Abstract
Objective
Interstitial pneumonia is common and has high short-term mortality in patients with PM and DM despite glucocorticoid (GC) treatment. Retrospective studies suggested that the early use of immunosuppressive drugs with GCs might improve its short-term mortality.
Methods
A multicentre, single-arm, 52-week-long clinical trial was performed to test whether the initial combination treatment with tacrolimus (0.075 mg/kg/day, adjusted for the target whole-blood trough levels between 5 and 10 ng/ml) and GCs (0.6–1.0 mg/kg/day of prednisolone followed by a slow taper) improves short-term mortality of PM/DM-interstitial pneumonia patients. The primary outcome was overall survival. We originally intended to compare, by using propensity-score matching, the outcome data of clinical trial patients with that of historical control patients who were initially treated with GCs alone.
Results
The 52-week survival rate with the combination treatment (N = 26) was 88.0% (95% CI, 67.3, 96.0). Safety profiles of the combination treatment were consistent with those known for tacrolimus and high-dose GCs individually. Serious adverse events occurred in 11 patients (44.0%), which included four opportunistic infections. Only 16 patients, including only 1 deceased patient, were registered as historical controls, which precluded meaningful comparative analysis against the clinical trial patients.
Conclusion
Our study provided findings which suggest that initial treatment with tacrolimus and GCs may improve short-term mortality of PM/DM-interstitial pneumonia patients with manageable safety profiles. This was the first prospective clinical investigation conducted according to the Good Clinical Practice Guideline of the International Conference on Harmonization for the treatment of this potentially life-threatening disease.
Trial registration
ClinicalTrials.gov, http://clinicaltrials.gov, NCT00504348.
Keywords: tacrolimus, polymyositis, dermatomyositis, interstitial lung disease, interstitial pneumonia
Rheumatology key messages
This is the first study conducted according to the International Conference on Harmonization Good Clinical Practice Guideline that prospectively investigated treatments for PM/DM-interstitial pneumonia.
The study indicated that the initial treatment with tacrolimus and glucocorticoids improves short-term mortality of PM/DM-interstitial pneumonia patients.
Safety profiles of the combination treatment were consistent with those known for tacrolimus and high-dose glucocorticoids individually.
Introduction
Interstitial pneumonia is a common complication of, and has a significant impact on the prognosis of patients with, PM and DM. Its reported prevalence in PM/DM patients varies between 23 and 65% [1–7] depending on criteria applied, as well as on clinical settings of studied cohorts, and an earlier overview [8] and a later study [6] reported its high short-term mortality.
Treatment for this grave complication had not yet been established or even been prospectively investigated. Glucocorticoids (GCs), while long being considered as the first-line drugs, are effective in <50% of patients [2, 9, 10] and the mortality of these GCs-resistant patients does not improve even if immunosuppressive drugs are later added [10].
Recently, we [11] and others [12, 13] reported that either an early addition of immunosuppressive drugs to GCs or the initial combined use of immunosuppressive drugs with GCs might improve the survival of PM/DM-interstitial pneumonia patients, and desperate treating physicians had started using these approaches. Among the immunosuppressive drugs used, tacrolimus had been suggested to be effective even for those patients who are resistant to ciclosporin or CYC [14–17]. While these results urged prospective studies to investigate the superiority of this approach over GCs alone, it was considered ethically inappropriate to conduct a prospective study with a concurrent control group that receives GCs alone given the presence of the PM/DM-interstitial pneumonia subtype with rapidly progressive course and high short-term mortality if treated with GCs alone, which could not be distinguished from other subtypes early in the course due to the absence of useful demographic markers or bio-markers.
To investigate whether the initial combination treatment with tacrolimus and GCs improves the short-term mortality of PM/DM-interstitial pneumonia patients, we conducted the IMPPACT (Investigation in Myositis-associated Pneumonitis of Prednisolone and Concomitant Tacrolimus) study, a multicentre, single-arm clinical trial of this combination treatment in patients with newly developed active PM/DM-interstitial pneumonia or with its relapse, and compared its data with the clinical outcomes of historical control patients who were treated with GCs alone as an initial treatment.
Methods
Prospective investigation group patients
We enrolled patients with PM and DM (‘definite’ or ‘probable’ by criteria of Bohan et al. [18, 19] or clinically amyopathic DM (CADM) by criteria proposed by Sontheimer et al. [20]) if they had evidence of active interstitial pneumonia. The active interstitial pneumonia was defined as (i) high-resolution CT findings consistent with interstitial pneumonia, (ii) serum levels of Krebs von den Lungen 6 (KL-6), a mucinous high molecular weight glycoprotein that is produced by type II pneumocytes and has been reported to increase in patients with active interstitial pneumonia [21, 22], above the upper normal limit (500 U/ml) and (iii) at least one of the following four findings within 2 weeks prior to the initiation of the study drug: presence of dyspnoea on exertion, PaO2 of <80 mmHg while breathing ambient air at rest not accompanied by abnormal increase of PaCO2, vital capacity <80% predicted or diffusing capacity for carbon monoxide <65% predicted. Patients were excluded if they received GCs at doses equivalent to or higher than prednisolone 0.6 mg/kg/day for 8 days or longer within 4 weeks (28 days) or immunosuppressive agents within 12 weeks (84 days) prior to the study. Detailed inclusion and exclusion criteria are given in the supplementary material, section Inclusion and Exclusion Criteria and Screening Process, available at Rheumatology online.
Historical control group patients
At the institutions that participated in this clinical trial (supplementary material, section Participated Institutions, available at Rheumatology online), consecutive patients were systematically searched and were enrolled as historical control patients if they were treated with high-dose GCs alone as an initial treatment for PM/DM-interstitial pneumonia between 1 January 2000 and the date of the institutional approval of this clinical trial, and satisfied all the inclusion and exclusion criteria described in the supplementary material, section Inclusion and Exclusion Criteria for Historical Control Group Patients, available at Rheumatology online.
Treatments
Tacrolimus was initiated at 0.075 mg/kg/day divided into two doses, subsequently adjusted to maintain its whole-blood trough levels between 5 and 10 ng/ml and to keep total daily doses ⩽0.3 mg/kg, and was continued for 52 weeks. Tacrolimus was discontinued temporarily or permanently if there was evidence of drug-related toxic effects, at the discretion of treating physicians. All patients received GCs with starting doses equivalent to between 0.6 and 1.0 mg/kg/day of prednisolone, which were continued for the first 28 days unless they were deemed clinically inappropriate by treating physicians. Up to two courses of i.v. pulse GC treatment were allowed during this period. GCs were subsequently tapered according to a predefined guideline for GC tapering and then were kept at the lowest possible dose, although a dose increase up to 1.0 mg/kg/day of prednisolone or its equivalent was allowed if deemed clinically necessary (supplementary material, section Glucocorticoid Use During Study, available at Rheumatology online). Pneumocystis jirovecii pneumonia (PCP) prophylaxis was recommended according to the guideline (supplementary material, section PCP Prophylaxis Recommendation, available at Rheumatology online).
Concomitant use of the following was not permitted: other immunosuppressive drugs, GCs at doses equivalent to >1 mg/kg/day of prednisolone, potassium-sparing diuretics, live vaccines, bosentan hydrate, IVIG or plasma exchange. If administration of these treatments was deemed necessary by treating physicians for any reason, the patient was withdrawn from the study. If the reason was a progression of interstitial pneumonia, treating physicians were strongly encouraged to refer to the following criteria as a guideline for using the disallowed treatments; (i) ⩾10% decline from baseline forced vital capacity or ⩾15 mmHg increase in baseline resting alveolar-arterial oxygen gradient and (ii) a worsening of interstitial pneumonia findings by chest CT compared with the most recent study, confirmed by a radiologist. Drugs known to interact with the same CYP450 enzyme (3A4) were used with caution and recorded.
Baseline and serial monitoring
Study visits were at baseline, 3 days, and 1, 2 and 4 weeks after the start of the study, and at 4-week intervals thereafter until the end of the 52nd week or the time of study withdrawal. The details of safety and efficacy evaluation at these visits are described in the supplementary material, section Details of Safety and Efficacy Evaluation at Each Visit, available at Rheumatology online. Treating physicians of those patients who were withdrawn from the study were contacted at the end of the 52nd week to acquire the information on their vital status.
Primary and secondary outcomes
Primary outcome was overall survival, and key secondary outcome was progression-free survival. Patients were considered to have reached progression if they died, or if they met all of the following criteria: (i) ⩾10% decline from baseline forced vital capacity or ⩾15 mmHg increase in baseline resting alveolar-arterial oxygen gradient, (ii) a worsening of interstitial pneumonia findings by chest CT compared with the most recent study, confirmed by a radiologist and (iii) exclusion of PCP, CMV pneumonia and other pulmonary infection on clinical ground. If non-protocol immunosuppressive treatments were used to treat a deterioration of interstitial pneumonia before performing spirometry or an arterial blood gas analysis, the cases were reviewed by an independent committee (the Endpoint Committee, described in the supplementary material, section Endpoint Committee, available at Rheumatology online) to decide whether the degree of deterioration was at least equivalent to or worse than those defined in the first criterion.
Safety was evaluated by adverse event reports. All adverse events were categorized according to the Medical Dictionary for Regulatory Affairs. During protocol development, the study team and the Pharmaceuticals and Medical Devices Agency in Japan decided to report all the worsening of respiratory symptoms or respiratory findings as adverse events regardless of causes, given the recent reports of the new development or worsening of interstitial pneumonia in RA patients in whom causal relationships with tacrolimus were suspected [23, 24].
Statistical analysis
Given the rarity of the disease, a sample size for the prospective investigation group of 20 was determined, not based on statistical calculation, but on the predicted enrolment rate. Statistical analyses both for the primary and secondary outcomes were performed on the full analysis set, comprising all registered patients who had received any amount of the study drug and had at least one post-baseline measurement.
For overall and progression-free survival, Kaplan–Meier estimates of the survivor function at different time points were presented together with the 95% CIs calculated using Greenwood’s formula. For progression-free survival, those patients who were withdrawn for reasons other than the deterioration of interstitial pneumonia were censored at the time of withdrawal unless they died by the end of the 52nd week, in which case they were not censored but were counted as having the event. We planned to use propensity-score matching to compare the Kaplan–Meier survival curves of the two groups. However, because the historical control group ended up being very small in size (N = 16), it was concluded that the study data would allow neither reasonable matching nor meaningful statistical comparison of survivals, and therefore that we would not compare but show the results of both groups independently. Other pre-specified efficacy parameters and statistical approaches are described in the supplementary material, section Other Pre-specified Efficacy Parameters and Statistical Approaches, available at Rheumatology online. High-resolution CT scan images were used for histopathology prediction and for semi-quantitative analysis of the extent of ground-glass opacity and air-space consolidation (supplementary material, section Analysis of HRCT Images, available at Rheumatology online). The statistical analyses were performed with SAS software (version 9.1, SAS Institute Inc., Cary, NC, USA), where appropriate.
This clinical trial was carried out in accordance with the Declaration of Helsinki as well as the ethical guidelines for clinical research and the ethical guidelines for epidemiological research by the Ministry of Health, Labour and Welfare in Japan, and according to a protocol prepared in accordance with the Japanese Good Clinical Practice (GCP) standards, which conform with the International Conference on Harmonization (ICH) guidelines for GCP. The protocol was approved at all participating institutions by their governing institutional review boards or equivalent, and all patients provided voluntary written informed consent.
Results
Enrolment and baseline characteristics
Twenty-six patients were registered to the prospective investigation group between July 2007 and December 2009, and 25 eligible patients underwent the protocol treatment, among whom 8 patients (32%) withdrew (supplementary Fig. S1, available at Rheumatology online). Only 16 patients were enrolled to the historical control group. Baseline characteristics of the patients in both groups were listed in Table 1. As it was concluded that the study data would not allow meaningful between-group comparison for outcomes, no statistical analysis was performed to compare baseline and outcome values between the groups. In the prospective investigation group, 18 patients (72%) had either DM or CADM, all 25 eligible patients enrolled in the study for the initial treatment for interstitial pneumonia, and 23 patients (92%) had non-specific interstitial pneumonia by radiographic prediction based on CT images. None had lung biopsy.
Table 1.
Baseline characteristics of ‘prospective investigation group’ patients and ‘historical control group’ patients
| Characteristic | Prospective investigation group (N = 25) | Historical control group (N = 16) |
|---|---|---|
| Age (years), mean (s.d.) | 55.4 (12.0) | 51.2 (12.0) |
| Female sex, n (%) | 19 (76.0) | 14 (87.5) |
| Duration of IIM (months), mean (s.d.) | 1.47 (4.03) | 0.49 (0.89) |
| Idiopathic inflammatory myopathies, n (%) | ||
| PM | 7 (28.0) | 7 (43.8) |
| DM | 12 (48.0) | 8 (50.0) |
| Clinically amyopathic DM | 6 (24.0) | 1 (6.3) |
| Anti-Jo-1 antibody, n (%) | 6 (24.0) | 13 (81.3) |
| CK (IU/l), mean (s.d.) | 948.2 (1259.0) | 2407.0 (3060.9) (N = 15)a |
| KL-6 (U/l), mean (s.d.) | 1118.8 (740.3) | 1119.2 (764.0) (N = 14)a |
| SP-D (μg/ml), mean (s.d.) | 182.8 (132.7) | 118.0 (56.0) (N = 9)a |
| Duration of interstitial pneumonitis (months), mean (s.d.) | 2.51 (7.33) | 1.24 (1.26) |
| FVC (% of predicted), mean (s.d.) | 69.1 (14.0) | 70.7 (19.1) (N = 13)a |
| DLCO (% of predicted), mean (s.d.) | 43.7 (8.7) | 50.1 (12.1) (N = 10)a |
| PaO2 at rest (room air) (mmHg), mean (s.d.) | 79.5 (14.2) | 82.4 (8.9) (N = 13)a |
| AaDO2 at rest (mmHg), mean (s.d.) | 29.5 (33.0) | 20.9 (18.7) (N = 13)a |
| Score on Mahler Baseline Dyspnoea Index, mean (s.d.) | 6.3 (2.5) | NA |
| Score for HAQ disability index, mean (s.d.) | 0.95 (0.74) | NA |
| SF-36v2 Health Survey score, mean (s.d.) | ||
| Physical functioning score | 48.8 (22.9) | NA |
| Role-physical score | 42.0 (27.8) | NA |
| Bodily pain score | 48.5 (29.2) | NA |
| General health perception score | 50.1 (14.3) | NA |
| Vitality score | 35.8 (23.3) | NA |
| Social functioning score | 46.0 (28.1) | NA |
| Role-emotional score | 50.0 (30.4) | NA |
| Mental health score | 49.6 (23.4) | NA |
| Whole-lung high attenuation scoreb | 52.2 (26.2) | 38.9 (23.0) (N =14)a |
| Radiographically speculated histological type, n (%)c | ||
| UIP | 1 (4.0) | 0 (0.0) |
| NSIP | 23 (92.0) | 15 (93.8) |
| OP | 1 (4.0) | 0 (0.0) |
| DAD | 0 (0.0) | 0 (0.0) |
Scores for the Mahler Baseline Dyspnoea Index can range from 0 to 12, with lower scores indicating worse dyspnoea. Scores for the HAQ disability index can range from 0 to 3, with higher numbers indicating greater disability. Scores for SF-36v2 Health Survey can range from 0 to 100, with lower scores indicating worse health status.
Some baseline clinical data for historical control group patients were not available.
Detailed description of the analytic method for high-resolution CT images are provided in the supplementary material, section Analysis of HRCT Images, available at Rheumatology online.
Some patients had more than one histopathological subtype speculated by independent radiologists. For example, one patient had OP and NSIP speculated. This table shows only the most dominant subtype in each patient. IIM: idiopathic inflammatory myopathies; CK: creatine kinase; KL-6: Krebs von den Lungen 6; SP-D: surfactant protein D; FVC: forced vital capacity; DLCO: diffusing capacity for carbon monoxide; AaDO2: alveolar-arterial oxygen gradient; UIP: usual interstitial pneumonia; NSIP: non-specific interstitial pneumonia; OP: organizing pneumonia; DAD: diffuse alveolar damage; NA: not available.
Overall survival
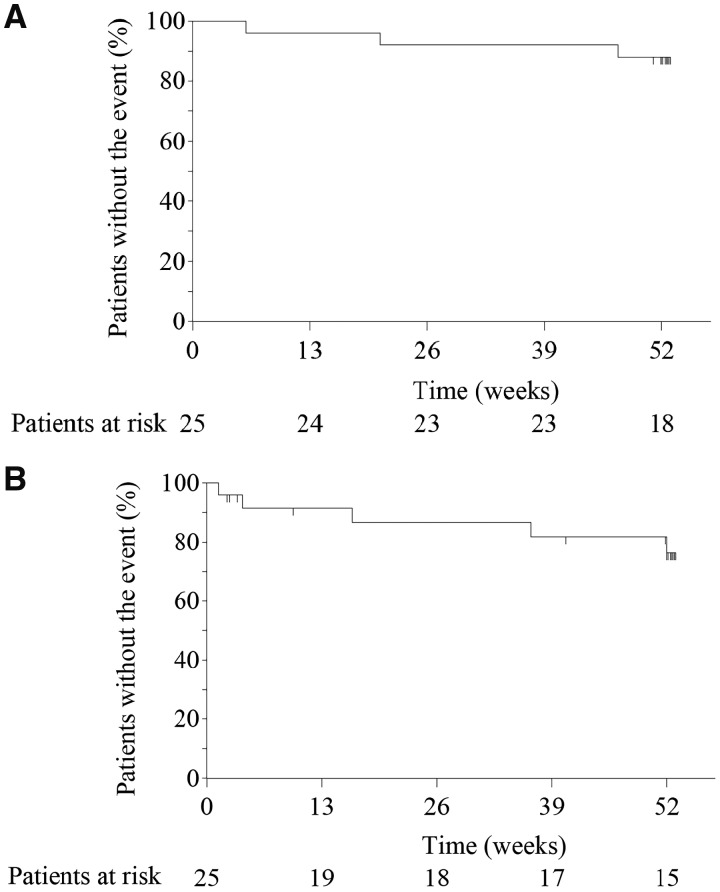
The 52-week survival rate in the prospective investigation group was 88.0% (95% CI, 67.3, 96.0) (Fig. 1A). Three patients died during the 52-week treatment period; two (one DM and one CADM) due to the exacerbation of interstitial pneumonia and one (CADM) due to liver cirrhosis. An additional patient (DM) died 92 days after the end of the 52-week study treatment due to the exacerbation of interstitial pneumonia that developed during the study period (on the 172nd day of the study). For the historical control group, informed consent could be obtained from only one family out of four deceased patients identified.
Fig. 1.
Overall and progression-free survival in prospective investigation group patients
(A) Overalla and (B) progression-free survivalb. aOne patient died 92 days after the end of 52-week tacrolimus treatment due to the exacerbation of interstitial pneumonitis that developed during the study period (on the 172nd day of the study), although it is not reflected in this figure. bKaplan–Meier estimates of time to death or ‘progression’ up to the end of the 52nd week in 25 prospective investigation group patients.
Progression-free survival
A 52-week progression-free survival rate in the prospective investigation group was 76.4% (95% CI, 51.8, 89.5) (Fig. 1B) (supplementary material, section Sensitivity Analysis for Progression-free Survival, available at Rheumatology online). Three patients who died from the exacerbation of interstitial pneumonia had fulfilled the criteria for progression earlier in their courses and two additional patients (both DM) reached progression but survived. One of the latter two patients reached progression on the fourth day of the study and survived after replacing tacrolimus with ciclosporin, while the other reached on the 364th day of study and survived continuously using tacrolimus and increasing the dose of GC. Four patients in the historical control group reached progression [52-week progression-free survival rate of 66.2% (95% CI, 32.4, 86.0)].
Association between baseline characteristics and clinical outcomes
All patients who died and/or reached progression in the prospective investigation group had either DM or CADM and were negative for anti-Jo-1 antibody (supplementary Table S1, available at Rheumatology online). One patient with radiographically predicted histology of usual interstitial pneumonia reached progression and died, and four and two patients with non-specific interstitial pneumonia reached progression and died, respectively (supplementary Table S1, available at Rheumatology online).
Other secondary outcomes
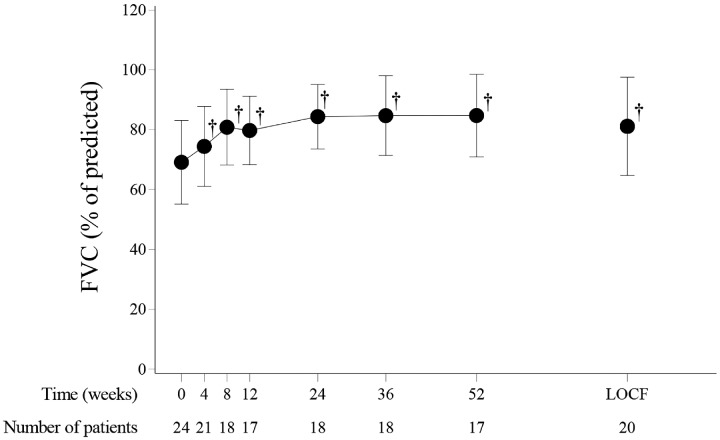
In the prospective investigation group, statistically significant improvement was observed between baseline values and the values at the end of the 52nd week, or at the last observation for those patients who withdrew before the end of the 52nd week, in spirometry, arterial blood gas analyses, chest CT findings, serum creatine kinase and surfactant protein D, the Japanese version of the HAQ Disability Index, SF-36v2 Health Survey, and daily GC doses (Fig. 2, Table 2). The mean score according to the transitional dyspnoea index showed clinically meaningful improvement in dyspnoea (by >1 unit) (Table 2) [25, 26].
Fig. 2.
Changes in FVC (% of predicted) in prospective investigation group patients
Points and bars represent the mean and s.d., respectively. †P < 0.001, paired t-test, compared against baseline. FVC: forced vital capacity; LOCF: the end-of-the-study data for all patients using the last-observation-carried-forward method.
Table 2.
Change in values from baseline to the end of the 52nd week in the prospective investigation group
| Characteristic | Baseline value | Value at week 52a | P-value |
|---|---|---|---|
| FVC (% of predicted), mean (s.d.) | 69.1 (14.0) | 81.2 (16.4) | 0.0023 |
| DLCO (% of predicted), mean (s.d.) | 43.7 (8.7) | 49.5 (10.2) | 0.1428 |
| PaO2 at rest (room air) (mmHg), mean (s.d.) | 79.5 (14.2) | 90.7 (20.7) | 0.0118 |
| AaDO2 at rest (mmHg), mean (s.d.) | 29.5 (33.0) | 27.2 (48.6) | 0.8409 |
| Score on Mahler Dyspnoea Index | |||
| Baseline Dyspnoea Index, mean (s.d.) | 6.3 (2.5) | ||
| Transition Dyspnoea Index, mean (s.d.) | 3.0 (5.1) | ||
| CK (IU/l), mean (s.d.) | 948.2 (1259.0) | 115.3 (181.7) | 0.0020 |
| KL-6 (U/l), mean (s.d.) | 1118.8 (740.3) | 957.2 (580.6) | 0.3744 |
| SP-D (μg/ml), mean (s.d.) | 182.8 (132.7) | 124.4 (81.5) | 0.0265 |
| Score for HAQ disability index, mean (s.d.) | 0.95 (0.74) | 0.69 (0.84) | 0.1336 |
| Whole-lung high attenuation score, mean (s.d.) | 52.2 (26.2) | 33.9 (20.4) | 0.0005 |
| SF-36v2 Health Survey score, mean (s.d.) | |||
| Physical functioning score | 48.8 (22.9) | 59.6 (30.6) | 0.0757 |
| Role—physical score | 42.0 (27.8) | 59.1 (31.8) | 0.0377 |
| Bodily pain score | 48.5 (29.2) | 66.3 (24.2) | 0.0214 |
| General health perception score | 50.1 (14.3) | 51.5 (13.3) | 0.7957 |
| Vitality score | 35.8 (23.3) | 59.1 (22.0) | 0.0010 |
| Social functioning score | 46.0 (28.1) | 69.3 (29.0) | 0.0054 |
| Role—emotional score | 50.0 (30.4) | 67.0 (34.5) | 0.0505 |
| Mental health score | 49.6 (23.4) | 67.5 (21.5) | 0.0045 |
| Glucocorticoid dose (mg/kg/day of prednisolone), mean (s.d.)b | 0.81 (0.21) | 0.21 (0.16) (N = 17) | <0.0001 |
The analysis included 25 patients in the prospective investigation group. Scores for the Mahler Transition Dyspnoea Index can range from –9 to +9, with the plus/minus sign indicating improvement/worsening, respectively.
The last-observation-carried-forward method was employed for patients who withdrew before the end of the 52nd week or with missing values for all parameters except for glucocorticoid dose, as explained below.
Average daily glucocorticoid doses during the first 2 weeks of study (for baseline values) and during the 52nd week (for week 52 values) were presented, which do not include pulse glucocorticoid treatment. FVC: forced vital capacity; DLCO: diffusing capacity for carbon monoxide; AaDO2: alveolar-arterial oxygen gradient; CK: creatine kinase; KL-6: Krebs von den Lungen 6; SP-D: surfactant protein D.
Tacrolimus trough level
Whole-blood tacrolimus trough levels were maintained within the targeted range of 5 and 10 ng/ml throughout the study period (average 6.6 ± 2.2 ng/ml), with an average daily dose of 3.81 ± 1.75 mg/day (0.072 ± 0.032 mg/kg/day) (supplementary Fig. S3, available at Rheumatology online).
Adverse events
Adverse events (AEs) were reported in all 25 patients in the prospective investigation group (supplementary Table S2, available at Rheumatology online). Among 475 AEs reported, 357 events (75.2%) were considered either possibly, probably or definitely related to tacrolimus or GCs by treating physicians. As mentioned above, all the worsening of respiratory symptoms or findings were reported as AEs regardless of causes, and ‘interstitial lung disease’ was the second most commonly observed adverse events (nine patients, 36.0%). Six of the nine ‘interstitial lung disease’ events were reported as serious AEs (SAEs). Five of them reached progression, three of which resulted in death. In two of these nine events, a causal relationship with tacrolimus was reported as ‘probable’ or higher. One of them developed on the fourth day of the study and recovered after tacrolimus was switched to ciclosporin, while the other developed on the 28th day of the study simultaneously with CMV pneumonitis and, despite the replacement of tacrolimus with ciclosporin, resulted in death on the 41st day of the study.
Sixteen SAEs (Table 3) were reported in 11 patients (44.0%), among which 14 events (9 patients) were considered possibly, probably or definitely related to tacrolimus by treating physicians, and included six interstitial lung diseases, four infections (zoster, nocardiosis, CMV pneumonitis and PCP), cataract and pneumomediastinum. Four of the 16 SAEs developed in one patient (liver cirrhosis, ascites, thrombocytopenia and acute respiratory failure). The patient developed clinical findings consistent with the decompensation of liver cirrhosis during the study and the findings of non-invasive liver investigation was compatible with liver cirrhosis, which was not known or obvious at the time of enrolment. Eight (five patients) of the 16 SAEs required discontinuation of tacrolimus (three events of interstitial lung disease, nocardiosis, CMV pneumonitis, PCP, ascites and thrombocytopenia).
Table 3.
Adverse events observed in prospective investigation group patients
| Event | Prospective investigation group patients who experienced the event (N = 25) |
|---|---|
| Serious adverse eventsa, no. of patients (%) | |
| Total serious adverse events, no. of patients (%) | 11 (44.0) |
| Specific events | |
| Interstitial lung disease | 6 (24.0) |
| Zoster | 1 (4.0) |
| Nocardiosis | 1 (4.0) |
| CMV pneumonitis | 1 (4.0) |
| Pneumocystis jiroveci pneumonia | 1 (4.0) |
| Thrombocytopenia | 1 (4.0) |
| Cataract | 1 (4.0) |
| Acute respiratory failure | 1 (4.0) |
| Pneumomediastinum | 1 (4.0) |
| Ascites | 1 (4.0) |
| Liver cirrhosis | 1 (4.0) |
| Infections and infestations, no. of patients (%) | |
| Any | 19 (76.0) |
| Reported as serious adverse event | 3 (12.0) |
| Exacerbation of interstitial pneumonitisb, no. of patients (%) | |
| Any | 9 (36.0) |
| Reported as serious adverse event | 6 (24.0) |
| Led to treatment discontinuation | 3 (12.0) |
| Resulted in death | 3 (12.0) |
| Deathsc, no. of patients (%) | 4 (16.0)c |
| Adverse events leading to treatment discontinuation, no. of patients (%) | 5 (20.0) |
All adverse events that resulted in death; life-threatening illness; persistent or clinically significant disability, incapacity or both; hospitalization or prolongation of hospitalization; a congenital abnormality or birth defect; or cancer.
During protocol development, the study team and the Pharmaceuticals and Medical Devices Agency in Japan decided to report all the worsening of respiratory symptoms or findings as adverse events regardless of cause.
Includes one patient who died 92 days after the end of the study due to the exacerbation of interstitial pneumonitis that developed during the study (on the 172nd day of the study).
Discussion
Although the data on patients treated with GCs alone, collected retrospectively under this study, were few and did not serve as a meaningful control for comparative analysis, our prospective study indicated that the initial treatment with tacrolimus and GCs improves the short-term mortality of PM/DM-interstitial pneumonia patients, with a manageable safety profile. To our knowledge, this is the first prospective study of treatment for PM/DM-interstitial pneumonia.
Compared with previously published data [2, 10–13], the initial treatment with tacrolimus and GCs appears to achieve survival that is better than GCs alone or the late addition of immunosuppressive drugs to GCs, although the difference in disease severity of studied cohorts and various biases such as indication bias and non-contemporaneous treatment bias preclude strict comparisons. Nawata et al. [2] retrospectively analysed the survival of PM/DM-interstitial pneumonia patients who were treated with GCs alone between 1975 and 1995 and reported that the 1-year survival was poor, especially among DM-interstitial pneumonia patients (50%). Fujisawa et al. [10] reported similarly poor survival of PM/DM-interstitial pneumonia patients even with the late addition of immunosuppressive drugs. Their patients, who were treated between 1985 and 2001, initially received GCs alone and immunosuppressive drugs were later added if GCs alone did not result in a favourable response. The 5-year survival rates of DM-interstitial pneumonia and PM-interstitial pneumonia patients were 55.6% and 87.1%, respectively [10]. In contrast, 1-year survival rates of DM-interstitial pneumonia (including CADM-interstitial pneumonia) and PM-interstitial pneumonia patients in the present study were 83.3% and 100.0%, respectively (supplementary Table S1, available at Rheumatology online).
The limited enrolment in the historical control group and the presence of various biases precluded meaningful statistical comparison of survival rates between the two groups in our study. Whereas the historical control group needed to enrol at least twice the number of patients of the prospective investigation group for reasonable propensity-score matching in comparing the survival rates of the two groups meaningfully, the final size of the former was only 16 despite systematic search efforts at each participating institution. This is due in large part to the rarity of PM/DM-interstitial pneumonia patients whose initial treatment consisted of GCs only. The nation-wide survey we conducted along with the present study (supplementary material, section Description of Nation-wide Survey on Treatments for PM/DM-interstitial pneumonia, available at Rheumatology online) supports this by indicating that many physicians had already been using immunosuppressive drugs as part of initial treatment in their practice before such approach was prospectively investigated. In addition, the number of deceased patients enrolled to the historical control group was limited (only one consent obtained from four families approached), generating a critical selection bias for overall survival. Furthermore, the universally better trend in baseline spirometry and arterial blood gas parameters in the historical control group patients strongly suggests indication bias.
The present study possibly suggests the limit of the initial treatment with tacrolimus and GCs in some patients with DM/CADM-interstitial pneumonia. Two DM patients continued to progress acutely. One of them later developed CMV pneumonitis and died, while the other survived after switching to ciclosporin. In addition, one CADM patient, after having stabilized and prednisolone being tapered to 30 mg/day, developed acute exacerbation on the 115th day of the study and died 35 days later. Notably, all of these three patients were negative for anti-Jo-1 antibody. The poor prognosis and treatment-refractory nature of the acute/subacute subtype of DM/CADM-interstitial pneumonia has been reported [27, 28], especially in a Jo-1-negative population [29, 30]. Recently, several serum bio-markers were identified that were strongly associated with this subtype, an anti- melanoma differentiation-associated gene 5 (MDA-5) antibody [31–33] and ferritin [34]. While these bio-markers were not measured here because our study started before these discoveries, their pre-treatment measurement may help identify those patients whose survival may improve by adding a second immunosuppressive drug to the combination of tacrolimus and GCs if severity and clinical course indicates their use.
The present study did not strongly support the causal role of tacrolimus in the exacerbation of interstitial pneumonia. Given the reports of the new development or worsening of interstitial pneumonia in RA patients who were receiving tacrolimus [23, 24], we carefully explored the causal relationship between tacrolimus and interstitial pneumonia exacerbation in the present study by having all the worsening of respiratory symptoms or findings reported as AEs regardless of cause. Among nine such reported events, a causal relationship with tacrolimus was reported ‘probable’ or higher in two cases that continued to progress acutely, although it is also possible that these two cases did so as a natural, treatment-resistant, disease course [27, 28]. While we recommend vigilance, we believe that the potential life-saving benefit of tacrolimus outweighs the causal concern of the drug in the exacerbation of interstitial pneumonia.
The overall AE profiles in the prospective investigation group were consistent with the previous studies that used tacrolimus at similar doses and were conducted according to GCP Guideline of the ICH [35–37], and AEs were generally manageable with only 20 events (4.2%) requiring any changes in tacrolimus doses. While the incidence of infections and of a few tacrolimus-related AEs (tremor, elevation of blood pressure and glucose intolerance) was higher than reported in those studies [35–37], it is very likely that the concomitant use of high-dose GCs contributed to this. A recently conducted study in GCA that used high-dose GCs alone in the control group and was conducted according to GCP Guideline of the ICH [38] reported incidence rates of all infections and of infections reported as SAEs that were similar to those in the present study.
The present study has several limitations. Firstly, the conclusion is not based on meaningful statistical comparison. As explained earlier, it was considered ethically inappropriate to conduct a study with a concurrent control group with GCs alone. We therefore planned to compare against historical control patients, but their limited enrolment and the presence of various biases precluded meaningful statistical comparison. Secondly, our results will not apply to those patients with either asymptomatic or subclinical interstitial pneumonia, since we recruited PM/DM patients with active interstitial pneumonia that warranted immediate treatment. Thirdly, it may not be possible to extrapolate the findings of our study to treatment for the exacerbation of once-stabilized PM/DM-interstitial pneumonia, since our cohort consisted of only those patients who required initial treatment for PM/DM-interstitial pneumonia. Fourthly, our study does not separately provide the efficacy of the initial combination treatment in the PM/DM-interstitial pneumonia subgroup defined by anti-MDA-5 antibodies, which has high short-term mortality. Finally, our results do not indicate comparative efficacy of tacrolimus over other immunosuppressive drugs as an additional agent to GCs in initial treatment for PM/DM-interstitial pneumonia.
In summary, our prospective study provided findings which suggest that initial combination treatment with tacrolimus and GCs may improve the short-term mortality of PM/DM-interstitial pneumonia patients, with manageable safety profiles. This was the first prospective clinical investigation conducted according to GCP Guideline of the ICH for the treatment, which had been widely used by treating physicians based on limited and retrospective experiences. Based on the results of this study, tacrolimus became the first immunosuppressive drug, apart from GSs, to be approved for PM/DM-interstitial pneumonia by the Ministry of Health, Labour and Welfare in Japan.
Supplementary Material
Acknowledgements
We thank the members of the Endpoint Committee (Tsutomu Takeuchi, MD, PhD and Yasuyuki Yoshizawa, MD, PhD), Suminobu Ito, MD, PhD, for strategic advice, Taro Shibata, MS, for statistical consultation, Kazuhiko Yamamoto, MD, PhD, for support in coordination, and the patients, their family members and participating staff at all the study sites. For this investigator-initiated study, we commissioned statistical analysis by EPS Corporation (Tokyo, Japan) and an audit by Astellas Pharma Inc. (Tokyo, Japan), the manufacturer of tacrolimus, which also donated the drug. Astellas Pharma Inc., however, had no role in the design or conduct of the study, or the acquisition or analysis of the data. We thank Astellas Pharma Inc. for kindly donating the drug for this clinical trial, and for submitting an application for and obtaining an approval of new indication (PM/DM-interstitial pneumonia) for the drug from the Ministry of Health, Labour and Welfare in Japan following the trial completion.
Funding: This work was supported by the Japan Medical Association under the Large Scale Clinical Trial Network Project by the Ministry of Health, Labour and Welfare, Japan.
Disclosure statement: K.T. has received consulting fees, speaking fees and/or honoraria from Abbvie, Asahi Kasei, Astellas, Bristol-Myers, Chugai, Daiichi-Sankyo, Eisai, Janssen, Mitsubishi-Tanabe, Otsuka, Pfizer and Takeda, and has received research grants/support from Abbvie, Astellas, Chugai, Eisai, Mitsubishi-Tanabe and Takeda. S.I. has received speaking fees from Abbvie, Asahi Kasei, Bristol-Myers, Chugai, Eisai, Janssen, Mitsubishi-Tanabe and Takeda. K.I. has received research grants from Asahi Kasei, Astellas, Chugai, Daiichi-Sankyo, Eisai, Mitsubishi-Tanabe and Teijin. M.H. has received research grants/support from Asahi Kasei, Astellas, Chugai, Daiichi-Sankyo, Eisai, Mitsubishi-Tanabe and Takeda. K.K. has received speaking fees from Abbvie, Asahi Kasei, Astellas, Bristol-Myers, Chugai, Daiichi-Sankyo, Eisai, Janssen, Mitsubishi-Tanabe, Otsuka, Pfizer and Takeda, and has received research grants/support from Abbvie, Asahi Kasei, Astellas, Bristol-Myers, Chugai, Eisai, Mitsubishi-Tanabe, Pfizer and Takeda. A.K. has received consulting fees, speaking fees and research grants from Abbvie, Actelion, Alexion, Asahi Kasei, Astellas, AstraZeneca, Boehringer, Bristol-Myers, Chugai, Cosmic, Daiichi-Sankyo, Dainippon-Sumitomo, Eisai, Eli Lilly, Janssen, Kissei, Kowa, Kyowa-Kirin, Mitsubishi-Tanabe, Mochida, MSD, Nihon Medi-Physics, Nippon Kayaku, Novartis, Ono, Otsuka, Pfizer, Sanofi, Taisho-Toyama, Takeda, Teijin, UCB and YL Biologics. N.W. has received consulting fees, speaking fees and/or honoraria from Bristol-Myers, Eisai, Kowa, Mitsubishi-Tanabe, MSD, Pfizer and Takeda, and has received research grants/support from Asahi Kasei, Astellas, Eisai, Mitsubishi-Tanabe and MSD. T.A. has accepted honoraria for educational meetings from Abbvie, Astellas, Bristol-Myers, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, Mitsubishi-Tanabe, Pfizer, Takeda and UCB, and has received a research grant from Alexion, Astellas, Chugai, Daiichi-Sankyo, Mitsubishi-Tanabe, Otsuka, Pfizer and Takeda.
Y.T. has received research grants from Asahi Kasei, Astellas, AstraZeneca, Bristol-Myers, Chugai, Daiichi-Sankyo, Eisai, Janssen, Mitsubishi-Tanabe and MSD. N.M. has received research grants from Abbvie, Astellas, Chugai, Eisai, Mitsubishi-Tanabe, Pfizer and Takeda.
References
- 1. Marie I, Hatron PY, Hachulla E. et al. Pulmonary involvement in polymyositis and in dermatomyositis. J Rheumatol 1998;25:1336–43. [PubMed] [Google Scholar]
- 2. Nawata Y, Kurasawa K, Takabayashi K. et al. Corticosteroid resistant interstitial pneumonitis in dermatomyositis/polymyositis: prediction and treatment with cyclosporine. J Rheumatol 1999;26:1527–33. [PubMed] [Google Scholar]
- 3. Marie I, Hachulla E, Cherin P. et al. Interstitial lung disease in polymyositis and dermatomyositis. Arthritis Rheum 2002;47:614–22. [DOI] [PubMed] [Google Scholar]
- 4. Schnabel A, Reuter M, Biederer J, Richter C, Gross WL.. Interstitial lung disease in polymyositis and dermatomyositis: clinical course and response to treatment. Semin Arthritis Rheum 2003;32:273–84. [DOI] [PubMed] [Google Scholar]
- 5. Fathi M, Dastmalchi M, Rasmussen E, Lundberg IE, Tornling G.. Interstitial lung disease, a common manifestation of newly diagnosed polymyositis and dermatomyositis. Ann Rheum Dis 2004;63:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kang EH, Lee EB, Shin KC. et al. Interstitial lung disease in patients with polymyositis, dermatomyositis and amyopathic dermatomyositis. Rheumatology (Oxford) 2005;44:1282–6. [DOI] [PubMed] [Google Scholar]
- 7. Selva-O’Callaghan A, Labrador-Horrillo M, Muñoz-Gall X. et al. Polymyositis/dermatomyositis-associated lung disease: analysis of a series of 81 patients. Lupus 2005;14:534–42. [DOI] [PubMed] [Google Scholar]
- 8. Arsura EL, Greenberg AS.. Adverse impact of interstitial pulmonary fibrosis on prognosis in polymyositis and dermatomyositis. Semin Arthritis Rheum 1988;18:29–37. [DOI] [PubMed] [Google Scholar]
- 9. Schwarz MI, Matthay RA, Sahn SA. et al. Interstitial lung disease in polymyositis and dermatomyositis: analysis of six cases and review of the literature. Medicine (Baltimore) 1976;55:89–104. [DOI] [PubMed] [Google Scholar]
- 10. Fujisawa T, Suda T, Nakamura Y. et al. Differences in clinical features and prognosis of interstitial lung diseases between polymyositis and dermatomyositis. J Rheumatol 2005;32:58–64. [PubMed] [Google Scholar]
- 11. Takada K, Kishi J, Miyasaka N.. Step-up versus primary intensive approach to the treatment of interstitial pneumonia associated with dermatomyositis/polymyositis: a retrospective study. Mod Rheumatol 2007;17:123–30. [DOI] [PubMed] [Google Scholar]
- 12. Nagasaka K, Harigai M, Tateishi M. et al. Efficacy of combination treatment with cyclosporin A and corticosteroids for acute interstitial pneumonitis associated with dermatomyositis. Mod Rheumatol 2003;13:231–8. [DOI] [PubMed] [Google Scholar]
- 13. Kotani T, Makino S, Takeuchi T. et al. Early intervention with corticosteroids and cyclosporin A and 2-hour postdose blood concentration monitoring improves the prognosis of acute/subacute interstitial pneumonia in dermatomyositis. J Rheumatol 2008;35:254–9. [PubMed] [Google Scholar]
- 14. Wilkes MR, Sereika SM, Fertig N, Lucas MR, Oddis CV.. Treatment of antisynthetase-associated interstitial lung disease with tacrolimus. Arthritis Rheum 2005;52:2439–46. [DOI] [PubMed] [Google Scholar]
- 15. Ochi S, Nanki T, Takada K. et al. Favorable outcomes with tacrolimus in two patients with refractory interstitial lung disease associated with polymyositis/dermatomyositis. Clin Exp Rheumatol 2005;23:707–10. [PubMed] [Google Scholar]
- 16. Takada K, Nagasaka K, Miyasaka N.. Polymyositis/dermatomyositis and interstitial lung disease: a new therapeutic approach with T-cell-specific immunosuppressants. Autoimmunity 2005;38:383–92. [DOI] [PubMed] [Google Scholar]
- 17. Kurita T, Yasuda S, Oba K. et al. The efficacy of tacrolimus in patients with interstitial lung diseases complicated with polymyositis or dermatomyositis. Rheumatology (Oxford) 2015;54:39–44. [DOI] [PubMed] [Google Scholar]
- 18. Bohan A, Peter JB.. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344–7. [DOI] [PubMed] [Google Scholar]
- 19. Bohan A, Peter JB.. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975;292:403–7. [DOI] [PubMed] [Google Scholar]
- 20. Sontheimer RD Dermatomyositis: an overview of recent progress with emphasis on dermatologic aspects. Dermatol Clin 2002;20:387–408. [DOI] [PubMed] [Google Scholar]
- 21. Kohno N, Kyoizumi S, Awaya Y. et al. New serum indicator of interstitial pneumonitis activity. Sialylated carbohydrate antigen KL-6. Chest 1989;96:68–73. [DOI] [PubMed] [Google Scholar]
- 22. Nakajima H, Harigai M, Hara M. et al. KL-6 as a novel serum marker for interstitial pneumonia associated with collagen diseases. J Rheumatol 2000;27:1164–70. [PubMed] [Google Scholar]
- 23. Koike R, Tanaka M, Komano Y. et al. Tacrolimus-induced pulmonary injury in rheumatoid arthritis patients. Pulm Pharmacol Ther 2011;24:401–6. [DOI] [PubMed] [Google Scholar]
- 24. Sasaki T, Nakamura W, Inokuma S, Matsubara E.. Characteristic features of tacrolimus-induced lung disease in rheumatoid arthritis patients. Clin Rheumatol 2016;35:541–5. [DOI] [PubMed] [Google Scholar]
- 25. Mahler DA, Weinberg DH, Wells CK, Feinstein AR.. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest 1984;85:751–8. [DOI] [PubMed] [Google Scholar]
- 26. Witek TJ, Mahler DA.. Minimal important difference of the transition dyspnoea index in a multinational clinical trial. Eur Respir J 2003;21:267–72. [DOI] [PubMed] [Google Scholar]
- 27. Fujisawa T, Hozumi H, Kono M. et al. Prognostic factors for myositis-associated interstitial lung disease. PLoS One 2014;9:e98824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kameda H, Nagasawa H, Ogawa H. et al. Combination therapy with corticosteroids, cyclosporin A, and intravenous pulse cyclophosphamide for acute/subacute interstitial pneumonia in patients with dermatomyositis. J Rheumatol 2005;32:1719–26. [PubMed] [Google Scholar]
- 29. Takizawa H, Shiga J, Moroi Y. et al. Interstitial lung disease in dermatomyositis: clinicopathological study. J Rheumatol 1987;14:102–7. [PubMed] [Google Scholar]
- 30. High WA, Cohen JB, Murphy BA, Costner MI.. Fatal interstitial pulmonary fibrosis in anti-Jo-1-negative amyopathic dermatomyositis. J Am Acad Dermatol 2003;49:295–8. [DOI] [PubMed] [Google Scholar]
- 31. Chen Z, Cao M, Plana MN. et al. Utility of anti-melanoma differentiation-associated gene 5 antibody measurement in identifying patients with dermatomyositis and a high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a meta-analysis. Arthritis Care Res (Hoboken) 2013;65:1316–24. [DOI] [PubMed] [Google Scholar]
- 32. Moghadam-Kia S, Oddis CV, Sato S, Kuwana M, Aggarwal R.. Anti-melanoma differentiation-associated gene 5 is associated with rapidly progressive lung disease and poor survival in US patients with amyopathic and myopathic dermatomyositis. Arthritis Care Res (Hoboken) 2016;68:689–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Selva-O’Callaghan A, Pinal-Fernandez I, Trallero-Araguás E. et al. Classification and management of adult inflammatory myopathies. Lancet Neurol 2018;17:816–28. [DOI] [PubMed] [Google Scholar]
- 34. Isoda K, Takeuchi T, Kotani T. et al. Pre-treatment ferritin level and alveolar-arterial oxygen gradient can predict mortality rate due to acute/subacute interstitial pneumonia in dermatomyositis treated by cyclosporine a/glucocorticosteroid combination therapy: a case control study. PLoS One 2014;9:e89610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miyasaka N, Kawai S, Hashimoto H.. Efficacy and safety of tacrolimus for lupus nephritis: a placebo-controlled double-blind multicenter study. Mod Rheumatol 2009;19:606–15. [DOI] [PubMed] [Google Scholar]
- 36. Ogata H, Matsui T, Nakamura M. et al. A randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut 2006;55:1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ogata H, Kato J, Hirai F. et al. Double-blind, placebo-controlled trial of oral tacrolimus (FK506) in the management of hospitalized patients with steroid-refractory ulcerative colitis. Inflamm Bowel Dis 2012;18:803–8. [DOI] [PubMed] [Google Scholar]
- 38. Stone JH, Tuckwell K, Dimonaco S. et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017;377:317–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.