ABSTRACT
The SARS-CoV-2 (COVID-19) pandemic has caused unprecedented morbidity, mortality and global disruption. Following the initial surge of infections, focus shifted to managing the longer-term sequelae of illness in survivors. ‘Post-acute COVID’ (known colloquially as ‘long COVID’) is emerging as a prevalent syndrome. It encompasses a plethora of debilitating symptoms (including breathlessness, chest pain, palpitations and orthostatic intolerance) which can last for weeks or more following mild illness. We describe a series of individuals with symptoms of ‘long COVID’, and we posit that this condition may be related to a virus- or immune-mediated disruption of the autonomic nervous system resulting in orthostatic intolerance syndromes. We suggest that all physicians should be equipped to recognise such cases, appreciate the symptom burden and provide supportive management. We present our rationale for an underlying impaired autonomic physiology post-COVID-19 and suggest means of management.
KEYWORDS: orthostatic, dizziness, dysautonomia, COVID-19, long COVID
Introduction
The SARS-CoV-2 (COVID-19) pandemic has caused unprecedented morbidity, mortality and disruption across the world. Following the initial phase, healthcare services are focusing now on controlling new cases and planning rehabilitation strategies. ‘Post-acute COVID’1 refers to persistent symptoms 3 weeks after COVID-19 infection, while ‘Chronic COVID’ describes symptoms lasting more than 12 weeks.2
Results of the COVID Symptom Study (based on a smartphone tracker app where users enter their symptoms, which had over 2.8 million recorded users by May 2020)3,4 are awaited, but smaller studies reveal that protracted symptoms following COVID-19 infection are common. In Italian inpatients, 53% had fatigue, 43% were dyspnoeic and 22% were experiencing chest pain after 2 months.2 Halpin et al reported that after 4–8 weeks, ongoing fatigue is present in more than two thirds, followed by breathlessness and symptoms of post-traumatic stress disorder.5 Mainstream media are giving publicity to patient-coined terms ‘long COVID’6 and ‘long-haul COVID’.7 The popularity and visibility of patient support groups such as wearebodypolitic.com (who according to their website have 14,000 subscribers) and the Long COVID SOS group (longcovidsos.org), and the frequent trending of the hashtag #longcovid on Twitter, suggest that patient need is high, and under-recognised by clinicians.
‘Long COVID’ and orthostatic intolerance syndromes
In our tertiary syncope unit, we have encountered patients with debilitating symptoms following viral infections who were subsequently found to have orthostatic intolerance syndromes. A summary is shown in Table 1. While only one case in this series had confirmed COVID-19, the others had symptoms consistent with a debilitating viral illness in early 2020 and were suspected cases during a period where COVID testing was not recommended or widely available. Of note, all of the individuals in this short series were female and were between the ages of 26 and 50 years old. They all had orthostatic intolerance with either resting or postural hypotension and/or tachycardia. We propose that some symptoms of the so-called ‘long COVID’ infection may be related to a virus- or immune-mediated disruption to the autonomic nervous system, resulting in transient or long-term orthostatic intolerance syndromes.
Table 1.
Symptom profile of individuals attending our clinic with autonomic symptoms following viral infection during the COVID-19 pandemic
| Patient | Symptoms | Timing of antecedent illness | Observations in clinic | Other investigations | Clinical diagnosis |
|---|---|---|---|---|---|
| 26-year-old female | Palpitations on standing Dyspnoea Fatigue | Gastrointestinal symptoms 5 days prior to symptoms (suspected viral illness) | Postural BP 104/82 to 120/79 mmHg Postural HR 121–150 bpm | Echocardiogram normal ECG normal | Viral induced postural orthostatic tachycardia syndrome |
| 43-year-old female | Palpitations Fatigue Breathlessness | Upper respiratory tract symptoms 1 month previously (suspected COVID-19) | Postural BP 121/92 to 129/96 mmHg Postural HR 86–106 bpm | Ambulatory BP monitor: average BP 101/66 mmHg 24-hour Holter: sinus rhythm, HRB 68–159, average 86 bpm. Diurnal sinus tachycardia | Viral induced reactive tachycardia, with sympathetic overactivity |
| 50-year-old female | Palpitations Chest pain | Chesty cough March 2020 (suspected COVID-19) | Postural BP 136/48 to 115/91 mmHg Postural HR 48–60 bpm | 24-hour Holter: sinus rhythm, rate 37–134 bpm, average 51 bpm | Post viral orthostatic intolerance |
| 30-year old female | Aches Dizziness Diarrhoea Dizziness and palpitations | Flu-like symptoms March 2020 (confirmed COVID-19) | Postural HR 80–118 bpm Postural BP 112/79 to 123/103 mmHg | Head-up tilt: starting BP 106/69 and HR 67 bpm, BP 72/52 mmHg and HR 99 bpm after 14 minutes | Post viral orthostatic intolerance with reactive tachycardia |
| 50-year-old female | Recurrent presyncopal episodes Fatigue Panic attacks | Suspected COVID-19 infection March 2020 | Postural HR 88–113 bpm Postural BP 137/85 mmHg to 122/88 mmHg | Echocardiogram normal Ambulatory BP monitor: average 101/65 mmHg; Holter monitor: sinus tachycardia Head up tilt: postural drop of 17 mmHg and HR rise to 132 bpm after 24 minutes | Orthostatic intolerance with a tendency to vasovagal presyncope |
| 44-year-old female | Dizziness on walking Fatigue Irritable bowel symptoms Anxiety | Upper respiratory tract symptoms for 5 weeks in March 2020 (suspected COVID-19) | Telephone consultation so only readings from patient's BP monitor: 88/67 mmHg | Nil | Orthostatic intolerance |
BP = blood pressure; bpm = beats per minute; ECG = electrocardiogram; HR = heart rate.
Orthostatic intolerance syndromes include orthostatic hypotension (OH), vasovagal syncope (VVS) and postural orthostatic tachycardia syndrome (POTS). The pathophysiology hinges on an abnormal autonomic response to orthostasis (standing up). When a healthy person stands, blood pools in the pelvis and legs, reducing venous return to the heart. This is detected by baroreceptors in the heart and aorta, which respond by increasing sympathetic neural and adrenergic tone (mediated by norepinephrine and epinephrine respectively). This results in tachycardia (thus compensating for reduced stroke volume). This is then followed by vasoconstriction in the splanchnic vascular bed, which increases venous return to the heart.
In orthostatic intolerance, the release of epinephrine and norepinephrine causes pronounced tachycardia, which is experienced as palpitations, breathlessness and chest pain (common symptoms of ‘long COVID’). Very high catecholamine levels can lead to paradoxical vasodilatation, sympathetic activity withdrawal and activation of the vagus nerve resulting in hypotension, dizziness and ultimately syncope.8–11
These syndromes may be exacerbated by hypovolaemia resulting from the initial infection or due to deconditioning by bedrest. Prolonged bedrest (there have been extensive studies of head-down bedrest simulating chronic weightlessness in astronauts) leads to reduced cardiac output and stroke volume, hypovolaemia, baroreflex impairment, and withdrawal of the sympathetic neural response.12–15
COVID-19, autoimmunity and the autonomic nervous system
It has been hypothesised that COVID-19 infection affects the autonomic nervous system.16 The relationship between the two is complex: the well-documented cytokine response storm of COVID-1917 results from sympathetic activation inducing pro-inflammatory cytokine release.18,19 Conversely, vagal stimulation results in an anti-inflammatory responses,17 suggesting possible therapeutic targets in the autonomic nervous system.
Alternatively, COVID-19 related autonomic dysfunction could be mediated by the virus itself. Immune-mediated neurological syndromes have been described.20 It is also well established that autonomic disorders such as OH and POTS are associated with autoantibodies,21 for example to α-/β-adrenoceptors and muscarinic receptors.22–25 Cohort studies describe commonly preceding infections in POTS,26 as well as a link with autoimmune biomarkers and autoimmune disorders.27 Thus, we speculate that there is an underlying autoimmune component to the post-COVID syndromes that we report.
Identifying autonomic dysfunction following COVID-19 infection
Any individuals presenting with breathlessness, palpitations, fatigue, chest pain, presyncope or syncope should be evaluated carefully. Cardiovascular, respiratory and neurological examination with vital signs and pulse oximetry are essential. Electrocardiogram, blood tests and imaging should be considered to identify other important diagnoses such as organising pneumonia, pulmonary embolism and myocarditis.
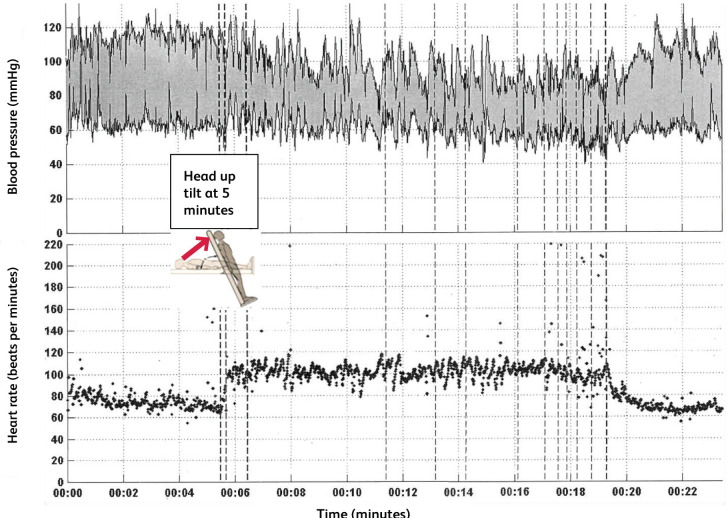
An active stand test should be undertaken, measuring blood pressure and heart rate after 5 minutes lying supine, and then 3 minutes after standing. Orthostatic hypotension is defined as a fall of >20 mmHg systolic and >10 mmHg diastolic after standing for 3 minutes.28 POTS is characterised by orthostatic symptoms (in the absence of orthostatic hypotension) with an increase in heart rate of 30 beats per minute or more when standing for more than 30 seconds, or 40 beats per minute or more in those aged 12–19 years.29 A continuous blood pressure and heart rate trace during a tilt table test of an individual with orthostatic intolerance post-COVID is shown in Fig 1. After adopting the upright position, a marked and continuous rise in heart rate was seen, corresponding with the onset of clinical symptoms and mirrored by blood pressure oscillations in keeping with an adrenergic response. The average heart rate rise was under 30 beats per minute, thus not fulfilling criteria for POTS.
Fig 1.
Continuous heart rate and blood pressure monitoring during a head-up tilt table test from an individual with long COVID and orthostatic intolerance following COVID-19 infection in March 2020.
Management of orthostatic intolerance
We suggest the following methods of management. This is based on published international guidance, consensus statements, and the authors’ own experience and practice in managing orthostatic intolerance syndromes.8,29–31
Education
Education, explanation and reassurance provide the cornerstone of management of orthostatic intolerance syndromes. Managing the uncertainty associated with COVID-19 and explaining the underlying physiological changes can reassure the patient that their symptoms are not sinister. Reproducing symptoms, for example while on a tilt table, can be effective for explaining symptom correlations with heart rate and blood pressure variations. The patient should be directed to further education sources, such as those from the STARS initiative (Syncope Trust and Reflex Anoxic Seizures) from the Heart Rhythm Alliance (www.heartrhythmalliance.org/stars/uk/), and the authors’ free educational website, www.stopfainting.com.
Exercise
After medical examination, we suggest a regular, structured exercise programme with both aerobic and resistance elements. As orthostasis (the upright position) may be problematic, non-upright exercise such as cycling on a recumbent exercise bike and swimming are encouraged.
Fluid and salt repletion
Ensuring fluid repletion (2–3 litres water per day and avoiding caffeine and alcohol) and ensuring one to two teaspoons of salt supplementation per day helps maintain plasma volume and avoid hypovolaemia.
Avoiding exacerbating factors
The patient should be advised on rising cautiously from a lying or seated position and avoiding exacerbating factors such as prolonged standing, warm environments and dehydration. Additionally, patients can be advised to consume small and frequent rather than large meals to avoid splanchnic vasodilatation.
Isometric exercises
Simple physical isometric counter-manoeuvres can be taught to those with orthostatic hypotension. These simple exercises involve sustained tensing of muscles which increases venous return to the heart and raises blood pressure. These counter-pressure manoeuvres include tensing thigh and buttock muscles, crossing arms and legs, folding arms and leaning forward, squatting, or raising a leg and placing the foot on a stool.
Compression garments
In orthostatic hypotension, compression garments extending up to the waist, or abdominal binders, are effective and tolerable.
Pharmacological treatment
If POTS features are present, norepinephrine reuptake inhibitors such as duloxetine, nortryptiline and tapentadol should be discontinued if possible.29
If symptoms persist despite full compliance with conservative measures, pharmacological therapy may be considered.
Fludrocortisone, a fluid expander, can be used if hypovolaemia is considered to be a dominant symptom. It is associated with side effects and not particularly well tolerated. Monitoring should take place for fluid retention and hypokalaemia.
Midodrine, a sympathomimetic α-1-agonist, increases vasoconstriction and venous return to the heart. It can effectively treat orthostatic hypotension and tachycardia in patients who have a baseline normal or low-normal blood pressure. Patients should be advised to avoid the supine position as much as possible, thereby reducing the tendency to excessive blood pressure rise in this position. Urinary retention may be seen in male symptoms with prostatic symptoms and scalp itch are common side effects.
For prominent hyperadrenergic symptoms caused by the catecholamine surge on standing, clonidine and methyldopa may alleviate symptoms. Similarly, propranolol can attenuate palpitations and tachycardia.8,29–31 However, none of these agents is well tolerated.
Conclusion
Several months on from the declaration of the COVID-19 pandemic, new symptom patterns and syndromes such as ‘long COVID’ are emerging. These patterns may be explained by autonomic instability and may result from deconditioning, hypovolaemia or immune- or virus-mediated neuropathy. We anticipate that these syndromes will represent a large proportion of primary and secondary care consultations in coming months. Clinicians must be aware that prompt and correct diagnosis with careful management are essential for recovery.
References
- 1.Greenhalgh T, Knight M, A'Court C, Buxton M, Husain L. Management of post-acute Covid-19 in primary care. BMJ 2020;370:m3026. [DOI] [PubMed] [Google Scholar]
- 2.Chan AT, Drew DA, Nguyen LH, et al. The Coronavirus Pandemic Epidemiology (COPE) Consortium: A call to action. Cancer Epidemiol Biomarkers Prev 2020;29:1283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carfi A, Bernabei R, Landi F, et al. Persistent symptoms in patients after acute COVID-19. JAMA 2020;324:603–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drew DA, Nguyen LH, Steves CJ, et al. Rapid implementation of mobile technology for real-time epidemiology of COVID-19. Science 2020;368:1362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J Med Virol 2020, in press ( 10.1002/jmv.26368). [DOI] [PubMed] [Google Scholar]
- 6.Gallagher S. What is long Covid? Three quarters of people hospitalised suffer symptoms for three months or more. Independent, 12 October 2020. independent.co.uk/life-style/health-and-families/long-covid-symptoms-hospitalised-long-haulers-three-months-study-a9679876.html. [Google Scholar]
- 7.Gross A. Fatigue plagues thousands suffering post-coronavirus symptoms. Financial Times, 3 August 2020. [Google Scholar]
- 8.Freeman R, Abuzinadah AR, Gibbons C, et al. Orthostatic hypotension: JACC State-of-the-Art Review. J Am Coll Cardiol 2018;72:1294–309. [DOI] [PubMed] [Google Scholar]
- 9.Jardine DL, Wieling W, Brignole M, et al. The pathophysiology of the vasovagal response. Heart Rhythm 2018;15:921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenton AM, Hammill SC, Rea RF, Low PA, Shen WK. Vasovagal syncope. Ann Intern Med 2000;133:714–25. [DOI] [PubMed] [Google Scholar]
- 11.Fedorowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med 2019;285:352–66. [DOI] [PubMed] [Google Scholar]
- 12.Barbic F, Heusser K, Minonzio M, et al. Effects of prolonged head-down bed rest on cardiac and vascular baroreceptor modulation and orthostatic tolerance in healthy individuals. Front Physiol 2019;10:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck L, Baisch F, Gaffney FA, et al. Cardiovascular response to lower body negative pressure before, during and after ten days head-down tilt bedrest. Acta Physiol Scand Suppl 1992;604:43–52. [PubMed] [Google Scholar]
- 14.Goldstein DS, Vernikos J, Holmes C, Convertino VA. Catecholaminergic effects of prolonged head-down bed rest. J Appl Physiol 1985;78: 1023–9. [DOI] [PubMed] [Google Scholar]
- 15.Kamiya A, Michikami D, Fu Q, et al. Pathophysiology of orthostatic hypotension after bed rest: paradoxical sympathetic withdrawal. Am J Physiol Heart Circ Physiol 2003;285:H1158–67. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein DS. The extended autonomic system, dyshomeostasis, and COVID-19. Clin Auton Res 2020;30:299–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fudim M, Qadri YJ, Ghadimi K, et al. Implications for neuromodulation therapy to control inflammation and related organ dysfunction in COVID-19. J Cardiovasc Transl Res 2020, in press ( 10.1007/s12265-020-10031-6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konig MF, Powell M, Staedtke V, et al. Preventing cytokine storm syndrome in COVID-19 using alfa-1 adrenergic receptor antagonists. J Clin Invest 2020;137:3345–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staedtke V, Bai R-Y, Kim K, et al. Disruption of a self-amplifying catecholamine loop reduced cytokine release syndrome. Nature 2018;564:273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guilmot A, Maldonado Slootjes S, Sellimi A, et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J Neurol 2020, in press ( 10.1007/s00415-020-10108-x). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruzieh M, Batizy L, Dasa O, et al. The role of autoantibodies in the syndromes of orthostatic intolerance: a systematic review. Scand Cardiovasc J 2017;51:243–7. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Kem DC, Reim S, et al. Agonistic autoantibodies as vasodilators in orthostatic hypotension: a new mechanism. Hypertension 2012;59:402–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fedorowski A, Li H, Yu X, et al. Antiadrenergic autoimmunity in postural tachycardia syndrome. Europace 2017;19:1211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Yu X, Liles C, et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc 2014;3:e000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu X, Stavrakis S, Hill MA, et al. Autoantibody activation of beta- adrenergic and muscarinic receptors contributes to an “autoimmune” orthostatic hypotension. J Am Soc Hypertens 2012;6:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watari M, Nakane S, Mukaino A, et al. Autoimmune postural orthostatic tachycardia syndrome. Ann Clin Transl Neurol 2018;5:486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blitshteyn S, Brinth L, Hendrickson JE, Martinez-Lavin M. Autonomic dysfunction and HPV immunization: an overview. Immunol Res 2018;66:744–54. [DOI] [PubMed] [Google Scholar]
- 28.Lahrmann H, Cortelli P, Hilz M, et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol 2006;13:930–6. [DOI] [PubMed] [Google Scholar]
- 29.Sheldon RS, Grubb BP, 2nd, Olshansky B, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015; 12:e41–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: executive summary. Circulation 2017;136:e25–e59. [DOI] [PubMed] [Google Scholar]
- 31.Brignole M, Moya A, de Lange FJ, et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J 2018;39:1883–48. [DOI] [PubMed] [Google Scholar]