Abstract
Background
Little is known about the risk of hepatitis C virus (HCV) reinfection among people with HIV (PWH) in the direct-acting antiviral (DAA) era. We evaluate HCV reinfection rates in the DAA era and characterize presustained virologic response (SVR) behavioral risk factors associated with reinfection among PWH at the University of California, San Diego (UCSD).
Methods
Observational longitudinal cohort of PWH treated with DAAs between 2014 and July 2019 who achieved SVR and had at least 1 subsequent HCV viral load measurement. HCV reinfection was defined as new HCV viremia after SVR. We examined whether screening for sexually transmitted infections (STIs) and substance use during the pre-SVR period could identify patients at greater risk for reinfection using exact Poisson regression to compare reinfection incidence rates between those with and without pre-SVR STIs and positive urine drug screens.
Results
Eight out of 200 PWH were reinfected with HCV a median ~26 weeks after SVR over 328.1 person-years of follow-up (PYFU), for an incidence rate of 2.44/100 PYFU. The observed HCV reinfection rate was highest among men who have sex with men who inject drugs (MSM IDU; 4.63/100 PFYU) and those aged 30–39 years (6.80/100 PYFU). Having a positive gonorrhea/chlamydia test during the pre-SVR period was a predictor of HCV reinfection.
Conclusions
The HCV reinfection rate in the DAA era is similar to the rate observed in the interferon era in San Diego in PWH. STI screening during HCV treatment may help determine those at higher risk for HCV reinfection.
Keywords: direct-acting antivirals, hepatitis C, HIV, reinfection
We previously reported on the expanding hepatitis C virus (HCV) epidemic among people with HIV (PWH) in San Diego in the interferon era between 2000 and early 2014 [1]. We observed a HCV reinfection rate of 2.89 (95% CI, 0.60–8.44) per 100 person-years of follow-up (PYFU) among HIV-positive men who had sex with men (MSM), which was 2.5-fold higher than the primary HCV infection rate among that group. Given that transmission risk behavior and the HCV and HIV epidemics are syndemic, especially among MSM, some have suggested that increased condomless sexual practices associated with the expansion of pre-exposure prophylaxis programs could increase HCV reinfection during the DAA era among PWLH [2–4]. If HCV reinfection rates increase during the era of direct-acting antivirals (DAAs), one of the critical HCV elimination goals outlined by the World Health Organization by 2030, a reduction of 90% in HCV incidence, could be compromised. Data on HCV reinfection among PWH during the DAA era are mostly available from European hotspots [5–7]. In the United States, Carollo et al. observed a HCV reinfection rate of 4.0 per 100 PYFU among 160 PWH who had achieved sustained viral response (SVR) after DAA therapy [8]. Prior studies addressing DAA reinfection among PWH have focused on MSM with known high-risk behaviors, but without a specific description of risk behavior pattern modification during or after DAA therapy. Therefore, we conducted this study to (1) describe the differential HCV reinfection rates during the HCV DAA era by transmission risk group and (2) examine whether screening for STI and substance use during the pre-SVR period could identify PWH at greater risk for HCV reinfection at the University of California, San Diego (UCSD).
METHODS
Cohort and Study Variables
We conducted an observational, prospective cohort analysis of adult PWH treated with DAAs at UCSD who achieved SVR between January 2014 and July 2019. SVR was defined as having a documented undetectable HCV viral load 12 weeks after finishing DAA treatment. HCV reinfection was defined as having a detectable HCV viral load after achieving SVR. Patients were eligible for the study if they had at least 1 subsequent follow-up HCV viral load after SVR, unless there was a change in HCV genotype that occurred after completing HCV treatment if HCV viral load was detectable before achieving SVR. Baseline covariates included demographics, HIV/HCV transmission risk factors, CD4+ lymphocyte count, HIV plasma viral load within 3 months of DAA initiation, HCV genotype and baseline HCV viral load, DAA regimen type, duration, and dates of DAA initiation and termination.
Longitudinal Monitoring of Risk Behaviors
All patients were counseled by treating providers and clinical pharmacists regarding risk behaviors associated with HCV reinfection, focusing on both sexual risk behaviors and ongoing injection drug use (IDU). Counseling was conducted before DAA treatment and re-enforced whenever patients tested positive during DAA treatment for a sexually transmitted infection (STI) or urine drug screen (UDS) [9]. Patients with active IDU were encouraged to provide their registration card to a needle exchange program unless it was documented that their primary HIV provider prescribed clean needles routinely for them. However, access to DAA treatment was never restricted to those with ongoing IDU. Our standard-of-care HCV treatment protocol requires completion of STI and UDS screening at baseline, week 4 of treatment, end of treatment, and 12 weeks post-treatment for monitoring of ongoing HCV reinfection risk and the need for ongoing risk counseling (Supplementary Table 1). STI screening included gonorrhea–chlamydia nucleic acid amplification tests (G-C) from 3 anatomical sites (anorectal, pharyngeal, and urine) as well as reverse algorithm (treponemal followed by nontreponemal) syphilis screening. Every component of our standard-of-care HCV treatment protocols requires the voluntary participation of our patients. As such, G-C testing was performed while the patient attended their clinical visits, and the patient could decline to be screened at any clinical visit. In comparison, we relied on patients’ willingness to complete a urine sample when going to the laboratory for blood collection for UDS analysis. A test for G-C screenings was considered positive if either urine, pharyngeal, or anorectal result screened positive. The UDS screen included amphetamines, barbiturates, cocaine, opioids, methadone, phencyclidine, and tetrahydrocannabinol. A UDS was considered positive if it showed detectability of any illegal substance or of prescription opioids if these were not being prescribed at the time of DAA treatment. Based on the clinical judgment of the primary HIV medical provider, additional STI testing could occur during the pre-SVR period. The frequency of follow-up testing for STI and UDS after achieving SVR was at the discretion of the patient’s HIV medical provider.
Statistical Analyses
Descriptive statistics were used to compare baseline demographics. Person-time incidence rates (95% CI) per 100 PYFU were estimated using the Poisson distribution. Follow-up time was calculated from the date of SVR to either the first positive HCV RNA or the last negative HCV RNA after SVR. HCV reinfection was defined as having detectable HCV viremia after achieving SVR or having viremia with a change in HCV genotype between the end of treatment and SVR. We defined time to HCV reinfection as the midway point between the date of first detectable HCV viremia and date of SVR. We divided the timing of STI and UDS collection into 2 periods. The pre-SVR period included test collection following DAA initiation until the date of SVR ascertainment. The post-SVR period consisted of any screening test collected after documented SVR until the time of the first new detectable HCV viral load or the last available follow-up result. We compared person-time screening rates for G-C, syphilis, and UDS in the pre-SVR period by reinfection status using Wilcoxon rank-sum tests. Patients with multiple positive screening tests during the defined time period were only counted once. The effect of pre-SVR screen positivity on the post-SVR person-time HCV reinfection rate was evaluated using exact Poisson regression with robust standard errors. Finally, we evaluated potential ascertainment bias due to differential screening by comparing the unstratified incidence rate ratio (IRR) for HCV reinfection as a function of G-C, syphilis, and UDS positivity in the pre-SVR period with an IRR stratified by screening rate (binary stratification at the median screening rate among the reinfected). As a sensitivity analysis for the effects of residual ascertainment bias in estimating the effect of having a positive G-C, syphilis, or UDS result, we calculated the e-value. The higher the e-value, the stronger the confounder associates must be to explain away an effect [10]. We also performed another sensitivity analysis using a survival model and depicted the Kaplan-Meier curve to demonstrate time to reinfection based on pre-SVR G/C status. Statistical analysis was conducted using Stata, version 16.1.
Patient Consent Statement
The design of the work was approved by the UCSD Human Subjects Research Protection Program (#171954).
RESULTS
Our HCV population prevalence varied from 14.9% to 16.1% during the study period, and we enrolled 200 consecutive PWH treated with DAAs. The median age was 52 years, with 15% being cis-gender female and 23.5% non-White. Most (92%) had an undetectable HIV viral load before DAA initiation. By HCV risk factor, 56% were MSM, of whom 36% also had either a history of or were actively using IDU (Table 1). A total of 48 patients (24%) had cirrhosis diagnosed by liver biopsy or fibroscan, of whom 16 had decompensated cirrhosis. The median follow-up time (interquartile range [IQR]) after achieving SVR was 1.38 (0.50–2.59) years. Eight patients became reinfected with HCV after a median time (IQR) of 26 (1.7–39.1) weeks from SVR, with 3 patients acquiring a different HCV genotype. The cohort was followed over 328.1 PYFU, resulting in an overall HCV reinfection rate of 2.44/100 PYFU (95% CI, 1.05–4.80/100 PYFU). The median number of HCV RNA assays after SVR (range) was 2 (1–7), and there was no difference in the frequency of HCV viral load ascertainment between those with and without HCV reinfection (1.53/PYFU vs 1.93/PYFU; P = .40). HCV transmission risk factors among those reinfected included MSM only (n = 5), MSM with IDU (n = 2), and heterosexual with IDU (n = 1). There were no observed HCV reinfections among the 30 treated cis-gender females, despite 83% of them having a history of IDU. All patients who were reinfected had an undetectable HIV viral load at the time of HCV treatment. The HCV pooled reinfection rate among MSM was 4.43/100 PFYU. Although there was no statistically significant difference in reinfection rate by HCV risk factor (P = .46), we observed a trend for the highest HCV reinfection rates among younger patients (age 30–39 years) and MSM regardless of history of IDU (Table 2).
Table 1.
Baseline Characteristics of PWH Treated With DAAs Between January 2014 and July 2019 (n = 200)
| Median age (range), y | 52 (20–72) |
|---|---|
| Sex, No. (%) | |
| Female | 30 (15) |
| Male | 170 (85) |
| Gender, No. (%) | |
| Cis-gender female | 30 (15) |
| Cis-gender male | 168 (84) |
| Transgender female | 2 (1) |
| Race/ethnicity, No. (%) | |
| White | 153 (76.5) |
| Non-White | 47 (23.5) |
| HIV/HCV risk factor, No. (%) | |
| MSM only | 72 (36) |
| MSM+IDU | 40 (20) |
| IDU only | 78 (39) |
| Heterosexual only | 5 (2.5) |
| Other/unknown | 5 (2.5) |
| HCV genotype, No. (%) | |
| 1/1a | 139 (69.5) |
| 1b | 19 (9.5) |
| 2 | 9 (4.5) |
| 3 | 24 (12) |
| 4 | 8 (4) |
| Other | 1 (0.5) |
| Median CD4 count (range) | 503 (26–2010) |
| HIV VL <50 copies/mL, No. (%) | 184 (92) |
Abbreviations: DAA, direct-acting antiviral; HCV, hepatitis C virus; IDU, intravenous drug use; MSM, men who have sex with men; PWH, people with HIV.
Table 2.
HCV Reinfection Rates Among PWH Treated at UCSD Between January 2014 and July 2019
| Reinfection Rate per 100 PYFU (95% CI) | PYFU | Incidence Rate Ratio (95% CI) | |
|---|---|---|---|
| HIV/HCV risk factor | |||
| All (n = 200) | 2.44 (1.05–4.80) | 328.10 | - |
| MSM only (n = 72) | 4.36 (1.42–10.17) | 114.76 | 1 |
| MSM+IDU (n = 40) | 4.63 (0.56–16.74) | 43.17 | 1.06 (0.10–6.50) |
| IDU only (n = 78) | 0.64 (0.02–3.59) | 155.33 | 0.15 (0.003–1.32) |
| Heterosexual only (n = 5) | 0.00 | 5.78 | - |
| Other/unknown (n = 5) | 0.00 | 9.07 | - |
| Gender | |||
| Cis-gender female (n = 30) | 0.00 | 46.46 | - |
| Cis-gender male (n = 168) | 2.50 (1.01–5.16) | 279.73 | 1 |
| Transgender female (n = 2) | 52.36 (1.33–291.71) | 1.91 | 20.92 (0.46–162.86) |
| Race/ethnicity | |||
| White (n = 153) | 2.41 (0.88–5.24) | 249.25 | 1 |
| Non-White (n = 47) | 2.54 (0.31–9.16) | 78.85 | 1.05 (0.10–5.89) |
| Age category | |||
| <30 y (n = 10) | 0.00 | 13.53 | - |
| 30–39 y (n = 24) | 6.80 (0.82–24.55) | 29.43 | 2.72 (0.25–18.99) |
| 40–49 y (n = 41) | 1.52 (0.04–8.47) | 65.81 | 0.61 (0.01–6.15) |
| 50–59 y (n = 88) | 2.50 (0.68–6.39) | 160.18 | 1 |
| >59 y (n = 37) | 1.69 (0.04–9.42) | 59.16 | 0.68 (0.01–6.84) |
Abbreviations: HCV, hepatitis C virus; IDU, intravenous drug use; MSM, men who have sex with men; PWH, people with HIV; PYFU, persons-years of follow-up.
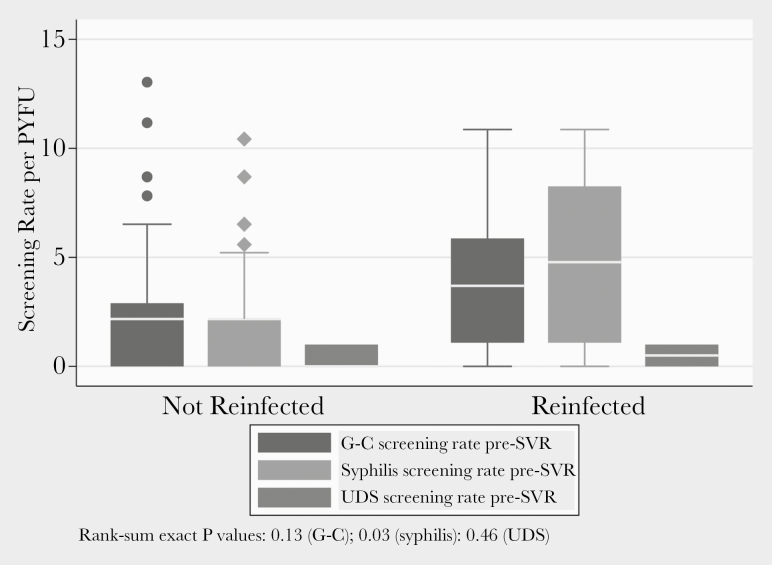
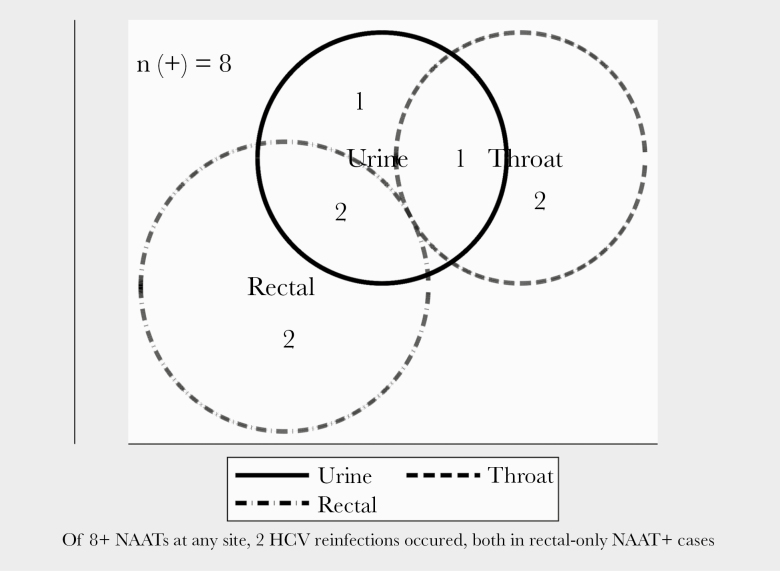
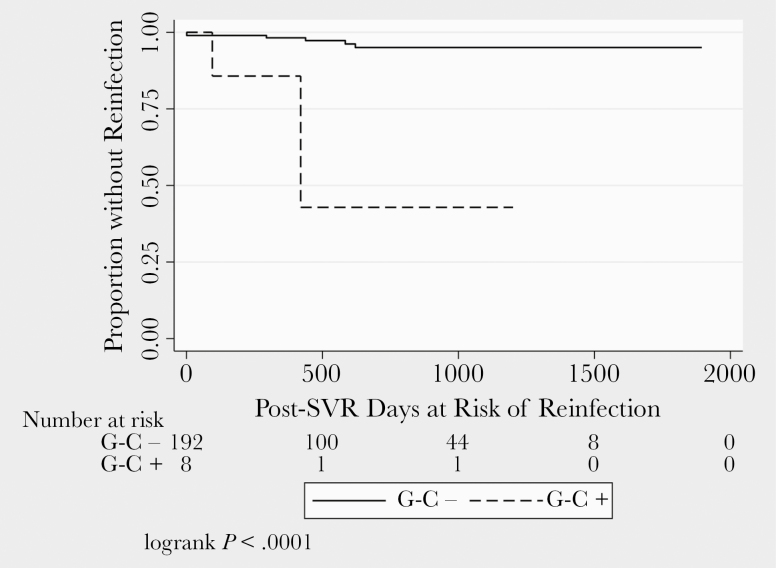
Overall among the 200 patients, 79 (39.5%), 120 (60%), and 122 (61%) had at least 1 test result available for UDS, G-C, and syphilis, respectively, in the pre-SVR period. There was no difference in the screening rate for UDS or G-C among those who were and were not reinfected with HCV in the pre-SVR period. However, patients reinfected with HCV were tested for syphilis more regularly than those not reinfected (Figure 1). There was no significant difference in the percentage of patients with pre-SVR positive UDS (0 vs 10.4%; exact P = .42) or syphilis (0 vs 0.5%; exact P = .96) in those who were and were not reinfected, respectively. Among those who were reinfected, there was a higher percentage of patients with a pre-SVR positive G-C test as compared with those not reinfected (25% vs 3.1%; exact P = .04). Inference is limited regarding bodily site of G-C sampling because of small numbers (n = 8 G-C positives). Two of 4 rectal NAAT positives in the pre-SVR period were associated with post-SVR HCV reinfection. No reinfections occurred among those with positive NAAT in only throat or urine (Figure 2). Of the 3 evaluated potential pre-SVR laboratory predictors of post-SVR HCV reinfection, only screening positive for G-C was predictive of HCV reinfection (Figure 3). Having a positive G-C result in the pre-SVR period remained a significant predictor of HCV reinfection after stratification by G-C screening rate (Table 3). The e-value for the observed estimate of having a positive G-C result was 27.95.
Figure 1.
G-C, syphilis, and UDS screening rates pre-SVR by reinfection status. Abbreviations: G-C, gonorrhea–chlamydia; PYFU, person-years of follow-up; SVR, sustained virologic response; UDS, urine drug screen.
Figure 2.
Venn diagram showing distribution by anatomical site of positive pre-SVR G-C NAAT. Abbreviations: G-C, gonorrhea–chlamydia; HCV, hepatitis C virus; NAAT, nucleic acid testing; SVR, sustained virologic response.
Figure 3.
Time to reinfection after SVR by pre-SVR G-C NAAT status. Abbreviations: G-C, gonorrhea–chlamydia; NAAT, nucleic acid testing; SVR, sustained virologic response.
Table 3.
Unstratified and Stratified Incidence Rate Ratio by Screening Rate for HCV Reinfection vs No Reinfection as a Function of Positive G-C, Syphilis, or UDS Results in the Pre-SVR Period
| Unstratified IRR (95% CI) | P Value | Stratified IRR (95% CI) | Screening Rate Cutoff for Stratification/PYFU | P Value | |
|---|---|---|---|---|---|
| G-C (n = 120) | 21.41 (1.94–149.39) | .01 | 14.23 (1.11–121.88) | 3.69 | .04 |
| Syphilis (n = 122) | 26.16 (0–181.43) | 1.0 | 52.27 (0–486.70) | 4.78 | 1.0 |
| UDS (n = 79) | 1.22 (0–8.49) | 1.0 | 0.60 (0–5.61) | 1.30 | .68 |
Abbreviations: G-C, gonorrhea–chlamydia; HCV, hepatitis C virus; IRR, incidence rate ratio; SVR, sustained virologic response; UDS, urine drug screen.
DISCUSSION
After 5 years of DAA availability, we found that the HCV reinfection rate in PWH was highest among MSM as compared with those with IDU only as a risk factor in San Diego, but was comparable to the rates we previously reported during the interferon era [1]. When evaluating the pre-SVR predictors of HCV reinfection, we noted that having a positive G-C test in the pre-SVR period was a predictor of HCV reinfection. In the model stratified on binary grouped pre-SVR G-C screening rate, the IRR effect estimate was attenuated from 21.4 to 14.2. There is the possibility of further within-strata ascertainment bias; however, the magnitude of the stratified IRR suggests that regular G-C screening during the pre-SVR period may identify patients at higher risk of HCV reinfection in the post-SVR period due to ongoing sexual risk behaviors. This is in comparison to the lack of positive UDS as a predictor of HCV reinfection. However, the small proportion of patients who completed UDS limits our ability to draw categorical conclusions relative to its utility as a potential predictor of HCV reinfection.
Our median time of HCV reinfection was 26 weeks, which is within the range of what others have reported [5–8]. However, our overall reinfection rate was 5-fold higher than a large Spanish study of PWH, and differences can be explained in part because the Spanish study was primarily composed of people with a history of IDU and because only 7% were MSM, whereas in our study 36% were MSM and another 20% were MSM IDUs [5]. Our results are in alignment with other reports in that the HCV reinfection rate is highest among MSM [5–8]. Interestingly, our reinfection rate was not as high as the rates seen in Dutch and Thai studies of PWH with treated or spontaneously cleared acute HCV infection. Unlike the Thai and Dutch studies, our study included primarily those with chronic HCV infection and was not restricted to MSM [7, 11]. Although our numbers of reinfected people were small, we did not observe a similar trend among non-MSM IDU patients. However, we do not have data regarding active needle sharing in those with IDU, an identified risk factor for HCV reinfection [12].
The high proportion of positive G-C tests during HCV treatment in those who were reinfected and the identification of positive G-C testing as a potential predictor of HCV reinfection highlight the lack of adequate sexual risk reduction strategies and the limited effectiveness of counseling to decrease HCV sexual reacquisition risk. This is supported by previous results from the Thai study, which observed higher reinfection rates in those with syphilis, and in the Dutch study, which identified higher reinfection rates in those with receptive condomless anal intercourse and other high-risk sexual practices [7, 11]. Although specific STI rates may differ in different sexual networks, these studies support that STI testing may help identify those at higher risk of HCV reinfection. Notably, the 2 patients with a pre-SVR G-C positive test who subsequently became reinfected were positive on the rectal site, indicating ongoing condomless anal receptive sexual practices. We did not see a similar trend in UDS results, and interventions to prevent IDU HCV reinfection remain critical, as opioid substitution therapy and needle exchange programs have been shown to reduce the risk for HCV acquisition [13, 14]. The absence of a statistically significant association between HCV transmission risk factors and reinfection rate contrasts with the potent effect of STIs (a marker of current sexual risk behavior) as a predictor of reinfection. Women comprised only a small fraction of our cohort, and no definitive conclusions can be made. However, it is noteworthy that no females became reinfected with HCV. We note that other studies have shown that women are at higher risk of HCV acquisition, but a recent population study on reinfection in Canada found females at a lower risk of reinfection, and as such further research on this area is warranted [15, 16]. To our knowledge, no previous study has described HCV reinfection rates specifically among HIV-infected women.
Our study has important limitations. Although the amount of follow-up time was similar to prior studies, a median follow-up time of <2 years may have limited the number of cumulative reinfections that would have been observed with longer follow-up [5–8]. We also were unable to capture further information regarding the presence or absence of high-risk sexual practices such as condom use, receptive vs insertive anal sex, and the use of chemsex. In addition, as we evaluated outcomes during routine clinical practice, we were not able to control the frequency of STI screening, potentially leading to ascertainment bias. However, because clinicians did not know during the pre-SVR period which patients would be subsequently reinfected, any differential ascertainment of STI or UDS by reinfection status must have been due to other pre-SVR known or implicitly inferred factors such as patient self-reported condomless behaviors that prompted a standard-of-care STI assessment [17]. We attempted to control for this by stratifying our analysis by screening rates, although residual imbalance in within-stratum screening rates must be acknowledged. Nonetheless, having a positive G-C test in the pre-SVR period remained a significant predictor of HCV reinfection in the stratified exact Poisson regression model. The observed G-C stratified screening rate IRR of 14.2 could be explained by an unmeasured confounder that was associated with both having a positive G-C screen and the outcome of HCV reinfection by an IRR of 27.9-fold each, above and beyond the stratification factor, but weaker associations could not explain this. However, the magnitude of this e-value (27.95) provides some degree of support that the observed effect is not due to residual bias or confounding [10]. This suggests that there may be value in regularly monitoring for STIs during HCV treatment to target interventions aiming to prevent HCV reinfection. Our proportion of completed urine toxicology tests was low. This illustrates challenges in clinical practice that may be due to patient risk aversion or other factors such as stigma and mistrust that need to be taken into account when working toward HCV elimination efforts [18]. We did not use phylogenetic analysis to differentiate whether patients without HCV genotype switch, in reality, could have been late HCV relapses. However, after 12 weeks of finishing DAAs, HCV relapse occurs at around 0.1%, making it unlikely that these were late relapses, particularly in patients with ongoing risk of reinfection [19]. Finally, generalizability is limited due to our single-site geographical location; however, our observed DAA reinfection rates among MSM of 4.43 PYFU is comparable to the reinfection rates in HIV-infected MSM in New York City [8].
In conclusion, the HCV reinfection rate among MSM in the DAA era in PWH was similar to that observed in the interferon era in San Diego, and the observed reinfection rate remained highest among those who are young and MSM. Having a positive G-C test in the pre-SVR time period was a predictor of HCV reinfection.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This work was supported in part by the NoCo Grant funded by Gilead Sciences (IN-US-334–4481), the University of California San Diego Center for AIDS Research (AI036214), and the Pacific AIDS Education and Training Center (PAETC). N.M. was additionally supported by NIAID/NIDA grant R01AI147490.
Disclaimer. The funders had no role in the study design, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. L.A.H. is a member of the speakers’ bureau for Gilead Sciences. E.R.C. has received research grants paid to UC Reagents from Gilead Sciences and Merck & Co., Inc., and has been on an advisory board for Gilead Sciences. N.K.M. has received unrestricted research grants paid to UC Reagents from Gilead Sciences and Merck & Co., Inc. F.J.T., S.J., H.Q., and W.M.C. have no conflicts of interest to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Chaillon A, Sun X, Cachay E, et al. Primary incidence of hepatitis C virus infection among HIV-infected men who have sex with men in San Diego, 2000–2015. Open Forum Infect Dis 2019; 6:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoornenborg E, Achterbergh RCA, Schim van der Loeff MF, et al. ; Amsterdam PrEP Project Team in the HIV Transmission Elimination AMsterdam Initiative, MOSAIC Study Group MSM starting preexposure prophylaxis are at risk of hepatitis C virus infection. AIDS 2017; 31:1603–10. [DOI] [PubMed] [Google Scholar]
- 3. Falade-Nwulia O, Sulkowski MS, Merkow A, et al. Understanding and addressing hepatitis C reinfection in the oral direct-acting antiviral era. J Viral Hepat 2018; 25:220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han WM, Colby DJ, Khlaiphuengsin A, et al. Large transmission cluster of acute hepatitis C identified among HIV-positive men who have sex with men in Bangkok, Thailand. Liver Int 2020; 40:2104–9. [DOI] [PubMed] [Google Scholar]
- 5. Berenguer J, Gil-Martin Á, Jarrin I, et al. ; Madrid-CoRe Study Group Reinfection by hepatitis C virus following effective all-oral direct-acting antiviral drug therapy in HIV/hepatitis C virus coinfected individuals. AIDS 2019; 33:685–9. [DOI] [PubMed] [Google Scholar]
- 6. Ingiliz P, Wehmeyer M, Boesecke C, et al. Reinfection with the hepatitis C virus in men who have sex with men after successful treatment with direct acting antivirals in Germany: current incidence rates, compared with rates during the interferon era. Clin Infect Dis 2020; 71:1248–54. [DOI] [PubMed] [Google Scholar]
- 7. Newsum A, Matser A, Schinkel J, et al. Incidence of HCV reinfection among HIV-positive MSM and its association with sexual risk behavior: a longitudinal analysis. [published online ahead of print May 27, 2020] Clin Infect Dis. 2020. doi:; doi: 10.1093/cid/ciaa645. [DOI] [PubMed] [Google Scholar]
- 8. Carollo J, Factor S, Rodriguez-Caprio G, et al. HCV reinfection among HIV-infected MSM in New York City. Abstract #86. Paper presented at: 2019 Conference on Retroviruses and Opportunistic Infections; 4–7 Marcy 2019; Seattle, WA. [Google Scholar]
- 9. AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. Available at: http://www.hcvguidelines.org. Accessed 1 June 2020.
- 10. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017; 167:268–74. [DOI] [PubMed] [Google Scholar]
- 11. Huang MH, Chang SY, Liu CH, et al. HCV reinfections after viral clearance among HIV-positive patients with recent HCV infection in Taiwan. Liver Int 2019; 39:1860–7. [DOI] [PubMed] [Google Scholar]
- 12. Cunningham E, Hajarizadeh B, Amin J, et al. Reinfection following successful direct-acting antiviral therapy for hepatitis C infection among people who inject drugs [published online ahead of print March 12, 2020]. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa253 [DOI] [PubMed] [Google Scholar]
- 13. Page K, Morris MD, Hahn JA, et al. Injection drug use and hepatitis C virus infection in young adult injectors: using evidence to inform comprehensive prevention. Clin Infect Dis 2013; 57(Suppl 2):S32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev 2017; 9:CD012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tracy D, Hahn JA, Fuller Lewis C, et al. Higher risk of incident hepatitis C virus among young women who inject drugs compared with young men in association with sexual relationships: a prospective analysis from the UFO Study cohort. BMJ Open 2014; 4:e004988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Islam N, Krajden M, Shoveller J, et al. ; British Columbia Hepatitis Testers Cohort (BC-HTC) team Incidence, risk factors, and prevention of hepatitis C reinfection: a population-based cohort study. Lancet Gastroenterol Hepatol 2017; 2:200–10. [DOI] [PubMed] [Google Scholar]
- 17. Li J, Armon C, Palella F, et al. Chlamydia and gonorrhea incidence and testing among patients in the Human Immunodeficiency Virus Outpatient Study (HOPS), 2007–2017. Clin Infect Dis 2020; 71:1824–35. [DOI] [PubMed] [Google Scholar]
- 18. Cachay E Do we need to address stigma and mistrust to facilitate hepatitis C elimination among people living with HIV? AIDS 2020; 34:325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sarrazin C, Isakov V, Svarovskaia ES, et al. Late relapse versus hepatitis C virus reinfection in patients with sustained virologic response after sofosbuvir-based therapies. Clin Infect Dis 2017; 64:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.