Abstract
Background
Leptomeningeal metastases (LM) are associated with limited survival and treatment options. While involved-field radiotherapy is effective for local palliation, it lacks durability. We evaluated the toxicities of proton craniospinal irradiation (CSI), a treatment encompassing the entire central nervous system (CNS) compartment, for patients with LM from solid tumors.
Methods
We enrolled patients with LM to receive hypofractionated proton CSI in this phase I prospective trial. The primary endpoint was to describe treatment-related toxicity, with dose-limiting toxicity (DLT) defined as any radiation-related grade 3 non-hematologic toxicity or grade 4 hematologic toxicity according to the Common Terminology Criteria for Adverse Events that occurred during or within 4 weeks of completion of proton CSI. Secondary endpoints included CNS progression-free survival (PFS) and overall survival (OS).
Results
We enrolled 24 patients between June 2018 and April 2019. Their median follow-up was 11 months. Twenty patients were evaluable for protocol treatment–related toxicities and 21 for CNS PFS and OS. Two patients in the dose expansion cohort experienced DLTs consisted of grade 4 lymphopenia, grade 4 thrombocytopenia, and/or grade 3 fatigue. All DLTs resolved without medical intervention. The median CNS PFS was 7 months (95% CI: 5–13) and the median OS was 8 months (95% CI: 6 to not reached). Four patients (19%) were progression-free in the CNS for more than 12 months.
Conclusion
Hypofractionated proton CSI using proton therapy is a safe treatment for patients with LM from solid tumors. We saw durable disease control in some patients.
Keywords: craniospinal irradiation, leptomeningeal metastases, proton radiation
Key Points.
1. Proton CSI is safe for patients with LM.
2. Proton CSI is potentially effective for LM.
Importance of the Study.
This is the first prospective trial evaluating hypofractionated proton CSI for patients with LM from solid tumors. We found that hypofractionated proton CSI is well tolerated with limited bone marrow effects, which is critical in this often heavily pretreated population. We also found promising durable CNS control and survival in a subset of patients.
Leptomeningeal metastasis (LM), the spread of a malignancy into the cerebrospinal fluid (CSF), is a dreaded complication of solid tumors. Although approximately 20% of patients with cancer with neurologic symptoms harbor LM at autopsy,1,2 it is clinically detected in only 5–8% of patients with solid tumors.3 The incidence of LM is rising, likely due to improved MRI4 and better systemic treatments.5–7 The current incidence of LM varies widely depending on primary site, with lung cancer and breast cancers most commonly associated with LM (5–25%), followed by melanoma (6–18%) and gastrointestinal malignancies (4–14%).2,4,8,9
Within the subarachnoid space, tumor cells disseminate throughout the central nervous system (CNS). As a result, patients with LM develop headache, pain, and multifocal neurologic deficits, including cranial neuropathies, cerebellar dysfunction, radiculopathy, and cauda equina syndrome. Treatments for LM are palliative, with the goals of stabilizing or improving neurologic symptoms. The prognosis for patients with LM is poor. Untreated, LM can lead to death within 4–6 weeks.10 Therapy prolongs overall survival (OS) to 3–11 months.9,11–14 Responses to therapies and outcomes vary widely, impacted by performance status, tumor histology, and disease outside the CNS.
Radiation therapy (RT) effectively relieves local symptoms due to LM and, in the form of involved-field RT, is commonly used to treat symptomatic or bulky disease sites.15–17 Because LM disseminates throughout the entire CSF compartment, involved-field RT cannot halt the progression of LM along the entire neuroaxis. Given RT’s effectiveness in treating symptomatic LM, craniospinal irradiation (CSI), treatment of the entire leptomeningeal compartment, may be advantageous for symptom and disease control.
CSI is standard of care treatment in pediatric patients with leptomeningeal spread of some CNS primary tumors such as medulloblastoma18,19 and germinomas,20,21 and for select patients with leukemia and lymphoma.22,23 In solid tumors, the role of CSI is less clearly defined. Small studies have demonstrated its effectiveness in alleviating neurologic symptoms caused by LM but have also revealed significant side effects associated with photon CSI.24–27 These are the result of the gradual dose fall-off of the exit dose of photon radiation.28–30 When delivering photon radiation to the entire neuroaxis, photons exit the body anteriorly, exposing the entire spinal column and anterior organs to radiation. In contrast, protons deposit the bulk of their energy at the last few millimeters of their range. This results in diminished delivery of RT beyond the neuroaxis with proton CSI. The vertebral column and the anterior structures are largely spared. This unique property of proton radiation plans translates into dosimetric superiority compared with photon radiation for CSI, resulting in significantly less gastrointestinal and hematologic toxicity.31
In this phase Ib study, we evaluated the safety of proton CSI for patients with LM due to solid tumors. In addition, we explored efficacy, survival, and patient-reported symptoms of this approach.
Materials and Methods
Patient Eligibility
Patients aged 10 or above with pathologically proven solid tumor malignancies with LM established radiographically and/or through CSF cytology were eligible for this study. Additional eligibility criteria included a Karnofsky or Lansky performance score of 60; no serious major neurologic deficits including encephalopathy; no previous RT that precluded developing a treatment plan respective of normal tissue tolerance (Supplement 1); available systemic treatment options; and adequate bone marrow function (hemoglobin 9 g/dL, absolute neutrophil count 1500/mm3, and platelet count 100 000/mm3). Systemic therapy was held 7 days prior to initiation and during proton CSI treatment, with the exception of endocrine therapy and trastuzumab for breast cancer patients. Patients resumed their previous systemic therapy after the completion of proton CSI. Patients were not allowed to initiate a new systemic therapy until the end of the dose-limiting toxicity (DLT) evaluation period, 4 weeks after completion of proton CSI. Patients with parenchymal brain metastases and with radiographically/CSF established LM were eligible. No restriction on steroid use was required for the study.
The protocol was reviewed and approved by the institutional review board and registered with ClinicalTrials.gov (NCT03520504). The study was conducted in accordance with the principles of the World Medical Association Declaration of Helsinki. Informed consent was obtained from all participants.
Study Design and Protocol Therapy
This was a phase Ib study with a dose expansion cohort evaluating hypofractionated proton CSI for patients with LM from solid tumors. The primary endpoint was to determine the frequency of treatment-related toxicity. Secondary endpoints included duration of CNS disease control, CNS progression-free survival (PFS), OS, and patient-reported symptoms.
DLT was defined as any radiation-related grade 3 non-hematologic toxicity or grade 4 hematologic toxicity according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4 that occurred during or within 4 weeks of completion of proton CSI. We employed a 3 + 3 design with de-escalation. The first 3 patients received a dose of 30 Gy (relative biological effectiveness [RBE]) in 3 GyRBE fractions. If 1 or no patients experienced a DLT, 3 additional patients would be enrolled at this dose of proton CSI. If 1 or none of the 6 patients experienced a DLT, the trial would proceed to the dose expansion cohort at 30 GyRBE in 3 GyRBE fractions. In contrast, if 2 or more patients experienced a DLT, 3 patients would then be enrolled at a dose level of 25 GyRBE in 2.5 GyRBE fractions. If 1 or no patients experienced a DLT, an additional 3 patients would be enrolled at 25 GyRBE. If 2 or more patients experienced a DLT at 25 GyRBE, the study would stop and treatment would be declared too toxic. If 1 or no patients developed a DLT among the 6 patients who received 25 GyRBE, the trial would proceed to the dose expansion cohort at 25 GyRBE in 2.5 GyRBE fractions.
Patients were simulated supine using standard CSI immobilization including a customized rigid mask. The clinical target volume (CTV) of the proton CSI treatment fields consisted of the entire brain, thecal sac, and proximal sacral nerve roots. The planning target volume (PTV) was the CTV plus margin to ensure that the prescribed dose was delivered to the CTV, accounting for variation in treatment delivery and range uncertainties in the proton beam direction. The PTV was a 4 mm margin from the brain CTV and 5 mm margin from the spine CTV. The radiation planning time for all patients was 2 weeks or less as mandated by the protocol. Dose volume constraint guidelines for organs at risk (Supplement 1) were used in the optimization process during treatment planning. Patients were treated with pencil beam scanning proton therapy.32
Pretreatment evaluations included a history and physical exam, including standardized neurological examination per Response Assessment in Neuro-Oncology Leptomeningeal Metastases (RANO-LM) neurological assessment33; complete blood count (CBC), electrolytes, renal and liver function tests; lumbar puncture for CSF assessment; MRI of brain and MRI of total spine with and without contrast; patient-reported symptoms using MD Anderson Symptom Inventory‒Brain (MDASI-BT) and MDASI‒Spine (MDASI-SP); and baseline adverse event evaluation using CTCAE. During proton CSI, patients underwent history and physical examination including standardized neurological examination, weekly CBC, and adverse event evaluation. At 1 month, 3 months, 6 months, and/or at time of disease progression after the completion of protocol treatment, patients underwent history and physical examination including standardized neurological examination, CBC, lumbar puncture for CSF assessment, MRI brain and MRI total spine, MDASI-BT, MDASI-SP, and adverse event evaluation. CNS disease progression was defined by a combination of clinical, radiographic, and cytologic examinations. Patients with new neurologic deficit(s) and/or progressive radiographic change not related to therapeutic intervention, and/or new positive cytology, were considered to have progressive LM.
Statistical Considerations
Treatment-related toxicities were evaluated during and after proton CSI by CTCAE value and grade. CNS responses at 1 month, 3 months, and 6 months were estimated along with 95% confidence intervals. Patients with improved or stable disease were considered as having a response. Patients who progressed earlier were counted in the denominator; patients who died without progression were excluded from the denominator. CNS PFS was evaluated from the first day of proton CSI until CNS progression or death. Patients who were alive and progression-free at the end of study were censored. OS was evaluated from the first day of proton CSI until death. Patients alive at the end of study were censored. Survival outcomes were estimated with Kaplan–Meier methods and plots. Median follow-up time was calculated using the reverse Kaplan–Meier method.
Overall MDASI-BT and MDASI-SP symptom scores were described with medians and ranges and visualized with box plots. One-month, 3-month, and 6-month scores were compared with baseline scores with the Wilcoxon signed rank test. Changes from baseline for individual symptoms were visualized with bar charts of median and interquartile range. In these bar charts, a score difference of 0 corresponded to symptom stability, a negative score difference corresponded to symptom improvement, and a positive score difference corresponded to symptom worsening. Due to the exploratory nature of this study, no adjustment for multiple comparisons was performed.
Two-sided P-values less than 0.05 were considered statistically significant. All analyses were performed with SAS 9.4 TS1 M6.
Results
Dose Determination
Between June 2018 and April 2019, twenty-four patients enrolled. The majority of patients had non-small-cell lung cancer, and patients with molecularly driven disease all had CNS progression on small-molecule tyrosine kinase inhibitors at the time of enrollment. The median age was 52 and the median KPS was 70. Patient characteristics are presented in Table 1. Four patients were inevaluable for protocol-related toxicity assessment: 1 patient did not start protocol treatment due to systemic progression, 2 patients did not complete protocol treatment due to systemic progression, and 1 patient completed protocol treatment outside of the allowed treatment window (this patient was included exclusively in efficacy and survival analyses). Of the 3 evaluable patients enrolled at 30 GyRBE in 3 GyRBE fractions, no patient experienced DLT and therefore 3 additional evaluable patients were enrolled at the 30 GyRBE dose again with no further DLTs observed. The study went on to enroll 14 additional evaluable patients to the expansion cohort at the same dose of 30 GyRBE in 3 GyRBE fractions, for a total of 20 patients evaluable for all analyses, including toxicity, and 21 patients evaluable for efficacy and survival analyses.
Table 1.
Characteristics of all evaluable patients (N = 21)
| Characteristics | Median (range) | Number (%) |
|---|---|---|
| Age, y | 52 (30–67) | |
| Sex Female Male |
15 (71) 6 (29) |
|
| KPS | 70 (60–90) | |
| Histology Non-small-cell lung cancer ALK+ EGFR+ ROS1+ Breast carcinoma ER + HER2− ER + HER2+ ER-HER2+ TN Esophageal adenocarcinoma Rectal adenocarcinoma Adenoid cystic carcinoma of parotid |
11 (52) 1 (5) 7 (33) 1 (5) 7 (33) 3 (14) 1 (5) 1 (5) 2 (10) 1 (5) 1 (5) 1 (5) |
|
| LM Diagnosis at Enrollment Newly diagnosed LM LM progression on prior therapies |
9 (43) 12 (57) |
|
| Number of Prior Metastatic Disease Therapies at Enrollment Systemic Therapies Afatinib Alectinib Brigatinib Enctrectinib Erlotinib Lorlatinib Osimertinib Local Therapies (surgery, radiation therapy) |
2 (0*-5) 1 (5) 1 (5) 1 (5) 1 (5) 4 (19) 1 (5) 7 (33) 1 (0-6) |
|
| Baseline Evaluation Positive MRI Positive cytology |
21 (100) 16 (76) |
|
| With Parenchymal Brain Metastases at Enrollment Yes No |
11 (52) 10 (48) |
Abbreviations: EGFR = epidermal growth factor receptor; ALK = anaplastic lymphoma kinase; ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; KPS = Karnofsky performance status; LM = leptomeningeal metastasis; MRI = magnetic resonance imaging; ROS1 = ROS proto-oncogene 1; TN = triple negative breast cancer
*One patient with metastatic adenoid cystic carcinoma of parotid did not receive prior systemic therapy prior to enrollment
Proton CSI Toxicities
Of the 20 patients evaluable for protocol treatment–related toxicities, 2 patients in the dose expansion cohort experienced DLTs: 1 patient (5%) experienced grade 4 lymphopenia; another patient experienced grade 4 lymphopenia, grade 4 thrombocytopenia, and grade 3 fatigue. All DLTs resolved without medical intervention. Protocol treatment–related toxicities that occurred within the DLT period in the majority of patients include:
• fatigue (19 patients [95%]: 10 [50%] grade 1, 8 [40%] grade 2, 1 [5%] grade 3),
• lymphopenia (18 patients [90%]: 1 [5%] grade 2, 15 [75%] grade 3, 2 [10%] grade 4), and
• thrombocytopenia (10 patients [50%]: 7 [35%] grade 1, 2 [10%] grade 2, 1 [5%] grade 4).
Other protocol treatment–related grade 3 toxicities included grade 3 leukopenia within 1 month of protocol therapy in 3 patients (15%), grade 3 anemia within 1 month of protocol therapy in 1 (5%), grade 3 anorexia at 6 months after protocol therapy in 1 (5%), and grade 3 back pain at 6 months after protocol therapy in 1 patient (5%). All protocol treatment–related toxicities during the DLT period and at 3 months and 6 months after protocol therapy are listed in Table 2.
Table 2.
Proton CSI-related toxicities (N = 20)
| # Patients (%) | ≤1 Month | 3 Months | 6 Months | |
|---|---|---|---|---|
| General Disorders | ||||
| Dry Skin | Grade 1 | 1 (5) | 1 (5) | 0 (0) |
| Fatigue | Grade 1 | 10 (50) | 2 (10) | 1 (5) |
| Grade 2 | 8 (40) | 2 (10) | 1 (5) | |
| Grade 3 | 1* (5) | 0 (0) | 0 (0) | |
| Pain | Grade 2 | 1 (5) | 0 (0) | 0 (0) |
| Radiation Dermatitis | Grade 1 | 6 (30) | 0 (0) | 0 (0) |
| Grade 2 | 1 (5) | 0 (0) | 0 (0) | |
| Gastrointestinal Disorders | ||||
| Anorexia | Grade 2 | 1 (5) | 1 (5) | 0 (0) |
| Grade 3 | 0 (0) | 0 (0) | 1 (5) | |
| Dysgeusia | Grade 1 | 0 (0) | 1 (5) | 0 (0) |
| Dyspepsia | Grade 1 | 1 (5) | 0 (0) | 0 (0) |
| Vomiting | Grade 1 | 2 (10) | 0 (0) | 0 (0) |
| Neurologic Disorders | ||||
| Back Pain | Grade 3 | 0 (0) | 0 (0) | 1 (5) |
| Gait Disturbance | Grade 2 | 1 (5) | 0 (0) | 0 (0) |
| Headache | Grade 1 | 1 (5) | 1 (5) | 1 (5) |
| Grade 2 | 0 (0) | 1 (5) | 0 (0) | |
| Memory Impairment | Grade 1 | 0 (0) | 1 (5) | 0 (0) |
| Nausea | Grade 1 | 8 (40) | 0 (0) | 1 (5) |
| Grade 2 | 0 (0) | 1 (5) | 0 (0) | |
| Sensory Impairment | Grade 1 | 0 (0) | 1 (5) | 0 (0) |
| Hematologic Disorders | ||||
| Anemia | Grade 1 | 2 (10) | 0 (0) | 0 (0) |
| Grade 2 | 1 (5) | 0 (0) | 0 (0) | |
| Grade 3 | 1 (5) | 0 (0) | 0 (0) | |
| Leukopenia | Grade 1 | 3 (15) | 0 (0) | 0 (0) |
| Grade 2 | 2 (10) | 0 (0) | 0 (0) | |
| Grade 3 | 3 (15) | 0 (0) | 0 (0) | |
| Lymphopenia | Grade 2 | 1 (5) | 0 (0) | 0 (0) |
| Grade 3 | 15 (75) | 0 (0) | 0 (0) | |
| Grade 4 | 2* (10) | 0 (0) | 0 (0) | |
| Neutropenia | Grade 2 | 5 (25) | 0 (0) | 0 (0) |
| Thrombocytopenia | Grade 1 | 7 (35) | 0 (0) | 0 (0) |
| Grade 2 | 2 (10) | 0 (0) | 0 (0) | |
| Grade 4 | 1* (5) | 0 (0) | 0 (0) |
* = Dose limiting toxicity.
CNS Progression-Free Survival and Overall Survival
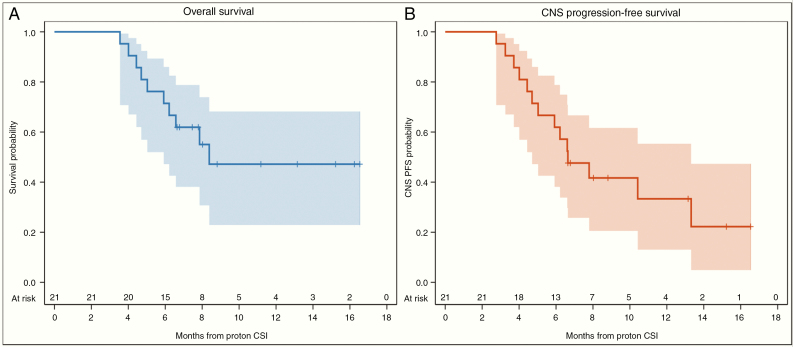
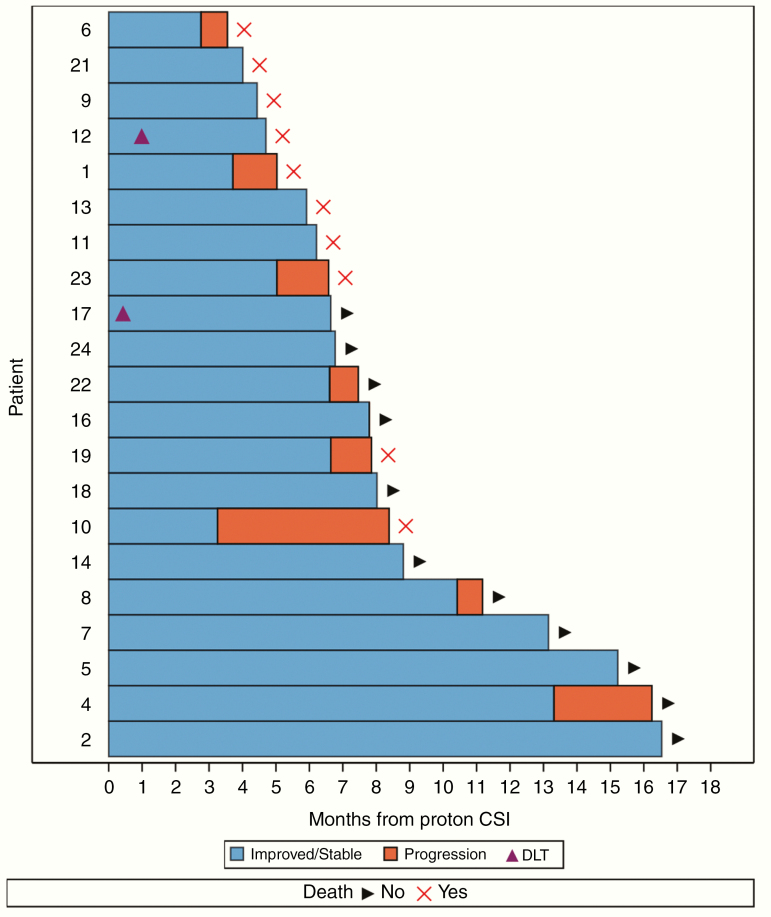
The median follow-up was 11 months (95% CI: 8–15 mo). The median OS of the study cohort was 8 months (95% CI: 6–not reached), with estimated OS of 71% (95% CI: 47–86%) at 6 months and 47% (95% CI: 23–68%) at 12 months (Figure 1A). The median CNS PFS was 7 months (95% CI: 5–13), with estimated CNS PFS of 62% (95% CI: 38–79%) at 6 months and 33% (95% CI: 13–55%) at 12 months (Figure 1B). Of the 21 patients evaluable for treatment outcomes, 9 patients had progressed in the CNS and 10 patients had died: 5 from concurrent systemic progression and CNS progression, 2 from pneumonia, 1 from CNS progression, 1 from liver failure, and 1 from a pulmonary embolism. CNS response rate to protocol therapy was 100% (95% CI: 84–100%, N = 21/21) at 1 month, 86% (95% CI: 64–97%, N = 18/21) at 3 months, and 63% (95% CI: 35–85%, N = 10/16) at 6 months. At the time of this analysis, 4 patients (19%) achieved CNS disease control for more than 12 months after protocol therapy (Figure 2).
Fig. 1.
Kaplan–Meier plot of (A) overall survival of the study cohort (B) central nervous system progression-free survival of the study cohort.
Fig. 2.
Event history plot of the 21 evaluable patients for treatment outcomes. Progression in the figure represents CNS progression.
Patient-Reported Symptoms
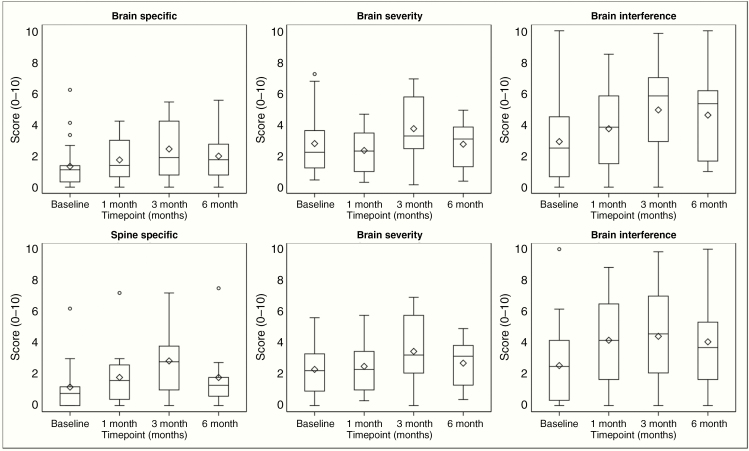
Patients completed MDASI-BT and MDASI-SP as assessments for patient reported symptoms: 21 patients completed assessments at baseline, 18 at 1 month, 16 at 3 months and 11 at 6 months. Compared with baseline graphically, a trend toward worsening of patient reported symptoms was found at 3 months with stabilization or improvement of symptoms at 6 months (Figure 3). Specifically, patients reported gradually worsening spine-specific symptoms—such as weakness and change in bowel pattern—that are statistically significant at 1 month (median 0.9, P = 0.041) and 3 months (median 2.4, P = 0.002), but such symptoms improved by 6 months (median = 0.4, P = 0.43) compared with baseline (Table 3). While no significant difference was observed at 1 month (median = 1.0, P = 0.25) or 3 months (median = 1.0, P = 0.058), patients also reported worse brain-interference symptoms, such as mood or ability to work, at 6 months (median = 2.2, P = 0.009). Changes in individual specific symptoms compared with baseline are depicted through a bidirectional bar graph and can be found in Supplement 2–3.
Figure 3.
MDASI score distribution overtime. These plots are a schematic for the distribution of values. The ends of the box represent the 25th and 75th percentiles (or 1st and 3rd quartiles), while the center line and diamond represent the median and mean, respectively. The 75th minus the 25th percentile equals the interquartile range (IQR), and the ends of the whiskers are placed at 1.5 times the IQR. Any values lying outside these boundaries are considered outliers. Higher values indicate worsening symptoms.
Table 3.
MDASI and RANO neurologic examination differences between follow-up timepoints and baseline*
| Score | 1 Month | 3 Months | 6 Months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Median (range) | P = | N | Median (range) | P = | N | Median (range) | P = | |
| Brain Specific Score | 18 | 0.6 (−3.2–2.9) | 0.45 | 15 | 0.7 (−2.1–5.2) | 0.14 | 11 | 0.7 (−1.8–4.0) | 0.07 |
| Brain Severity Score | 17 | −0.2 (−4.8–3.4) | 0.30 | 16 | 1.0 (−2.1–5.5) | 0.08 | 11 | 0.8 (−2.5–4.3) | 0.13 |
| Brain Interference Score | 17 | 1.0 (−5.7–6.0) | 0.25 | 16 | 1.0 (−4.2–6.2) | 0.06 | 11 | 2.2 (−0.8–5.8) | 0.009 |
| Spine Specific Score | 14 | 0.9 (−3.0–3.0) | 0.04 | 13 | 2.4 (−0.8–4.2) | 0.002 | 9 | 0.4 (−3.5–5.5) | 0.43 |
| Spine Severity Score | 18 | 0.0 (−4.8–4.5) | 0.85 | 16 | 1.5 (−3.0–5.5) | 0.06 | 10 | 0.8 (−2.5–4.3) | 0.19 |
| Spine Interference Score | 17 | 1.5 (−4.2–6.0) | 0.08 | 16 | 1.0 (−4.2–7.0) | 0.07 | 10 | 1.7 (−1.4–5.5) | 0.08 |
Numbers represent difference from baseline, with a higher score signifying worsening of reported symptoms.
Discussion
Patients with LM who receive CSI traditionally receive X-ray–based photon RT. While photon CSI alleviates neurologic symptoms, it is associated with significant side effects.25,26 Here, we have demonstrated that proton CSI of 30 Gy in 10 fractions can be safely delivered to patients with LM due to solid tumors with limited toxicity. It is important to note that 30 Gy in 10 treatments or 3 Gy per fraction of RT is a hypofractionated approach. Traditionally CSI is given in 1.8 Gy or 2 Gy per fraction requiring 20 or more treatments. For patients with LM, hypofractionated RT would be preferable to long-course RT. It is more convenient for patients with functional decline, and it minimizes interruptions in systemic therapy for patients with active systemic disease. Furthermore, it is unclear whether long-course RT would result in superior outcomes in patients with significant CNS disease.34 As 30 Gy in 10 treatments is considered the standard involved-field RT dose for patients with LM, it was used as the starting dose for this study.
In our study, only 2 patients developed DLTs (grade 3 fatigue, grade 4 lymphopenia, grade 4 thrombocytopenia), which did not interrupt therapy and resolved without medical intervention. No unexpected adverse event was observed on study. The most common protocol treatment–related toxicity was fatigue (95% of patients), a known side effect of CNS radiation, which persisted at 3 months (20%) and 6 months (10%) in some patients. Another common toxicity was lymphopenia (90% of patients), which was resolved by 3 months. No infections were observed. No patient experienced grade 3 gastrointestinal toxicities or required blood product transfusions.
We found that the toxicity profile of proton CSI was lower compared with what has been reported on X-ray–based photon CSI. Hermann et al retrospectively examined 16 patients with solid tumor LM who received 36 Gy photon CSI in 18 fractions between 1995 and 2000 and found that the majority of patients experienced significant myelosuppression and dysphagia.24 In another study, Harada et al evaluated 17 patients with LM from solid tumors who received X-ray–based photon CSI to a median of 41.4 Gy between 2008 and 2013 and found that the majority of patients had grade 3 or above hematologic and/or gastrointestinal side effects.25 Involved-field photon RT can be associated with severe, persistent lymphopenia, which has been shown to negatively impact survival.35 Given that LM is a late complication in patients with solid tumors, and patients commonly have compromised bone marrow reserve and abnormal organ function from systemic and local therapies, proton CSI may potentially be a safer approach in this patient population.
In addition, we found that CSI may be effective in achieving durable CNS disease control in patients with LM from solid tumors. The majority of evaluable patients (63%) achieved CNS disease control 6 months after proton CSI, and at the time of this analysis, 19% of patients maintained CNS disease control over 12 months. We also found favorable survival outcomes in our study cohort, with a median CNS PFS and OS of 7 months and 8 months, respectively. Although it is difficult to draw conclusions given the small number of patients in our study, our survival outcomes compared favorably with earlier reports with median survivals of 3–5 months.26,36,37
Our study is the first to demonstrate patient-reported neurologic symptom stability after CSI. Using MDASI-BT and MDASI-SP, we also found that patients reported worsening of spine symptoms at 1 and 3 months and worsening of brain symptoms at 6 months after protocol therapy. The change in patient-reported symptoms can be confounded by a variety of factors, including initiation of new systemic therapy after study DLT period, performance status, and systemic disease progression leading to patients coming off the study. Nevertheless, decline in neurologic function at 2–4 months after CNS RT has been reported; Saito et al reported worsening of Hopkins Verbal Learning Test–Revised scores at 4 months after whole-brain RT compared with baseline but not at 8 months,38 and Brown et al reported decline of function at 2 months which persisted at 6 months.39 This suggests that subacute radiation-induced toxicities can occur at 2–4 months after completion of RT and should be taken into consideration in patient evaluation and explored in a larger study.
There are several limitations to this study. Given the small number of patients enrolled in the study, the array of primary cancers included and the many systemic therapies received, we are not able to draw robust conclusions on efficacy, survival outcomes, or patient-reported symptoms. Changes in patient-reported symptoms are confounded by multiple clinical factors, including change in therapy and change in disease status, and, given the limited number of participants, should be interpreted with caution. We plan to evaluate the effects of proton CSI on patient reported symptoms in an upcoming randomized trial.
In conclusion, this is the first prospective study evaluating proton CSI with 30 Gy in 10 fractions for patients with LM from solid tumors. We have determined that hypofractionated proton CSI appears safe for patients with LM from solid tumors, a population that would benefit significantly from shorter courses of RT to minimize interruptions to systemic therapy. We have seen early evidence that hypofractionated proton CSI is an effective treatment in many patients and observed durable disease control on this study. We plan a phase II randomized trial to further determine the efficacy of hypofractionated proton CSI.
Supplementary Material
Acknowledgments
We thank all patients and their family members in participating in this trial. We also thank the physicians and staff at ProCure Proton Therapy Center, Somerset, New Jersey in providing excellent care and treatments for our patients on trial. This work was presented at the 2019 annual meeting of the Society of Neuro-Oncology (SNO), November 23, 2019 in Phoenix, AZ.
Conflict of interest statement. TJY: research funding from AstraZeneca and Kazia Therapeutics. DAG: stock ownership in Johnson & Johnson. RJY: research funding from Agios; consultant for Agios, Puma, NodicNeuroLab, and ICON. MGK: consultant for AstraZeneca, Daiichi-Sankyo, and Pfizer. HAY: research funding from AstraZeneca, Daiichi, Pfizer, Novartis, Lily, and Cullinan Oncology; consultant for AstraZeneca and Daiichi. ADS: consultant and lecturer for Genomic Health, Genentech, Novartis, Lily, and Eisai; consultant for Puma, Odonate, and Nektar; lecturer for Pfizer and Celgene. CG: consultant for Ono, Kite, and BTG. AB: consultant for Arix Biosciences 2018; member of Scientific Advisory Board for Evren Scientific (unpaid); patents 62/258,044 pending November 20, 2015, United States 10413522, awarded September 17, 2019.
Authorship statement. Experimental design of the study: TJY, LMD, AB, EP. Implementation of the study: TJY, JY, SW, MM, RJY, MGK, HAY, ADS, ITG, AL, BS, CG, AFP, LS, JBS, AB, EP. Data analysis and interpretation: TJY, NAW, DAG, ZZ, AB, EP. Manuscript preparation: TJY, NAW, JY, SW, MM, DAG, ZZ, RJY, MGK, HAY, ADS, ITG, AL, BS, CG, AFP, LS, JBS, LMD, AB, EP.
Funding
This work was supported by grants from Cycle for Survival Equinox Innovation Initiative Award and the National Institutes of Health/National Cancer Institute (P30-CA008748).
References
- 1. Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982;49(4):759–772. [DOI] [PubMed] [Google Scholar]
- 2. Kaplan JG, DeSouza TG, Farkash A, et al. Leptomeningeal metastases: comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J Neurooncol. 1990;9(3):225–229. [DOI] [PubMed] [Google Scholar]
- 3. Beauchesne P Intrathecal chemotherapy for treatment of leptomeningeal dissemination of metastatic tumours. Lancet Oncol. 2010;11(9):871–879. [DOI] [PubMed] [Google Scholar]
- 4. Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM. Leptomeningeal metastases in the MRI era. Neurology. 2010;74(18):1449–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lai R, Dang CT, Malkin MG, Abrey LE. The risk of central nervous system metastases after trastuzumab therapy in patients with breast carcinoma. Cancer. 2004;101(4):810–816. [DOI] [PubMed] [Google Scholar]
- 6. Omuro AM, Kris MG, Miller VA, et al. High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer. 2005;103(11):2344–2348. [DOI] [PubMed] [Google Scholar]
- 7. Emoto S, Ishigami H, Yamaguchi H, Yamashita H, Kaisaki S, Kitayama J. Frequent development of leptomeningeal carcinomatosis in patients with peritoneal dissemination of gastric cancer. Gastric Cancer. 2011;14(4):390–395. [DOI] [PubMed] [Google Scholar]
- 8. Kesari S, Batchelor TT. Leptomeningeal metastases. Neurol Clin. 2003;21(1):25–66. [DOI] [PubMed] [Google Scholar]
- 9. Mack F, Baumert BG, Schäfer N, et al. Therapy of leptomeningeal metastasis in solid tumors. Cancer Treat Rev. 2016;43:83–91. [DOI] [PubMed] [Google Scholar]
- 10. Shapiro WR, Johanson CE, Boogerd W. Treatment modalities for leptomeningeal metastases. Semin Oncol. 2009;36(4Suppl 2): S46–S54. [DOI] [PubMed] [Google Scholar]
- 11. Morikawa A, Jordan L, Rozner R, et al. Characteristics and outcomes of patients with breast cancer with leptomeningeal metastasis. Clin Breast Cancer. 2017;17(1):23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Remon J, Le Rhun E, Besse B. Leptomeningeal carcinomatosis in non-small cell lung cancer patients: a continuing challenge in the personalized treatment era. Cancer Treat Rev. 2017;53:128–137. [DOI] [PubMed] [Google Scholar]
- 13. Lassman AB, Abrey LE, Shah GD, et al. Systemic high-dose intravenous methotrexate for central nervous system metastases. J Neurooncol. 2006;78(3):255–260. [DOI] [PubMed] [Google Scholar]
- 14. Morris PG, Reiner AS, Szenberg OR, et al. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol. 2012;7(2):382–385. [DOI] [PubMed] [Google Scholar]
- 15. Brem SS, Bierman PJ, Black P, et al. ; National Comprehensive Cancer Network Central nervous system cancers: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2005;3(5):644–690. [DOI] [PubMed] [Google Scholar]
- 16. Buszek SM, Chung C. Radiotherapy in leptomeningeal disease: a systematic review of randomized and non-randomized trials. Front Oncol. 2019;9:1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El Shafie RA, Böhm K, Weber D, et al. palliative radiotherapy for leptomeningeal carcinomatosis-analysis of outcome, prognostic factors, and symptom response. Front Oncol. 2018;8:641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Central Nervous System Cancers Version 2.2020. 2020. https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf [Google Scholar]
- 19. Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. [DOI] [PubMed] [Google Scholar]
- 20. Kretschmar C, Kleinberg L, Greenberg M, Burger P, Holmes E, Wharam M. Pre-radiation chemotherapy with response-based radiation therapy in children with central nervous system germ cell tumors: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2007;48(3):285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calaminus G, Kortmann R, Worch J, et al. SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol. 2013;15(6):788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pinnix CC, Yahalom J, Specht L, Dabaja BS. Radiation in central nervous system leukemia: guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2018;102(1):53–58. [DOI] [PubMed] [Google Scholar]
- 23. Gunther JR, Rahman AR, Dong W, et al. Craniospinal irradiation prior to stem cell transplant for hematologic malignancies with CNS involvement: effectiveness and toxicity after photon or proton treatment. Pract Radiat Oncol. 2017;7(6):e401–e408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hermann B, Hültenschmidt B, Sautter-Bihl ML. Radiotherapy of the neuroaxis for palliative treatment of leptomeningeal carcinomatosis. Strahlenther Onkol. 2001;177(4):195–199. [DOI] [PubMed] [Google Scholar]
- 25. Harada HM, Asakura K, Ogawa H, et al. Cranio-spinal irradiation for leptomeningeal carcinomatosis: a pilot study. Int J Radiat Oncol Biol Phys. 2014;90(1):S310. [Google Scholar]
- 26. El Shafie RA, Böhm K, Weber D, et al. Outcome and prognostic factors following palliative craniospinal irradiation for leptomeningeal carcinomatosis. Cancer Manag Res. 2019;11:789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De B, Kinnaman MD, Wexler LH, Kramer K, Wolden SL. Central nervous system relapse of rhabdomyosarcoma. Pediatr Blood Cancer. 2017;65(1):e26710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yuh GE, Loredo LN, Yonemoto LT, et al. Reducing toxicity from craniospinal irradiation: using proton beams to treat medulloblastoma in young children. Cancer J. 2004;10(6):386–390. [DOI] [PubMed] [Google Scholar]
- 29. Howell RM, Giebeler A, Koontz-Raisig W, et al. Comparison of therapeutic dosimetric data from passively scattered proton and photon craniospinal irradiations for medulloblastoma. Radiat Oncol. 2012;7:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Song S, Park HJ, Yoon JH, et al. Proton beam therapy reduces the incidence of acute haematological and gastrointestinal toxicities associated with craniospinal irradiation in pediatric brain tumors. Acta Oncol. 2014;53(9):1158–1164. [DOI] [PubMed] [Google Scholar]
- 31. Brown AP, Barney CL, Grosshans DR, et al. Proton beam craniospinal irradiation reduces acute toxicity for adults with medulloblastoma. Int J Radiat Oncol Biol Phys. 2013;86(2):277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin H, Ding X, Kirk M, et al. Supine craniospinal irradiation using a proton pencil beam scanning technique without match line changes for field junctions. Int J Radiat Oncol Biol Phys. 2014;90(1):71–78. [DOI] [PubMed] [Google Scholar]
- 33. Chamberlain M, Junck L, Brandsma D, et al. Leptomeningeal metastases: a RANO proposal for response criteria. Neuro Oncol. 2017;19(4):484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rades D, Bohlen G, Dunst J, et al. Comparison of short-course versus long-course whole-brain radiotherapy in the treatment of brain metastases. Strahlenther Onkol. 2008;184(1):30–35. [DOI] [PubMed] [Google Scholar]
- 35. Grossman SA, Ellsworth S, Campian J, et al. Survival in patients with severe lymphopenia following treatment with radiation and chemotherapy for newly diagnosed solid tumors. J Natl Compr Canc Netw. 2015;13(10):1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Le Rhun E, Taillibert S, Zairi F, et al. A retrospective case series of 103 consecutive patients with leptomeningeal metastasis and breast cancer. J Neurooncol. 2013;113(1):83–92. [DOI] [PubMed] [Google Scholar]
- 37. Lee SJ, Lee JI, Nam DH, et al. Leptomeningeal carcinomatosis in non-small-cell lung cancer patients: impact on survival and correlated prognostic factors. J Thorac Oncol. 2013;8(2): 185–191. [DOI] [PubMed] [Google Scholar]
- 38. Saito H, Tanaka K, Kanemoto A, Nakano T, Abe E, Aoyama H. Factors affecting the baseline and post-treatment scores on the hopkins verbal learning test-revised japanese version before and after whole-brain radiation therapy. Int J Mol Sci. 2016;17(11):1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brown PD, Pugh S, Laack NN, et al. ; Radiation Therapy Oncology Group (RTOG) Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.