffuse midline glioma, H3 K27M-mutant” (which does not typically feature EGFR alterations).3 This new group of diffuse midline gliomas with uniform H3K27me3 loss and frequent EGFR alteration can also harbor H3 K27M mutation in a subset. Distinction between these 2 tumor types cannot be reliably determined by histologic features or H3 K27M mutation status alone, and thus requires assessment of EGFR copy number and mutation status or DNA methylation signature at present. Notably, not all H3 K27M mutations were comparably covered by the different panel sequencing approaches integrated in the present study, so the frequency of alterations might be underestimated.
A small subset of cases harbored MAPK alterations in a similar manner as described for typical H3 K27-mutant diffuse midline glioma. Interestingly, all of the 4 MAPK alterations were found in cases without obvious EGFR alteration. This might indicate that other MAPK alterations represent an alternative to EGFR activation in this group of tumors.
A major limitation of our study is the relatively low number of tumor samples with available tissue for a more comprehensive molecular workup. Further analyses are needed to reveal the full spectrum of genetic alterations within this group of tumors. Considering the features identified in the recent previous study9 and evaluating the overlaps and additional findings in the current set, we propose the broader designation of “diffuse midline glioma, H3K27me3-lost and EGFR-altered,” which would include the majority of bithalamic diffuse gliomas as well as a subset of unilateral thalamic gliomas. However, this will need confirmation in following studies.
Intriguingly, loss of H3K27me3 can now be regarded as unifying feature of 3 molecular classes of diffuse astrocytic glioma with predominant midline location: (i) the “typical” diffuse midline glioma with H3K27M mutation, (ii) the closely related diffuse midline glioma with EZHIP overexpression (which additionally shows a high frequency of ACVR1 mutations), and (iii) the series presented here which can show either H3 K27M or EZHIP overexpression together with a strong enrichment for EGFR alterations. Thus, one logical approach may be to integrate these 3 into a novel overarching tumor type, “diffuse midline glioma with H3K27me3 loss,” with the 3 molecular classes (DMG-K27, DMG-EZHIP, and DMG-EGFR) as subtypes. Important to note is that the H3 K27-mutant tumors remain by far the most common subtype, with the remaining 2 classes representing rare exceptions. Besides that, our data further support the recently described concept that cXorf67/EZHIP expression is a mutually exclusive alternative to H3 K27M histone mutation for driving massive loss of H3K27me3.12,16,25 These relationships, however, will need further prospective validation.
In summary, we provide further evidence for a subset of pediatric-type malignant glioma that is preferentially located in the thalamic region and shows frequent alteration of EGFR, which might provide future options for targeted therapeutic approaches. The occurrence of H3 K27M mutations in this glioma subgroup has important implications for classification. Assessment of H3 mutation status alone is not sufficient to distinguish the more frequent “typical” diffuse midline glioma, H3 K27M-mutant tumors from this novel glioma subtype with loss of nuclear H3K27me3 expression and frequent EGFR alteration. Although this diagnostic distinction has limited implications for patient outcome at present, our results suggest that a more comprehensive molecular workup of diffuse gliomas (particularly in the thalamus) may eventually become clinically relevant (eg, if shown to be associated with distinct responses to molecularly targeted therapeutic approaches).
Supplementary Material
Acknowledgments
We thank L. Dörner, L. Hofmann, and M. Schalles for skillful technical assistance and the microarray unit of the DKFZ Genomics and Proteomics Core Facility for providing Illumina DNA methylation array-related services.
Funding
This study was supported by the Hertie Network of Excellence in Clinical Neuroscience and the SFB 1389 UNITE glioblastoma by the Deutsche Forschungsgemeinschaft. PS is a fellow of the Hertie Academy of Excellence in Clinical Neuroscience, FS is a fellow of the Else Kröner Excellence Program of the Else Kröner-Fresenius Stiftung (EKFS, EKES.24). DC, DTWJ, JG, and M-AD gratefully acknowledge funding from the KickCancer-ITCC (Innovative Therapies for Children with Cancer) initiative.
Conflict of interest statement. There are no conflicts of interest for any authors.
Authorship statement. Experimental design: PS, MS, SMP, AvD, DTWJ, FS. Implementation: PS, MS, DS, DTWJ, FS. Data analysis/interpretation: PS, MS, DS, DSt, BB, DTWJ, FS. Sample collection: PS, DER, DStu, JH, SF, LK, AV, MZ, DC, JG, M-AD, PNH, MSn, CMK, GR, AK, NJ, PW, WW, DAS, AP, TSJ, CJ, OW, AvD, FS. Approved final manuscript: all authors.
References
- 1. Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21(Suppl 5):v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jones C, Baker SJ. Unique genetic and epigenetic mechanisms driving paediatric diffuse high-grade glioma. Nat Rev Cancer. 2014;14(10):651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mackay A, Burford A, Carvalho D, et al. Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell. 2017;32(4):520–537 e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paugh BS, Qu C, Jones C, et al. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J Clin Oncol. 2010;28(18):3061–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sturm D, Bender S, Jones DT, et al. Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat Rev Cancer. 2014;14(2):92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–231. [DOI] [PubMed] [Google Scholar]
- 7. Wu G, Broniscer A, McEachron TA, et al. ; St Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome Project Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44(3):251–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425–437. [DOI] [PubMed] [Google Scholar]
- 9. Mondal G, Lee JC, Ravindranathan A, et al. Pediatric bithalamic gliomas have a distinct epigenetic signature and frequent EGFR exon 20 insertions resulting in potential sensitivity to targeted kinase inhibition. Acta Neuropathol. 2020;139(6):1071–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Korshunov A, Schrimpf D, Ryzhova M, et al. H3-/IDH-wild type pediatric glioblastoma is comprised of molecularly and prognostically distinct subtypes with associated oncogenic drivers. Acta Neuropathol. 2017;134(3):507–516. [DOI] [PubMed] [Google Scholar]
- 12. Castel D, Kergrohen T, Tauziède-Espariat A, et al. Histone H3 wild-type DIPG/DMG overexpressing EZHIP extend the spectrum diffuse midline gliomas with PRC2 inhibition beyond H3-K27M mutation. Acta Neuropathol. 2020;139(6):1109–1113. [DOI] [PubMed] [Google Scholar]
- 13. Aryee MJ, Jaffe AE, Corrada-Bravo H, et al. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics. 2014;30(10):1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sahm F, Schrimpf D, Jones DT, et al. Next-generation sequencing in routine brain tumor diagnostics enables an integrated diagnosis and identifies actionable targets. Acta Neuropathol. 2016;131(6):903–910. [DOI] [PubMed] [Google Scholar]
- 15. International Cancer Genome Consortium PedBrain Tumor P. Recurrent MET fusion genes represent a drug target in pediatric glioblastoma. Nat Med. 2016;22(11):1314–1320. [DOI] [PubMed] [Google Scholar]
- 16. Pajtler KW, Wen J, Sill M, et al. Molecular heterogeneity and CXorf67 alterations in posterior fossa group A (PFA) ependymomas. Acta Neuropathol. 2018;136(2):211–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hübner JM, Müller T, Papageorgiou DN, et al. EZHIP/CXorf67 mimics K27M mutated oncohistones and functions as an intrinsic inhibitor of PRC2 function in aggressive posterior fossa ependymoma. Neuro Oncol. 2019;21(7):878–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jain SU, Do TJ, Lund PJ, et al. PFA ependymoma-associated protein EZHIP inhibits PRC2 activity through a H3 K27M-like mechanism. Nat Commun. 2019;10(1):2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Piunti A, Smith ER, Morgan MAJ, et al. CATACOMB: an endogenous inducible gene that antagonizes H3K27 methylation activity of Polycomb repressive complex 2 via an H3K27M-like mechanism. Sci Adv. 2019;5(7):eaax2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Binder ZA, Thorne AH, Bakas S, et al. Epidermal growth factor receptor extracellular domain mutations in glioblastoma present opportunities for clinical imaging and therapeutic development. Cancer Cell. 2018;34(1):163–177 e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brabetz S, Leary SES, Gröbner SN, et al. A biobank of patient-derived pediatric brain tumor models. Nat Med. 2018;24(11):1752–1761. [DOI] [PubMed] [Google Scholar]
- 22. Lassman AB, Rossi MR, Raizer JJ, et al. Molecular study of malignant gliomas treated with epidermal growth factor receptor inhibitors: tissue analysis from North American Brain Tumor Consortium Trials 01-03 and 00-01. Clin Cancer Res. 2005;11(21):7841–7850. [DOI] [PubMed] [Google Scholar]
- 23. Hegi ME, Diserens AC, Bady P, et al. Pathway analysis of glioblastoma tissue after preoperative treatment with the EGFR tyrosine kinase inhibitor gefitinib–a phase II trial. Mol Cancer Ther. 2011;10(6):1102–1112. [DOI] [PubMed] [Google Scholar]
- 24. Weller M, Butowski N, Tran DD, et al. ; ACT IV trial investigators Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10): 1373–1385. [DOI] [PubMed] [Google Scholar]
- 25. Pratt D, Quezado M, Abdullaev Z, et al. Diffuse intrinsic pontine glioma-like tumor with EZHIP expression and molecular features of PFA ependymoma. Acta Neuropathol Commun. 2020;8(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.