Abstract
Multidrug resistance-associated protein 4 (Mrp4) is an efflux transporter involved in the active transport of several endogenous and exogenous chemicals. Previously, we have shown that hepatic Mrp4 expression increases following acetaminophen overdose. In mice, these increases in Mrp4 expression are observed specifically in hepatocytes undergoing active proliferation. From this, we hypothesized that Mrp4 plays a key role in hepatocyte proliferation and that lack of Mrp4 impedes liver regeneration following liver injury and/or tissue loss. To evaluate the role of Mrp4 in these processes, we employed two-third partial hepatectomy (PH) as an experimental liver regeneration model. In this study, we performed PH-surgery on male wildtype (C57BL/6J) and Mrp4 knockout mice. Plasma and liver tissues were collected at 24, 48, and 72 h postsurgery and evaluated for liver injury and liver regeneration endpoints, and for PH-induced hepatic lipid accumulation. Our results show that lack of Mrp4 did not alter hepatocyte proliferation and liver injury following PH as evaluated by Ki-67 antigen staining and plasma alanine aminotransferase levels. To our surprise, Mrp4 knockout mice exhibited increased hepatic lipid content, in particular, di- and triglyceride levels. Gene expression analysis showed that lack of Mrp4 upregulated hepatic lipin1 and diacylglycerol O-acyltransferase 1 and 2 gene expression, which are involved in the synthesis of di- and triglycerides. Our observations indicate that lack of Mrp4 prolonged PH-induced hepatic steatosis in mice and suggest that Mrp4 may be a novel genetic factor in the development of hepatic steatosis.
Keywords: partial hepatectomy, Mrp4, drug transporters, hepatic steatosis
The remarkable regenerative capacity of the liver is essential for its recovery following acute or chronic liver injury caused by toxicants, alcohol consumption, and various liver diseases. Partial hepatectomy (PH) is a well-known surgical procedure used in animal models to understand the process and determinants of tissue mass recovery during liver regeneration in donor and recipient patients following liver transplantation. In humans, hepatic steatosis is associated with poor outcomes following PH and liver transplantation (Chu et al., 2015; Kele et al., 2013; Zezos and Renner, 2014). The development of nonalcoholic fatty liver disease (NAFLD) following liver transplantation surgery is a major concern in patients (Shaker et al., 2014; Zezos and Renner, 2014). In rodents, the impact of hepatic steatosis on liver regeneration processes is still unclear, as some studies have shown that fatty liver impedes liver regeneration, whereas other studies showed that transient hepatic lipid accumulation following PH is essential for liver regeneration (Gazit et al., 2010; Rudnick and Davidson, 2012). In contrast, persistent hepatic steatosis observed in obese and diabetic mice has been shown to be detrimental to tissue regeneration (DeAngelis et al., 2005; Yamauchi et al., 2003; Yang et al., 2001). Several factors associated with the development of transient hepatic steatosis post-PH-surgery have been identified (Rudnick and Davidson, 2012). However, the identity of the molecular mechanism(s) and potential therapeutic targets to minimize persistent hepatic lipid accumulation during liver regeneration processes remain elusive. New knowledge in this area of investigation will aid in devising new treatment modalities aimed at improving liver transplantation success and lead to the development of novel therapeutic interventions for various forms of fatty liver disease. These studies have identified multidrug resistance-associated protein (Mrp4) as a novel target and determinant of lipid accumulation in liver regeneration.
Mrp4 belongs to the ATP-binding cassette class of plasma membrane transport proteins involved in the efflux of chemicals and endogenous substances from cells. Specifically, Mrp4 is involved in the transport of several antiviral, anticancer and nonsteroid antiinflammatory drugs, conjugated bile acids, signaling molecules such as prostaglandins, cyclic adenosine monophosphate (cAMP), and cyclic guanosine monophosphate (Russel et al., 2008). Mrp4 is expressed in several tissues, with the highest expression in kidneys and choroid plexus (Leggas et al., 2004; Maher et al., 2005). In the liver, Mrp4 has minimal expression, which increases dramatically under several liver pathological conditions such as fatty liver diseases, cholestasis, and drug-induced liver injury (Aleksunes et al., 2008; Barnes et al., 2007; Donepudi et al., 2016, 2019; Klaassen and Aleksunes, 2010; Maher et al., 2005). In mice, Mrp4 deficiency is linked to increased risk of cholestatic liver injury, altered intestinal fluid secretion, pulmonary hypertension, platelet aggregation, and altered drug disposition in brain and kidneys (Cheepala et al., 2015; Hara et al., 2011; Klaassen and Aleksunes, 2010; Li et al., 2007; Russel et al., 2008). Our laboratory previously reported that hepatic Mrp4 expression increases in the regenerating liver after acetaminophen (APAP)-induced liver injury. Increased hepatic Mrp4 expression with APAP intoxication is specifically observed in proliferating hepatocytes and localized to centrilobular regions (Aleksunes et al., 2008). Treatment with colchicine, an antimitotic agent, increases susceptibility toward APAP toxicity along with inhibition of APAP-mediated Mrp4 induction. This suggests that increased Mrp4 expression in proliferating hepatocytes may have an important role in liver regeneration processes post-APAP toxicity. Recent studies in our laboratory with Mrp4 knockout (KO) mice showed that lack of Mrp4-altered kinetics of APAP-induced liver injury, but does not aggravate overall hepatotoxicity (Donepudi et al., 2019). Together, these studies suggest that not basal, but induction of Mrp4 expression may be an important event during hepatocyte proliferation. The significance of increases in Mrp4 expression in proliferating hepatocytes has not been thoroughly studied.
To elucidate the role of Mrp4 in hepatocyte proliferation and liver regeneration processes, we performed PH-surgery on wildtype (WT) mice and Mrp4 KO and collected tissue 24, 48, and 72 h postsurgery. Plasma and liver tissues were collected at specified time points and analyzed for liver regeneration endpoints, hepatic lipids and gene expression analysis. To our surprise, the lack of Mrp4 did not alter hepatocyte proliferation rates; however, it prolonged PH-induced hepatic steatosis and caused persistent hepatic lipid accumulation post-PH-surgery. These observations served to establish a novel role for Mrp4 as a gene that regulates hepatic lipid metabolism and that lack of, or impaired Mrp4 activity may increase the risk of NAFLD development when other risk factors (eg, genetic, environmental stressors) are also in place.
MATERIALS AND METHODS
Animals studies
Wildtype (C57BL/6J) mice were purchased from Jackson Laboratories (Bar Harbor, Maine). Mrp4 KO mice were generated by the Schuetz Laboratory on a C57BL6/129-SVJ background (Leggas et al., 2004), and subsequently backcrossed for > 10 generations into a C57BL/6J genetic background (Cheepala et al., 2015). Mrp4−/− mice were bred at the University of Connecticut, Storrs. In this study, 12- to 14-week-old male WT and KO mice (n = 4–5 mice per group) underwent either sham or two-third PH-surgery as previously described in Mitchell and Willenbring (2008). Briefly, mice were anesthetized using isoflurane and the liver was exposed through an abdomen midline incision. Median and left lobes of the liver were tied using silk sutures and were resected. Buprenorphine hydrochloride (0.05 mg/kg, SC) was used as an analgesic during and after surgery. Mice were provided free access to food and water before and after surgical procedure. Plasma and liver samples were collected at 24, 48, and 72 h postsurgery. Sham-operated mice were used as controls and euthanized 72 h after surgery. All animal experiments were approved by the institutional animal care and use committee at the University of Connecticut, Storrs.
Plasma alanine aminotransferase analysis
Plasma alanine aminotransferase (ALT) levels were analyzed using Infinity ALT Liquid Reagent (Catalog: TR71121; Thermo Fisher Scientific, Waltham, Massachusetts).
Histology
Liver samples were fixed in 10% neutral buffered zinc formalin and processed for sectioning [17]. Liver sections (5 µm) were stained with hematoxylin and eosin and Ki-67 immunohistochemistry for hepatocyte proliferation (Gerlach et al., 1997). Lipid accumulation in livers was characterized using Oil Red O (ORO) staining as described previously (Donepudi et al., 2017). At the end of the staining, cells were counterstained with hematoxylin. Ki-67 staining was quantified using Image J and plotted as the average number of stained cells.
Hepatic and plasma lipid content analysis
Hepatic lipids were extracted using the Folch method as previously described (Lee et al., 2019). Hepatic triglyceride and cholesterol levels were quantified from isolated lipid extracted using L‐Type Triglyceride M kit (Wako, Richmond, Virginia) and Cholesterol Reagent Set (Pointe Scientific, Canton, Michigan), respectively, as previously mentioned (Lee et al., 2019). Plasma triglyceride and cholesterol levels were analyzed using Total Triglyceride (Catalog: TR22421) and Cholesterol (Catalog: TR13421) Reagents (Thermo Scientific, Waltham, Massachusetts) according to the manufacturer’s protocol.
Lipidomics analysis
Hepatic lipidomics and metabolomics analysis were performed as previously mentioned (Tran et al., 2016, 2017). Liver samples (n = 4 per group) from sham and 72-h time point WT and Mrp4 KO mice were analyzed. Sample extraction and quantification were performed by the West Coast Center for Metabolomics at the University of California (UC) Davis as described previously (Tran et al., 2016, 2017). Raw data (peak heights) were quantified and normalized and plotted as fold changes compared with WT mice (Tran et al., 2017).
RNA isolation and RT-qPCR analysis
Total RNA was isolated using a phenol-chloroform isolation method. cDNA was synthesized according to manufacturer protocol using iScript cDNA synthesis kit (Catalog: 170-8891, Bio-Rad Laboratories Inc., Hercules, California). Gene expression analysis was performed with qPCR analysis using iTaq Universal SYBR Green Supermix (Catalog: 172-5121, Bio-Rad Laboratories Inc.). β-actin was used as a housekeeping gene for analysis and data was presented as a relative mRNA expression compared with the WT Sham group. Primer sequences of genes analyzed using RT-qPCR are tabulated in Supplementary Table 1.
Quantigene multiplex analysis
Hepatic lipid metabolism gene mRNA expression was determined using a QuantiGene Plex 2.0 assay (Thermo Scientific). Total mRNA was isolated from livers and 500 ng of total RNA was loaded into each well and assay was performed as mentioned previously (Donepudi et al., 2016). The assay was performed on a Bio-Plex System array reader with Luminex 100 xMAP technology, and data were acquired using Bio-Plex Software Data Manager (Bio-Rad Laboratories Inc.). The list of genes analyzed in this assay is presented in Supplementary Table 2. Gene expression data are plotted as fold change, with cyclophilin A used as a housekeeping gene for this analysis.
Tissue fractionation and protein isolation
Liver homogenates and plasma membrane fractions were prepared using sucrose-Tris (ST) buffer (0.25 M sucrose, 10 mM Tris–HCl, pH 7.4; Donepudi et al., 2012). Briefly, frozen livers were homogenized in ST buffer using Dounce homogenizer and the homogenates were centrifuged at 10 000 × g for 20 min at 4°C. The resulting supernatants were again centrifuged at 100 000 × g for 60 min at 4°C. The pellet fraction was resuspended and analyzed for membrane proteins.
Cytosolic and nuclear protein fractions for lipin1 protein analysis were extracted using NE-PER Nuclear and Cytoplasmic Extraction kit (Catalog: 78833; Thermo Scientific) according to the manufacturer’s protocol.
Western blot analysis
Membrane, cytosol, and nuclear sub-cellular fraction proteins (75, 40, and 25 μg protein/lane, respectively) were electrophoretically resolved using 8% polyacrylamide gels and trans-blotted onto PVDF membrane (Donepudi et al., 2012). Immunochemical detection of proteins was performed with anti-Mrp4 (M4I-10, Abcam, Cambridge, Massachusetts), anti-Lipin1 (donated from Dr Brian Finck lab at Washington University in St Louis), anti-Lamin B1 (13435s, Cell Signaling, Danver, Massachusetts) and β-actin (ab8227, Abcam) primary antibodies (Aleksunes et al., 2008). Protein-antibody complexes were detected using an Immobilon Western chemiluminescent kit (Millipore, Billerica, Massachusetts) and exposed to CL-Xposure X-ray film (Thermo Scientific).
Statistical analysis
Results are expressed as means + SEM of 4–5 mice per group. Data were analyzed by 2-way ANOVA followed by Bonferroni post hoc test using Graph pad Prism5 software (GraphPad Software, Inc., La Jolla, California). Differences were considered significant at p ≤ .05.
RESULTS
Phenotypic Characterization of WT and Mrp4 KO Mice Following PH-Surgery
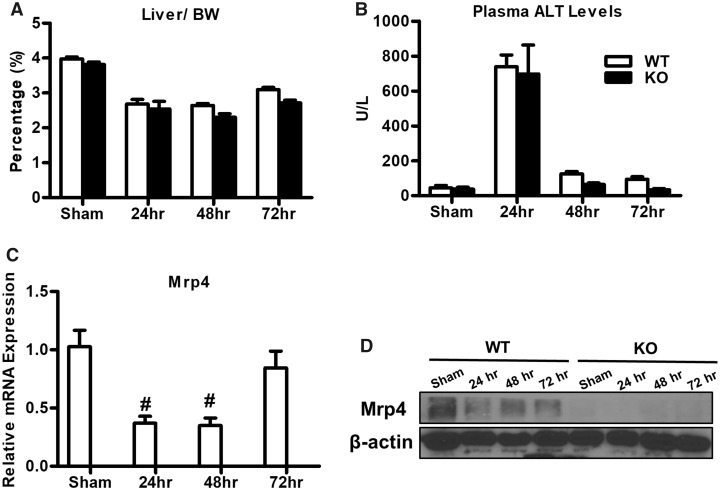
The liver-to-bodyweight ratio is known to decrease after PH-surgery, with values reverting back to normal as the liver regenerates. Accordingly, in both WT and Mrp4 KO mice, the liver-to-body weight ratio decreased after PH-surgery with no difference between genotypes (Figure 1A). Plasma ALT, a biomarker for liver injury, was elevated following PH in both WT and Mrp4 KO mice compared with sham controls. The peak ALT values were observed at 24 h with no apparent difference WT and Mrp4 KO mice. ALT levels then declined to near sham values at 48 and 72 h (Figure 1B). Hepatic Mrp4 expression is known to increase during liver injury and metabolic diseases. In rats, 90% PH-surgery (not two-third) resulted in an increase in hepatic Mrp4 expression (Miura et al., 2011), whereas in mice, two-third PH-surgery hepatic resulted in no or decreasing trend in Mrp4 gene expression (Csanaky et al., 2009). In contrast to this previous report in mice, hepatic Mrp4 mRNA expression in our mouse model decreased following PH only at 24 and 48 h, but not at 72 h (Figure 1C). In agreement with the observed mRNA expression values, hepatic Mrp4 protein expression also decreased after PH (Figure 1D).
Figure 1.
Phenotypic characterization of WT and Mrp4 KO mice following PH-surgery. The liver-to-body weight ratio (A); plasma ALT activity (B) of WT and Mrp4 KO mice following either sham or PH-surgery; (C) hepatic Mrp4 mRNA levels in WT mice (D) hepatic Mrp4 protein expression in WT and Mrp4 KO mice. Data are presented as mean ± SEM (p ≤ .05). The symbol “#” denotes significant difference between sham and PH.
Lack of Mrp4 Does Not Alter Hepatocyte Proliferation during Liver Regeneration Processes
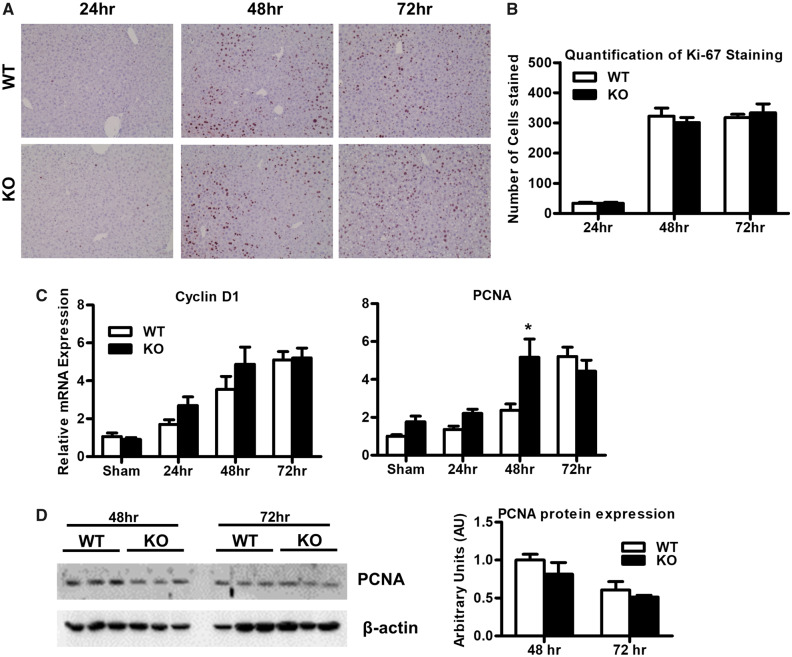
To investigate the role of Mrp4 on hepatocyte proliferation, liver sections were immunostained for Ki-67 antigen, a marker for cell proliferation (Figure 2A). In both WT and Mrp4 KO mice, Ki-67 staining was significantly increased at 48 and 72 h with no apparent differences between genotypes (Figure 2A and 2B). Additionally, we analyzed the mRNA expression of genes involved in cell proliferation such as cyclin D1 and proliferating cell nuclear antigen (PCNA; Figure 2C). Expression of both genes increased following PH-surgeries in both genotypes. Except for the 48-h time point, which showed greater PCNA expression in Mrp4 KO mice, there were no other changes in PCNA expression between genotypes. Similarly, no differences in cyclin D1 expression between Mpr4 KO and WT were noted at any of the time points examined. Although PCNA mRNA levels were increased in Mrp4 KO mice at 48-h time point, protein expression analysis showed no apparent significant changes between Mrp4 KO and WT mice at 48 or 72 h (Figure 2D).
Figure 2.
Mrp4 has no role in the regulation of hepatocyte proliferation during liver regeneration processes. A, Representative images of WT and Mrp4 KO liver sections stained for Ki-67 antigen (200× magnification. B, Quantification of Ki-67 staining. C, mRNA levels of 2 additional hepatocyte proliferation markers, cyclin D, and PCNA. D, Western blot images of PCNA and β-actin protein in total protein lysates. Data are presented as mean ± SEM (p ≤ .05). An asterisk “*” denotes significant difference between WT and Mrp4 KO mice.
Lack of Mrp4 Alters Hepatic Lipid Metabolism and Results in Persistent Hepatic Lipid Accumulation
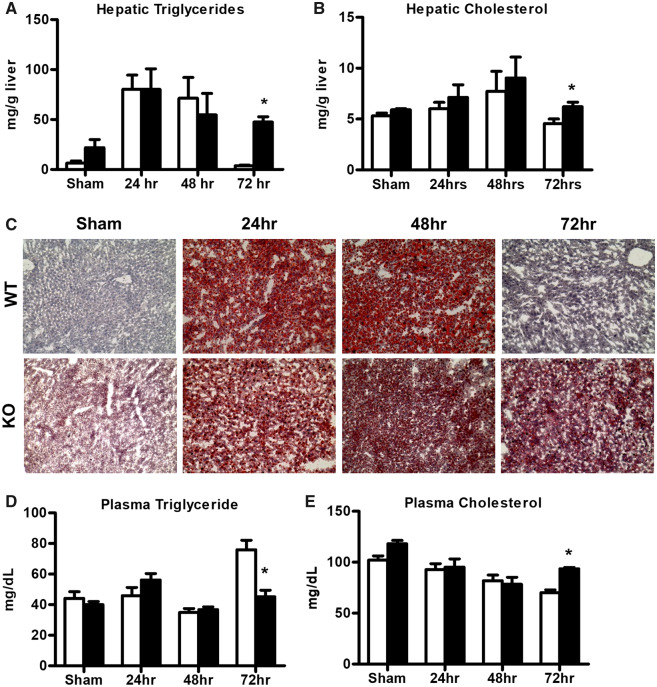
In mice, hepatic lipid accumulation is observed during liver regeneration as a transient phenomenon, which is known to occur between 12 and 48 h following PH (Tijburg et al., 1991). In our WT mice, we observed increased hepatic triglyceride accumulation compared with sham mice at 24 and 48 h time points following PH-surgery, whereas at 72 h, hepatic triglyceride levels reached normal values as those detected in sham WT mice (Figure 3A). In Mrp4 KO mice, sham controls had higher but not statistically significant levels of hepatic triglycerides compared with the respective WT mice. Similar to WT mice, hepatic triglyceride levels increased at 24 and 48 h in Mrp4 KO mice compared with respective sham controls. At the same time points, no significant differences in hepatic triglyceride levels were observed in Mrp4 KO mice compared with WT mice. However, at 72 h, Mrp4 KO mice had significantly higher hepatic triglycerides levels (12-fold) compared with their WT counterparts (Figure 3A). These increases in hepatic triglyceride levels were also observed in additional group of mice at 96 h postsurgery (Supplementary Figure 1). The extended prolongation of hepatic steatosis in Mrp4 KO mice following PH further confirms that the lack of Mrp4 results in persistent hepatic lipid accumulation following PH-surgery in mice. Hepatic cholesterol levels were not altered after PH- surgery in both WT and Mrp4 KO mice, except for 72 h, where Mrp4 KO mice had higher hepatic cholesterol levels compared with WT mice (Figure 3B). These changes in hepatic lipid accumulation were also evidenced by ORO staining, a stain for neutral lipids. Similar to the observed hepatic triglycerides changes, ORO staining also showed persistent hepatic lipid accumulation in Mrp4 KO mice compared with WT mice (Figure 3C). In contrast to hepatic lipid accumulation, plasma triglycerides and cholesterol levels were not significantly different between WT and Mrp4 KO mice following PH, except for the 72-h time point when plasma triglycerides levels were decreased, whereas cholesterol values were increased in Mrp4 KO compared with their respective WT mice (Figure 3C and 3D).
Figure 3.
Lack of Mrp4 results in persistent hepatic lipid accumulation following PH-surgery. Biochemical quantification of hepatic triglyceride (A) and cholesterol (B) in WT and Mrp4 KO mice; (C) Representative images of WT and Mrp4 KO liver sections stained with ORO for detection of neutral lipids (200× magnification); Biochemical quantification of plasma triglyceride (D) and cholesterol (E) in WT and Mrp4 KO mice. Data are presented as mean ± SEM (p ≤ .05). An asterisk “*” denotes significant difference between WT and Mrp4 KO mice.
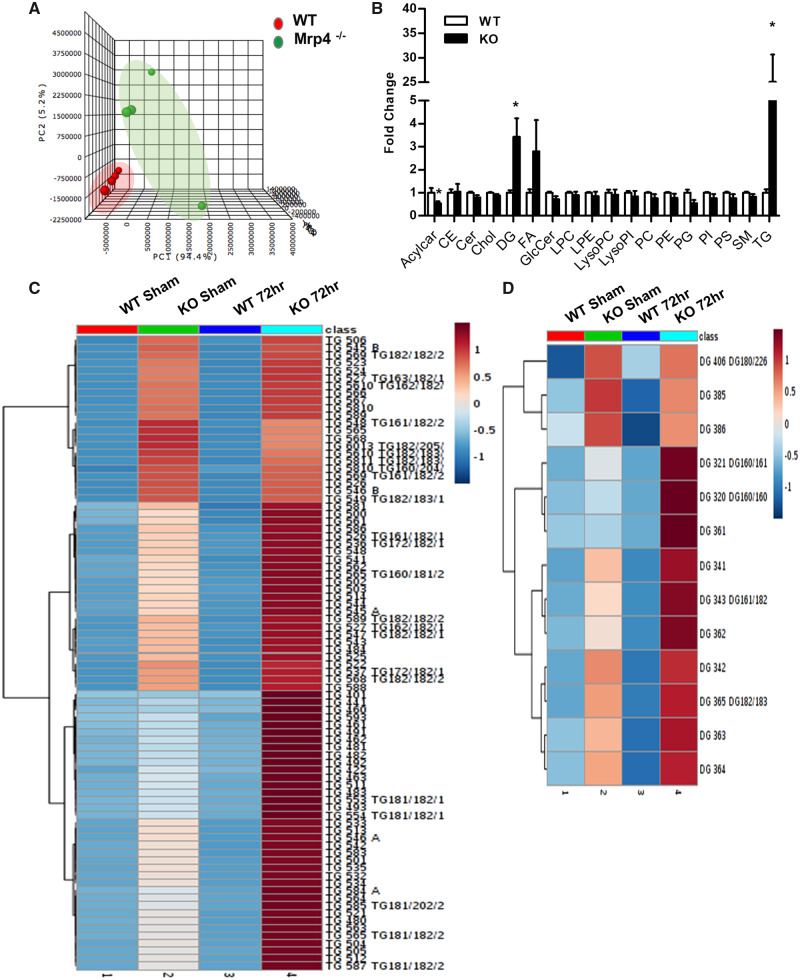
These changes in hepatic lipid accumulation are surprising and unexpected considering the well-documented role of Mrp4 as a drug transporter and not as a functional mediator of lipid homeostasis. To better understand the nature and extent of this increased hepatic lipid accumulation in Mrp4 KO mice, we performed detailed lipidomics analysis in both WT and KO mouse livers. For this study, we specifically selected livers from sham controls and the 72-h time point following PH, since these set of samples showed the most dramatic differences in hepatic lipid accumulation between genotypes (Figs. 3A and 3C). Principal component analysis showed that 72 h Mrp4 KO liver samples have a distinct lipid profile compared with the 72 h WT mice (Figure 4A). The lack of Mrp4 resulted in a significant increase in hepatic di- and triglyceride (3.4- and 25-fold, respectively) accumulation following PH-surgery compared with their WT counterparts. Elevated hepatic di- and triglycerides were also observed in sham Mrp4 KO mice compared with sham WT mice, but of a lesser magnitude (1.7- and 9-fold) (Supplementary Figure 2). Hepatic free fatty acids levels showed an increasing trend in Mrp4 KO mice compared with WT mice that was not statistically significant. To better decipher the di- and triglycerides composition, we generated heat maps from the lipidomics data, with blue color indicative of low levels and red color indicative of high levels of analytes. The heat map data show that even in sham controls, Mrp4 KO mice have increased levels of several hepatic triglyceride species compared with their respective WT controls (Figure 4C). At 72 h post-PH, the content of all triglyceride species analyzed is increased in Mrp4 KO mice compared with WT mice. Not surprisingly, these increases in hepatic triglyceride levels in Mrp4 KO mice are much more profound at 72-h time point than in sham controls. Diglycerides, a precursor of triglycerides also showed a similar pattern as triglycerides (Figure 4D). These data suggest that in mice, lack of Mrp4 worsens hepatic lipid accumulation following PH-surgery.
Figure 4.
Mrp4 regulates hepatic lipid metabolism by altering di-triglyceride levels. Lipidomics analysis was performed on WT and Mrp4 KO mice. A, Principal component analysis was plotted for liver samples obtained at 72 h postPH-surgery from WT and Mrp4 KO mice; (B) Hepatic lipid species such as acylcarnitine (AcylCar), cholesterol ester (CE), cholesterol (Chol), diglyceride (DG), Free fatty acids (FA), ceramide (Cer), lysophosphatidylethanolamine (LPE), lysophosphatidylcholine (LysoPC), lysophosphatidylinositol (LysoPI), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI), phosphatidylserine (PS), sphingomyelin (SM), and triglyceride (TG), were also quantified in the 72 h post-PH liver samples from WT and Mrp4 KO mice. Data are presented as fold change compared with WT mice and p ≤ .05 is considered as statistically significant. An asterisk “*” indicates significance difference between WT and Mrp4 KO mice; Heatmaps for hepatic triglycerides (C) and diglyceride (D) content are plotted with high lipid species content denoted in red and low content as blue.
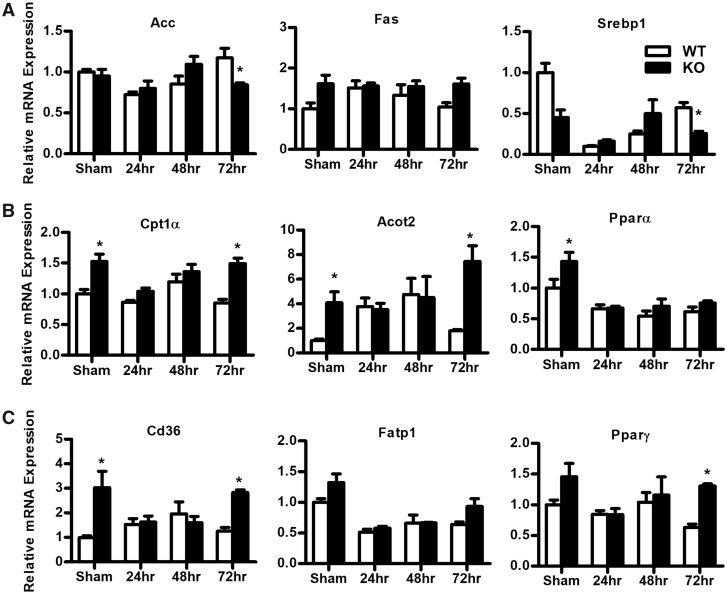
Mrp4 Regulates Hepatic Lipid Metabolism by Altering Lipid Synthesis and Fatty Acid Uptake Gene Expression
Gene expression studies demonstrate that hepatic lipid metabolism genes are altered in mice lacking Mrp4. In sham controls, the mRNA levels of fatty acid synthesis genes, such as acetyl-CoA carboxylase (Acc) and fatty acid synthase (Fas), were not significantly different between WT and Mrp4 KO mice (Figure 5A). However, Acc mRNA levels following PH are significantly decreased in Mrp4 KO mice only at 72 h, whereas Fas gene expression remained unchanged at all time points. Similar to Acc expression, hepatic sterol regulatory element-binding protein-1 (Srebp1), a key transcription factor involved in the regulation of fatty acid synthesis (Shimomura et al., 1998), also showed decreased mRNA levels only at the 72-h time point in Mrp4 KO mice (Figure 5A).
Figure 5.
Lack of Mrp4 alters hepatic fatty acid metabolism gene expression. Genes involved in fatty acid synthesis (A), hepatic β-oxidation (B), and fatty acid uptake (C) processes were analyzed in WT and Mrp4 KO mice following at 72 h either sham or PH-surgery. Data are presented as mean ± SEM (p ≤ .05). An asterisk “*” denotes significant difference between WT and Mrp4 KO mice.
Hepatic mitochondrial β-oxidation plays an important role in lipid catabolism. Impaired hepatic β-oxidation also leads to hepatic lipid accumulation (Koo, 2013). Interestingly, a lack of Mrp4 in mice resulted in increased expression of genes involved in hepatic β-oxidation (Figure 5B). In sham controls, the mRNA levels of carnitine palmitoyltransferase 1 alpha (Cpt1α), a rate-limiting gene in the β-oxidation processes (Koo, 2013), are upregulated (by 1.5-fold) in Mrp4 KO mice compared with WT mice. This change in gene expression for Cpt1α is also observed in Mrp4 KO mice at 72 h post-PH-surgery. Similar to Cpt1α gene expression, hepatic mRNA levels of acyl-CoA thioesterase 2 (Acot2), a mitochondrial thioesterase that catalyzes the breakdown of long-chain fatty acid CoA (Moffat et al., 2014), are also increased in Mrp4 KO sham controls (4-fold) and also at 72 h following PH (7.8-fold) compared with their corresponding WT mice. The same gene expression pattern was detected for the peroxisome proliferator-activated receptor alpha (Pparα), a key transcription factor involved in the regulation of hepatic β-oxidation genes (Reddy and Hashimoto, 2001). Pparα mRNA levels are also higher in Mrp4 sham-operated KO mice but not at any of the time points examined following PH. Changes in gene expression of hepatic β-oxidation genes in Mrp4 KO mice suggest that lack of Mrp4 in mice results in increased hepatic lipid catabolism pathways along with an increase in hepatic di- and triglyceride synthesis.
Hepatic lipid accumulation can be the outcome of increased fatty acid and lipid synthesis, impaired β-oxidation and increased fatty acid uptake. Hepatic fatty acid translocase (Fat/Cd36) is known to play a key role in hepatic fatty acid uptake. In NAFLD patients, hepatic Cd36 gene expression is positively correlated with hepatic triglyceride accumulation, highlight the importance of Cd36 function in the pathogenesis of hepatic steatosis (Greco et al., 2008; Koo, 2013). In Mrp4 KO mice, hepatic Cd36 mRNA levels are increased in the sham group and at 72 h post-PH by at least 2.5-fold compared with their WT counterparts (Figure 5C). Increased hepatic Cd36 gene expression in the Mrp4 null mice correlates with the increased hepatic triglyceride accumulation determined in these mice. Hepatic fatty acid transport protein 1 did not show any significant changes due to Mrp4 absence. On the other hand, Pparγ, a transcription factor known to regulate hepatic Cd36 expression (Zhou et al., 2008), also showed a similar expression pattern as Cd36 in Mrp4 KO mice compared with WT mice.
Apart from the above-mentioned genes, we have analyzed several other genes that are involved in inflammatory and oxidative stress and lipid metabolism pathways on the multiplex platform. mRNA expression levels of these genes are presented in Supplementary Figures 3 and 4.
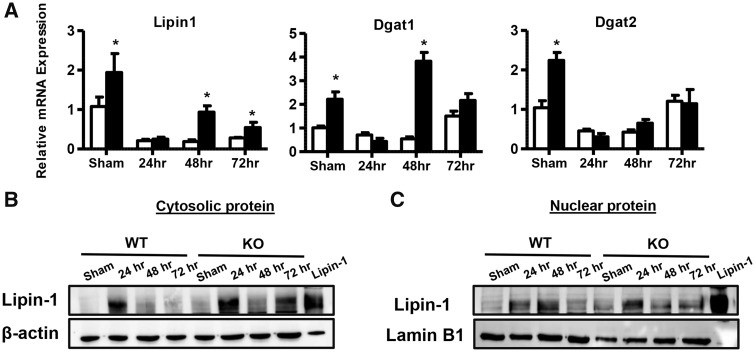
Although the expression of genes involved in fatty acid synthesis did not increase in Mrp4 KO mice, the lack of Mrp4 resulted in the upregulation of hepatic di- and triglyceride genes (Figure 6). For lipin1, a key gene involved in the synthesis of diacylglycerol (Finck et al., 2006), mRNA levels were significantly upregulated by 1.8-fold in Mrp4 KO sham-operated mice compared with WT shams. This induction in lipin1 gene expression was also observed in Mrp4 KO mice at 48 and 72 h post-PH-surgery in comparison to their respective WT counterparts (Figure 6A). These increases in lipin1 expression are in agreement with the increased hepatic diglyceride levels observed in these mice (Figs. 4B and 6A). Lipin1 is known to have dual function depending on the subcellular localization. In the cytosol, lipin1 acts as lipid synthesis enzyme due to its phosphatidic acid phosphatase (PAP) activity, whereas in the nucleus it is known to act as a transcriptional coregulatory protein (Hu et al., 2013; Reue and Zhang, 2008). Lipin1 protein expression analysis in the cytosolic and nuclear subcellular protein fractions showed that in contrast to mRNA expression, lipin1 protein levels increased after PH-surgery in both WT and KO mice with the highest expression observed at 24 h. In line with increased mRNA expression and hepatic diglycerol content, lipin1 protein expression in both cytosol and nucleus increased in Mrp4 KO mice compared with WT mice (Figs. 6B and 6C). Along with the increase in lipin1 gene expression, hepatic diacylglycerol O-acyltransferase 1 and 2 (Dgat1 and 2), key enzymes that are known to catalyze terminal steps in triglycerides synthesis from diacylglycerols (Choi and Diehl, 2008), mRNA levels are increased by at least 2-fold in sham Mrp4 KO compared with WT mice (Figure 6A). Furthermore, in the Mrp4 KO mice, the mRNA levels of Dgat1 were increased at only 48 h postsurgery compared with WT mice, whereas Dgat2 expression showed no significant changes post-PH-surgery due to genotype. These results suggest that the lack of Mrp4 increases hepatic di- and triglyceride synthesis gene expression without an increase in de novo fatty acid synthesis, which is a precursor for di- and triglycerides.
Figure 6.
Expression of hepatic di-and triglyceride synthesis genes are increased in Mrp4 KO mice. A, mRNA expression of genes involved in hepatic di-and triglycerides synthesis such as lipin-1, Dgat1 and 2 were analyzed using RT-qPCR. Hepatic lipin-1 protein expression in cytosolic (B) and nuclear fractions (C) was analyzed using Western blot analysis. A total cell protein lysate of HEK-293 cells overexpressing HA-tagged lipin-1 was used as positive control. Data are presented as mean ± SEM (p ≤ .05). An asterisk “*” denotes significant difference between WT and Mrp4 KO mice.
DISCUSSION
PH-induced hepatic steatosis is a common phenomenon observed due to metabolic alterations caused by hepatic insufficiency (Rudnick and Davidson, 2012). Several factors such as glucocorticoid receptors, leptin, lipin1, and adipose tissue are involved in the regulation of this phenomenon (Gazit et al., 2010; Rudnick and Davidson, 2012; Shteyer et al., 2004). In general, hepatic fat accumulation is due to imbalances in either hepatic lipid synthesis, uptake, or lipolysis (Koo, 2013). The role of Mrp4 in regulating these lipid metabolism pathways was unknown. Our studies are the first to provide evidence that Mrp4 function regulates liver lipid homeostasis. Previously, we have shown that in both humans and mice, hepatic Mrp4 expression increased during the development of fatty liver diseases and other metabolic diseases such as obesity and diabetes (Donepudi et al., 2016; More and Slitt, 2011; More et al., 2013 ). The significance of increases in hepatic Mrp4 expression and the role of Mrp4 in hepatic steatosis has not been considered because investigations on Mrp4 have primarily centered on its function as a drug transporter with a lesser emphasis on the transport of endogenous molecules. Some of these endogenous molecules that are transported by Mrp4 are known to alter several cell signaling pathways, which are involved in regulating cell physiological functions. Studies in our laboratory indicate for the first time that mice lacking Mrp4 have higher hepatic lipid accumulation than WT mice and that this hepatic lipid accumulation worsens considerably following PH-surgery.
This study also shows that a lack of Mrp4 in mice upregulates lipid synthesis, specifically di- and triglyceride and that the activity of lipid uptake pathways is similarly increased. Studies using fatty liver dystrophy (fld) mice indicate that defective lipin1 expression results in decreased PH-induced hepatic steatosis (Gazit et al., 2010). In line with these observations in fld mice, Mrp4 KO mice have increased expression of hepatic lipin1 and also hepatic di- and triglycerides levels after PH (Figure 5B). Hepatic lipin-1 expression is shown to be upregulated by cAMP (Manmontri et al., 2008), a well-known, substrate of Mrp4 (Russel et al., 2008). Based on these observations, we now hypothesize that the lack of Mrp4 increases intracellular cAMP in hepatocytes, which in turn upregulates hepatic lipin1 expression in Mrp4 KO mice. Lipin1 gene has pleiotropic functions, known to regulate lipid metabolism by altering different molecular pathways (Reue and Zhang, 2008). Lipin1 possesses PAP enzyme activity, which is known to catalyze the synthesis of diacylglycerol from phosphatidic acid (PA, Reue and Zhang, 2008). Increases in hepatic diglycerol levels in Mrp4 KO mice can be attributed to increased hepatic lipin1 activity (Figure 3). Additionally, the increase in hepatic Dgat-1 and 2 expression can be linked to hepatic triglyceride accumulation as both enzymes are involved in catalyzing triglyceride synthesis (Choi and Diehl, 2008). In Mrp4 KO mice, hepatic β-oxidation genes such as Cpt1α and Acot2 expression increased along with the increase in Pparα gene expression (Figure 5C). Increased expression of these genes in KO mice can be attributed to the increase in lipin1 expression, as lipin1 has a role in amplifying Pparα-regulated transcription of hepatic β-oxidation genes (Finck et al., 2006). Lipin1 transcriptional activity has also been shown to stimulate Pparγ activity and promote adipogenesis in adipocytes (Koh et al., 2008). As Pparγ is known to upregulate Cd36 expression (Zhou et al., 2008), a key transporter in fatty acid uptake, increases in both hepatic Cd36 and Pparγ expression in KO mice can also be the outcome of increased lipin1 expression. Cd 36 is known to play an important role in regulating hepatic lipid metabolism, as overexpression of hepatic Cd36 contributes to dyslipidemia by increasing hepatic triglyceride synthesis and fatty acid uptake (Koonen et al., 2007). Similarly, increases in hepatic Cd36 expression in Mrp4 KO mice may have contributed to increased hepatic triglyceride levels observed in our studies.
Although hepatic di- and triglycerides levels and expression of genes involved in their synthesis increased, hepatic fatty acid synthesis genes such as Acc and Srebp1 expression decreased. These decreases can be due to compensatory mechanisms activated in Mrp4 KO mice in response to increased hepatic lipid accumulation. Studies with liver-specific Fas and global Srebp1c KO mice showed that hepatic lipogenesis, specifically fatty acid synthesis, has no role in PH-induced hepatic triglyceride accumulation (Newberry et al., 2008; Peng et al., 2018). Moreover, the lack of Srebp1c in liver resected mice increases hepatic cholesterol levels, which in turn enhances liver regeneration (Peng et al., 2018). In our studies, the lack of Mrp4 decreased Srebp1c expression along with increases in hepatic cholesterol levels at 72 h post-PH-surgery. These results suggest that Srebp1 plays a role in increasing hepatic cholesterol levels in the Mrp4 KO mice following PH-surgery.
Studies using ob/ob, db/db, and diet-induced obese models of fatty liver and metabolic diseases suggest that hepatic steatosis impairs the liver regeneration processes (DeAngelis et al., 2005; Yamauchi et al., 2003; Yang et al., 2001). In contrast to these observations, studies using liver-specific Fabp1 and intestine-specific microsomal triglyceride transfer protein KO mice, and methionine and choline-deficient diet-fed mice, suggest that hepatic lipid accumulation following PH-surgery by itself does not alter liver regeneration capacity (Newberry et al., 2008; Picard et al., 2002). These studies also suggested that other factors such as leptin receptor deficiency and inflammation drive hepatocyte proliferation following PH-surgery (Fausto, 2000; Michalopoulos, 2007; Newberry et al., 2008; Picard et al., 2002; Rudnick and Davidson, 2012). Pro-inflammatory genes such as Interleukin 6 (Il6) and tumor necrosis factor-alpha (Tnfα) are known to play a significant role in the initiation of hepatocyte proliferation (Cressman et al., 1996; Yamada et al., 1997). Lack of Mrp4 in mice did not alter the expression of pro-inflammatory genes such as Tnfα, Il6, and chemokine (C-C motif) ligand 2 following PH (Supplementary Figure 4), indicating that the absence of Mrp4 do not regulate hepatic inflammation and thereby hepatocyte proliferation. Our observations do not challenge existing knowledge about the effects of PH-induced hepatic lipid accumulation on liver regeneration processes, as hepatic fat accumulation by itself is not sufficient to impair the liver regeneration processes. For example, studies using fld mice suggests lack of lipin-1 hampers for liver regeneration processes but recent studies from McGill group suggest that the accumulation of PA a precursor of di- and triglycerides due to the inhibition of hepatic lipin-1 enhances liver regeneration processes in drug-induced liver injury (Clemens et al., 2019; Lutkewitte et al., 2018). Although lack of Mrp4 in Mrp4 KO mice prolonged hepatic lipid accumulation following PH-surgery, hepatocyte proliferation, and liver injury markers did not show any significant changes compared with WT mice.
In summary, our study indicates that Mrp4 is a novel genetic factor regulating PH-induced hepatic steatosis and is the first to document that Mrp4 function influences hepatic lipid metabolism and the expression of various genes involved in lipid homeostasis, such as lipin 1, Dgat1 and 2. Our observations suggest that the lack of Mrp4 drives the development of hepatic steatosis through the upregulation of hepatic triglyceride synthesis and fatty acid uptake pathways. The detailed molecular mechanisms through which Mrp4 regulates such genes and the subsequent effects on lipid metabolism pathways are still unknown, which warrants further investigation. Finally, our study suggests that the impairment of Mrp4 activity exacerbates hepatic lipid accumulation in the presence of other stressor such as PH, genetic, and environmental factors.
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge the Mechanisms Specialty Section of the Society of Toxicology for highlighting our work at 2017 SOT annual conference at Baltimore, MD. We would also like to acknowledge Dr Brian Finck and Andrew Lutkewitte from Washington University in St Louis for providing anti-lipin-1 antibody and lipin-1 positive protein control.
FUNDING
This study was funded by University of Connecticut Research Excellence Program 2018 (J.E.M.). This study was also supported by P30 CA021765 Cancer Center Support grant (5R01CA194206), and ALSAC (J.D.S).
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Aleksunes L. M., Campion S. N., Goedken M. J., Manautou J. E. (2008). Acquired resistance to acetaminophen hepatotoxicity is associated with induction of multidrug resistance-associated protein 4 (Mrp4) in proliferating hepatocytes. Toxicol. Sci. 104, 261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S. N., Aleksunes L. M., Augustine L., Scheffer G. L., Goedken M. J., Jakowski A. B., Pruimboom-Brees I. M., Cherrington N. J., Manautou J. E. (2007). Induction of hepatobiliary efflux transporters in acetaminophen-induced acute liver failure cases. Drug Metab. Dispos. 35, 1963–1969. [DOI] [PubMed] [Google Scholar]
- Cheepala S. B., Pitre A., Fukuda Y., Takenaka K., Zhang Y., Wang Y., Frase S., Pestina T., Gartner T. K., Jackson C., et al. (2015). The ABCC4 membrane transporter modulates platelet aggregation. Blood 126, 2307–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. S., Diehl A. M. (2008). Hepatic triglyceride synthesis and nonalcoholic fatty liver disease. Curr. Opin. Lipidol. 19, 295–300. [DOI] [PubMed] [Google Scholar]
- Chu M. J., Dare A. J., Phillips A. R., Bartlett A. S. (2015). Donor hepatic steatosis and outcome after liver transplantation: A systematic review. J. Gastrointest. Surg. 19, 1713–1724. [DOI] [PubMed] [Google Scholar]
- Clemens M. M., Kennon-McGill S., Apte U., James L. P., Finck B. N., McGill M. R. (2019). The inhibitor of glycerol 3-phosphate acyltransferase FSG67 blunts liver regeneration after acetaminophen overdose by altering GSK3beta and Wnt/beta-catenin signaling. Food Chem. Toxicol. 125, 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman D. E., Greenbaum L. E., DeAngelis R. A., Ciliberto G., Furth E. E., Poli V., Taub R. (1996). Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274, 1379–1383. [DOI] [PubMed] [Google Scholar]
- Csanaky I. L., Aleksunes L. M., Tanaka Y., Klaassen C. D. (2009). Role of hepatic transporters in prevention of bile acid toxicity after partial hepatectomy in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G419–G433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelis R. A., Markiewski M. M., Taub R., Lambris J. D. (2005). A high-fat diet impairs liver regeneration in C57BL/6 mice through overexpression of the NF-kappaB inhibitor IkappaBalpha. Hepatology 42, 1148–1157. [DOI] [PubMed] [Google Scholar]
- Donepudi A. C., Aleksunes L. M., Driscoll M. V., Seeram N. P., Slitt A. L. (2012). The traditional ayurvedic medicine, Eugenia jambolana (Jamun fruit), decreases liver inflammation, injury and fibrosis during cholestasis. Liver Int. 32, 560–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donepudi A. C., Boehme S., Li F., Chiang J. Y. (2017). G-protein-coupled bile acid receptor plays a key role in bile acid metabolism and fasting-induced hepatic steatosis in mice. Hepatology 65, 813–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donepudi A. C., Cheng Q., Lu Z. J., Cherrington N. J., Slitt A. L. (2016). Hepatic transporter expression in metabolic syndrome: Phenotype, serum metabolic hormones, and transcription factor expression. Drug Metab. Dispos. 44, 518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donepudi A. C., Goedken M. J., Schuetz J. D., E Manautou J. (2019). Lack of multidrug resistance-associated protein 4 (Mrp4) alters the kinetics of acetaminophen toxicity. Toxicol. Rep. 6, 841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto N. (2000). Liver regeneration. J. Hepatol. 32(1 Suppl.), 19–31. [DOI] [PubMed] [Google Scholar]
- Finck B. N., Gropler M. C., Chen Z., Leone T. C., Croce M. A., Harris T. E., Lawrence J. C. Jr,, Kelly D. P. (2006). Lipin 1 is an inducible amplifier of the hepatic PGC-1alpha/PPARalpha regulatory pathway. Cell Metab. 4, 199–210. [DOI] [PubMed] [Google Scholar]
- Gazit V., Weymann A., Hartman E., Finck B. N., Hruz P. W., Tzekov A., Rudnick D. A. (2010). Liver regeneration is impaired in lipodystrophic fatty liver dystrophy mice. Hepatology 52, 2109–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach C., Sakkab D. Y., Scholzen T., Daßler R., Alison M. R., Gerdes J. (1997). Ki-67 expression during rat liver regeneration after partial hepatectomy. Hepatology 26, 573–578. [DOI] [PubMed] [Google Scholar]
- Greco D., Kotronen A., Westerbacka J., Puig O., Arkkila P., Kiviluoto T., Laitinen S., Kolak M., Fisher R. M., Hamsten A., et al. (2008). Gene expression in human NAFLD. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G1281–G1287. [DOI] [PubMed] [Google Scholar]
- Hara Y., Sassi Y., Guibert C., Gambaryan N., Dorfmuller P., Eddahibi S., Lompre A. M., Humbert M., Hulot J. S. (2011). Inhibition of MRP4 prevents and reverses pulmonary hypertension in mice. J. Clin. Investig. 121, 2888–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M., Yin H., Mitra M. S., Liang X., Ajmo J. M., Nadra K., Chrast R., Finck B. N., You M. (2013). Hepatic-specific lipin-1 deficiency exacerbates experimental alcohol-induced steatohepatitis in mice. Hepatology 58, 1953–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kele P. G., van der Jagt E. J., Gouw A. S., Lisman T., Porte R. J., de Boer M. T. (2013). The impact of hepatic steatosis on liver regeneration after partial hepatectomy. Liver Int. 33, 469–475. [DOI] [PubMed] [Google Scholar]
- Klaassen C. D., Aleksunes L. M. (2010). Xenobiotic, bile acid, and cholesterol transporters: Function and regulation. Pharmacol. Rev. 62, 1–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh Y. K., Lee M. Y., Kim J. W., Kim M., Moon J. S., Lee Y. J., Ahn Y. H., Kim K. S. (2008). Lipin1 is a key factor for the maturation and maintenance of adipocytes in the regulatory network with CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma 2. J. Biol. Chem. 283, 34896–34906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo S. H. (2013). Nonalcoholic fatty liver disease: Molecular mechanisms for the hepatic steatosis. Clin. Mol. Hepatol. 19, 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonen D. P., Jacobs R. L., Febbraio M., Young M. E., Soltys C. L., Ong H., Vance D. E., Dyck J. R. (2007). Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes 56, 2863–2871. [DOI] [PubMed] [Google Scholar]
- Lee Y., Pham T. X., Bae M., Hu S., O’Neill E., Chun O. K., Han M. J., Koo S. I., Park Y. K., Lee J. Y. (2019). Blackcurrant (Ribes nigrum) prevents obesity-induced nonalcoholic steatohepatitis in mice. Obesity 27, 112–120. [DOI] [PubMed] [Google Scholar]
- Leggas M., Adachi M., Scheffer G. L., Sun D., Wielinga P., Du G., Mercer K. E., Zhuang Y., Panetta J. C., Johnston B., et al. (2004). Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol. Cell. Biol. 24, 7612–7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Krishnamurthy P. C., Penmatsa H., Marrs K. L., Wang X. Q., Zaccolo M., Jalink K., Li M., Nelson D. J., Schuetz J. D., et al. (2007). Spatiotemporal coupling of cAMP transporter to CFTR chloride channel function in the gut epithelia. Cell 131, 940–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkewitte A. J., Schweitzer G. G., Kennon-McGill S., Clemens M. M., James L. P., Jaeschke H., Finck B. N., McGill M. R. (2018). Lipin deactivation after acetaminophen overdose causes phosphatidic acid accumulation in liver and plasma in mice and humans and enhances liver regeneration. Food Chem. Toxicol. 115, 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher J. M., Slitt A. L., Cherrington N. J., Cheng X., Klaassen C. D. (2005). Tissue distribution and hepatic and renal ontogeny of the multidrug resistance-associated protein (Mrp) family in mice. Drug Metab. Dispos. 33, 947–955. [DOI] [PubMed] [Google Scholar]
- Manmontri B., Sariahmetoglu M., Donkor J., Bou Khalil M., Sundaram M., Yao Z., Reue K., Lehner R., Brindley D. N. (2008). Glucocorticoids and cyclic AMP selectively increase hepatic lipin-1 expression, and insulin acts antagonistically. J. Lipid Res. 49, 1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos G. K. (2007). Liver regeneration. J Cell Physiol. 213, 286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C., Willenbring H. (2008). A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat. Protoc. 3, 1167–1170. [DOI] [PubMed] [Google Scholar]
- Miura T., Kimura N., Yamada T., Shimizu T., Nanashima N., Yamana D., Hakamada K., Tsuchida S. (2011). Sustained repression and translocation of Ntcp and expression of Mrp4 for cholestasis after rat 90% partial hepatectomy. J. Hepatol. 55, 407–414. [DOI] [PubMed] [Google Scholar]
- Moffat C., Bhatia L., Nguyen T., Lynch P., Wang M., Wang D., Ilkayeva O. R., Han X., Hirschey M. D., Claypool S. M., et al. (2014). Acyl-CoA thioesterase-2 facilitates mitochondrial fatty acid oxidation in the liver. J. Lipid Res. 55, 2458–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More V. R., Cheng Q., Donepudi A. C., Buckley D. B., Lu Z. J., Cherrington N. J., Slitt A. L. (2013). Alcohol cirrhosis alters nuclear receptor and drug transporter expression in human liver. Drug Metab. Dispos. 41, 1148–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More V. R., Slitt A. L. (2011). Alteration of hepatic but not renal transporter expression in diet-induced obese mice. Drug Metab. Dispos. 39, 992–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry E. P., Kennedy S. M., Xie Y., Luo J., Stanley S. E., Semenkovich C. F., Crooke R. M., Graham M. J., Davidson N. O. (2008). Altered hepatic triglyceride content after partial hepatectomy without impaired liver regeneration in multiple murine genetic models. Hepatology 48, 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J., Yu J., Xu H., Kang C., Shaul P. W., Guan Y., Zhang X., Su W. (2018). Enhanced liver regeneration after partial hepatectomy in sterol regulatory element-binding protein (SREBP)-1c-null mice is associated with increased hepatocellular cholesterol availability. Cell Physiol. Biochem. 47, 784–799. [DOI] [PubMed] [Google Scholar]
- Picard C., Lambotte L., Starkel P., Sempoux C., Saliez A., Van den Berge V., Horsmans Y. (2002). Steatosis is not sufficient to cause an impaired regenerative response after partial hepatectomy in rats. J. Hepatol. 36, 645–652. [DOI] [PubMed] [Google Scholar]
- Reddy J. K., Hashimoto T. (2001). Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: An adaptive metabolic system. Annu. Rev. Nutr. 21, 193–230. [DOI] [PubMed] [Google Scholar]
- Reue K., Zhang P. (2008). The lipin protein family: Dual roles in lipid biosynthesis and gene expression. FEBS Lett. 582, 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick D. A., Davidson N. O. (2012). Functional relationships between lipid metabolism and liver regeneration. Int. J. Hepatol. 2012, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel F. G., Koenderink J. B., Masereeuw R. (2008). Multidrug resistance protein 4 (MRP4/ABCC4): A versatile efflux transporter for drugs and signalling molecules. Trends in Pharmacol. Sci. 29, 200–207. [DOI] [PubMed] [Google Scholar]
- Shaker M., Tabbaa A., Albeldawi M., Alkhouri N. (2014). Liver transplantation for nonalcoholic fatty liver disease: New challenges and new opportunities. World J. Gastroenterol. 20, 5320–5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I., Shimano H., Korn B. S., Bashmakov Y., Horton J. D. (1998). Nuclear sterol regulatory element-binding proteins activate genes responsible for the entire program of unsaturated fatty acid biosynthesis in transgenic mouse liver. J. Biol. Chem. 273, 35299–35306. [DOI] [PubMed] [Google Scholar]
- Shteyer E., Liao Y., Muglia L. J., Hruz P. W., Rudnick D. A. (2004). Disruption of hepatic adipogenesis is associated with impaired liver regeneration in mice. Hepatology 40, 1322–1332. [DOI] [PubMed] [Google Scholar]
- Tijburg L. B., Nyathi C. B., Meijer G. W., Geelen M. J. (1991). Biosynthesis and secretion of triacylglycerol in rat liver after partial hepatectomy. Biochem. J. 277(Pt 3), 723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran M., Lee S. M., Shin D. J., Wang L. (2017). Loss of miR-141/200c ameliorates hepatic steatosis and inflammation by reprogramming multiple signaling pathways in NASH. JCI Insight 2, e96094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran M., Yang Z., Liangpunsakul S., Wang L. (2016). Metabolomics analysis revealed distinct cyclic changes of metabolites altered by chronic ethanol-plus-binge and Shp deficiency. Alcohol Clin. Exp. Res. 40, 2548–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Kirillova I., Peschon J. J., Fausto N. (1997). Initiation of liver growth by tumor necrosis factor: Deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc. Natl. Acad. Sci. U.S.A. 94, 1441–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi H., Uetsuka K., Okada T., Nakayama H., Doi K. (2003). Impaired liver regeneration after partial hepatectomy in db/db mice. Exp. Toxicol. Pathol. 54, 281–286. [DOI] [PubMed] [Google Scholar]
- Yang S. Q., Lin H. Z., Mandal A. K., Huang J., Diehl A. M. (2001). Disrupted signaling and inhibited regeneration in obese mice with fatty livers: Implications for nonalcoholic fatty liver disease pathophysiology. Hepatology 34(4 Pt 1), 694–706. [DOI] [PubMed] [Google Scholar]
- Zezos P., Renner E. L. (2014). Liver transplantation and non-alcoholic fatty liver disease. World J. Gastroenterol. 20, 15532–15538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Febbraio M., Wada T., Zhai Y., Kuruba R., He J., Lee J. H., Khadem S., Ren S., Li S., et al. (2008). Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology 134, 556–567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.