ABSTRACT
Background
Recently, we showed that there are higher protein, lysine, and phenylalanine requirements in late stages of pregnancy compared with early stages. Animal studies have suggested an increased dietary need for specific dispensable amino acids in pregnancy; whether such a need exists in human pregnancies is unknown.
Objective
The objective of the current study was to examine whether healthy pregnant women at midgestation (20–29 wk) and late gestation (30–40 wk) have a dietary demand for glycine, a dispensable amino acid, using the indicator amino acid oxidation method and measurement of plasma 5-oxoproline concentrations.
Methods
Seventeen healthy women (aged 26–36 y) randomly received different test glycine intakes (range: 5–100 mg·kg−1·d−1) during each study day in midgestation (∼26 wk, n = 17 observations in 9 women) and late gestation (∼35 wk, n = 19 observations in 8 women). Diets were isocaloric with energy at 1.7 × resting energy expenditure. Protein was given as a crystalline amino acid mixture based on egg protein composition at current estimated average requirement (EAR; 0.88 g·kg−1·d−1). Breath samples were collected at baseline and isotopic steady state to measure oxidation of L-[1–13C]phenylalanine to 13CO2 (F13CO2). Plasma was collected at the sixth hour of the study day. Linear regression crossover analysis and simple linear regression were used to assess responses in F13CO2 and plasma 5-oxoproline concentrations to different glycine intakes.
Results
No statistically significant responses were observed in midgestation. However, in late gestation, lower glycine intakes resulted in higher rates of F13CO2 (suggesting low protein synthesis) with a breakpoint for phenylalanine oxidation at >37 mg glycine·kg−1·d−1 and higher plasma 5-oxoproline (suggesting low glycine availability) with a breakpoint >27 mg glycine·kg−1·d−1.
Conclusions
The findings suggest that glycine should be considered a “conditionally” indispensable amino acid during late gestation, especially when protein intakes are at 0.88 g·kg−1·d−1, the current EAR. This trial was registered at clinicaltrials.gov as NCT02149953.
Keywords: glycine, pregnancy, requirements, human, stable isotopes
See corresponding commentary on page 275.
Introduction
Pregnancy is accompanied by changes in energy and nutrient requirements due to profound body adaptations and high rates of tissue synthesis (1). We determined the protein requirements during early (∼16 wk) and late (∼36 wk) gestation in healthy singleton pregnant women using the indicator amino acid oxidation (IAAO) method. The estimated average requirements (EARs) were determined to be 1.22 g·kg−1·d−1 (upper 95% CI: 1.66 g·kg−1·d−1) and 1.52 g·kg−1·d−1 (upper 95% CI: 1.77 g·kg−1·d−1) during early and late gestation, respectively (2). These values are considerably higher than the current EAR and RDA of 0.88 and 1.1 g·kg−1·d−1, respectively (2, 3), although we have reported that current median intakes in healthy pregnant women are 1.3 and 1.5 g·kg−1·d−1 (3). Further, we recently demonstrated that the indispensable amino acid (IAA) lysine and phenylalanine requirements during late gestation are higher compared with early gestation (4, 5).
While the IAAs are clearly a major focus to ensure adequate dietary guidelines in pregnancy, the dispensable amino acids (DAAs) have recently been of focus in animal reproduction. Supplementing pig diets with arginine and/or glutamine has been shown to improve piglet birth weight and improve efficiency of nutrient utilization by optimizing placental growth (6). In a recent study, Tessari (7) estimated adult human DAA needs using factorial calculations. It was suggested that under some conditions, the endogenous synthesis of DAA may not be sufficient to meet body demands.
Glycine has traditionally been classified as a DAA, as it can be synthesized in the human body. Glycine is used for synthesis of glutathione, heme, creatine, nucleic acid, and uric acid (8). In addition, glycine is a main component of bile acids and makes up one-third of the amino acids in collagen, the most abundant protein in the human body (9). It has been suggested that de novo synthesis of glycine is insufficient to supply the metabolic demand (8, 9). Studies have shown decreased glycine flux in pregnant adolescents, suggesting an inability to maintain endogenous glycine production (10). With increasing stages of pregnancy, there is a progressive increase in excretion of 5-oxoproline (11). Conversion of γ-glutamylcysteine to glutathione (GSH) requires glycine, and when glycine availability is low, 5-oxoproline is formed (Supplemental Figure 1). Furthermore, glycine plays a central role in 1-carbon metabolism, and during pregnancy, perturbations of the transfer of methyl groups might affect cell proliferation and function (12).
Recently, we explored the role of 9 of the DAAs [alanine (Ala), arginine (Arg), asparagine (Asn), aspartate (Asp), glutamine (Gln), glutamate (Glu), glycine (Gly), proline (Pro), serine (Ser)] as an ideal nitrogen source to improve whole-body protein synthesis using the IAAO method in adults. We observed that 7 of the 9 DAAs (Ala, Arg, Asn, Asp, Glu, Gly, Ser) decreased IAAO significantly, except Gln and Pro (13). Whether such differences exist during pregnancy, especially during different stages of gestation, is unknown. We explored glycine's role in pregnancy first because of its implications in 1-carbon metabolism and because of the potential of using 5-oxoproline as a biomarker of glycine status. Therefore, the objectives of the current study were to determine whether there is a dietary demand for glycine during midgestation (20–29 wk) or late gestation (30–39 wk), examined using the IAAO, and measurement of plasma concentrations of 5-oxoproline and 1-carbon metabolites.
Methods
Participants
Healthy women aged between 20 and 40 y, pregnant with a single child, with reported prepregnancy BMI <30 (in kg/m2) were recruited for the current study. All participants provided written and informed consent and were screened to ensure they were not experiencing severe nausea or vomiting, gestational diabetes, preeclampsia, or any other health conditions. The women were also interviewed about prescription medication and supplement intake. Supplemental Figure 2 provides details on study flow, including screening and enrollment of pregnant participants. Financial compensation for transportation costs and an honorarium were offered to all participants upon completion of each study day. The study protocol was approved by the British Columbia Children's and Women's Hospital Research Ethics Board (H14–00495), and the study was registered at clinicaltrials.gov as NCT02149953.
Experimental design
The study design was based on the IAAO technique previously used to determine protein, lysine, and phenylalanine requirements in healthy pregnant women (2, 4, 5). Midgestation and late gestation were defined as 20–29 wk and 30–39 wk, respectively. The protocol included intakes ranging from 5 to 100 mg·kg−1·d−1, in 5-mg·kg−1·d−1 increments, selected randomly. Due to a combination of excluded study days (Supplemental Figure 2) and the selection method, not all intakes were used in each gestational stage. All study days completed by the same participant were separated by ≥5 d.
Preassessment
To determine eligibility, all potential participants attended a prestudy assessment at the Clinical Research and Evaluation Unit, BC Children's Hospital Research Institute. Participants arrived after a 10- to 12-h fast and were instructed to minimize physical exertion before the assessment. Fasted blood glucose concentrations were measured by finger prick (Ultra 2 LifeScan; One Touch) to screen for gestational diabetes, with a cutoff value of ≥5.3 mmol/L, based on the guidelines from the Canadian Diabetes Association (14). Protein and glucose in urine were assessed using Chemstrip 7 Urinalysis Strips (Roche Diagnostics) as potential indicators of gestational diabetes and preeclampsia, respectively. Body composition was measured by 3-site (biceps, triceps, and subscapular) skinfold assessments (Harpenden Calipers; Baty International) with site, sex, gestation stage, and age-specific factors used (15, 16) to calculate fat-free mass. Resting energy expenditure (REE) was assessed by continuous, open-circuit indirect calorimetry (Vmax Encore). Each woman's height and weight were measured using a stadiometer and digital scale, respectively.
A brief medical and pregnancy history was collected to screen for medication use, pregnancy complications, and general health. Two days prior to each study day, participants were prescribed a standardization diet, recommending protein intakes at 1.5 g·kg−1·d−1. The prescribed diets were based on food sources preferred by each participant, as indicated by a 2-d food record collected during the prestudy assessment. Participants also kept a 2-d food record 2 d prior to coming in for the study, in order to assess protein intake.
Study day protocol
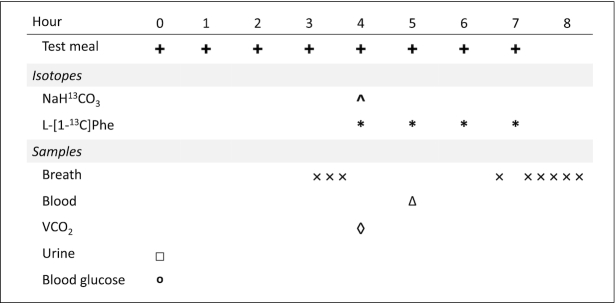
Each study day protocol is outlined in Figure 1. On the study day, participants arrived at the Clinical Research and Evaluation Unit after a 10- to 12-h fast. Height, weight, fasted blood glucose, and urinary measurements of glucose and protein were repeated at the beginning of each study day. Participants were randomly assigned to receive a test glycine intake (range: 5.0–100 mg·kg−1·d−1). The study day diet was consumed as 8-hourly isocaloric and isonitrogenous meals, each meal providing 1/12th of the daily energy requirement. The daily energy requirement was calculated as 1.7 × REE for each participant, as well as protein at 0.88 g·kg−1·d−1, based on the current EAR for healthy pregnant women (17). Each meal consisted of a small protein shake and protein-free cookies. The shakes contained a liquid formula made with protein-free powder (PFD1; Mead Johnson Nutrition), flavored drink crystals (Tang and Kool-Aid; Kraft Canada, Inc.), and corn oil (Mazola; ACH Food Companies, Inc.). The macronutrient composition of the diet was ∼53% from carbohydrate, ˜38% from fat, and ˜9% protein. The test protein was provided as a crystalline L-amino acid mixture (Ajinomoto, Inc.) based on the amino acid composition of egg protein, with the exception of glycine, and phenylalanine and tyrosine were provided at 31 and 61 mg·kg−1·d−1, respectively. As previously described by Elango et al. (18), the presence of excess tyrosine is necessary to minimize retention of the 13C label in the tyrosine pool. This ensures partitioning of the carboxyl carbon from phenylalanine and thus incorporation into protein or oxidation (18). With the exception of water, participants did not consume anything besides the experimental diets during the study day.
FIGURE 1.
Study day protocol. The experimental diets were given as hourly meals for 8 h. Priming doses of NaH13CO3 and L-[1–13C]phenylalanine were given with the fifth meal; hourly doses of L-[1–13C]phenylalanine were given with meals 5 through 8. Three breath samples were collected prior to the tracer protocol. Six plateau breath samples were collected after the tracer protocol. One venous blood sample was collected after the sixth hourly meal. Carbon dioxide production rate (VCO2) was measured by indirect calorimetry after the fifth hourly meal. A fasting blood glucose measurement and a urine sample were taken before the first meal.
Tracer protocol
During each 8-h study day, the participants initially consumed 4-hourly meals not containing L-[1–13C]phenylalanine to allow for baseline sample collection. An oral priming dose of 0.264 mg·kg−1 NaH13CO3 (99 atom percent excess; Cambridge Isotope Laboratories, Inc.) and 4.0 mg·kg−1 L-[1–13C]phenylalanine (99 atom percent excess; Cambridge Isotope Laboratories, Inc.) was provided at the fifth meal. Hourly oral doses of 3.0 mg·kg−1·h−1 L-[1–13C]phenylalanine were provided with the sixth to eighth meals until the end of the study (Figure 1).
Breath sample collection and analysis
During each study day, 3 and 6 breath samples were collected before (baseline) and after (isotopic steady state) the introduction of tracer amino acid, respectively, to measure the oxidation of L-[1–13C]phenylalanine to 13CO2 (F13CO2). Breath samples were collected using disposable exetainer tubes (Labco Limited) and rate of carbon dioxide production was measured using open-circuit indirect calorimetry (VMAX Encore; Viasys). Baseline breath samples were collected 45, 30, and 15 min before the tracer protocol began at hour 5, and isotopic steady-state samples were collected 150, 165, 180, 195, 210, and 240 min after the tracer protocol (Figure 1). Breath samples were stored at room temperature until analysis. Breath 13CO2 enrichment was determined using continuous-flow isotope ratio mass spectrometry (Isoprime Ltd) and expressed as atom percent excess (APE) (19).
Plasma amino acids and metabolites
Glycine interacts with the methionine cycle and in metabolism with several 1-carbon metabolites (12) as outlined in Supplemental Figure 1. Therefore, plasma concentrations of related amino acids and metabolites were measured and compared between mid and late stages of pregnancy in response to glycine intakes.
Plasma collection
Venous blood samples were collected at BC Children's and Women's Hospital blood collection laboratory by a certified phlebotomist, using EDTA as the anticoagulant. The plasma was separated by centrifugation at 3000 × g at 4°C for 10 min, sampled, and stored immediately at −80°C until analyzed. Plasma samples were collected at the sixth hour of the study to ensure a steady-state period of amino acid enrichment.
Plasma analytical methods
Plasma free amino acid concentrations were determined by ion exchange chromatography, using an amino acid analyzer (Hitachi L8900), as previously described (20). 5-Oxoproline was quantified by LC-MS/MS, as described previously (21). Choline, betaine, dimethylglycine (DMG), methionine, cysteine, and total homocysteine were analyzed by HPLC-MS/MS as previously described in detail by Friesen et al. (21) and Innis and Hasman (22).
S-adenosylmethionine (SAM) and S-adenosylhomocysteine (SAH) were analyzed by Waters H-class UHPLC and Waters Xevo tandem mass spectrometer (Waters Corp.), using a Zorbax SB-Aqua 2.1 × 100-mm column 3.5-micron particle size column with guard column (Agilent). Mobile phase A was composed of deionized water with 0.2% heptafluorobutyric acid (HFBA) and 0.1% formic acid. Mobile phase B consisted of methanol, LCMS grade; 0.2% HFBA; and 0.1% formic acid. Gradient separation was performed at a flow rate of 300 μL/min starting at 100% A. Then, 50 μL plasma was added to an Eppendorf tube containing 10 μL internal standard. Next, 50 μL 20% HFBA was added, and the sample was vortexed and left to stand at room temperature for 10 min, then centrifuged at 20,000 × g for 10 min at 4°C. The supernatant was removed and injected directly into the instrument.
Tracer oxidation
The rate of phenylalanine tracer oxidation (F13CO2, µmol·kg−1·h−1) was calculated as follows:
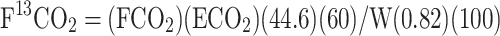 |
(1) |
where FCO2 represents carbon dioxide production (mL/min), ECO2 is the 13CO2 enrichment in breath at plateau isotopic steady state (APE), W is the weight (kg) of the subject, 44.6 (µmol/L) and 60 (min/h) are constants used to convert FCO2 to µmol/h, 0.82 is the correction factor for carbon dioxide retained by the body due to bicarbonate fixation, and 100 is used to convert APE to a fraction (23).
Statistical analysis
Results are reported as means ± SDs. Breakpoint analysis was performed using a 2-phase linear regression crossover model of the F13CO2, 5-oxoproline, SAM, and SAH data from late stages of pregnancy. The method selects the model with minimum residual standard error in a stepwise partitioning of glycine intake values (x) between 2 regression lines. The lines are estimated for each selected candidate breakpoint using mixed models (Proc Mixed, Statistical Analysis Systems—SAS/STAT version 9.4; SAS Institute) to account for variability in number of completed study days per participant (24). The final model that best fit the data with the lowest SE, lowest root mean square error, and the highest R2 identified the breakpoint. The 95% CI was calculated using Fieller's theorem (25): 95% CI = breakpoint ± tdf, α/2 × SE, where SE is the SE of the combined regression lines, df is the degrees of freedom associated with the residual mean square of the best-fit model, and α is the 95% CI level (2, 19). The effect of glycine intake on plasma concentrations of the remaining biomarkers was assessed using linear regression (GraphPad Prism 6; GraphPad Software). Significance was set at P < 0.05 for all analysis.
Results
Participants
A total of 17 (nmid = 8 and nlate = 9) women were studied, completing 36 individual study days (17 study days in midgestation and 19 study days in late gestation) (Table 1, Supplemental Figure 2). Three participants were studied in both midgestation and late gestation, each completing 2, 1, and 5 study days in midgestation and 2, 3, and 2 in late gestation, respectively (Supplemental Table 1). For these 3 women, a second prestudy assessment was performed prior to study days conducted during late gestation, to allow measurement for updated body weight and REE, as well as to reassess their health. All participants were otherwise unique between stages. Of the remaining participants recruited for midgestation, 3 participants came for 1 study day and 3 participants came for 2 study days. For late gestation among the remaining participants recruited, 1 participant came for 1 study day, 2 participants came for 2 study days, 1 participant came for 3 study days, and 1 participant came for 4 study days (Supplemental Table 1).
TABLE 1.
Characteristics of pregnant women at preassessment during mid and late gestation1
| Characteristic | Midgestation | Late gestation |
|---|---|---|
| Participants,2n | 9 | 8 |
| Gestational age, wk | 23.3 ± 4.2 | 31.8 ± 2.3 |
| Age, y | 30.7 ± 3.1 | 31.4 ± 3.8 |
| Prepregnancy weight, kg | 61.7 ± 13.2 | 59.0 ± 9.7 |
| Height, m | 1.64 ± 0.08 | 1.62 ± 0.06 |
| Prepregnancy BMI,3 kg/m2 | 22.7 ± 3.2 | 22.2 ± 2.9 |
| Fat mass,4 % | 26.7 ± 4.9 | 22.3 ± 3.1 |
| Resting energy expenditure,5 kcal/d | 1483 ± 195 | 1484 ± 210 |
Values are means ± SDs, n = 17 observations in 9 women (mid) or 19 observations in 8 women (late).
Three women participated in both mid- and late-gestation studies.
Based on participant reported prepregnancy weight.
Determined by skinfold measurements (Harpenden Skinfold Caliper; Baty International).
Determined by open-circuit indirect calorimetry (VMax Encore; Viasys).
Women who participated in this study were not experiencing nausea or vomiting during the study days and reported no pregnancy complications. One woman reported having Crohn disease; however, the disease was inactive while participating in the study. Two women reported using SynthyroidTM (levothyroxine, AbbVie Inc.) for hypothyroidism and 1 woman reported using DiclectinTM (doxylamine succinate – pyridoxine, Duchesnay Inc.) for pregnancy-related nausea in midpregnancy. One woman had finished consuming antibiotics the day prior to coming in for a study day due to a urinary tract infection. No medications were consumed on the study day. None of the women studied reported consuming alcohol or illicit substances at any time during their pregnancy. All participants were consuming daily prenatal multivitamin supplements for the duration of enrollment in this study.
The mean ± SD prepregnancy BMIs were within normal range for both mid (22.7 ± 3.2) and late (22.2 ± 2.9) pregnancy stages (26), and fasting blood glucose concentrations were ≤5.3 mmol/L (Table 2) (14, 26). The mean ± SD protein intakes for the 2 d prior to each study day were lower than prescribed (1.2 ± 0.3 and 1.3 ± 0.3 g·kg−1·d−1 for midgestation and late gestation, respectively) but similar to our recent pregnancy study (5).
TABLE 2.
Study day assessments of healthy pregnant women during midgestation and late gestation1
| Variable | Midgestation (n = 17) | Late gestation (n = 19) |
|---|---|---|
| Weight, kg | 64.9 ± 11.6 | 72.4 ± 10.4 |
| Fasting blood glucose, mmol/L | 4.4 ± 0.4 | 4.6 ± 0.5 |
| VCO2, mL/min | 225 ± 32 | 257 ± 38 |
| Gestational age, wk | 25.9 ± 3.3 | 34.6 ± 2.7 |
| Energy intake, kcal/d | 2438 ± 338 | 2620 ± 335 |
| Protein intake before study day,2 g·kg−1·d−1 | 1.2 ± 0.3 | 1.3 ± 0.3 |
Values are means ± SDs, n = 17 observations in 9 women (mid) or 19 observations in 8 women (late). VCO2, volume of carbon dioxide production per minute.
Amount of protein consumed by participants in the 2 d before the study day, as indicated by dietary records.
L-[1–13C]phenylalanine oxidation
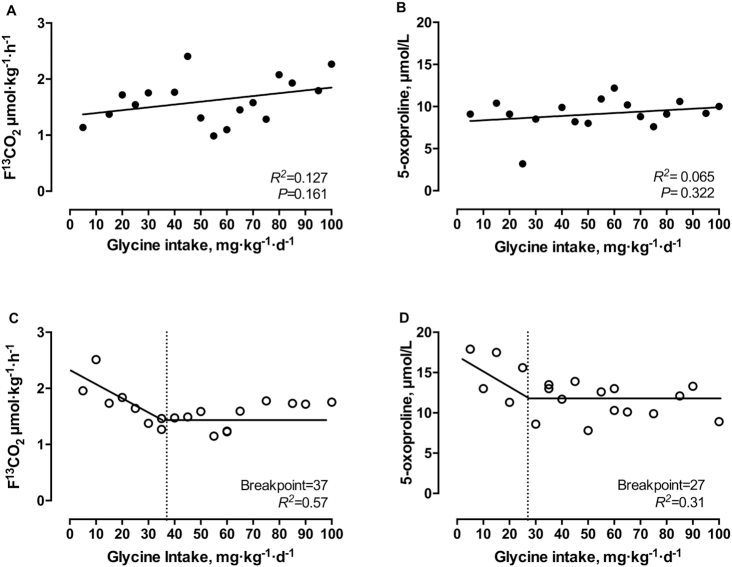
With increasing intakes of glycine (5–100 mg·kg−1·d−1) in midgestation, linear regression analysis showed no significant relation (R2 = 0.127, P = 0.161; Figure 2A). In late gestation, the oxidation of L-[1–13C]phenylalanine (F13CO2) was higher with low glycine intakes and declined with increasing glycine intakes. Two-phased linear regression analysis of the F13CO2 data identified a breakpoint at 37 mg·kg−1·d−1(R2 = 0.57; 95% CI: 17, 58 mg·kg−1·d−1; Figure 2C).
FIGURE 2.
Effect of graded glycine intakes on F¹³CO2 (A, C) and plasma 5-oxoproline (B, D) during midgestation (A, B) and late (C, D) gestation in healthy pregnant women. There were 17 observations in 9 women (mid) or 19 observations in 8 women (late). Linear regression analysis was performed on midgestation data. Biphase linear regression crossover analysis was performed on late gestation data.
5-Oxoproline
With increasing glycine intakes (5–100 mg·kg−1·d−1) in midgestation, no pattern was observed in plasma 5-oxoproline concentrations (R2 = 0.065, P = 0.322; Figure 2B). However, plasma 5-oxoproline concentrations decreased with increasing intakes of glycine (5–100 mg·kg−1·d−1) in late gestation (Figure 2D). Two-phased linear regression analysis identified a breakpoint at 27 mg·kg−1·d−1 (R2 = 0.31; 95% CI: 14, 40 mg·kg−1·d−1; Figure 2D).
Plasma concentrations of amino acids and metabolites
Glycine and serine
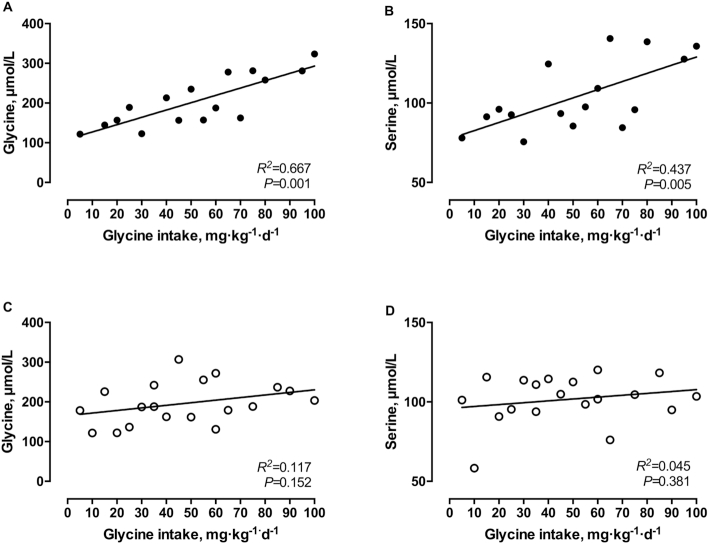
During midgestation, plasma glycine and serine concentrations increased significantly with increasing glycine intakes (5–100 mg·kg−1·d−1) (R2 = 0.667, P = 0.0001 and R2 = 0.437, P = 0.0053, respectively; Figure 3A,B), whereas no significant increase in plasma glycine or serine concentrations was observed in late gestation (R2 = 0.117, P = 0.152 and R2 = 0.045, P = 0.381, respectively; Figure 3C,D).
FIGURE 3.
Plasma concentrations of glycine (A, C) and serine (B, D) in response to graded intakes of glycine during midgestation (A, B) and late gestation (C, D) in healthy pregnant women. There were 17 observations in 9 women (mid) or 19 observations in 8 women (late). Linear regression analysis was performed on all data.
Methionine, homocysteine, and cysteine
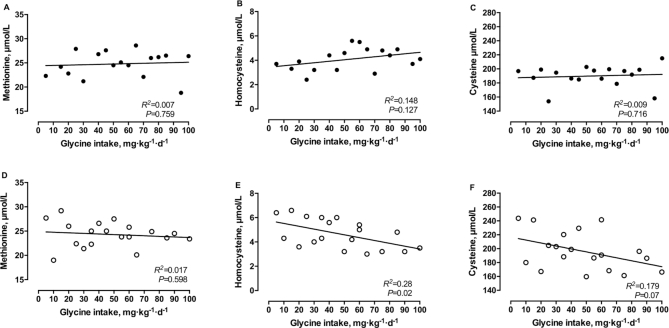
No significant change in plasma methionine concentration was observed for either midgestation (R2 = 0.007, P = 0.759; Figure 4A) or late gestation (R2 = 0.017, P = 0.598; Figure 4D) with increasing glycine intakes (5–100 mg·kg−1·d−1). Similarly, no significant changes in plasma concentrations of cysteine were observed for either midgestation or late gestation (R2 = 0.009, P = 0.716 and R2 = 0.179, P = 0.071, respectively; Figure 4C,F). Interestingly, plasma concentrations of homocysteine decreased significantly with increasing intakes of glycine (5–100 mg·kg−1·d−1) in late gestation (R2 = 0.28, P = 0.0197; Figure 4E), which was not observed in midgestation (R2 = 0.148, P = 0.127; Figure 4B).
FIGURE 4.
Plasma concentrations of methionine (A, D), homocysteine (B, E), and cysteine (C, F) in response to graded intakes of glycine during midgestation (A, B, C) and late gestation (D, E, F) in healthy pregnant women. There were 17 observations in 9 women (mid) or 19 observations in 8 women (late). Linear regression analysis was performed on all data.
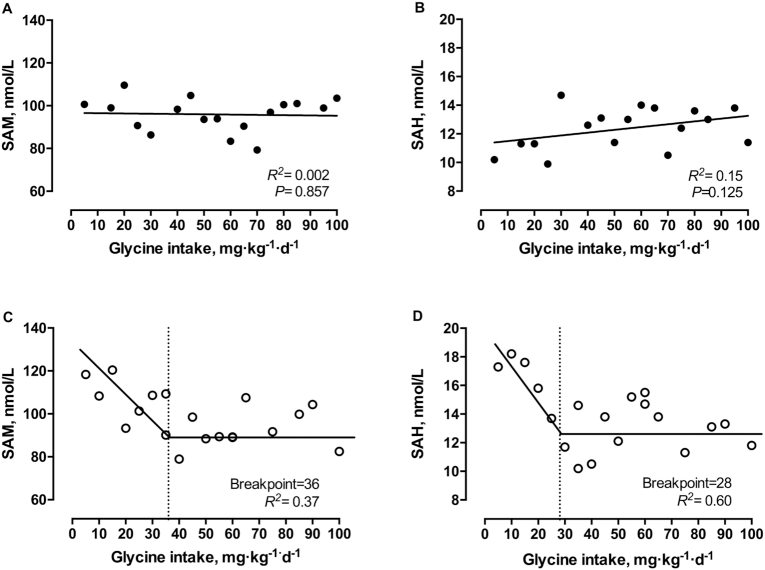
SAM and SAH
SAM and SAH concentrations decreased with increasing intakes of glycine (5–100 mg·kg−1·d−1) in late gestation. Two-phased linear regression analysis identified breakpoints at 36 mg·kg−1·d−1 (95% CI: 21, 51 mg·kg−1·d−1; R2 = 0.37; Figure 5C) for SAM and at 28 mg·kg−1·d−1 (95% CI: 22, 35 mg·kg−1·d−1; R2 = 0.60; Figure 5D) for SAH in late gestation. In contrast, for midgestation, there were no significant changes in plasma concentrations of SAM (R2 = 0.002, P = 0.857) or SAH (R2 = 0.15, P = 0.13), with increasing glycine intakes (5–100 mg·kg−1·d−1) (Figure 5A,B).
FIGURE 5.
Plasma concentrations of S-adenosylmethionine (SAM) (A, C) and S-adenosylhomocysteine (SAH) (B, D) in response to graded intakes of glycine in healthy pregnant women during midgestation (A, B) and late gestation (C, D) in healthy pregnant women. There were 17 observations in 9 women (mid) or 19 observations in 8 women (late). Linear regression analysis was performed on midgestation data. Biphase linear regression crossover analysis was performed on late gestation data.
Choline, betaine, DMG, and sarcosine
There were no significant responses in plasma choline, betaine, or sarcosine to increasing glycine intakes (5–100 mg·kg−1·d−1) during midgestation (R2 = 0.0005, P = 0.934, R2 = 0.0003; P = 0.957, R2 = 0.046, P = 0.425; Supplemental Figure 3A,B,D) and late gestation (R2 = 0.137, P = 0.119, R2 = 0.005; P = 0.772, R2 = 0.08, P = 0.239; Supplemental Figure 3E,F,H). In midgestation, DMG concentrations tended to increase (R2 = 0.22, P = 0.055), which was not observed in late gestation (R2 = 0.048, P = 0.36) (Supplemental Figure 3C,G).
Ornithine, citrulline, arginine, urea, and histidine
In both midgestation and late gestation, no significant changes were observed in plasma citrulline (R2 = 0.196, P = 0.086 and R2 = 0.059, P = 0.318; Supplemental Figure 5B,G) and arginine (R2 = 3.14E-07, P = 0.998 and R2 = 0.012, P = 0.657; Supplemental Figure 5C,H) in response to increased glycine intakes (5–100 mg·kg−1·d−1) in both midgestation and late gestation. However, ornithine (R2 = 0.289, P = 0.018), histidine (R2 = 0.258, P = 0.027), and urea (R2 = 0.543, P = 0.003) significantly decreased with increasing glycine intakes in late gestation (Supplemental Figure 4F,J,I) but not in midgestation (R2 = 0.002, P = 0.888, R2 = 0.0002; P = 0.962, R2 = 0.004, P = 0.807; Supplemental Figure 4A,E,D).
Discussion
To our knowledge, this is the first study to directly address the impact of a range of glycine intakes (low to high) in pregnant women at 2 distinct gestational stages (mid and late). Our results suggest that restriction of glycine in acute dietary studies during midgestation (∼26 wk) does not have significant effects on whole-body protein synthesis, plasma 5-oxoproline, and 1-carbon metabolite concentrations. However, in late stages of gestation (∼35 wk), restriction of glycine reduced whole-body protein synthesis (as observed by high 13C-phenylalanine oxidation, F13CO2) and increased plasma concentrations of 5-oxoproline, which plateaued with increasing intakes of glycine (>37 mg·kg−1·d−1). Earlier 5-oxoproline excretion has been suggested as a marker for glycine status in pregnant women (27). Synthesis of GSH, a tripeptide of cysteine, glutamate, and glycine, is initiated when glutamate is combined with cysteine to form γ-glutamyl cysteine (Supplemental Figure 1). With the addition of glycine to γ-glutamyl cysteine, GSH is produced. However, when glycine is limiting, γ-glutamyl cysteine is metabolized to 5-oxoproline. Previous studies have shown that in late pregnancy, plasma 5-oxoproline is increased by 128% compared with nonpregnant women, possibly due to glycine insufficiency, which may limit GSH synthesis (21). Furthermore, Jackson et al. (11) showed that compared with nonpregnant women, urinary excretion of 5-oxoproline in late pregnancy was 365% higher when standardized against creatinine, suggesting that endogenous synthesis of glycine may be insufficient during late pregnancy. To our knowledge, our findings are the first to provide evidence of a direct relation between glycine intake and plasma 5-oxoproline concentrations during pregnancy. Taken together, our results suggest that glycine is conditionally indispensable during late stages of pregnancy.
In late but not midpregnancy, we found that low glycine intakes are associated with increased plasma SAM and SAH concentrations, which plateaued with increasing glycine intakes (>37 mg·kg−1·d−1). The exact mechanisms behind these findings are unknown. We hypothesize that with our study design supplying adequate methionine (and most 1-carbon nutrients involved in the methionine cycle) with low glycine intakes, it is possible that glycine N-methyltransferase, which catalyzes glycine to sarcosine, is downregulated (28). However, phosphatidylethanolamine N-methyltransferase, the enzyme responsible for converting phosphatidylethanolamine to phosphatidylcholine, which also converts SAM to SAH, is upregulated (29, 30). Similar to our results, recently, van Riet et al. (31) followed a set of sows throughout gestation and observed that by late stages of gestation, plasma concentrations of SAM and SAH were positively correlated with plasma methionine concentrations and negatively correlated with plasma glycine concentrations. Finkelstein (32) has stated earlier that plasma concentrations of SAM and SAH need to be interpreted with caution, as they do not reflect intracellular concentrations. However, since we observed changes in SAM and SAH plasma concentrations in late gestation and not in midgestation in response to glycine intakes using the same study design, the relative differences are comparable and suggest that glycine availability is insufficient in later stages of pregnancy.
Structurally, glycine is the simplest amino acid and is a key component of proteins, such as collagen, and important for nucleic acids, heme, and creatine synthesis (8). Thus, a developing fetus has an increased demand for glycine. Previously, how the fetus meets the demand for glycine has been explored (12). Glycine can be synthesized from serine via serine hydroxymethyltransferase (SHMT), and since sheep placenta express a significant amount of SHMT, it was hypothesized that maternal serine is converted to glycine in the placenta and transferred to the fetus (33). But this theory has been questioned due to low SHMT activity in human placentas, and studies of amino acid uptake and interconversions suggest that the serine-glycine transfers may not be that significant (34). In the current study, plasma concentrations of glycine and serine increased significantly with increasing glycine intakes in midgestation but not in late gestation. The women received adequate serine in the diet, and thus our results suggest that in human late-stage pregnancies, de novo synthesis of glycine was inadequate and there might be a demand for maternal preformed glycine.
From a physiologic viewpoint, all of the amino acids that occur in proteins, whether they are synthesized in the body or not, are essential for tissue protein synthesis and various other functions (35). Recently, there have been several discussions on whether a dietary need can be determined for DAA (7, 36, 37). Tessari (7) uses the term nonessential amino acid usage, and based on factorial estimates of whole-body amino acid composition and obligatory nitrogen losses, has suggested estimates for all amino acids. Glycine has been suggested to have a usage of 46 to 59 mg·kg−1·d−1 in healthy nonpregnant adults (7). Our results suggest that during late stages of pregnancy, there is a potential need for preformed glycine at 37 mg·kg−1·d−1. One key aspect in the current study is that the protein intake on study days was at the current recommended EAR of 0.88 g·kg−1·d−1 for protein during pregnancy (17). This is considerably lower than the EAR during late stages of pregnancy, as determined recently by us (1.52 g·kg−1·d−1) (2). The women in our study received protein at 57–67 g/d and 64–73 g/d in midgestation and late gestation, respectively. These are lower than normally reported by pregnant mothers in Canada (∼99 g/d) (3). Consequently, while the moderate protein intake in the current study during midgestation did not affect de novo glycine synthesis, in late gestation, glycine synthesis was not sufficient. On average, high-quality protein diets provide ∼33 mg/kg protein, suggesting that an intake of ∼1.1 g·kg−1·d−1 would be needed to satisfy a glycine need of 37 mg·kg−1·d−1 in pregnancy. While these intakes are normally achieved in developed countries (3), our findings have implications for pregnant populations who consume diets with low protein quantity and quality (38, 39). In developing countries, total protein intakes in pregnancy is ∼50 g/d, which translates to ∼0.83 g·kg−1·d−1 for a 60-kg body weight (38). Thus, glycine needs are unlikely to be met at these protein intakes, emphasizing the “conditional essential” nature of this amino acid. Similar to our study, Yu et al. (40), using 15N-tracers, showed earlier in adults that when dietary nitrogen was provided as IAA alone with a total protein at 0.6 g·kg−1·d−1 (EAR for adults) (17), alanine synthesis was not affected, but glycine synthesis declined (40). A later longer-term study in young adults, using L-5-[1–13C]oxoproline and diets either free of glycine or methionine + cysteine, showed by day 6 significantly higher urinary excretion and oxidation of 5-oxoproline (41). Thus, even in well-nourished adult men, glycine supply is crucial in the context of length of dietary restriction or when restricted in dispensable amino acid nitrogen.
Our study has a few limitations with respect to small sample size and the acute study design. However, the strength of the study is the use of a study design earlier applied during pregnancy to determine protein/amino acid requirements and measurement of several plasma metabolites related to glycine metabolism at 2 gestational stages in pregnancy (2, 4, 5). It should be emphasized, however, that our objective in the study was not to determine a “requirement” for glycine during pregnancy but rather test the hypothesis whether a dietary need for glycine exists during stages of pregnancy. Future studies need to be conducted with a range of glycine intakes with adequate protein and measurement of other markers, including synthesis of glutathione and creatine during pregnancy (42).
In summary, the current study showed an increased rate of IAAO when glycine intakes were low, suggesting low protein synthesis in late gestation but not in midgestation. Plasma 5-oxoproline concentrations, an independent biomarker of glycine status, were also elevated with low glycine intakes only in late stages of pregnancy. Patterns of response in plasma 1-carbon metabolites, including SAM, SAH, and, to a lesser extent, choline, were also different in late gestation. Taken together, these findings suggest that glycine de novo synthesis may be insufficient during late pregnancy and is conditionally indispensable, especially when protein intakes are at the current recommended pregnancy EAR of 0.88g·kg−1·d−1.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all of the pregnant mothers who participated in the study and Erin Gilbert at the BC Children's Hospital Research Institute for technical assistance in the laboratory.
The authors’ responsibilities were as follows—RE: study design; BFR and MAE: recruitment, consent, data and sample collection; KL: recruitment and clinical support; BFR, MAE, and RAD: sample analyses; BFR, MAE, and RE: data analysis; BFR, MAE, RAD, KL, and RE: manuscript writing; RE: had primary responsibility for final content; all authors: read and approved the final manuscript.
Notes
Sources of support: Canadian Institutes of Health Research (FRN-134069).
Author disclosures: The authors report no conflicts of interest.
Supplemental Table 1 and Supplemental Figures 1–4 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://jn.nutrition.org.
Abbreviations used: APE, atom percent excess; DAA, dispensable amino acid; DMG, dimethylglycine; EAR, estimated average requirement; F13CO2, rate of appearance of (13C) labeled carbon dioxide in breath; GSH, glutathione; HFBA, heptafluorobutyric acid; IAA, indispensable amino acids; IAAO, indicator amino acid oxidation; REE, resting energy expenditure; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; SHMT, serine hydroxymethyltransferase.
Contributor Information
Betina F Rasmussen, BC Children's Hospital Research Institute, Vancouver, British Columbia, Canada; Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada.
Madeleine A Ennis, BC Children's Hospital Research Institute, Vancouver, British Columbia, Canada; Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada.
Roger A Dyer, BC Children's Hospital Research Institute, Vancouver, British Columbia, Canada; Analytical Core for Metabolomics and Nutrition (ACMaN), BC Children's Hospital Research Institute, Vancouver, British Columbia, Canada.
Kenneth Lim, BC Children's Hospital Research Institute, Vancouver, British Columbia, Canada; Department of Obstetrics and Gynecology, University of British Columbia, Vancouver, British Columbia, Canada.
Rajavel Elango, BC Children's Hospital Research Institute, Vancouver, British Columbia, Canada; Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada; School of Population and Public Health, University of British Columbia, Vancouver, British Columbia, Canada.
References
- 1. Elango R, Ball RO.. Protein and amino acid requirements during pregnancy. Adv Nutr. 2016;7:839S–44S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stephens TV, Payne M, Ball RO, Pencharz PB, Elango R. Protein requirements of healthy pregnant women during early and late gestation are higher than current recommendations. J Nutr. 2015;145:73–8. [DOI] [PubMed] [Google Scholar]
- 3. Stephens TV, Woo H, Innis SM, Elango R. Healthy pregnant women in Canada are consuming more dietary protein at 16- and 36-week gestation than currently recommended by the Dietary Reference Intakes, primarily from dairy food sources. Nutr Res. 2014;34:569–76. [DOI] [PubMed] [Google Scholar]
- 4. Payne M, Stephens T, Lim K, Ball RO, Pencharz PB, Elango R. Lysine requirements of healthy pregnant women are higher during late stages of gestation compared to early gestation. J Nutr. 2018;148:94–9. [DOI] [PubMed] [Google Scholar]
- 5. Ennis MA, Rasmussen BF, Lim K, Ball RO, Pencharz PB, Courtney-Martin G, Elango R. Dietary phenylalanine requirements during early and late gestation in healthy pregnant women. Am J Clin Nutr. 2020;111:351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Li XL, Satterfield MC, Spencer TE. Impacts of amino acid nutrition on pregnancy outcome in pigs: mechanisms and implications for swine production. J Anim Sci. 2010;88:E195–204. [DOI] [PubMed] [Google Scholar]
- 7. Tessari P. Nonessential amino acid usage for protein replenishment in humans: a method of estimation. Am J Clin Nutr. 2019;110:255–64. [DOI] [PubMed] [Google Scholar]
- 8. Wang W, Wu Z, Dai Z, Yang Y, Wang J, Wu G. Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids. 2013;45:463–77. [DOI] [PubMed] [Google Scholar]
- 9. Meléndez-Hevia E, De Paz-Lugo P, Cornish-Bowden A, Cárdenas ML. A weak link in metabolism: the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis. J Biosci. 2009;34:853–72. [DOI] [PubMed] [Google Scholar]
- 10. Hsu JW, Thame MM, Gibson R, Baker TM, Tang GJ, Chacko SK, Jackson AA, Jahoor F. Unlike pregnant adult women, pregnant adolescent girls cannot maintain glycine flux during late pregnancy because of decreased synthesis from serine. Br J Nutr. 2016;115:759–63. [DOI] [PubMed] [Google Scholar]
- 11. Jackson AA, Persaud C, Werkmeister G, McClelland IS, Badaloo A, Forrester T. Comparison of urinary 5-L-oxoproline (L-pyroglutamate) during normal pregnancy in women in England and Jamaica. Br J Nutr. 1997;77:183–96. [DOI] [PubMed] [Google Scholar]
- 12. Kalhan SC. One carbon metabolism in pregnancy: impact on maternal, fetal and neonatal health. Mol Cell Endocrinol. 2016;435:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cooper L, Ball RO, Pencharz PB, Sakai R, Elango R. Dispensable amino acids, except glutamine and proline, are ideal nitrogen sources for protein synthesis in the presence of adequate indispensable amino acids in adult men. J Nutr. 2020;150(9):2398–404. [DOI] [PubMed] [Google Scholar]
- 14. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee, Thompson D, Berger H, Feig D, Gagnon R, Kader T, Keely E, Kozak S, Ryan E, Sermer Met al. Diabetes and pregnancy. Can J Diabetes. 2013;37(Suppl 1):S168–83. [DOI] [PubMed] [Google Scholar]
- 15. Durnin JV, Womersley J.. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. [DOI] [PubMed] [Google Scholar]
- 16. Soltani H, Fraser RB.. A longitudinal study of maternal anthropometric changes in normal weight, overweight and obese women during pregnancy and postpartum. Br J Nutr. 2000;84:95–101. [DOI] [PubMed] [Google Scholar]
- 17. Institute of Medicine, editors. Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington (DC): National Academies Press; 2005. [Google Scholar]
- 18. Elango R, Ball RO, Pencharz PB. Recent advances in determining protein and amino acid requirements in humans. Br J Nutr. 2012;108(Suppl 2):S22–30. [DOI] [PubMed] [Google Scholar]
- 19. Elango R, Humayun MA, Ball RO, Pencharz PB. Protein requirement of healthy school-age children determined by the indicator amino acid oxidation method. Am J Clin Nutr. 2011;94:1545–52. [DOI] [PubMed] [Google Scholar]
- 20. Rasmussen B, Gilbert E, Turki A, Madden K, Elango R. Determination of the safety of leucine supplementation in healthy elderly men. Amino Acids. 2016;48:1707–16. [DOI] [PubMed] [Google Scholar]
- 21. Friesen RW, Novak EM, Hasman D, Innis SM. Relationship of dimethylglycine, choline, and betaine with oxoproline in plasma of pregnant women and their newborn infants. J Nutr. 2007;137:2641–6. [DOI] [PubMed] [Google Scholar]
- 22. Innis SM, Hasman D.. Evidence of choline depletion and reduced betaine and dimethylglycine with increased homocysteine in plasma of children with cystic fibrosis. J Nutr. 2006;136:2226–31. [DOI] [PubMed] [Google Scholar]
- 23. Hoerr RA, Yu YM, Wagner DA, Burke JF, Young VR. Recovery of 13C in breath from NaH13CO3 infused by gut and vein: effect of feeding. Am J Physiol. 1989;257:E426–38. [DOI] [PubMed] [Google Scholar]
- 24. Wang Z, Goonewardene LA.. The use of mixed models in the analysis of animal experiments with repeated measures data, Can J Anim Sci. 2004;84:1–11. [Google Scholar]
- 25. Seber GAF Linear regression analysis. New York: Wiley; 1977. [Google Scholar]
- 26. Institute of Medicine, National Research Council Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight gain during pregnancy: reexamining the guidelines. [Internet] Washington (DC): National Academies Press; 2009; [cited 2020 Jun 4]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK32813/. [Google Scholar]
- 27. Persaud C, McDermott J, De Benoist B, Jackson AA. The excretion of 5-oxoproline in urine, as an index of glycine status, during normal pregnancy. Br J Obstet Gynaecol. 1989;96:440–4. [DOI] [PubMed] [Google Scholar]
- 28. Luka Z, Mudd SH, Wagner C. Glycine N-methyltransferase and regulation of S-adenosylmethionine levels. J Biol Chem. 2009;284:22507–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martínez‐Uña M, Varela‐Rey M, Cano A, Fernández‐Ares L, Beraza N, Aurrekoetxea I, Martínez‐Arranz I, García‐Rodríguez JL, Buqué X, Mestre Det al. Excess S-adenosylmethionine reroutes phosphatidylethanolamine towards phosphatidylcholine and triglyceride synthesis. Hepatology. 2013;58:1296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yan J, Jiang X, West AA, Perry CA, Malysheva OV, Brenna JT, Stabler SP, Allen RH, Gregory JF, Caudill MA. Pregnancy alters choline dynamics: results of a randomized trial using stable isotope methodology in pregnant and nonpregnant women. Am J Clin Nutr. 2013;98:1459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Riet MMJ, Millet S, Langendries KCM, van Zelst BD, Janssens GPJ. Association between methylation potential and nutrient metabolism throughout the reproductive cycle of sows. J Anim Physiol Anim Nutr (Berl). 2019;103:858–67. [DOI] [PubMed] [Google Scholar]
- 32. Finkelstein JD. Metabolic regulatory properties of S-adenosylmethionine and S-adenosylhomocysteine. Clin Chem Lab Med. 2007;45:1694–9. [DOI] [PubMed] [Google Scholar]
- 33. Lewis RM, Godfrey KM, Jackson AA, Cameron IT, Hanson MA. Low serine hydroxymethyltransferase activity in the human placenta has important implications for fetal glycine supply. J Clin Endocrinol Metab. 2005;90:1594–8. [DOI] [PubMed] [Google Scholar]
- 34. Holm MB, Bastani NE, Holme AM, Zucknick M, Jansson T, Refsum H, Mørkrid L, Blomhoff R, Henriksen T, Michelsen TM. Uptake and release of amino acids in the fetal-placental unit in human pregnancies. PLoS One. 2017;12:e0185760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harper AE. Editorial: “Nonessential” amino acids. J Nutr. 1974;104:965–7. [DOI] [PubMed] [Google Scholar]
- 36. Tessari P. Are there dietary requirements for dispensable amino acids and if so, how do we assess requirements?. Curr Opin Clin Nutr Metab Care. 2019;22:329–36. [DOI] [PubMed] [Google Scholar]
- 37. Watford M Glutamine and glutamate: nonessential or essential amino acids? Anim Nutr. 2015;1:119–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Swaminathan S, Vaz M, Kurpad AV. Protein intakes in India. Br J Nutr. 2012;108(Suppl 2):S50–8. [DOI] [PubMed] [Google Scholar]
- 39. Ghosh S, Suri D, Uauy R. Assessment of protein adequacy in developing countries: quality matters. Br J Nutr. 2012;108(Suppl 2):S77–87. [DOI] [PubMed] [Google Scholar]
- 40. Yu YM, Yang RD, Matthews DE, Wen ZM, Burke JF, Bier DM, Young VR. Quantitative aspects of glycine and alanine nitrogen metabolism in postabsorptive young men: effects of level of nitrogen and dispensable amino acid intake. J Nutr. 1985;115:399–410. [DOI] [PubMed] [Google Scholar]
- 41. Metges CC, Yu YM, Cai W, Lu XM, Wong S, Regan MM, Ajami A, Young VR. Oxoproline kinetics and oxoproline urinary excretion during glycine- or sulfur amino acid-free diets in humans. Am J Physiol Endocrinol Metab. 2000;278:E868–76. [DOI] [PubMed] [Google Scholar]
- 42. Brosnan JT, da Silva RP, Brosnan ME. The metabolic burden of creatine synthesis. Amino Acids. 2011;40:1325–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.