Abstract
Background
The aim of the project was to identify risk factors associated with visual progression and treatment indications in pediatric patients with neurofibromatosis type 1 associated optic pathway glioma (NF1-OPG).
Methods
A multidisciplinary expert group consisting of ophthalmologists, pediatric neuro-oncologists, neurofibromatosis specialists, and neuro-radiologists involved in therapy trials assembled a cohort of children with NF1-OPG from 6 European countries with complete clinical, imaging, and visual outcome datasets. Using methods developed during a consensus workshop, visual and imaging data were reviewed by the expert team and analyzed to identify associations between factors at diagnosis with visual and imaging outcomes.
Results
Eighty-three patients (37 males, 46 females, mean age 5.1 ± 2.6 y; 1–13.1 y) registered in the European treatment trial SIOP LGG-2004 (recruited 2004–2012) were included. They were either observed or treated (at diagnosis/after follow-up).
In multivariable analysis, factors present at diagnosis associated with adverse visual outcomes included: multiple visual signs and symptoms (adjusted odds ratio [adjOR]: 8.33; 95% CI: 1.9–36.45), abnormal visual behavior (adjOR: 4.15; 95% CI: 1.20–14.34), new onset of visual symptoms (adjOR: 4.04; 95% CI: 1.26–12.95), and optic atrophy (adjOR: 3.73; 95% CI: 1.13–12.53). Squint, posterior visual pathway tumor involvement, and bilateral pathway tumor involvement showed borderline significance. Treatment appeared to reduce tumor size but improved vision in only 10/45 treated patients. Children with visual deterioration after primary observation are more likely to improve with treatment than children treated at diagnosis.
Conclusions
The analysis identified the importance of symptomatology, optic atrophy, and history of vision loss as predictive factors for poor visual outcomes in children with NF1-OPG.
Keywords: neurofibromatosis 1, optic pathway glioma, treatment, vision, visual outcome
Key Points.
1. This analysis of risk factors for visual deterioration in patients with NF1-OPG was carried out using data from a European trial of chemotherapy.
2. Symptomatology, optic atrophy, and history of vision loss predict poor visual outcomes in NF1-OPG.
3. Treatment early after visual deterioration is more likely to salvage visual acuity.
Importance of the Study.
As prospective data on an appropriate risk stratification for vision loss in children with NF1-OPG are lacking, an international panel of experts in the field analyzed risk factors for visual deterioration in a large European trial cohort. The identification of patients at risk for vision loss will help to discern children with NF1-OPG to be observed from those in need of treatment. Symptomatology, optic atrophy, and history of vision loss could be identified as predictive factors for poor visual outcomes in children with NF1-OPG. Children with visual deterioration after primary observation are more likely to improve after treatment than children treated at diagnosis. This suggests a benefit for close monitoring and early intervention.
Neurofibromatosis type 1 (NF1) is a genetic tumor predisposition syndrome with an incidence of less than 1:3000.1 About 15% of NF1-affected children develop optic pathway gliomas (OPGs) during childhood, usually presenting during the first decade of life and occasionally in the second.2 Approximately 40% of OPG patients develop visual symptoms, but only about 15% of all OPG patients are treated.3–7 This visual risk is unpredictable, justifying regular ophthalmic screening during infancy and early childhood, although asymptomatic screening with MRI remains controversial.2,5,6,8–13 Data on the natural history and visual risk factors in children with NF1-OPG are scarce.3,5,6,10,14
Factors influencing visual outcome have been investigated only retrospectively, identifying age, tumor extension to the optic tracts, optic disc pallor, and young age as possible risk factors correlated with poor visual outcome.6,8,14,15 Other data suggest that girls with isolated optic nerve glioma have a higher risk for visual loss; however, visual outcomes after treatment do not differ from boys.6,16
Fisher et al reported a retrospective US multicenter analysis of visual outcome in 88 children treated with chemotherapy for NF1-OPG, showing improvement of visual acuity (VA) in 32%, worsening in 28%, while 40% remained stable.6 Visual and imaging outcomes were dissociated, consistent with previous observations.17–19
The SIOP-LGG-2004 trial recorded visual data in <25% of children with NF1-OPG; no visual data were reported in the Children’s Oncology Group (COG) A9952 study.20,21 This justifies consideration of developing criteria for treatment indication, outcome assessments, and effectiveness of treatments,6,8,18,22 which have now been acknowledged in upcoming trials in NF1 where methods for measuring visual outcomes are now specified using the experience of this project as basis (eg, ACNS1831, LOGGIC).
We report the results of a multidisciplinary international workshop held in Nottingham, UK, April 10–11, 2014 and focusing on childhood NF1-OPG. The European Society for Paediatric Oncology (SIOPE) LGG NF1 subgroup addressed previous inconsistencies of trial methods including their experience of the SIOP LGG 2004 trial. It was decided to validate current methods of assessing vision and imaging and to develop a European consensus on criteria for treatment indication and patient selection for future trials. The overall purpose was to use this experience to refine trial design for studying the natural history of this disease, identify possible risk factors for visual progression, and allow direct comparison of new drug treatments directed at preserving vision.
Methods
Trial Patient Cohort
A convenience cohort of trial’s patient data was assembled and analyzed to discern factors determining the risk of tumor and visual response/progression.
Workshop Methodology
The workshop was attended by 28 participants (ophthalmologists, pediatric neuro-oncologists, neurofibromatosis specialists, and neuro-radiologists) from 9 SIOPE centers (Austria: Vienna; Denmark: Copenhagen; France: Villejuif; Germany: Berlin, Hamburg; Italy: Padua; UK: Leeds, London, Nottingham). Participating centers were asked to retrieve data on children with NF1-OPG who had previously been enrolled in the institutional review board–approved LGG 2004 trial and for whom a complete clinical, visual outcome, and imaging dataset was available. It was intended to obtain a balanced mix of patients of different age groups (<3 y, 3–6 y, and >7 y) and various clinical course (observation only/observation followed by treatment/treatment at diagnosis). Mandatory visual data included VA and fundoscopic examination at the following time points: (i) OPG diagnosis, (ii) at start of treatment (if treated), (iii) at the end of treatment (if treated), or (iv) after 18 months from diagnosis if only observed. Presence of optic atrophy and further ophthalmologic signs (squint, proptosis, nystagmus, papilledema, abnormal visual behavior) as well neurologic signs and symptoms (eg, elevated intracranial pressure) were also noted. The term “abnormal visual behavior” used in the LGG 2004 trial protocol reflects the clinical overall impression of disturbed vision based upon history and parental observation in the absence of objective VA measurements due to the young age or poor cooperation.
Radiology
The tumors were identified using a combination of T2/fluid attenuated inversion recovery (FLAIR) and post gadolinium T1-weighted imaging. As contrast enhancement was variable and sometimes absent, and tumoral enhancement often fluctuates during the course of the disease both with and without treatment, T2/FLAIR data were used to define the full extent of the tumor. The T2 and FLAIR images were also the constant set of sequences available in all patients, and were therefore the basis for sequential study evaluations. Progression was determined by combining T2/FLAIR information with new or extending/enlarging T1 enhancing abnormality.
Where lesions were measurable, the measurements were used for sequential evaluations. More diffuse and ill-defined tumor involving the posterior visual pathways was less amenable for reliable and repeatable linear measurements. This is recognized as a methodological flaw. Tumor volumetric evaluation was not undertaken due to inherent difficulties defining tumor margins on T2/FLAIR images (often ill-defined) and separating the tumor from contiguous NF1-related focal areas of signal intensity. In addition, for the T2/FLAIR imaging, the slice thickness (usually 5 mm or, in some cases, 6 mm) and slice gap (usually 1.5 mm or more) precluded useful volumetric evaluation. Volumetric techniques for assessing ill-defined multifocal T2/FLAIR lesions have as yet not been fully validated, with ongoing work at developing appropriate software programs to enable reliable and auditable volumetric measurement. Involvement of the hypothalamus and presence of hydrocephalus were routinely recorded.
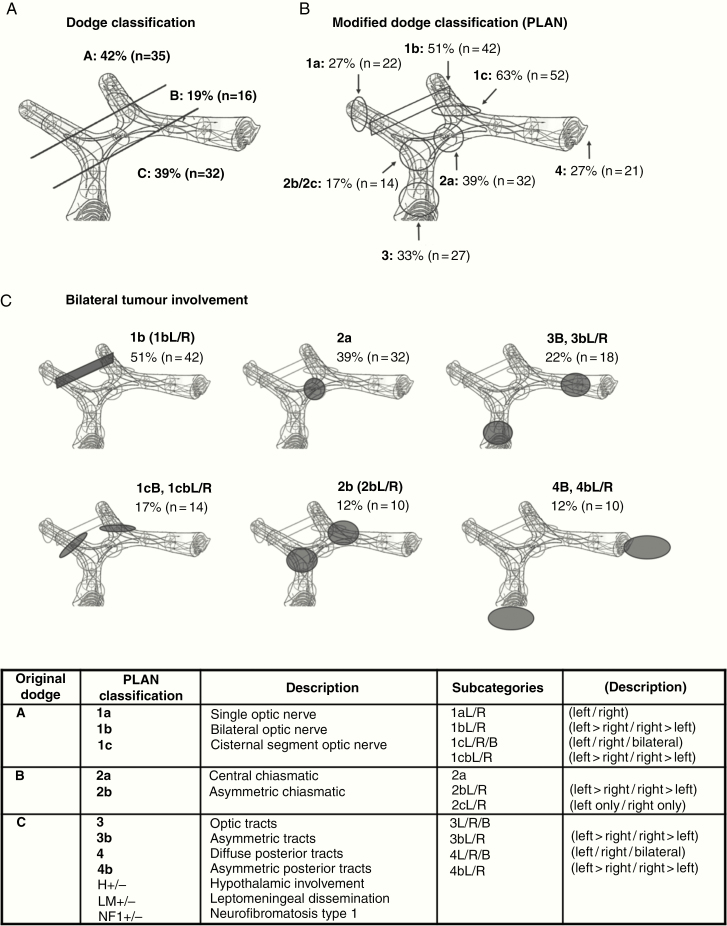
The radiology consensus work group also unified the terminology used to describe the anatomic pattern of NF1-OPG by using the classic Dodge and modified Dodge classification (MDC).
Ophthalmologic Data
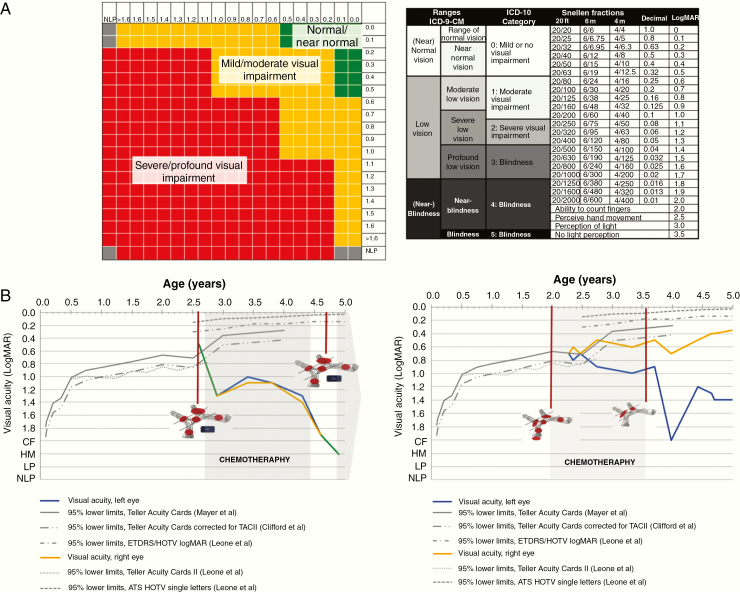
Visual acuity data were reported as LogMAR units, in order to ensure a quantitative and continuous measure as a surrogate marker of visual function, with higher LogMAR values corresponding to worse VA. As the VA testing methods varied, VA was converted from different grading systems (eg, decimal) to LogMAR. Prespecified values were used to describe qualitative VA (eg, “hand movement,” “light perception”). VA was depicted on a chart with axes corresponding to the right/left eye respectively (Fig. 1A). A graphical representation of different colors corresponding to different visual risk zones in both eyes was introduced and adapted subsequently by one of the authors (E.O.) from World Health Organization (WHO) categories of visual impairment.23,24 Changes over time were evaluated using this proposed visual risk assessment system and by analysis of VA data of single eyes as proposed previously.6 It was not possible to include visual field data as part of the workshop due to the young age of the patients and the lack of observation data for review.
Fig. 1.
Proposed graphical scheme for visualizing visual acuity measurement. (A) Visual function classification and a schematic for recording VA results.24 (B) Clinical follow-up record for NF1-OPG patients. Left: This graph portrays the longitudinal visual acuities of a young boy diagnosed at the age of 2 years 7 months old. Where the line is green, both eyes have the same visual acuity. Right: Longitudinal visual acuities of a young patient diagnosed at the age of 2 years and 2 weeks old. The red circles on the graph indicate the structures involved and the blue squares indicate hypothalamic involvement. The 95% lower limits of VA testing in young children are provided as published previously.35–37 CF: count fingers; HM: hand movement; LP: light perception; NLP: no light perception.
Appropriate methods adjusting for the age-related visual maturation are lacking. A clinical follow-up chart was also developed and shared with the review group and gained support as it permitted recording of VA of the right and left eye over time, comparison against age-appropriate normal values across infancy and early childhood, and grading of vision loss (Fig. 1B). In addition, for data analysis, visual function at the end of follow-up was classified by clinicians as “better,” “same,” or “worse” reflecting the subjective impact of VA change. The trial did not specify the requirement for visual field data because of the difficulties of making such measurements reliably in the very young age group who typically present with this tumor type.
Statistical Analysis
The online datasets were reviewed and approved by participants of the radiology and ophthalmology working groups. Descriptive analysis was used to characterize the study population. As appropriate, t-test, chi-square, or Fisher’s exact test was used for comparison between groups. Odds ratios (ORs) with 95% confidence intervals were calculated for each variable using logistic regression to identify potential predictors. All analyses were performed with SPSS 23 for Windows, and P < 0.05 was considered statistically significant in all analyses.
Results
The NF1-OPG Workshop Cohort
The cohort for analysis consisted of 83 patients (37 males, 46 females) with a mean age (at first imaging assessment) of 5.1 ± 2.6 years (range, 1–13.1 y). Patient characteristics, clinical history, and symptoms preceding diagnosis are shown in Supplementary Table 1. In approximately half of the patients (39/83, 47%) an OPG had been detected during MRI screening. All patients had a visual and imaging assessment before commencement of treatment or observation. Mean follow-up time, defined as the interval between the first and last assessment (either vision or imaging) was 3.4 ± 2.7 years.
Visual Assessment at Baseline
Forty-three children (52%) were asymptomatic, while 25 (30%) had 1 symptom and 15 children (18%) had multiple ophthalmologic symptoms. Common signs and symptoms presented in over 10% of patients included squint (22/83, 27%), abnormal visual behavior (19/83, 23%), and proptosis (11/83, 13%). Based on the visual function classification (Fig. 1A) agreed by the workshop participants, 44% (n = 36) of patients were classed as normal/near normal vision, 36% (n = 29) as mild/moderate, and 20% (n = 16) as severe/profound visual impairment. About a third (29/83, 35%) had optic atrophy (14 unilateral and 15 bilateral).
Imaging Assessment at Baseline
Distribution of anatomic site at diagnosis followed central review. Using the classic Dodge system,25 there were 42% (n = 35) stage A; 19% (n = 16) stage B, and 39% (n = 32) stage C (Fig. 2A). When classified according to the MDC26 in 31 children (37%) tumor extended posterior to the chiasm (MDC 3/4), and in 76% (63/83) the OPG was bilateral (Fig. 2B, C and Supplementary Table 1).
Fig. 2.
Distribution of anatomic site at diagnosis following central review. (A) Dodge27 and (B) MDC28; (C) bilateral involvement PLAN 1b, 1cB, 1cb, 2a, 2b, 3B, 3b, 4B, and 4b. In the MDC, all involved locations are stated, most tumors have more than one involved location, therefore percentages add up to over 100%.
Management Strategy
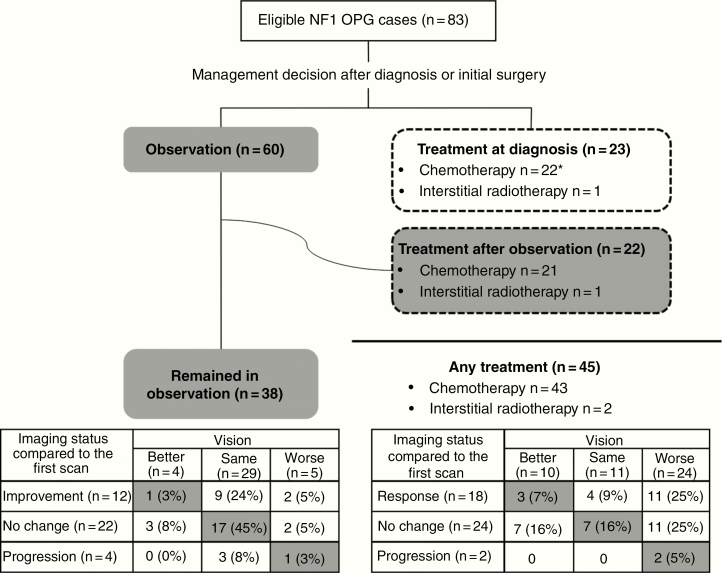
Overall management strategy and visual and radiological outcomes are summarized in Fig. 3. Sixty patients (72%) were initially observed after diagnosis, of whom 38 remained in observation throughout the follow-up period and 22 subsequently started treatment. Twenty-three patients (28%) started nonsurgical treatment at diagnosis, while 1 changed to second chemotherapy due to progressive vision loss.
Fig. 3.
Summary of management strategy, visual, and radiological outcomes of the Nottingham Workshop cohort (n = 83). Vision at last follow-up was judged as better, same, or worse by clinical judgment of trial physicians in the trial center. *One patient changed initial treatment to second chemotherapy due to progressive vision loss.
Justification of Selecting Treatment versus Observation
Among the 45 patients receiving treatment at diagnosis or after observation, the reasons to treat as well as prior visual and imaging assessments are summarized in Supplementary Tables 1 and 2. The most common indication for initial observation was normal or acceptable vision (n = 32), followed by lack of other visual symptoms (n = 16). Other reasons (n < 10) included unilateral visual deficit (n = 7), no threat to vision (n = 5), and stable vision (n = 4). Conversely, reasons to treat at diagnosis were preexisting severe vision loss and/or actual threat of vision loss, while indications after initial observation were progressive vision loss and/or radiological tumor progression (Supplementary Table 2).
In order to identify clinical characteristics associated with the decision to treat, we compared patients in the observation group throughout the follow-up period (n = 38) and those who had treatment (n = 45) (Supplementary Figure 1). Patients in the treatment group were more likely to be young children aged 2–5 years at diagnosis (27/45), to have new-onset visual symptoms (17/45), visual impairment classed as severe/profound (16/45), abnormal visual behavior (15/45), multiple visual symptoms (13/45), bilateral optic atrophy (12/45), and/or proptosis (10/45) (Supplementary Figure 1). A similar pattern was also observed when we further divided the subgroup according to the time point of treatment, ie, at diagnosis or after observation (Supplementary Table 1). Among patients who were originally observed after diagnosis (n = 60), 2 or more visual symptoms and severe visual impairment at diagnosis were factors which were significantly overrepresented in those who ultimately started treatment at a later stage (Supplementary Table 1). At the start of treatment, VA was better in patients being observed first compared with patients initially treated at diagnosis (P = 0.046).
Visual Outcome
Among the study population, 19 patients (23%) presented with VA of LogMAR ≥ 1.0 at diagnosis (16 unilateral and 3 bilateral), and this number increased to 28 (21 unilateral and 7 bilateral) at last follow-up.
For overall visual changes, as assessed clinically (Fig. 3), at the end of follow-up/treatment 54 out of 83 patients (65%) had better (n = 14) or the same vision (n = 40) compared with baseline assessment and 29 (35%) became worse (5 in observation group and 24 in treatment group; Fig. 3). Visual outcomes did not correlate with radiological changes (Fig. 3); for instance, 18/45 patients showed improvement in MRI after treatment but only 3 actually had better vision. The strongest correlation was static vision and stable imaging in the observation group. Age at which patients started treatment was not associated with difference in visual outcome (P = 0.88).
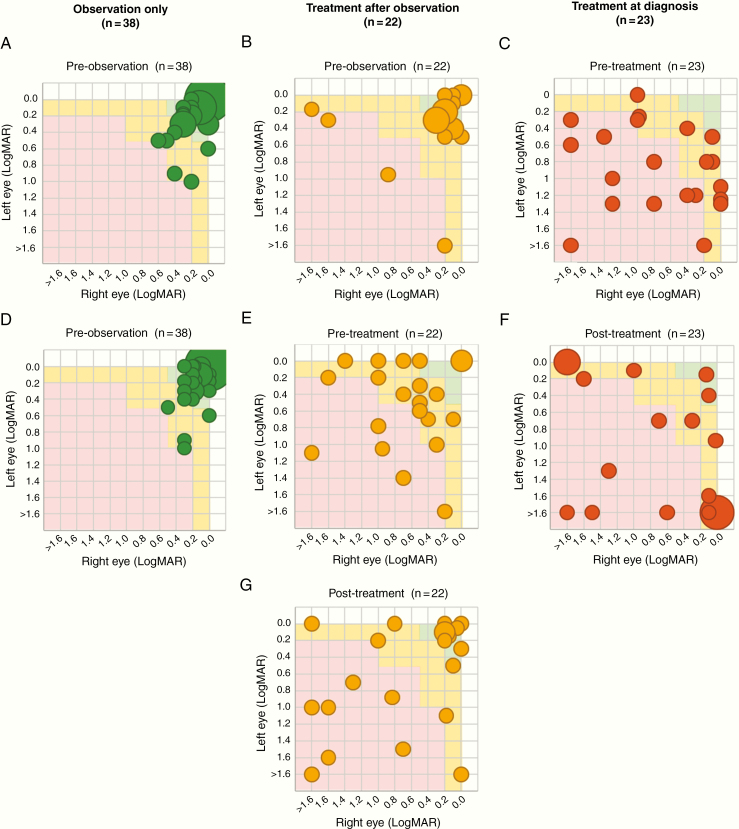
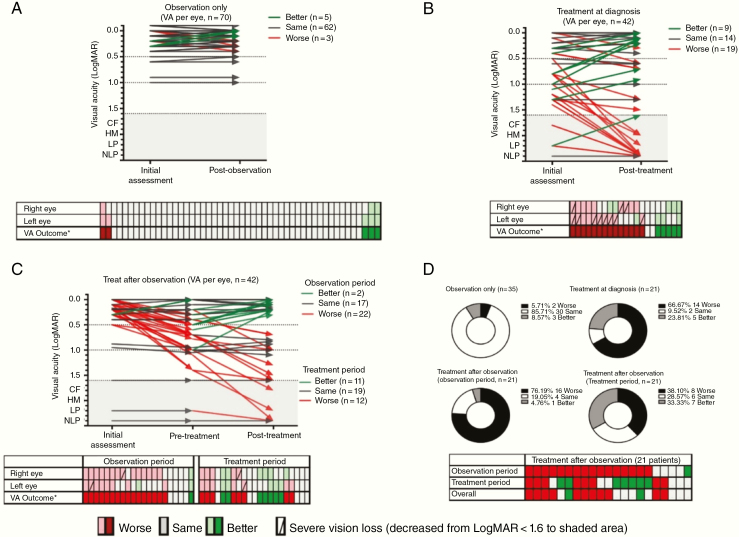
Classification by visual risk zones at diagnosis, pretreatment, and post-observation or end of treatment is shown in Fig. 4. Only 9 out of the 45 patients who received treatment moved up one (n = 8, 5 initially observed) or two (n = 1) risk zone categories compared with their pretreatment assessment (43 valid pairs), while the rest remained in the same category (n = 25, 12 initially observed) or became worse (n = 9, 4 initially observed).
Fig. 4.
Comparison of visual function classification at pre-observation, pretreatment, and post-observation/treatment. Visual function classification: normal/near normal (green); mild/moderate impairment (amber); severe profound impairment (red). Count fingers, hand movement; light perception and no light perception were combined into LogMAR > 1·6
At the individual level (Fig. 5), 154 eyes in 77 patients were eligible for analysis. Visual acuity remained unchanged in the observation only group (Fig. 5A). Compared with patients treated at diagnosis, children treated after observation less frequently developed further vision loss of 2 lines (0.2 LogMAR) or more (12 eyes in 8/21 patients vs 19 in 14/21) or dropped to near-blindness/blindness (2 eyes in 2/21 patients vs 11 in 10/21), indicating a better overall visual status at end of treatment (Fig. 5B, C. Of 16 patients worsening during observation, 7 had visual deterioration reversed with vincristine/carboplatin (VC) (Fig. 5D).
Fig. 5.
Visual acuity outcome between initial and post-observation/treatment assessment at individual level (154 evaluable eyes in 77 patients). Visual acuity outcome per eye for patients in (A) observation only, (B) treatment at diagnosis, (C) treatment after observation, and (D) summary of visual acuity outcome of all 77 patients. CF: count fingers, HM: hand movement, LP: light perception; NLP: no light perception. *“Improvement”/ “worsening” was defined as a 2-line change in VA, or decrease from LogMAR < 1·6 to shaded area, ie, CF, HM, LP, or NLP.
Potential Risk Factors for Visual Deterioration
Descriptive and univariate analyses were carried out to identify potential predictors of visual deterioration from the first assessment (Supplementary Table 3). Strong risk or protective factors with their crude ORs and adjusted ORs are summarized in Table 1. After adjustment for age at diagnosis, sex, and management strategy (ie, observation, treatment at diagnosis, and treatment after observation), variables remained significant (P < 0.05) and were: presence of more than one visual symptom (adjOR: 8.33; 95% CI: 1.9–36.45), abnormal visual behavior (adjOR: 4.15; 95% CI: 1.20–14.34), new onset of visual symptoms (adjOR: 4.04; 95% CI: 1.26–12.95), and optic atrophy (adjOR: 3.76; 95% CI: 1.13–12.53). Squint, posterior tumor involvement (MDC 3/4), and bilateral tumor involvement showed borderline significance (P-values between 0.05 and 0.1), with adjOR ranging between 2.9 and 3.50. Optic atrophy indicates neuronal loss, this study has not clarified its role in predicting vision change. We also tested a potential risk assessment model based on the variables selected from the workshop discussion (Supplementary Table 4). Although this model had an overall accuracy of 82.4%, the estimates were unstable due to the small sample size and number of variables in this model.
Table 1.
Risk factors for visual deterioration after observation or treatment
| Crude OR (95% CI) | Adjusted OR (95% CI)* | P-value | |||
|---|---|---|---|---|---|
| Risk factors, crude OR > 5 | |||||
| Two or more visual symptoms at initial visual assessment: squint, abnormal visual behavior, proptosis, nystagmus, papilledema | 17.50 | (3.98–76.88) | 8.33 | (1.90–36.45) | 0.005 |
| Bilateral optic atrophy | 9.75 | (2.62–36.34) | 5.15 | (1.21–21.96) | 0.027 |
| Optic atrophy (unilateral + bilateral) | 6.91 | (2.39–19.95) | 3.76 | (1.13–12.53) | 0.031 |
| Severe/profound visual impairment (red) | 6.91 | (1.87–25.49) | 1.74 | (0.32–9.35) | 0.518 |
| Abnormal visual behavior | 6.50 | (2.12–19.94) | 4.15 | (1.20–14.34) | 0.025 |
| New onset visual symptoms | 6.16 | (2.16–17.54) | 4.04 | (1.26–12.95) | 0.019 |
| Risk factors, crude OR 3–5 | |||||
| Unilateral optic atrophy | 4.88 | (1.34–17.79) | 2.68 | (0.61–11.79) | 0.191 |
| Squint | 4.06 | (1.46–11.31) | 3.19 | (0.99–10.28) | 0.052 |
| Bilateral tumor involvement | 3.98 | (1.06–15.00) | 3.50 | (0.81–15.06) | 0.092 |
| Proptosis | 3.98 | (1.06–14.99) | 2.05 | (0.48–8.87) | 0.336 |
| Posterior involvement (PLAN 3/4) | 3.20 | (1.25–8.22) | 2.90 | (0.99–8.53) | 0.053 |
| Protective factors, crude OR < 0.5 | |||||
| NF1 screening with imaging | 0.36 | (0.14–0.93) | 0.77 | (0.25–2.34) | 0.638 |
* Summary of crude and adjusted odds ratios for variables reached P < 0.1 in univariate analysis (Supplementary Table 3), ranked by effect size. Adjusted for age (as continuous variable), sex, and management strategy in 3 groups.
Discussion
The 2 major collaborative scientific groups, the SIOPE and the COG, like others, have been to date unsuccessful in gathering and adequately reporting visual outcome data from large prospective trials enrolling patients with NF1-associated OPG.20,21,27–29
Justification Workshop
Preliminary analysis of visual data from the SIOP-LGG 2004 trial followed by the process of designing the next generation of NF1-OPG trials highlighted this deficiency.20 The visual outcomes of the UK cohort of the trial have recently been published.14 Even with this significant effort, sufficient ophthalmological data could only be gathered for little more than half of all OPG patients. In response, this multidisciplinary workshop was conducted in order to discuss and develop a new consensus for visual and radiographic assessment criteria for future trials in Europe. Identification of visual risk factors and harmonization of appropriate eligibility criteria and outcome measures are of particular importance in view of the possible introduction of new targeted drugs (eg, MEK inhibitors), as potential first-line treatment in patients with NF1-associated LGG compared with standard chemotherapies in upcoming trials. A retrospective study conducted by Fisher et al among expert practitioners from several large neuro-oncology centers concluded that there was a lack of agreement on how to select patients for treatment or observation.6 In contrast to this retrospective data report, the present workshop was conducted with specific consensus methodology and was based on a selection of cases recruited within a prospective clinical trial at large international centers over a short time period. Furthermore, in the SIOP LGG-2004 trial, the reasons for initiating treatment including severe symptoms or vision loss, documented tumor progression on imaging, progressive vision loss, or threat to vision were prespecified. One notable difference between these 2 convenience cohorts is the median age at presentation. The Fisher cohort was 2.66 years, while this cohort median age was 4.7 years. Fisher’s cohort was recruited from institutional clinics indicative of current practice in the US. This cohort was recruited from a multicenter clinical trial according to a consensus-based selection criteria in Europe. The differences are likely to be due to referral bias and clinical practice norms in different health systems. The differences are important to consider in designing future trials, as such a significant difference in age at presentation will influence tumor behavior, suitability of drug preparations, and the capacity to comply with outcome assessments.
Study Cohort
The establishment of a convenience patient cohort, using prospectively collected complete clinical, visual, and imaging trial datasets overcame the inconsistencies of data for imaging and visual assessment methods in previous trials or retrospective studies. The use of trial patients harnessed existing ethical approvals for international collaboration and permitted representative case selection across age, vision, and imaging categories. The visual outcome datasets and images were reviewed centrally by specialists working in pairs. The process of central review of all imaging and visual outcome data permitted an in-depth assessment of the need for consistency of such data reporting and refinement of both the imaging and visual outcome methods. A consensus on both standardization of recording visual outcome as well as conversion to LogMAR scores (Fig. 1) was reached, and presentation of these data in a standardized format integrating WHO visual outcome criteria was agreed (see Appendix 1). The methods for outcome from a European perspective have already been fed into the transatlantic discussions of trial design that are active.30
Imaging Consensus
The imaging group tested the application of the MDC as a way of anatomically classifying the tumor distributions.26 Furthermore, the imaging group concluded that tumor response criteria could not be based upon tumor or optic pathway measurement according to progression of MDC stage (Supplementary Figure 2) and would need to be based upon overall opinion of the imaging appearances to represent “progression,” “stable disease,” or “response.” Experience gained in this way will set the foundation for future trial design and as the basis of plans for the next era of trials that will aim at improving functional outcomes.30
Cohort Analysis of Visual Risk
Finally, this comprehensive dataset permitted univariate and multivariate analyses of risk factors for visual and imaging outcomes in patients selected initially for observation versus those selected for initial treatment, based upon the LGG 2004 trial treatment. Compared with the results of Fisher’s US study6 and the national UK cohort,14 this European dataset confirmed that only a minority of patients (9/45, 20%) experience visual improvement after VC treatment. The previously recognized lack of correlation between vision and imaging outcomes has been replicated in this study. Factors that identify children with the greatest risk of vision loss and need for therapy include the presence of multiple visual symptoms, optic atrophy, abnormal visual behavior, and new onset of visual symptoms. In contrast to the study by Fisher, neither age at diagnosis nor anatomic features of modified Dodge categories 3 and 4 involving posterior tracts and radiations were of significance. The small sample size and short follow-up make this unique and contrasting finding worthy of further confirmation in future prospective studies to clarify its status as a predictive factor. The Fisher cohort and this cohort were both convenience cohorts. In Fisher’s cohort the age at diagnosis was a median of 2.66 years compared with a median age of 4.7 years in this cohort. Fisher’s cases were identified from participating hospitals from clinical databases where chemotherapy had been used (oncology, ophthalmology, neurology, and/or NF clinic) at each site. This cohort was derived from cases entered into an international trial. Their differences limit the validity of close comparison.
Treatment versus Observation Criteria
In accordance with literature, progressive vision loss, presence of multiple visual symptoms, and tumor progression on imaging were the main reasons to initiate treatment.6–8 Among 60 patients, selected initially for observation, 22 (37%) developed visual deterioration and were then treated. They showed a similar pattern of visual symptoms, but better VA than those selected initially for treatment. The multivariate analysis of factors associated with visual outcome helps to identify characteristics suitable for case selection for observation versus treatment, which need further validation. A strategy incorporating patient history, visual function, and imaging will be necessary to correctly select patients for treatment.31 Further work to be published has explored expert clinical justification of case selection for initial observation and immediate treatment (Walker et al manuscript in preparation). It is not possible from the data collected in the trial, and therefore available in this analysis, to identify whether the patients initially observed and who went on to be treated were identified with different imaging strategies or symptom types/severities compared with those who were initially treated. This was the local physician’s decision supported by the trial’s eligibility criteria.
Sight-Saving Therapy
New sight-saving/-preserving therapies need to be tested in NF1-OPG patients with greatest need for therapy to ensure that new treatments are truly tested for their vision-saving qualities. It is notable that in this workshop NF1 cohort, for those treated with VC, visual improvement was observed in a minority (Figures 4 and 5). Yet, the analysis of visual outcomes demonstrates that patients treated immediately or after observation had different patterns of visual response and severity of visual outcome. Those treated immediately had a smaller proportion of patients experiencing stable disease/visual improvement and a greater proportion with very severe vision loss compared with those treated after a period of observation This observation suggests that history of recent vision loss plays an important role in selecting cases with potential for visual stability or improvement and is associated with a lower risk of very severe visual outcome. VC would seem to be most effective in reversing vision loss when used before the vision loss is established. The poor outcome for patients with optic atrophy would support this observation indicating that vision recovery cannot be expected once neuronal loss becomes established.
As a consequence, we propose that eligibility criteria for future trials should include evidence, and timing, of prior visual decline, where it can be identified, as a factor for case selection for treatment and in analysis of visual outcomes. The role of optic atrophy could be better explored using optical coherence tomography, where retinal fiber layer thickness correlates with neuronal loss, offering a more objective measure of optic pathway injury as an outcome measure for early intervention trials.32,33
Our findings suggest that, for many, treatment may be starting when nerve damage has already occurred and become irreversible and that VC is only preserving, rather than improving, vision.18 It also justifies more detailed consideration of the mechanisms of vision loss and therapeutic impact of treatment(s) under trial. The goal is to restore, or at least preserve, optic nerve function.
One factor to be considered is the continued use of any drug with known neurotoxicity, such as weekly vincristine. Vincristine has a protracted half-life of about 5 days, making weekly scheduling lead to accumulation of tissue levels. Vincristine peripheral neuropathy is a recognized complication of weekly scheduling necessitating dose reduction or cessation of therapy. A recent case report and literature review identifies 12 cases, 9 in children (age <18 y) with optic atrophy and blindness after the use of vincristine for various cancer therapies.34 The COG A9952 study reported grade 3 and 4 neurotoxicity in 23% of NF1 patients receiving VC, which included weekly vincristine in induction.21 This drug combination, while standard, has never been tested in a randomized comparison and so is the de facto, rather than the tested, standard treatment. Reconsideration of vincristine dosing and scheduling may be justified.
Observation Strategy
The role of monitoring asymptomatic children with NF1 by prediagnostic MR imaging of the brain is disputed.2,9–13 The present analysis revealed that patients who had MRI as part of surveillance were at a lower risk of visual deterioration (Table 1) in univariate analysis (crude OR: 0.36; 95% CI: 0.14–0.93) but not in the multivariable analysis (adjOR: 0.77, 95% CI: 0.25–2.34). Novel MRI techniques such as fractional anisotropy and MRI volumetry of the optic pathway in conjunction with optical coherence tomography may help identify preclinical signs of neuronal loss and therefore those at higher risk for visual deterioration, which may justify changes to screening guidelines.31 Confirmation in population trials is needed especially if new treatments were demonstrated to be less toxic and more effective than current approaches with chemotherapy.
Conclusion
This workshop has refined understanding of risk factors for visual deterioration and therefore case selection for “observation” versus “treatment.” This work will assist with identifying criteria associated with the highest risk of visual deterioration and so the candidates most suitable for evaluating new drugs and their capacity to preserve or save vision.
Supplementary Material
Funding
This workshop was supported by the Children’s Brain Tumour Research Centre, University of Nottingham.
Conflict of interest statement. The authors report no disclosures relevant to this study.
Authorship statement. Amedeo A. Azizi: study design, manuscript writing, literature search, data collection, data interpretation. David A. Walker: correspondent author, study design, manuscript writing, data collection, data interpretation, Jo-Fen Liu: study design, figures, data analysis, manuscript writing. Astrid Sehested: study design, data collection, data interpretation. Timothy Jaspan: image analysis, data collection, data interpretation. Ian Simmons, Rosalie Ferner, Jacques Grill, Darren Hargrave, Pablo Hernáiz Driever: data collection, data interpretation. Berthold Pemp: data collection, data interpretation, manuscript writing. Gareth Evans: data interpretation. Enrico Opocher: study design, manuscript writing, data collection, data interpretation, figures. Ehlers-Hansen C, Grundy RG, Hammond C, Hampson L, Lucchetta M, Meijer L, O’Hare P, Pemp B, Picton S, Nissen KR, Thomas S, Schmook M, Simmons I, Warmuth-Metz M, Lischka T: data interpretation as workshop participants.
References
- 1. Evans DG, Howard E, Giblin C, et al. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A. 2010;152A(2):327–332. [DOI] [PubMed] [Google Scholar]
- 2. Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol. 2007;61(3):189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guillamo JS, Créange A, Kalifa C, et al. ; Réseau NF France Prognostic factors of CNS tumours in neurofibromatosis 1 (NF1): a retrospective study of 104 patients. Brain. 2003;126(Pt 1):152–160. [DOI] [PubMed] [Google Scholar]
- 4. Friedrich RE, Nuding MA. Optic pathway glioma and cerebral focal abnormal signal intensity in patients with neurofibromatosis type 1: characteristics, treatment choices and follow-up in 134 affected individuals and a brief review of the literature. Anticancer Res. 2016;36(8):4095–4121. [PubMed] [Google Scholar]
- 5. Hernáiz Driever P, von Hornstein S, Pietsch T, et al. Natural history and management of low-grade glioma in NF-1 children. J Neurooncol. 2010;100(2):199–207. [DOI] [PubMed] [Google Scholar]
- 6. Fisher MJ, Loguidice M, Gutmann DH, et al. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro Oncol. 2012;14(6):790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nicolin G, Parkin P, Mabbott D, et al. Natural history and outcome of optic pathway gliomas in children. Pediatr Blood Cancer. 2009;53(7):1231–1237. [DOI] [PubMed] [Google Scholar]
- 8. Dalla Via P, Opocher E, Pinello ML, et al. Visual outcome of a cohort of children with neurofibromatosis type 1 and optic pathway glioma followed by a pediatric neuro-oncology program. Neuro Oncol. 2007;9(4):430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caen S, Cassiman C, Legius E, Casteels I. Comparative study of the ophthalmological examinations in neurofibromatosis type 1. Proposal for a new screening algorithm. Eur J Paediatr Neurol. 2015;19(4):415–422. [DOI] [PubMed] [Google Scholar]
- 10. Thiagalingam S, Flaherty M, Billson F, North K. Neurofibromatosis type 1 and optic pathway gliomas: follow-up of 54 patients. Ophthalmology. 2004;111(3):568–577. [DOI] [PubMed] [Google Scholar]
- 11. Prada CE, Hufnagel RB, Hummel TR, et al. The use of magnetic resonance imaging screening for optic pathway gliomas in children with neurofibromatosis type 1. J Pediatr. 2015;167(4):851–856 e851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blanchard G, Lafforgue MP, Lion-François L, et al. ; NF France network Systematic MRI in NF1 children under six years of age for the diagnosis of optic pathway gliomas. Study and outcome of a French cohort. Eur J Paediatr Neurol. 2016;20(2):275–281. [DOI] [PubMed] [Google Scholar]
- 13. Schupper A, Kornreich L, Yaniv I, Cohen IJ, Shuper A. Optic-pathway glioma: natural history demonstrated by a new empirical score. Pediatr Neurol. 2009;40(6):432–436. [DOI] [PubMed] [Google Scholar]
- 14. Falzon K, Drimtzias E, Picton S, Simmons I. Visual outcomes after chemotherapy for optic pathway glioma in children with and without neurofibromatosis type 1: results of the International Society of Paediatric Oncology (SIOP) low-grade glioma 2004 trial UK cohort. Br J Ophthalmol. 2018;102(10):1367–1371. [DOI] [PubMed] [Google Scholar]
- 15. Balcer LJ, Liu GT, Heller G, et al. Visual loss in children with neurofibromatosis type 1 and optic pathway gliomas: relation to tumor location by magnetic resonance imaging. Am J Ophthalmol. 2001;131(4):442–445. [DOI] [PubMed] [Google Scholar]
- 16. Diggs-Andrews KA, Brown JA, Gianino SM, Rubin JB, Wozniak DF, Gutmann DH. Sex Is a major determinant of neuronal dysfunction in neurofibromatosis type 1. Ann Neurol. 2014;75(2):309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mitchell AE, Elder JE, Mackey DA, Waters KD, Ashley DM. Visual improvement despite radiologically stable disease after treatment with carboplatin in children with progressive low-grade optic/thalamic gliomas. J Pediatr Hematol Oncol. 2001;23(9):572–577. [DOI] [PubMed] [Google Scholar]
- 18. Kalin-Hajdu E, Décarie JC, Marzouki M, Carret AS, Ospina LH. Visual acuity of children treated with chemotherapy for optic pathway gliomas. Pediatr Blood Cancer. 2014;61(2):223–227. [DOI] [PubMed] [Google Scholar]
- 19. Kelly JP, Leary S, Khanna P, Weiss AH. Longitudinal measures of visual function, tumor volume, and prediction of visual outcomes after treatment of optic pathway gliomas. Ophthalmology. 2012;119(6):1231–1237. [DOI] [PubMed] [Google Scholar]
- 20. Opocher E, De Salvo GL, De Paoli A, et al. Visual outcome of children with Neurofibromatosis type 1 and progressive optic pathway glioma treated with chemotherapy: preliminary report from the SIOP-LGG 2004 study. Neuro-Oncology. 2012;14(suppl_1):i72–i72. [Google Scholar]
- 21. Ater JL, Xia C, Mazewski CM, et al. Nonrandomized comparison of neurofibromatosis type 1 and non-neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low-grade glioma: a report from the Children’s Oncology Group. Cancer. 2016;122(12):1928–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moreno L, Bautista F, Ashley S, Duncan C, Zacharoulis S. Does chemotherapy affect the visual outcome in children with optic pathway glioma? A systematic review of the evidence. Eur J Cancer. 2010;46(12):2253–2259. [DOI] [PubMed] [Google Scholar]
- 23. Colenbrander A Assessment of functional vision and its rehabilitation. Acta Ophthalmol. 2010;88(2):163–173. [DOI] [PubMed] [Google Scholar]
- 24. ICD-10 Version: 2016. World Health Organization; 2016. https://icd.who.int/browse10/2016/en. Accessed October 4, 2019.
- 25. Dodge HW Jr, Love JG, Craig WM, et al. Gliomas of the optic nerves. AMA Arch Neurol Psychiatry. 1958;79(6):607–621. [DOI] [PubMed] [Google Scholar]
- 26. Taylor T, Jaspan T, Milano G, et al. ; PLAN Study Group Radiological classification of optic pathway gliomas: experience of a modified functional classification system. Br J Radiol. 2008;81(970):761–766. [DOI] [PubMed] [Google Scholar]
- 27. Gnekow AK, Falkenstein F, von Hornstein S, et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol. 2012;14(10):1265–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gnekow AK, Walker DA, Kandels D, et al. ; Low Grade Glioma Consortium and participating centers A European randomised controlled trial of the addition of etoposide to standard vincristine and carboplatin induction as part of an 18-month treatment programme for childhood (≤16 years) low grade glioma—a final report. Eur J Cancer. 2017;81:206–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stokland T, Liu JF, Ironside JW, et al. A multivariate analysis of factors determining tumor progression in childhood low-grade glioma: a population-based cohort study (CCLG CNS9702). Neuro Oncol. 2010;12(12):1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jones DTW, Kieran MW, Bouffet E, et al. Pediatric low-grade gliomas: next biologically driven steps. Neuro Oncol. 2018;20(2):160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Blank PMK, Fisher MJ, Liu GT, et al. Optic pathway gliomas in neurofibromatosis type 1: an update: surveillance, treatment indications, and biomarkers of vision. J Neuroophthalmol. 2017;37(Suppl 1):S23–S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parrozzani R, Miglionico G, Leonardi F, et al. Correlation of peripapillary retinal nerve fibre layer thickness with visual acuity in paediatric patients affected by optic pathway glioma. Acta Ophthalmol. 2018;96(8):e1004–e1009. [DOI] [PubMed] [Google Scholar]
- 33. Zahavi A, Toledano H, Cohen R, et al. Use of optical coherence tomography to detect retinal nerve fiber loss in children with optic pathway glioma. Front Neurol. 2018;9:1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adhikari S, Dongol RM, Hewett Y, Shah BK. Vincristine-induced blindness: a case report and review of literature. Anticancer Res. 2014;34(11):6731–6733. [PubMed] [Google Scholar]
- 35. Clifford CE, Haynes BM, Dobson V. Are norms based on the original Teller Acuity Cards appropriate for use with the new Teller Acuity Cards II? J AAPOS. 2005;9(5):475–479. [DOI] [PubMed] [Google Scholar]
- 36. Leone JF, Mitchell P, Kifley A, Rose KA; Sydney Childhood Eye Studies Normative visual acuity in infants and preschool-aged children in Sydney. Acta Ophthalmol. 2014;92(7):e521–e529. [DOI] [PubMed] [Google Scholar]
- 37. Mayer DL, Beiser AS, Warner AF, Pratt EM, Raye KN, Lang JM. Monocular acuity norms for the Teller Acuity Cards between ages one month and four years. Invest Ophthalmol Vis Sci. 1995;36(3): 671–685. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.