Abstract
Mortality assessment in cohorts with high numbers of persons lost to follow-up (LTFU) is challenging in settings with limited civil registration systems. We aimed to assess mortality in a clinical cohort (the Kilombero and Ulanga Antiretroviral Cohort (KIULARCO)) of human immunodeficiency virus (HIV)-infected persons in rural Tanzania, accounting for unseen deaths among participants LTFU. We included adults enrolled in 2005–2015 and traced a nonrandom sample of those LTFU. We estimated mortality using Kaplan-Meier methods 1) with routinely captured data (method A), 2) crudely incorporating tracing data (method B), 3) weighting using tracing data to crudely correct for unobserved deaths among participants LTFU (method C), and 4) weighting using tracing data accounting for participant characteristics (method D). We investigated associated factors using proportional hazards models. Among 7,460 adults, 646 (9%) died, 883 (12%) transferred to other clinics, and 2,911 (39%) were LTFU. Of 2,010 (69%) traced participants, 325 (16%) were found: 131 (40%) had died and 130 (40%) had transferred. Five-year mortality estimates derived using the 4 methods were 13.1% (A), 16.2% (B), 36.8% (C), and 35.1% (D), respectively. Higher mortality was associated with male sex, referral as a hospital inpatient, living close to the index clinic, lower body mass index, more advanced World Health Organization HIV clinical stage, lower CD4 cell count, and less time since initiation of antiretroviral therapy. Adjusting for unseen deaths among participants LTFU approximately doubled the 5-year mortality estimates. Our approach is applicable to other cohort studies adopting targeted tracing.
Keywords: HIV, loss to follow-up, mortality, proportional hazards models, Tanzania
Abbreviations:
- ART
antiretroviral therapy
- CI
confidence interval
- HIV
human immunodeficiency virus
- IQR
interquartile range
- KIULARCO
Kilombero and Ulanga Antiretroviral Cohort
- LTFU
loss/lost to follow-up
- WHO
World Health Organization
Survival among human immunodeficiency virus (HIV)-infected persons receiving treatment is approaching that of the general population in resource-rich settings (1–3) but not yet in resource-limited settings (4). Estimation of mortality remains challenging in settings with high numbers of persons lost to follow-up (LTFU) and differential risk of mortality among those who remain in care versus those LTFU (5). In a 2009 meta-analysis assessing mortality among patients LTFU in antiretroviral therapy (ART) programs in resource-limited settings, Brinkhof et al. (6) found that 46% of those LTFU and traced had died. There was an inverse relationship between the proportion of patients LTFU and the mortality rate among those LTFU, with higher mortality being seen among those LTFU in cohorts with lower overall LTFU. In an updated 2017 meta-analysis, Zürcher et al. (7) found an overall mortality prevalence of 34% among those LTFU, with substantial declines as ART was scaled up over calendar time.
While passive surveillance of deaths captured in routine care is insufficient to estimate true mortality (8–13), active surveillance involves tracing a subset of patients LTFU, known as “double sampling” (14), and those found to be alive but not under care are encouraged to return to the clinic. In a 2013 systematic review, McMahon et al. (15) found that treatment programs with routine tracing had lower LTFU than those without it because of the determination of outcomes among those LTFU, and more importantly because of reengagement of patients in care after tracing. However, deaths determined through tracing cannot simply be pooled with those captured routinely: Appropriate statistical methods are required to adjust mortality estimates to account for the patients who were LTFU and not traced or who remained LTFU despite tracing efforts (8, 11, 12, 14).
Our objective in this study was to estimate mortality and associated factors among HIV-infected adults enrolled in an open cohort in Ifakara, rural Tanzania, incorporating additional information ascertained through tracing. We used previously developed methods (10, 12–14) plus a 2-step weighting approach which has previously been proposed but not yet implemented (16) and which accounts for the probabilities of being selected for tracing and of being found after tracing has been attempted. These methods allow unbiased estimation of mortality rates under the hypothetical scenario of being able to observe all outcomes, that is, without masking of outcomes due to LTFU.
METHODS
Study design and population
Since 2005, consenting HIV-infected persons visiting the Chronic Disease Clinic in Ifakara at St. Francis Referral Hospital in Ifakara, Tanzania, have been invited to enroll in the Kilombero and Ulanga Antiretroviral Cohort (KIULARCO), described previously (17, 18). Since 2013, comprehensive data have been systematically captured in electronic medical records, including information on demographic characteristics, ART use, and clinical outcomes (limited data were captured on paper previously). We included data from adults (ages ≥15 years) enrolled since 2005, with administrative censoring on November 15, 2015.
Outcomes and tracing
During the study period, participant clinical outcomes were assessed at every visit. Transfers to other clinics were captured if reported in advance by the participant, and deaths were reported by a relative or treatment supporter. In the 3-monthly data export, participants were identified as LTFU if they were more than 60 days late for their last scheduled appointment. Visits were scheduled 3-monthly for persons on ART and 6-monthly for persons not on ART. Nonrandom samples of LTFU participants were traced between February 2016 and July 2017. Participant selection for tracing was done pragmatically, based on logistical considerations (i.e., participants living in the same village were traced at the same time) and operational capacity, after administrative database closure. Tracing was attempted by making up to 3 telephone calls to the participant, household head, treatment supporter, and/or community (10-cell) leader, and if phone calls were unsuccessful, we communicated with community health workers for home visits. Participants who were found to be alive and not under care were counseled to return to the clinic. In this article, we refer to those participants who were LTFU and for whom tracing was attempted as “attempted traced” and those whose vital status was determined (regardless of outcome) as “successfully traced.” This information was used to define the participants’ vital status as of the date of administrative censoring for analysis under methods B–D (see below). In particular, 7 participants who were determined through tracing to have died a median of 8 months after administrative censoring were considered to have been alive at the time of censoring in November 2015.
Statistical methods
Our aim was to estimate mortality in the whole cohort population, under the hypothetical scenario that outcomes were observed in all participants. Notably, this is not the same estimand as mortality among the cohort population if all participants had (hypothetically) remained in care, which was not the objective of this analysis. We estimated mortality using Kaplan-Meier estimation, with 4 methods. Time was measured from enrollment. Participants with intermittent periods of LTFU or transfers to other clinics could reenter the risk set if they returned to the index clinic (the Chronic Disease Clinic in Ifakara) (19). The methods differed in the data used and the weights applied (Figure 1). Figure 1A illustrates data captured routinely and through tracing for 10 example participants. Method A used data captured routinely in the clinic, ignoring tracing data (Figure 1B). Method B used tracing data to update outcomes (and date last known to be alive or to have died) which had been determined through tracing, with no further statistical adjustment (Figure 1C). Method C used the weighting methods of Frangakis and Rubin (14) to correct for unobserved deaths among those who remained LTFU (9, 20). That is, participants not LTFU were assigned a weight of 1; those LTFU but not successfully traced were given a weight of 0 (i.e., excluded); and those LTFU and successfully traced were assigned a weight given by the number of participants who were LTFU divided by the number who were successfully traced (i.e., upweighted to account for those LTFU and not successfully traced) (Figure 1D). While a single weight is used, some authors refer to this as “time-dependent weighting” because of the way the risk set changes over time (9).
Figure 1.
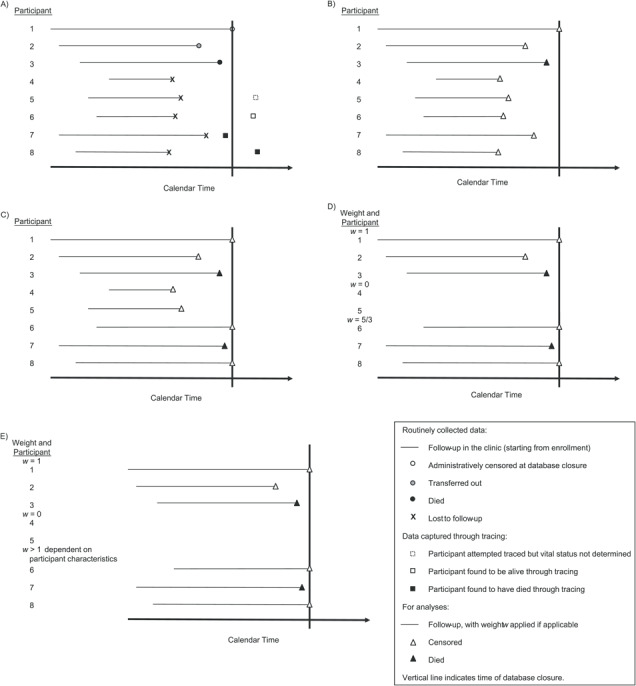
Data captured routinely and through tracing among KIULARCO participants and how it translated to analysis, Ifakara, Tanzania, 2005–2015. A) Illustration of data captured routinely and through tracing for 10 example participants. The horizontal lines indicate follow-up in the clinic starting from enrollment, and the vertical line indicates database closure. Participant 1 remained under active follow-up until administrative censoring (white circle) at database closure. Participant 2 transferred to another clinic, and this was captured in the routine database (gray circle). Participant 3 died, and this was captured in the routine database (black circle). Participants 4–8 were lost to follow-up from the routine database (“X”). Participant 4 was not selected for tracing. An attempt was made to trace participant 5, but vital status was not determined; therefore, no further data were available beyond those in the routine database (the dotted white square indicates the time at which tracing was attempted, but this has no other implication for data interpretation for this participant). Participant 6 was successfully traced and found to be alive (the white square indicates the time at which tracing was performed and therefore the time at which the participant was last known to be alive). Participant 7 was successfully traced and was found to have died before database closure (black square indicates date of death). Participant 8 was successfully traced and was found to have died after database closure (black square indicates date of death). B) Illustration of how the data were used in the analysis under method A (see text for methods) using data captured routinely (straightforward time-to-event analysis). The participants contributed follow-up time as indicated by the horizontal lines, and all participants were censored (white triangles), except for participant 3, who died (black triangle). C) Illustration of how the data were used in the analysis under method B, that is, updating the routinely collected data with information obtained through tracing. Participants 1–5 were included as for method A. Participants 6 and 8 were included as alive until censored at database closure. Participant 7 was included up to the point of death (before database closure) as indicated. D) Illustration of how the data were used in the analysis under method C. Participants 1–3 were included as for methods A and B (weight w = 1). Participants 4 and 5 were excluded because they were lost to follow-up and not successfully traced (weight w = 0). Participants 6–8 were included with weights w = 5/3, that is, upweighted to account for the exclusion of participants 4 and 5. As for method B, participants 6 and 8 were included as alive up to the date of database closure, and participant 7 was included up to the point of death. E) Illustration of how the data were used in the analysis under method D. The approach was the same as that for method C, except that the weights applied to participants 6–8 were based on participant characteristics, through the probabilities of being attempted traced and successfully traced. KIULARCO, Kilombero and Ulanga Antiretroviral Cohort.
In method D, we extended the Frangakis and Rubin approach to estimate the weights in a 2-step process based on the probabilities of being attempted traced and successfully traced, accounting for participant characteristics, as proposed previously (16). Firstly, we estimated the probability of a participant being attempted traced among those LTFU. Secondly, we estimated the probability of a participant being successfully traced among those attempted traced. For both steps, we used logistic regression models incorporating baseline covariates (see Web Table 1, available at https://academic.oup.com/aje) and variables determined at the time of LTFU: year of LTFU, time since ART initiation during follow-up, and number of gaps in care (transient periods of LTFU). Lastly, we included time between LTFU and attempted tracing in the model for successful tracing. The weights were calculated as the product of the inverse of the probabilities of being attempted traced and successfully traced and were applied to the participants who were successfully traced. The weights for those LTFU and successfully traced differed by participant, since they were based on participant characteristics. As for method C, participants not LTFU received a weight of 1, and participants LTFU but not successfully traced were excluded (Figure 1E). For methods C and D, 95% confidence intervals were estimated using a bootstrap of the weighted data with 1,000 replications.
If mortality were similar among participants LTFU and not LTFU, then all methods would unbiasedly estimate mortality in the cohort population. However, we found through tracing that mortality was higher among those LTFU than among those not LTFU (see Results section). Therefore, methods A and B will yield biased mortality estimates. Under the assumption that participants were randomly selected and successfully traced (i.e., the probability of being selected for tracing or successfully traced was not dependent on participant characteristics), method C would yield unbiased mortality estimates. Method D yields unbiased mortality estimates under the assumption that there are no unmeasured confounders for the probabilities of being selected for tracing or successfully traced.
For all 4 methods, we assessed factors associated with mortality using Cox proportional hazards models. We fitted univariable and multivariable models, with the latter including all covariates (no model selection performed). We included the same baseline covariates as in the weighting models, with some variables categorized to aid interpretation, plus time-dependent variables of time since ART initiation during follow-up and number of gaps in care (Web Table 1). In addition, models under methods C and D used weights calculated as described above. We performed sensitivity analyses with the weights truncated at a maximum of 20 (corresponding to the upper third percentile in our data) (21). Results are presented as hazard ratios and 95% confidence intervals. For the models, missing data on baseline covariates (Table 1) were imputed using multiple imputation with chained equations, assuming data were missing at random (22, 23). For the imputation, we used truncated regression with lower limits of 0 for square-root CD4 cell count and 3 for square-root body mass index, logistic regression for binary variables, and multinomial regression for the remaining categorical variables. Variables included in the imputation models were the baseline covariates plus indicators for each of the outcomes “attempted traced,” “successfully traced,” and “died”; the Nelson-Aalen estimator of the baseline cumulative hazard (23); time since ART initiation during follow-up; and number of gaps in care. We used 15 imputations, based on the approximate fraction of missing information (22). In sensitivity analyses, we fitted the same models only in participants with complete baseline data (“complete cases”). In further analyses, we used the same methods and multiply imputed data to assess factors associated with mortality among participants observed to initiate ART (with time at risk from ART initiation, and weights estimated among this subset of participants).
Table 1.
Characteristics of KIULARCO Participants by Outcome at Administrative Censoring on November 15, 2015, Ifakara, Tanzania, 2005–2015
| Outcome at Administrative Censoring in November 2015 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Died | Transferred Out | LTFU | In Active Care | Total | ||||||
| Participant Characteristic | No. | % a | No. | % a | No. | % a | No. | % a | No. | % a |
| Total | 646 | 9 | 883 | 12 | 2,911 | 39 | 3,020 | 40 | 7,460 | 100 |
| At Enrollment | ||||||||||
| Year of enrollment | ||||||||||
| 2005–2007 | 324 | 18 | 212 | 12 | 832 | 46 | 444 | 25 | 1,812 | 100 |
| 2008–2009 | 202 | 8 | 401 | 17 | 1,112 | 47 | 663 | 28 | 2,378 | 100 |
| 2010–2012 | 71 | 4 | 162 | 10 | 712 | 43 | 704 | 43 | 1,649 | 100 |
| 2013–2015 | 49 | 3 | 108 | 7 | 255 | 16 | 1,209 | 75 | 1,621 | 100 |
| Sex | ||||||||||
| Male | 263 | 10 | 306 | 12 | 1,102 | 42 | 932 | 36 | 2,603 | 100 |
| Female | 383 | 8 | 571 | 12 | 1,809 | 37 | 2,087 | 43 | 4,850 | 100 |
| Missing data | 0 | 0 | 6 | 86 | 0 | 0 | 1 | 14 | 7 | 100 |
| Age, years | ||||||||||
| 15–24 | 46 | 8 | 59 | 11 | 247 | 45 | 200 | 36 | 552 | 100 |
| 25–34 | 190 | 8 | 290 | 12 | 1,049 | 43 | 907 | 37 | 2,436 | 100 |
| 35–44 | 236 | 9 | 323 | 12 | 950 | 37 | 1,084 | 42 | 2,593 | 100 |
| ≥45 | 174 | 9 | 211 | 11 | 665 | 35 | 829 | 44 | 1,879 | 100 |
| Marital status | ||||||||||
| Married/cohabiting | 287 | 8 | 454 | 12 | 1,443 | 38 | 1,628 | 43 | 3,812 | 100 |
| Never married | 149 | 11 | 152 | 11 | 639 | 45 | 467 | 33 | 1,407 | 100 |
| Separated/divorced | 86 | 7 | 132 | 11 | 455 | 37 | 553 | 45 | 1,226 | 100 |
| Widowed/other | 68 | 9 | 107 | 14 | 287 | 37 | 316 | 41 | 778 | 100 |
| Missing data | 56 | 24 | 38 | 16 | 87 | 37 | 56 | 24 | 237 | 100 |
| HIV status of partner | ||||||||||
| Positive | 49 | 5 | 124 | 13 | 300 | 31 | 492 | 51 | 965 | 100 |
| Negative | 35 | 7 | 53 | 11 | 181 | 36 | 232 | 46 | 501 | 100 |
| Unknown | 517 | 10 | 629 | 12 | 2,210 | 44 | 1,703 | 34 | 5,059 | 100 |
| Not applicable | 23 | 4 | 43 | 8 | 77 | 14 | 405 | 74 | 548 | 100 |
| Missing data | 22 | 6 | 34 | 9 | 143 | 37 | 188 | 49 | 387 | 100 |
| Disclosure of HIV status | ||||||||||
| No | 109 | 6 | 205 | 11 | 855 | 45 | 749 | 39 | 1,918 | 100 |
| Yes | 282 | 7 | 501 | 12 | 1,431 | 35 | 1,866 | 46 | 4,080 | 100 |
| Missing data | 255 | 17 | 177 | 12 | 625 | 43 | 405 | 28 | 1,462 | 100 |
| Referral to clinic as hospital inpatient | ||||||||||
| No | 527 | 8 | 777 | 12 | 2,543 | 39 | 2,725 | 41 | 6,572 | 100 |
| Yes | 29 | 8 | 30 | 9 | 120 | 35 | 163 | 48 | 342 | 100 |
| Missing data | 90 | 16 | 76 | 14 | 248 | 45 | 132 | 24 | 546 | 100 |
| Distance of ward of residence from clinic, km | ||||||||||
| 1 | 295 | 10 | 223 | 7 | 1,071 | 35 | 1,511 | 49 | 3,100 | 100 |
| 2–49 | 58 | 5 | 69 | 6 | 384 | 35 | 602 | 54 | 1,113 | 100 |
| 50–79 | 88 | 11 | 105 | 14 | 385 | 50 | 195 | 25 | 773 | 100 |
| ≥80 | 82 | 7 | 277 | 23 | 496 | 40 | 373 | 30 | 1,228 | 100 |
| Missing data | 123 | 10 | 209 | 17 | 575 | 46 | 339 | 27 | 1,246 | 100 |
| Smoking status | ||||||||||
| Never/former smoker | 477 | 8 | 707 | 12 | 2,267 | 38 | 2,536 | 42 | 5,987 | 100 |
| Current smoker | 95 | 11 | 110 | 12 | 419 | 47 | 263 | 30 | 887 | 100 |
| Missing data | 74 | 13 | 66 | 11 | 225 | 38 | 221 | 38 | 586 | 100 |
| Pregnant | ||||||||||
| No | 375 | 8 | 547 | 12 | 1,699 | 37 | 1,980 | 43 | 4,601 | 100 |
| Yes | 8 | 3 | 24 | 10 | 110 | 44 | 107 | 43 | 249 | 100 |
| Body mass indexb | ||||||||||
| Underweight (<18.5) | 111 | 11 | 102 | 10 | 386 | 39 | 402 | 40 | 1,001 | 100 |
| Normal (18.5–24.9) | 142 | 6 | 273 | 11 | 857 | 35 | 1,209 | 49 | 2,481 | 100 |
| Overweight (25.0–29.9) | 13 | 3 | 40 | 8 | 154 | 33 | 266 | 56 | 473 | 100 |
| Obese (≥30.0) | 4 | 3 | 6 | 5 | 40 | 31 | 78 | 61 | 128 | 100 |
| Missing data | 376 | 11 | 462 | 14 | 1,474 | 44 | 1,065 | 32 | 3,377 | 100 |
| WHO HIV clinical stage | ||||||||||
| 1 | 116 | 5 | 286 | 12 | 1,033 | 42 | 1,041 | 42 | 2,476 | 100 |
| 2 | 102 | 7 | 174 | 12 | 548 | 38 | 628 | 43 | 1,452 | 100 |
| 3 | 182 | 10 | 242 | 13 | 756 | 42 | 636 | 35 | 1,816 | 100 |
| 4 | 169 | 19 | 116 | 13 | 372 | 42 | 221 | 25 | 878 | 100 |
| Missing data | 77 | 9 | 65 | 8 | 202 | 24 | 494 | 59 | 838 | 100 |
| CD4 cell count, cells/mm3 | ||||||||||
| <100 | 119 | 11 | 121 | 11 | 398 | 36 | 456 | 42 | 1,094 | 100 |
| 100–199 | 48 | 6 | 104 | 13 | 262 | 33 | 382 | 48 | 796 | 100 |
| 200–349 | 41 | 5 | 97 | 11 | 303 | 35 | 428 | 49 | 869 | 100 |
| ≥350 | 48 | 4 | 119 | 9 | 543 | 43 | 555 | 44 | 1,265 | 100 |
| Missing data | 390 | 11 | 442 | 13 | 1,405 | 41 | 1,199 | 35 | 3,436 | 100 |
| Tuberculosis | ||||||||||
| No | 585 | 9 | 804 | 12 | 2,708 | 40 | 2,728 | 40 | 6,825 | 100 |
| Yes | 61 | 10 | 79 | 12 | 203 | 32 | 292 | 46 | 635 | 100 |
| Initiated ART within 30 days of enrollment | ||||||||||
| No | 451 | 11 | 505 | 12 | 1,924 | 45 | 1,407 | 33 | 4,287 | 100 |
| Yes | 195 | 6 | 378 | 12 | 987 | 31 | 1,613 | 51 | 3,173 | 100 |
| At End of Follow-up | ||||||||||
| Time since ART initiation during follow-up, months | ||||||||||
| Not yet initiated | 302 | 15 | 274 | 13 | 1,260 | 62 | 194 | 10 | 2,030 | 100 |
| 0.1–5.9 | 185 | 15 | 141 | 12 | 555 | 46 | 333 | 27 | 1,214 | 100 |
| 6.0–11.9 | 39 | 6 | 86 | 13 | 277 | 42 | 250 | 38 | 652 | 100 |
| ≥12.0 | 120 | 3 | 382 | 11 | 819 | 23 | 2,243 | 63 | 3,564 | 100 |
| No. of gaps in care (periods of LTFU) | ||||||||||
| 0 | 448 | 17 | 376 | 14 | 0 | 0 | 1,783 | 68 | 2,607 | 100 |
| 1 | 162 | 5 | 368 | 11 | 2,064 | 62 | 731 | 22 | 3,325 | 100 |
| 2 | 27 | 3 | 90 | 9 | 568 | 58 | 301 | 31 | 986 | 100 |
| ≥3 | 9 | 2 | 49 | 9 | 279 | 51 | 205 | 38 | 542 | 100 |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; KIULARCO, Kilombero and Ulanga Antiretroviral Cohort; LTFU, lost to follow-up; WHO, World Health Organization.
a Row percentage.
b Weight (kg)/height (m)2. Data were missing for pregnant women.
Finally, we evaluated the impacts of tracing by assessing outcomes captured in the routine database by January 2018 among participants who were LTFU in November 2015. Analyses were conducted in Stata (24).
Ethical considerations
The institutional review board of the Ifakara Health Institute and the National Health Research Ethics Review Committee of the National Institute for Medical Research of Tanzania provided ethical approval for KIULARCO. Written informed consent was sought from all participants at registration at the Chronic Disease Clinic in Ifakara; those who refused were excluded. Data are stored on a secure server and were deidentified before analysis.
RESULTS
Among 7,460 adults, 4,850 (65% of participants with a response) were female and 5,029 (67%) were aged 25–44 years (Table 1). At enrollment, 5,059 (72% of those with a response) did not know their partner’s HIV status, 4,080 (68%) had disclosed their HIV status, 342 (5%) were inpatients, and 3,100 (50%) were living in the town of Ifakara. Large proportions of participants were underweight (n = 1,001; 25%), were classified as being in World Health Organization (WHO) HIV clinical stage 3/4 (n = 2,694; 41%), or had a CD4 cell count less than 200 cells/mm3 (n = 1,890; 47%), and 635 (9%) participants had tuberculosis. Overall, 11% of baseline covariate data were missing across all participants, mostly for body mass index, WHO stage, and CD4 cell count. ART was started within 30 days of enrollment in 3,173 (43%) participants. Overall, 5,471 (73%) participants initiated ART after a median of 0.5 months (interquartile range (IQR), 0.1–5).
Outcomes
In the routinely collected data, 646 (9%) participants died, 883 (12%) transferred to another clinic, 2,911 (39%) were LTFU, and 3,020 (40%) were in care at administrative censoring (Figure 2). The median follow-up times for each outcome category were 0.4 (IQR, 0.1–1.5), 1.6 (IQR, 0.5–3.1), 0.8 (IQR, 0.4–2.3), and 4.0 (IQR, 1.4–6.8) years, respectively. Participants living far away from the Chronic Disease Clinic in Ifakara were more likely to have transferred out or been LTFU than those living closer (Table 1). Women were more likely to remain in care than were men. Participants with poorer health status (body mass index, WHO stage, and CD4 cell count) were more likely to have died than those with better health. Those who initiated ART were more likely to have transferred to another clinic or remained in care than those who did not initiate ART. Overall, 4,853 (65%) participants had at least 1 LTFU episode (median, 1 LTFU episode per participant; maximum = 12). There were 7,227 LTFU episodes, following which participants returned to care 3,848 (53%) times, after a median of 2 months following the date on which they were declared LTFU (IQR, 0.4–5).
Figure 2.
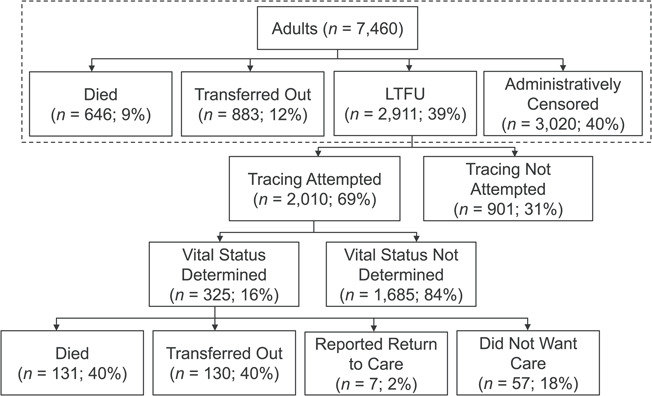
Outcomes among KIULARCO participants determined by means of routinely captured data and through tracing, Ifakara, Tanzania, 2005–2015. Data captured routinely are shown in the dashed box; the remaining data were determined through tracing. KIULARCO, Kilombero and Ulanga Antiretroviral Cohort; LTFU, lost to follow-up.
Tracing
Tracing attempts were made for 2,010 (69%) participants who were LTFU (Figure 2). Tracing attempts were more likely for participants who lived close to the clinic than for those living further away (Web Table 2). Among these 2,010 participants, 325 (16%) were successfully traced, with a median time between LTFU and attempted tracing of 6.0 years (IQR, 3.7–7.8). Comparing participants who were and were not successfully traced, characteristics were similar except that those living closer to the clinic were slightly less likely to have been successfully traced and those who did not initiate ART at enrollment were more likely to have been successfully traced (Web Table 3). The proportions of participants attempted traced and successfully traced were similar according to the year in which the participant was LTFU (Web Table 4).
Among the 325 participants successfully traced, 131 (40%) had died, 130 (40%) had transferred to another clinic, 7 (2%) were reported to have returned to the Chronic Disease Clinic in Ifakara, and 57 (18%) were alive but refused care (Figure 2). Those who were enrolled in earlier years were more likely to have died or transferred to another clinic; women and those living further from the clinic were more likely to have transferred; and those with poorer health status were more likely to have died (Web Table 5). Further, those who initiated ART at enrollment were more likely to have died, which is attributable to those with poorer health status being more likely to have initiated ART in the years before implementation of the test-and-treat strategy for HIV.
Weights
Under method C, the weight applied to those LTFU and successfully traced was 9.0 (2,911/325). Under method D, the weights applied to those LTFU and successfully traced varied by person (Table 2). Notably, for every additional month of delay in tracing (time between being lost and attempted traced), the odds of being successfully traced decreased by 2% (odds ratio = 0.98, 95% confidence interval (CI): 0.96, 1.00).
Table 2.
Weights Used Under Mortality Estimation Method Da for KIULARCO Participants Who Were Lost to Follow-up and Successfully Traced, Ifakara, Tanzania, 2005–2015
| Participant Outcome |
For Probability of Being Attempted Traced |
For Probability of Being Successfully Traced |
Overall b | |||
|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | |
| LTFU and successfully traced | 1.5 (0.6) | 1.0–6.0 | 6.0 (3.0) | 2.2–32 | 8.6 (4.8) | 3.0–43 |
| Died | 7.9 (4.1) | 3.6–27 | ||||
| Transferred to another clinic | 9.8 (5.8) | 3.0–43 | ||||
| Reported returning to care or did not want care | 7.7 (3.1) | 3.0–16 | ||||
Abbreviations: KIULARCO, Kilombero and Ulanga Antiretroviral Cohort; LTFU, lost to follow-up; SD, standard deviation.
a Extension to the Frangakis and Rubin (14) method; see Methods section of text.
b For attempted tracing and successful tracing combined.
Mortality
Under method A, mortality estimates were 6.8% (95% CI: 6.2, 7.4) at 1 year and 13.1% (95% CI: 12.1, 14.3) at 5 years (Figure 3). Under method B, mortality was slightly higher at 7.3% (95% CI: 6.7, 8.0) and 16.2% (95% CI: 15.0, 17.5), respectively. Under method C, mortality was substantially higher, at 11.5% (95% CI: 10.2, 12.8) and 36.8% (95% CI: 33.2, 40.3), respectively. Similar results were obtained under method D, with mortality of 11.1% (95% CI: 9.8, 12.4) and 35.1% (95% CI: 31.2, 38.6), respectively.
Figure 3.
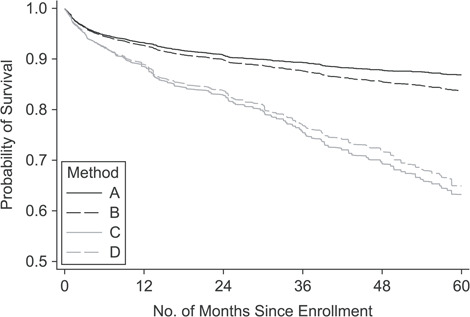
Probability of survival following enrollment in the Kilombero and Ulanga Antiretroviral Cohort, estimated according to 4 different methods, Ifakara, Tanzania, 2005–2015. Method A, routine data; method B, routine data plus tracing data; method C, Frangakis and Rubin (14) method; method D, extension to the Frangakis and Rubin method. See Methods section of text for more details.
Factors associated with mortality
In the multivariable model, higher mortality was associated with male sex (vs. being a nonpregnant female), HIV status disclosure (methods A and B only), referral as an inpatient (weaker evidence for method A), living in the town of Ifakara versus further away, lower body mass index, WHO stage 3/4, lower CD4 cell count, and less time on ART (Table 3). Associations between mortality and enrollment year differed by method: Mortality was higher in earlier years under method A, yet highest in 2010–2012 under methods C and D. Under methods A and B, any gaps in care were strongly associated with lower mortality risk, whereas under methods C and D, a single gap in care was strongly associated with higher risk (95% confidence intervals were wide for 2 or ≥3 gaps in care). Similar results were obtained after truncating weights at 20. In sensitivity analyses among complete cases, results were broadly similar except that 1) the 95% confidence intervals tended to be wider, 2) the point estimates for smoking reversed direction (but they remained nonsignificant across all methods), and 3) there was stronger evidence of both 1 and 2 gaps in care being associated with higher mortality under methods C and D (Web Table 6).
Table 3.
Factors Associated With Mortality in KIULARCO Participants Under 4 Different Methods of Mortality Estimationa, Ifakara, Tanzania, 2005–2015
| Participant Characteristic | Method A: Data as Captured in Database | Method B: Incorporating Tracing Outcomes | Method C: Frangakis and Rubin ( 14 ) Approach | Method D: Extension to Frangakis and Rubin ( 14 ) Approach | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Baseline Covariates | ||||||||
| Year of enrollment | ||||||||
| 2005–2007 | 0.94 | 0.67, 1.32 | 0.87 | 0.63, 1.21 | 0.58 | 0.37, 0.91 | 0.62 | 0.39, 1.00 |
| 2008–2009 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 2010–2012 | 0.69 | 0.51, 0.92 | 0.98 | 0.76, 1.26 | 1.86 | 1.32, 2.62 | 1.84 | 1.30, 2.63 |
| 2013–2015 | 0.37 | 0.23, 0.59 | 0.52 | 0.34, 0.80 | 1.18 | 0.55, 2.50 | 1.17 | 0.59, 2.33 |
| Sex | ||||||||
| Male | 1.24 | 1.03, 1.50 | 1.29 | 1.08, 1.53 | 1.32 | 1.00, 1.73 | 1.38 | 1.05, 1.80 |
| Female | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Age, years | ||||||||
| 15–24 | 1.05 | 0.72, 1.53 | 0.92 | 0.64, 1.33 | 0.56 | 0.31, 0.99 | 0.56 | 0.32, 0.99 |
| 25–34 | 0.85 | 0.69, 1.05 | 0.88 | 0.73, 1.07 | 0.85 | 0.62, 1.15 | 0.86 | 0.63, 1.18 |
| 35–44 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| ≥45 | 1.09 | 0.89, 1.34 | 1.12 | 0.93, 1.35 | 1.01 | 0.75, 1.36 | 0.98 | 0.73, 1.30 |
| Marital status | ||||||||
| Married/cohabiting | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Never married | 1.08 | 0.86, 1.35 | 1.03 | 0.84, 1.28 | 1.04 | 0.73, 1.47 | 0.99 | 0.70, 1.40 |
| Separated/divorced | 0.89 | 0.68, 1.16 | 0.92 | 0.72, 1.16 | 0.96 | 0.66, 1.39 | 1.00 | 0.69, 1.45 |
| Widowed/other | 0.88 | 0.66, 1.18 | 0.86 | 0.65, 1.13 | 0.82 | 0.50, 1.35 | 0.82 | 0.50, 1.36 |
| HIV status of partner | ||||||||
| Positive | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Negative | 1.29 | 0.82, 2.05 | 1.21 | 0.79, 1.85 | 1.08 | 0.55, 2.12 | 1.07 | 0.55, 2.10 |
| Unknown | 1.22 | 0.86, 1.74 | 1.35 | 0.98, 1.87 | 1.41 | 0.86, 2.31 | 1.45 | 0.87, 2.40 |
| Not applicable | 1.85 | 0.99, 3.46 | 1.95 | 1.08, 3.52 | 2.39 | 0.89, 6.41 | 2.01 | 0.78, 5.18 |
| Disclosure of HIV status | ||||||||
| No | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Yes | 1.34 | 1.06, 1.69 | 1.28 | 1.03, 1.59 | 1.03 | 0.77, 1.38 | 1.04 | 0.76, 1.42 |
| Referral to clinic as hospital inpatient | ||||||||
| No | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Yes | 1.49 | 0.99, 2.25 | 1.45 | 1.00, 2.11 | 1.76 | 1.09, 2.83 | 1.69 | 1.02, 2.80 |
| Distance of ward of residence from clinic, km | ||||||||
| 1 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 2–49 | 0.62 | 0.46, 0.84 | 0.61 | 0.46, 0.81 | 0.64 | 0.41, 1.00 | 0.66 | 0.42, 1.04 |
| 50–79 | 0.91 | 0.69, 1.18 | 0.80 | 0.62, 1.04 | 0.51 | 0.31, 0.86 | 0.53 | 0.33, 0.84 |
| ≥80 | 0.64 | 0.48, 0.84 | 0.65 | 0.50, 0.85 | 0.79 | 0.52, 1.18 | 0.75 | 0.49, 1.15 |
| Smoking status | ||||||||
| Never/former smoker | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Current smoker | 1.22 | 0.92, 1.61 | 1.23 | 0.94, 1.61 | 1.44 | 0.99, 2.08 | 1.39 | 0.95, 2.02 |
| Pregnant | ||||||||
| No | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Yes | 0.99 | 0.49, 2.03 | 1.26 | 0.71, 2.19 | 0.95 | 0.34, 2.59 | 1.11 | 0.41, 2.98 |
| Body mass indexb | ||||||||
| Underweight (<18.5) | 1.51 | 1.16, 1.98 | 1.56 | 1.22, 2.00 | 1.60 | 1.17, 2.20 | 1.58 | 1.15, 2.17 |
| Normal-weight (18.5–24.9) | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Overweight (25.0–29.9) | 0.66 | 0.41, 1.07 | 0.63 | 0.40, 1.00 | 0.66 | 0.32, 1.35 | 0.73 | 0.34, 1.56 |
| Obese (≥30.0) | 0.44 | 0.14, 1.36 | 0.32 | 0.11, 0.94 | 0.16 | 0.03, 0.81 | 0.15 | 0.03, 0.84 |
| WHO HIV clinical stage | ||||||||
| 1 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 2 | 1.44 | 1.08, 1.94 | 1.45 | 1.12, 1.89 | 1.52 | 1.05, 2.20 | 1.39 | 0.95, 2.02 |
| 3 | 1.90 | 1.46, 2.49 | 2.10 | 1.65, 2.68 | 2.43 | 1.69, 3.48 | 2.32 | 1.62, 3.32 |
| 4 | 3.31 | 2.47, 4.44 | 3.43 | 2.60, 4.53 | 3.16 | 2.01, 4.99 | 3.01 | 1.90, 4.77 |
| CD4 cell count, cells/mm3 | ||||||||
| <100 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 100–199 | 0.73 | 0.54, 0.97 | 0.73 | 0.55, 0.96 | 0.67 | 0.42, 1.07 | 0.65 | 0.41, 1.04 |
| 200–349 | 0.57 | 0.41, 0.79 | 0.59 | 0.45, 0.78 | 0.59 | 0.39, 0.90 | 0.58 | 0.39, 0.87 |
| ≥350 | 0.32 | 0.22, 0.46 | 0.33 | 0.23, 0.47 | 0.25 | 0.15, 0.43 | 0.24 | 0.14, 0.43 |
| Tuberculosis | ||||||||
| No | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Yes | 1.00 | 0.75, 1.33 | 0.90 | 0.69, 1.18 | 0.75 | 0.48, 1.19 | 0.71 | 0.46, 1.10 |
| Time-Dependent Covariates | ||||||||
| Time since ART initiation during follow-up, months | ||||||||
| Not yet initiated | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 0.1–5.9 | 0.23 | 0.18, 0.29 | 0.34 | 0.27, 0.42 | 0.63 | 0.47, 0.86 | 0.6 | 0.43, 0.84 |
| 6–11.9 | 0.04 | 0.02, 0.05 | 0.06 | 0.04, 0.08 | 0.16 | 0.10, 0.26 | 0.13 | 0.08, 0.22 |
| ≥12 | 0.03 | 0.02, 0.05 | 0.03 | 0.02, 0.04 | 0.02 | 0.01, 0.03 | 0.01 | 0.01, 0.02 |
| No. of gaps in care (periods of LTFU) | ||||||||
| 0 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1 | 0.17 | 0.13, 0.21 | 0.35 | 0.29, 0.43 | 2.30 | 1.72, 3.06 | 1.95 | 1.46, 2.61 |
| 2 | 0.18 | 0.11, 0.28 | 0.24 | 0.17, 0.36 | 0.71 | 0.36, 1.39 | 0.71 | 0.37, 1.37 |
| ≥3 | 0.17 | 0.08, 0.35 | 0.27 | 0.16, 0.46 | 1.27 | 0.58, 2.78 | 0.98 | 0.42, 2.28 |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; KIULARCO, Kilombero and Ulanga Antiretroviral Cohort; LTFU, lost to follow-up; WHO, World Health Organization.
a Results were derived from multivariable Cox proportional hazards models adjusting for all variables shown in the table, with multiple imputation for missing baseline covariates (see Methods section of text for details).
b Weight (kg)/height (m)2.
Mortality among persons who initiated ART
Among 5,430 participants with follow-up after ART initiation, 344 (6%) died, 609 (11%) transferred to another clinic, 1,651 (30%) were LTFU, and 2,826 (52%) were in care at administrative censoring. Among those LTFU, attempts were made to trace 1,144 (69%), and 186 (16%) were successfully traced. Of those successfully traced, 76 (41%) had died, 74 (40%) had transferred, 4 (2%) were reported to have returned to the index clinic, and 32 (17%) were alive but refused care. Mortality estimates were all somewhat lower than those in the whole population (when time was measured from enrollment) but displayed similar patterns (Web Figure 1). The factors associated with mortality were similar to those in the whole cohort, except for 1) some evidence of higher mortality risk at older ages, 2) current smoking being associated with higher mortality under methods A and B, 3) weaker associations of mortality with WHO stage and CD4 cell count, and 4) much higher mortality risk with a greater number of gaps in care under methods C and D (Web Table 7).
Impacts of tracing
Of the 2,911 participants LTFU at administrative censoring in November 2015, 13 were not included in the database by January 2018 (e.g., consent withdrawal). Routine data on the remaining 2,898 participants showed that the majority (2,421; 84%) remained LTFU, 53 (2%) returned for ≥1 visit but again became LTFU, 121 (4%) died, 143 (5%) transferred out, and 160 (6%) returned to care (Table 4). Among the deaths and transfers, 91 of 121 (75%) and 104 of 143 (73%), respectively, had been determined through the tracing efforts of this study. Among the 64 participants who had been traced and found to be alive but not under care, 15 (23%) returned for a clinic visit after 2015.
Table 4.
Subsequent Follow-up of KIULARCO Participants Who Were Lost to Follow-up at Administrative Censoring on November 15, 2015, Ifakara, Tanzania, 2005–2015
| Year of Last Visit | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005–2007 | 2008–2009 | 2010–2012 | 2013–2015 | 2016–2018 | Total b | |||||||
| Participant Outcome a | No. (n = 445) | % c (15d) | No. (n = 808) | % c (28d) | No. (n = 863) | % c (30d) | No. (n = 544) | % c (19d) | No. (n = 238) | % c (8d) | No. (n = 2,898) | % c (100d) |
| Died | 15 | 3 | 42 | 5 | 44 | 5 | 16 | 3 | 4 | 2 | 121 | 4 |
| Transferred to another clinic | 10 | 2 | 51 | 6 | 30 | 3 | 31 | 6 | 21 | 9 | 143 | 5 |
| LTFU | 420 | 94 | 715 | 88 | 789 | 91 | 497 | 91 | 53 | 22 | 2,474 | 85 |
| In active care | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 160 | 67 | 160 | 6 |
Abbreviations: KIULARCO, Kilombero and Ulanga Antiretroviral Cohort; LTFU, lost to follow-up.
a As captured at administrative censoring in January 2018.
b Excludes 13 participants who were LTFU in November 2015 but were not included in the January 2018 database (because of a change in consent, for example).
c Column percentage.
d Row percentage.
DISCUSSION
In this large cohort of people living with HIV in rural Tanzania, LTFU was high at 39%, in line with other estimates from the region (25, 26). Among participants who were successfully traced, mortality was much higher than that among those not LTFU. The substantial tracing efforts resulted in only 23% (n = 15) of those found alive through tracing actually returning to care, indicating that tracing alone was not successful in encouraging participants to reengage in care in this population, in contrast to other settings (15, 27). Among those LTFU, approximately 75% of outcomes captured within the following 2 years were a direct result of the tracing, with relatively few reports of death or transfers out being provided to the clinic directly. While outcome status was determined in only about 10% of those LTFU, incorporation of this information through appropriate statistical methods had substantial impacts on the mortality estimates, essentially doubling the uncorrected ones which underestimate death in those LTFU and not traced. Our 1-year (corrected) mortality estimates of around 11% (9% among those initiating ART) were in line with those of other studies (9, 11, 28, 29). Our 5-year mortality estimates of 27%–29% among persons initiating ART were in line with those of an analysis of 34 cohorts in sub-Saharan Africa (29) while markedly higher than those from another analysis of 57 cohorts in sub-Saharan Africa, but only from 2009 onwards (26).
There were a number of similarities in the determinants of survival across the methods. However, we found that gaps in care were associated with higher mortality risk after accounting for the unseen mortality among those LTFU, which was missed in the models which did not account for this. There were also some differences in the mortality risk by year of enrollment. However, we did not observe large differences in the results under our extended approach as compared with the Frangakis and Rubin method (14). As indicated in the Methods section, these results should be interpreted as factors associated with survival under the hypothetical scenario that all outcomes had been observed. Alternative approaches would be required if instead one wanted to address the hypothetical scenario that all participants could be retained in care (30).
A number of previous studies have attempted to correct mortality estimates for the unseen mortality among participants LTFU, using a range of approaches: by tracing participants LTFU and using weighted estimation as in this study (9–11, 14, 20, 31); by assuming that a proportion of those LTFU had died based on previously reported estimates (26, 32); by imputing survival times using results of a meta-regression (33); by using a nomogram (28, 29, 34); or by linking participants to national registries (5, 16, 35, 36). Assuming a mortality risk among those LTFU based on previously reported estimates is simple to implement but does not allow for changing LTFU and mortality risks over time or across different settings. The nomogram approach provides an estimate of mortality among those LTFU based on the observed LTFU within a particular cohort (28); this approach is also simple to apply but is limited to the estimation of mortality at 1 year following ART initiation. Few settings, especially in sub-Saharan Africa, have adequate civil registration and vital statistics systems in place to allow linkage (37). Other researchers have used a combination of these methods, while also accounting for a limited set of participant characteristics (38, 39). Other methods have been used to model the missingness mechanism—for example, by incorporating the “sensitivity” of routinely detecting a death (9) or using multiple imputation (16). An alternative approach is to use a multistage sampling approach to obtain a representative sample of those LTFU (31). In a recent paper, Jannat-Khah et al. (40) compared complete-case analysis with 2 principled approaches: 1) using multiple imputation for missing baseline covariates and outcomes and 2) using inverse probability weighting methods after tracing to account for unseen deaths among those LTFU, which is similar to our approach but based only on successful tracing. Further, the authors did not use multiple imputation to account for missing baseline covariates in the latter approach, as we have done. Our extension to the Frangakis and Rubin method explicitly models the 2 steps of the missingness mechanism, accounting for measured differences in the participant characteristics between those who were 1) attempted traced and 2) successfully traced versus those who remained LTFU.
This study had a number of limitations. Firstly, a low proportion of participants were successfully traced. This reflects the challenges involved in locating participants and leads us to advocate for prompt tracing. This low tracing success may partly explain the similar results between our 2-step approach and the Frangakis and Rubin method. Another reason may be the similarities in characteristics between persons attempted traced and not attempted traced and between those successfully traced and not successfully traced; the impact of our method may be greater in other cohorts with more pronounced differences in those traced, for example with targeted tracing. Secondly, our 2-step approach assumes that there is no residual unmeasured confounding in the models for attempted and successful tracing and for mortality, which is not straightforward to assess. Thirdly, we assumed that the risk of death among participants successfully traced was the same as that among those who remained LTFU despite tracing efforts, which is not typically possible to assess (8). Lastly, to implement the multiple imputation, we assumed that the data were missing at random. This assumption could not be tested directly in the data, but we increased the plausibility by including a broad range of covariates (22).
In conclusion, our study demonstrates the challenges associated with tracing participants who are LTFU, with a relatively low proportion being successfully traced and with little impact on participants subsequently returning to care at our HIV clinic. However, even with somewhat limited tracing information, incorporating tracing outcomes with appropriate statistical adjustment resulted in considerable changes in mortality estimates, demonstrating the importance of accounting for unseen mortality among those LTFU. The method of Frangakis and Rubin (14) has the advantage of being simple to apply and does not need measurement of covariates. However, it requires tracing to be performed in a random sample of participants LTFU. In practice, this may not be possible or even desirable. For example, individual clinics may wish to perform targeted tracing based on measured risk factors, and national programs may pool such clinic data for informing monitoring and evaluation assessments. In such circumstances, the Frangakis and Rubin method will yield biased estimates of mortality. We have demonstrated the application of a method which should be used to compensate for such nonrandom sampling to account for differential mortality among persons who have become LTFU from HIV programs. Our method can equally be applied in any setting where LTFU is of concern.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Swiss Tropical and Public Health Institute, Basel, Switzerland (Fiona Vanobberghen, Maja Weisser, Marcel Tanner, Tracy R. Glass); University of Basel, Basel, Switzerland (Fiona Vanobberghen, Maja Weisser, Manuel Battegay, Marcel Tanner, Tracy R. Glass); Ifakara Health Institute, Ifakara, Tanzania (Maja Weisser, Andrew Katende); Division of Infectious Diseases and Hospital Epidemiology, University Hospital Basel, Basel, Switzerland (Maja Weisser, Manuel Battegay); USAID Boresha Afya, Morogoro, Tanzania (Bryson Kasuga); and St. Francis Referral Hospital, Ifakara, Tanzania (Bryson Kasuga).
The Chronic Disease Clinic in Ifakara receives funding from the Ministry of Health and Social Welfare of the government of Tanzania; the government of the Canton of Basel Stadt, Switzerland; the Swiss Tropical and Public Health Institute (Basel, Switzerland); the Ifakara Health Institute (Ifakara, Tanzania); and USAID Boresha Afya (service and medication support, with funding provided by the US Agency for International Development through the President’s Emergency Plan for AIDS Relief).
We thank the staff of the Chronic Disease Clinic in Ifakara at St. Francis Referral Hospital (Ifakara, Tanzania). We thank Dr. Michael Schomaker for helpful discussions on statistical methods.
Members of the KIULARCO Study Group: Aschola Asantiel, Farida Bani, Manuel Battegay, Theonestina Byakuzana, Adolphina Chale, Anna Eichenberger, Sauli John Epimack, Gideon Francis, Hansjakob Furrer, Anna Gamell, Tracy R. Glass, Speciosa Hwaya, Aneth V. Kalinjuma, Joshua Kapunga, Bryson Kasuga, Andrew Katende, Namvua Kimera, Yassin Kisunga, Olivia Kitau, Thomas Klimkait, Emilio Letang, Ezekiel Luoga, Lameck B. Luwanda, Herry Mapesi, Ngisi Peter Masawa, Mengi Mkulila, Julius Mkumbo, Margareth Mkusa, Slyakus Mlembe, Dorcas K. Mnzava, Gertrud J. Mollel, Lilian Moshi, Germana Mossad, Dolores Mpundunga, Athumani Mtandanguo, Selerine Myeya, Sanula Nahota, Regina Ndaki, Robert C. Ndege, Omary Rajab Ngome, Agatha Ngulukila, Alex John Ntamatungiro, Amina Nyuri, James Okuma, Daniel H. Paris, Leila Samson, Elizabeth Senkoro, George Sikalengo, Jenifa Tarimo, Yvan Temba, Juerg Utzinger, Fiona Vanobberghen, Maja Weisser, John Wigay, and Herieth Wilson.
This work was presented in part at the 21st International Workshop on HIV and Hepatitis Observational Databases (IWHOD), Lisbon, Portugal, March 30–April 1, 2017, and the 16th European AIDS Conference, Milan, Italy, October 25–27, 2017.
Conflict of interest: none declared.
REFERENCES
- 1. Bhaskaran K, Hamouda O, Sannes M, et al. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA. 2008;300(1):51–59. [DOI] [PubMed] [Google Scholar]
- 2. Collaboration of Observational HIV Epidemiological Research Europe (COHERE) in EuroCoord, Lewden C, Bouteloup V, et al. All-cause mortality in treated HIV-infected adults with CD4 ≥500/mm3 compared with the general population: evidence from a large European observational cohort collaboration. Int J Epidemiol. 2012;41(2):433–445. [DOI] [PubMed] [Google Scholar]
- 3. The Antiretroviral Therapy Cohort Collaboration, Zwahlen M, Harris R, et al. Mortality of HIV-infected patients starting potent antiretroviral therapy: comparison with the general population in nine industrialized countries. Int J Epidemiol. 2009;38(6):1624–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brinkhof MWG, Boulle A, Weigel R, et al. Mortality of HIV-infected patients starting antiretroviral therapy in sub-Saharan Africa: comparison with HIV-unrelated mortality. PLoS Med. 2009;6(4):e1000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cornell M, Lessells R, Fox MP, et al. Mortality among adults transferred and lost to follow-up from antiretroviral therapy programmes in South Africa: a multicenter cohort study. J Acquir Immune Defic Syndr. 2014;67(2):e67–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brinkhof MWG, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4(6):e5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zürcher K, Mooser A, Anderegg N, et al. Outcomes of HIV-positive patients lost to follow-up in African treatment programmes. Trop Med Int Health. 2017;22(4):375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yiannoutsos CT, An M-W, Frangakis CE, et al. Sampling-based approaches to improve estimation of mortality among patient dropouts: experience from a large PEPFAR-funded program in western Kenya. PLoS One. 2008;3(12):e3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Henriques J, Pujades-Rodriguez M, McGuire M, et al. Comparison of methods to correct survival estimates and survival regression analysis on a large HIV African cohort. PLoS One. 2012;7(2):e31706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geng EH, Odeny TA, Lyamuya RE, et al. Estimation of mortality among HIV-infected people on antiretroviral treatment in East Africa: a sampling based approach in an observational, multisite, cohort study. Lancet HIV. 2015;2(3):e107–e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kiragga AN, Castelnuovo B, Musomba R, et al. Comparison of methods for correction of mortality estimates for loss to follow-up after ART initiation: a case of the Infectious Diseases Institute, Uganda. PLoS One. 2013;8(12):e83524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Geng EH, Glidden DV, Bangsberg DR, et al. A causal framework for understanding the effect of losses to follow-up on epidemiologic analyses in clinic-based cohorts: the case of HIV-infected patients on antiretroviral therapy in Africa. Am J Epidemiol. 2012;175(10):1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geng EH, Glidden DV, Emenyonu N, et al. Tracking a sample of patients lost to follow-up has a major impact on understanding determinants of survival in HIV-infected patients on antiretroviral therapy in Africa. Trop Med Int Health. 2010;15(suppl 1):63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frangakis CE, Rubin DB. Addressing an idiosyncrasy in estimating survival curves using double sampling in the presence of self-selected right censoring. Biometrics. 2001;57(2):333–342. [DOI] [PubMed] [Google Scholar]
- 15. McMahon JH, Elliott JH, Hong SY, et al. Effects of physical tracing on estimates of loss to follow-up, mortality and retention in low and middle income country antiretroviral therapy programs: a systematic review. PLoS One. 2013;8(2):e56047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schomaker M, Gsponer T, Estill J, et al. Non-ignorable loss to follow-up: correcting mortality estimates based on additional outcome ascertainment. Stat Med. 2014;33(1):129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vanobberghen F, Letang E, Gamell A, et al. A decade of HIV care in rural Tanzania: trends in clinical outcomes and impact of clinic optimisation in an open, prospective cohort. PLoS One. 2017;12(7):e0180983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Letang E, Vedastus Kalinjuma A, Glass TR, et al. Cohort profile: the Kilombero and Ulanga Antiretroviral Cohort (KIULARCO)—a prospective HIV cohort in rural Tanzania. Swiss Med Wkly. 2017;147:w14485. [DOI] [PubMed] [Google Scholar]
- 19. Johnson LF, Estill J, Keiser O, et al. Do increasing rates of loss to follow-up in antiretroviral treatment programs imply deteriorating patient retention? Am J Epidemiol. 2014;180(12):1208–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Geng EH, Emenyonu N, Bwana MB, et al. Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. JAMA. 2008;300(5):506–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cole S, Hernán M. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–399. [DOI] [PubMed] [Google Scholar]
- 23. White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med. 2009;28(15):1982–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. StataCorp LLC Stata Statistical Software, Release 14. College Station, TX: StataCorp LLC; 2015. [Google Scholar]
- 25. Fox MP, Rosen S. Retention of adult patients on antiretroviral therapy in low- and middle-income countries: systematic review and meta-analysis 2008–2013. J Acquir Immune Defic Syndr. 2015;69(1):98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haas AD, Zaniewski E, Anderegg N, et al. Retention and mortality on antiretroviral therapy in sub-Saharan Africa: collaborative analyses of HIV treatment programmes. J Int AIDS Soc. 2018;21(2):e25084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bershetyn A, Odeny TA, Lyamuya R, et al. The causal effect of tracing by peer health workers on return to clinic among patients who were lost to follow-up from antiretroviral therapy in eastern Africa: a randomized “natural experiment” arising from surveillance of lost patients. Clin Infect Dis. 2017;64(11):1547–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Egger M, Spycher BD, Sidle J, et al. Correcting mortality for loss to follow-up: a nomogram applied to antiretroviral treatment programmes in sub-Saharan Africa. PLoS Med. 2011;8(1):e1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Verguet S, Lim SS, Murray CJL, et al. Incorporating loss to follow-up in estimates of survival among HIV-infected individuals in sub-Saharan Africa enrolled in antiretroviral therapy programs. J Infect Dis. 2013;207(1):72–79. [DOI] [PubMed] [Google Scholar]
- 30. Robins J, Finkelstein D. Correcting for noncompliance and dependent censoring in an AIDS clinical trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56(3):779–788. [DOI] [PubMed] [Google Scholar]
- 31. Holmes CB, Sikazwe I, Sikombe K, et al. Estimated mortality on HIV treatment among active patients and patients lost to follow-up in 4 provinces of Zambia: findings from a multistage sampling-based survey. PLoS Med. 2018;15(1):e1002489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Farahani M, Vable A, Lebelonyane R, et al. Outcomes of the Botswana national HIV/AIDS treatment programme from 2002 to 2010: a longitudinal analysis. Lancet Glob Health. 2014;2(1):e44–e50. [DOI] [PubMed] [Google Scholar]
- 33. Brinkhof MWG, Spycher BD, Yiannoutsos C, et al. Adjusting mortality for loss to follow-up: analysis of five ART programmes in sub-Saharan Africa. PLoS One. 2010;5(11):e14149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Somi G, Keogh SC, Todd J, et al. Low mortality risk but high loss to follow-up among patients in the Tanzanian national HIV care and treatment programme. Trop Med Int Health. 2012;17(4):497–506. [DOI] [PubMed] [Google Scholar]
- 35. Boulle A, Schomaker M, May MT, et al. Mortality in patients with HIV-1 infection starting antiretroviral therapy in South Africa, Europe, or North America: a collaborative analysis of prospective studies. PLoS Med. 2014;11(9):e1001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hermanides H, Holman R, Gras L, et al. Loss to follow-up and mortality rates in HIV-1-infected patients in Curaçao before and after the start of combination antiretroviral therapy. AIDS Res Hum Retroviruses. 2013;29(10):1300–1305. [DOI] [PubMed] [Google Scholar]
- 37. Ye Y, Wamukoya M, Ezeh A, et al. Health and demographic surveillance systems: a step towards full civil registration and vital statistics system in sub-Sahara Africa? BMC Public Health. 2012;12:Article 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yiannoutsos CT, Johnson LF, Boulle A, et al. Estimated mortality of adult HIV-infected patients starting treatment with combination antiretroviral therapy. Sex Transm Infect. 2012;88(suppl 2):i33–i43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anderegg N, Johnson LF, Zaniewski E, et al. All-cause mortality in HIV-positive adults starting combination antiretroviral therapy: correcting for loss to follow-up. AIDS. 2017;31(suppl 1):S31–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jannat-Khah DP, Unterbrink M, McNairy M, et al. Treating loss-to-follow-up as a missing data problem: a case study using a longitudinal cohort of HIV-infected patients in Haiti. BMC Public Health. 2018;18(1):Article 1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


