Abstract
Background
For individuals with mild cognitive impairment (MCI) or dementia, elevated brain iron together with β-amyloid is associated with lower cognitive functioning. But this needs further investigation among cognitively normal older adults.
Purpose
To investigate via quantitative susceptibility mapping (QSM) in MRI and PET how cerebral iron together with β-amyloid affects cognition among cognitively normal older adults.
Materials and Methods
In this secondary analysis of a prospective study, cognitively normal older adults underwent QSM MRI to measure brain iron. A majority underwent PET to measure cerebral β-amyloid within 30 days of MRI. Multiple linear regression analyses were performed for 12 cortical and subcortical gray matter regions to assess the effect of brain iron on cognitive functions. Voxel-based analyses investigated the associations between tissue iron and β-amyloid load and their relationship to cognitive performance.
Results
Evaluated were 150 cognitively normal older adults (mean age, 69 years ± 8 [standard deviation]; 93 women). Of 150, 97 underwent PET; 22 of the 97 (mean age, 71 years ± 6; 13 women) were positive for β-amyloid. In all participants, brain iron content in the hippocampus negatively correlated with global cognitive composite score (standardized β = −0.24; 95% CI: −0.40, −0.07; P = .005). In the PET subgroup, brain iron in the hippocampus negatively correlated with episodic memory (β = −0.24; 95% CI: −0.40, −0.08; P = .004) and visuospatial score (β = −0.34; 95% CI: −0.56, −0.12; P = .003) independent of β-amyloid burden. Both negative and positive correlations between brain iron and β-amyloid were observed in the PET subgroup, revealing clusters where brain iron content negatively correlated with β-amyloid and global cognitive scores (eg, in the frontal cortex: β = −0.13; 95% CI: −0.23, −0.02; P = .02). No clusters showed associations between β-amyloid and global cognition.
Conclusion
Among cognitively normal older adults, quantitative susceptibility mapping in MRI and PET indicated that elevated cerebral iron load was related to lower cognitive performance independent of β-amyloid.
© RSNA, 2020
Online supplemental material is available for this article.
See also the editorial by Chiang in this issue.
Summary
Quantitative susceptibility mapping in MRI and PET indicated that elevated brain iron was related to lower cognitive performance independent of β-amyloid in cognitively normal older adults.
Key Results
■ Brain iron content in the hippocampus negatively correlated with episodic memory (β = −0.24; P = .004) and visuospatial scores (β = −0.34; P = .003), whereas β-amyloid showed no associations with cognition in cognitively normal older adults.
■ Voxel-based analyses also revealed clusters (eg, in the frontal cortex) where brain iron content negatively correlated with β-amyloid and global cognitive scores (β = −0.13; P = .02) with no associations between β-amyloid and cognition.
Introduction
It is clear that Alzheimer disease (AD) pathology, including the extracellular accumulation of β-amyloid plaques, occurs many years before the emergence of clinical symptoms of AD and can be detected noninvasively at PET imaging (1,2). However, large variability in the rate of cognitive decline among individuals with amyloid pathology (3,4) and ineffectiveness of antiamyloid therapeutics to slow cognitive decline (5,6) suggest that other factors, such as brain iron (7), may alter risk of cognitive decline (8,9).
As an essential transition metal, iron has many important roles in the brain biologic functions, including neurotransmitter synthesis, generation of myelin sheets, and metabolism (10). But animal models suggest elevated brain iron content can cause oxidative stress, promote β-amyloid toxicity and tau-protein dysfunction, enhance neuronal cell death, and lead to neurodegeneration and cognitive dysfunction (11). Although local brain iron increases during normal aging (12,13), a greater-than-normal iron accumulation among older adults is associated with neurodegeneration and amyloid plaque in AD (10,14).
Advances in quantitative susceptibility mapping (QSM) MRI techniques have made it possible to noninvasively measure tissue iron content with high spatial resolution and high sensitivity (15). Several studies that used QSM MRI reported elevated cerebral iron in subcortical nuclei and cortical regions among individuals with mild cognitive impairment (MCI) and AD dementia compared with cognitively normal individuals (16–18). Also, interactions between iron and amyloid among individuals with MCI or AD dementia (19,20) showed greater cognitive decline among those with both high iron and high amyloid load compared with those with only one of these pathologic conditions (20). A recent PET/MRI study among participants without dementia further suggested that in frontal and temporal regions, where iron and amyloid-β levels are locally correlated, higher regional amyloid is associated with lower global cognition. But the study did not show direct associations between iron and cognition (21).
In our study, the effects of elevated cerebral iron load on cognitive functions and its possible interaction with amyloid-β burden were examined with relevant clinical, genetic, and neurodegeneration factors, including age, sex, education, apolipoprotein E ε4 (APOE ε4) genotype, and brain atrophy in cognitively normal older adults. Elevated local cerebral iron load was hypothesized to relate to lower cognitive performance. To our knowledge, no study has examined such effects in a well-characterized sample of cognitively normal older adults. Unlike previous studies, participants with MCI were excluded.
Materials and Methods
This secondary analysis of a prospective study was approved by the Johns Hopkins University institutional review board. Written informed consent were obtained with compliance with the Health Insurance Portability and Accountability Act.
Study Participants
Participants were part of an ongoing, longitudinal study, known as Biomarkers for Older Controls at Risk for Dementia, or BIOCARD (22). The analyses herein are on the basis of cognitively normal older adults who underwent QSM MRI between January 2015 and September 2018. Exclusion criteria included corrupted image reconstruction or severe artifacts or diagnosis of MCI. The majority of participants underwent a PET examination using 11C-labeled Pittsburgh compound B tracer within 30 days (mean, 1 day ± 3 [standard deviation]) of their MRI to assess amyloid-β levels, and these participants are referred to as the PET group (see Appendix E1 [online] for details about participant recruitment and diagnostic procedures).
APOE Genotyping and Clinical and Cognitive Assessments
APOE ε4 carrier status was coded by a dichotomous indicator variable: ε4 carriers (ie, individuals with at least one APOE ε4 allele) were coded as 1, and noncarriers were coded as 0.
Each participant included in the present analyses received a consensus diagnosis by the staff of the Johns Hopkins University BIOCARD Clinical Core as (a) cognitively normal or (b) impaired not MCI (Appendix E1 [online]).
All participants underwent a comprehensive battery of neuropsychologic tests at the same visit as their MRI (22). Four domain-specific composite scores, reflecting verbal episodic memory, executive function, visuospatial processing, and language ability were calculated on the basis of 12 neuropsychologic test scores, as described previously (23) and summarized in Appendix E2 (online). In addition, global cognitive performance was measured by using a global cognitive composite score (24) (Appendix E2 [online]). For both the global and the domain-specific cognitive composite scores, higher scores mean better cognitive performance.
MRI Acquisition and Processing
MRI was conducted by using a 3.0-T scanner (Achieva; Philips Healthcare, Best, the Netherlands). A three-dimensional multiecho gradient-recalled echo sequence (1 × 1 × 1 mm3 resolution) was used for QSM, whereas a three-dimensional T1-weighted magnetization prepared rapid gradient-echo sequence (1 × 1 × 1.2 mm3 resolution) was used for anatomic referencing and automated image segmentation (Appendix E3 [online]).
For assessing brain iron content, QSM images were reconstructed by using the Johns Hopkins University/Kennedy Krieger Institute QSM Toolbox (version 3.0; http://godzilla.kennedykrieger.org/QSM/) (25). The phase preprocessing and QSM dipole inversion procedures are detailed in Appendices E4 and E5 (online).
For brain segmentation, T1-weighted magnetization prepared rapid gradient-echo images were coregistered to the QSM space. Twelve regions of interest (ROIs) including the superior and middle frontal cortex, inferior and orbital frontal cortex, parietal cortex, temporal cortex, occipital cortex, entorhinal cortex, cingulate cortex, amygdala, hippocampus, caudate, putamen, and globus pallidus were automatically segmented on the basis of human brain atlases for quantifying tissue magnetic susceptibility (iron measure) and structure volume in each region (Appendix E6 [online]).
PET Image Acquisition and Processing
The dynamic 11C-labeled Pittsburgh compound B tracer PET scans were performed on an Advance PET scanner (GE Healthcare, Milwaukee, Wis), and distribution volume ratio images were calculated in the native space of each PET image (26,27). Mean distribution volume ratio in each selected ROI was quantified and a global index of cortical distribution volume ratio value greater than a threshold of 1.06 was considered positive for Pittsburgh compound B (or positive for amyloid-β; Appendix E7 [online]).
Statistical Analysis
Multiple linear regression models were tested to assess the association between local cerebral iron load and the global cognitive composite score in each selected ROI. First, for all participants (model 1), the continuous global cognitive composite score was regressed on age, sex, APOE ε4 status, years of education, structure volume, and tissue magnetic susceptibility, with sex and APOE ε4 status as dichotomous variables. To test whether this association was independent of β-amyloid deposition, all models were rerun on the PET group (model 2) with local distribution volume ratio value as an extra predictor (see Appendix E8 [online] for details about sensitivity analyses). In addition, we reran the regression models in participants negative for amyloid-β to further investigate the association between brain iron and cognition in participants presumably at even lower risk of cognitive decline. Benjamini-Hochberg corrections for multiple comparisons were performed (12 tests for 12 ROIs; false discovery rate, 0.25). All regression analyses were then repeated by using the four domain-specific continuous cognitive composite scores as the outcome.
The Biologic Parametric Mapping toolbox (28) was used to estimate the voxel-based correlations between brain iron and β-amyloid in gray matter regions in the PET group (Appendix E9 [online], Fig 1). The associations of iron load and β-amyloid burden with cognition was further examined in clusters with significant positive or negative correlations between iron and β-amyloid. Ridge regressions were then conducted with the global or domain-specific cognitive score as an outcome. Benjamini-Hochberg correction for multiple comparisons was applied (number of tests determined by the number of clusters identified by Biologic Parametric Mapping; false discovery rate, 0.25).
Figure 1:
Diagram of the processing steps for performing voxel-based analysis on the local correlation between brain iron and amyloid-β load by using the corresponding quantitative susceptibility mapping (QSM) and PET distribution volume ratio (DVR) images with the Biologic Parametric Mapping toolbox. ANTs = Advanced Normalization Tools, APOE ε4 = apolipoprotein E ε4, FWHM = full width at half maximum, MNI = Montreal Neurologic Institute.
All regression analyses were performed by using software (SPSS version 25.0; SPSS, Chicago, Ill).
Results
Participant Characteristics
We initially identified 174 participants of the BIOCARD study. Two participants were excluded for severe imaging artifacts and 22 participants were excluded for diagnosis of MCI. We evaluated 150 cognitively normal older adults (mean age, 69 years ± 8 [standard deviation]; 93 women); 121 participants were cognitively normal and 29 participants were impaired not MCI (see Table E1 [online] for demographic information and see Appendix E8 [online] for information regarding how these participants were treated in the analysis). Of those 150 participants, 97 underwent a Pittsburgh compound B PET examination within 30 days of MRI to assess amyloid-β levels. Of the 97 participants with PET examinations, 22 were positive for β-amyloid (mean age, 71 years ± 6; 13 women); 75 participants were negative for amyloid-β.
The demographic information and neuropsychologic measurements for all participants and, separately, for the PET subgroup are summarized in Table 1. A higher percentage of APOE ε4 carriers was observed in β-amyloid positive versus the β-amyloid negative group (P < .001). Typical susceptibility maps with the macroscopic vein masked out are shown in Figure 2, A (see Fig E1 [online] for more slices), and the selected cortical and deep gray matter ROIs are shown in Figure 2, B.
Table 1:
Demographic Information and Neuropsychologic Testing Results
Figure 2:
A, Example quantitative susceptibility maps in a 72-year-old man with normal cognition in axial (left) and sagittal (right) view. Regions that contained macroscopic veins were masked out to better quantify the nonheme tissue iron content. B, The selected regions of interest in the cortex and basal ganglia are indicated on the susceptibility maps. Amyg = amygdala, Cingu = cingulate cortex, CN = caudate nuclei, GP = globus pallidus, Hippo = hippocampus, IOF = inferior and orbital frontal cortex, OcciCtx = occipital cortex, PT = putamen, SMF = superior and middle frontal cortex, TempCtx = temporal cortex.
Relationship between Brain Iron, Amyloid, and Global Cognitive Performance
Associations between the global cognitive composite score and regional brain iron in all participants and in the PET group are summarized in Table 2 and Table E2 (online). Older age, less education, and male sex were associated with lower global cognitive scores. A negative correlation between brain iron and global cognitive performance was found in the hippocampus (model 1: standardized β = −0.24; 95% CI: −0.40, −0.07; P = .005) (Fig 3, A). Similar analyses in the PET group (model 2) confirmed such negative associations in superior and middle frontal cortex (β = −0.22; 95% CI: −0.43, −0.11; P = .04) and globus pallidus (β = −0.26; 95% CI: −0.48,−0.05; P = .02), but not in hippocampus (β = −0.22; 95% CI: −0.45, 0.02; P = .07) (Fig 3, B), whereas β-amyloid showed no association with global cognition in all selected ROIs (eg, in the hippocampus [β = 0.05; 95% CI: −0.17, 0.27; P = .67]) (Table E2 [online]).
Table 2:
Results from Multiple Linear Regression of Global and Domain-specific Cognitive Composite Scores on Brain Iron Levels
Figure 3:
Scatterplot of the adjusted response data and adjusted response function of the multiple linear regression model between global cognitive composite score (continuous variable with z-score per unit) and tissue susceptibility values in the hippocampus of, A, all participants with normal cognition (n = 150), B, and in the PET subgroup (n = 97, blue) and in participants negative for amyloid-β (n = 75, orange). The reported β values are standardized coefficients.
In participants negative for amyloid-β, similar negative correlations between brain iron and global cognition were found in more ROIs including superior and middle frontal cortex (β = −0.34; 95% CI: −0.59, −0.10; P = .007), temporal cortex (β = −0.27; 95% CI: −0.52, −0.02; P = .03), entorhinal cortex (β = −0.33; 95% CI: −0.60, −0.06; P = .02), hippocampus (β = −0.43; 95% CI: −0.71, −0.16; P = .003) (Fig 3, B), globus pallidus (β = −0.30; 95% CI: −0.55, −0.06; P = .02) and others (Table 2, Table E3 [online]). Sensitivity analyses excluding the impaired-not-MCI group are shown in Table E4 (online).
Relationship between Brain Iron, Amyloid, and Domain-specific Cognitive Performance
Regarding episodic memory, older age and male sex were associated with lower scores (Table E5 [online]). Brain iron levels in the hippocampus were negatively associated with episodic memory score in all participants (β = −0.22; 95% CI: −0.38, −0.05; P = .01) and in the PET group (β = −0.24; 95% CI: −0.40, −0.08; P = .004) (Fig 4, A, B; Table 2), independent of β-amyloid levels (β = −0.03; 95% CI: −0.26, 0.19; P = .78) (Table E5 [online]). For participants negative for amyloid-β, negative correlations between iron and episodic memory score were found in hippocampus (β = −0.44; 95% CI: −0.72, −0.16; P = .002; Fig 4, B) and other ROIs including superior and middle frontal cortex (β = −0.27; 95% CI: −0.52, −0.02; P = .04), temporal cortex (β = −0.27; 95% CI: −0.52, −0.01; P = .04), and entorhinal cortex (β = −0.32; 95% CI: −0.59, −0.05; P = .02) (Table 2, Table E6 [online]). Sensitivity analyses excluding the impaired-not-MCI group are shown in Table E7 (online).
Figure 4:
Scatterplot of the adjusted response data and adjusted response function of the multiple linear regression model between, A, B, episodic memory score, C, D, executive function score, E, F, visuospatial score, G, H, language score, and tissue susceptibility values in the hippocampus of all participants with normal cognition (n = 150), in the PET group (n = 97), and of participants negative for amyloid-β in this group (n = 75). All cognitive scores are continuous variables and have z-score per unit. The reported β values are standardized coefficients.
For executive function, younger age and higher education was related to better performance (Table E8 [online]). Brain iron levels in hippocampus negatively correlated with executive function scores in all participants (β = −0.17; 95% CI: −0.34, −0.01; P = .04; Fig 4, C). Negative correlations between iron and executive function scores were also observed in globus pallidus (β = −0.23; 95% CI: −0.39, −0.08; P = .004) in all participants and in the PET group (β = −0.39; 95% CI: −0.58, −0.19; P < .001) (Table 2, Table E8 [online]). For participants negative for amyloid-β, similar negative correlations were observed in hippocampus (β = −0.31; 95% CI: −0.59, −0.04; P = .03; Fig 4, D) and globus pallidus (β = −0.44; 95% CI: −0.66, −0.21; P < .001; Table 2, Table E9 [online]). Sensitivity analyses excluding the impaired-not-MCI group are shown in Table E10 (online).
For visuospatial function, older age and female sex were associated with lower performance (Table E11 [online]). A negative correlation was observed between the visuospatial score and brain iron load in hippocampus in all participants (β = −0.25; 95% CI: −0.41, −0.09; P = .002) and in the PET group (β = −0.34; 95% CI: −0.56, −0.12; P = .003; Fig 4, E, F), independent of β-amyloid levels (β = −0.03; 95% CI: −0.24, 0.17; P = .75) (Table E11 [online]). For participants negative for amyloid-β, negative correlations were found in hippocampus (β = −0.45; 95% CI: −0.71, −0.20; P = .001; Fig 4, F) and globus pallidus (β = −0.29; 95% CI: −0.52, −0.06; P = .01) (Table 2, Table E12 [online]). Sensitivity analyses excluding the impaired-not-MCI group are shown in Table E13 (online).
For language function, higher education was related to higher scores (Table E14 [online]). Negative associations between the language scores and brain iron in globus pallidus (β = −0.27; 95% CI: −0.48, −0.07; P = .01] were observed in the PET group (Table 2, Table E14 [online]). For participants negative for amyloid-β, negative correlations were found in more ROIs including entorhinal cortex (β = −0.39; 95% CI: −0.65, −0.12; P = .005) and globus pallidus (β = −0.32; 95% CI: −0.56, −0.07; P = .01; Table E15 [online]). Sensitivity analyses excluding the impaired-not-MCI group are shown in Table E16 (online).
Voxel-level Associations between Brain Iron and Amyloid and Their Correlations with Cognition
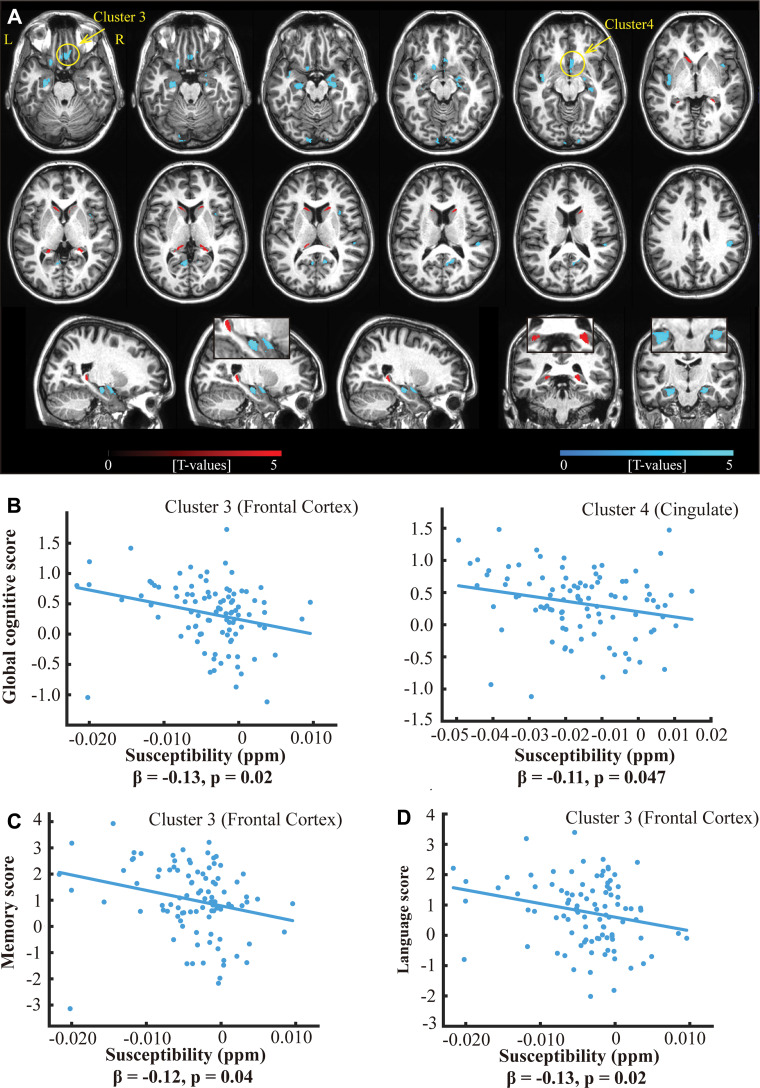
There were nine clusters (brain regions) of negative correlation and three clusters of positive correlation between iron and amyloid levels (Table 3, Fig 5, A). Two clusters among the nine clusters with negative amyloid-iron correlations demonstrated negative correlations between iron level and the global cognitive composite score: one in the frontal cortex (β = −0.13; 95% CI: −0.23, −0.02; P = .02) and one in the cingulate cortex area (β = −0.11; 95% CI: −0.21, −0; P = .047; Fig 5, B). The frontal cortex cluster also showed negative associations between iron levels and episodic memory scores (β = −0.12; 95% CI: −0.22, −0.01; P = .04 ; Fig 5, C) and language scores (β = −0.13; 95% CI: −0.23, −0.02; P = .02; Fig 5, D). No association was observed between β-amyloid and global cognitive composite score in any of these 12 clusters. Sensitivity analyses excluding the impaired-not-MCI group are shown in Table E17 (online) and Figure E2 (online).
Table 3:
Voxel-based Analysis Results of the PET Group Testing Associations between Iron Load and β-Amyloid Load
Figure 5:
A, Overlay of regions with positive (red) and negative (cyan) correlations between iron load (ie, quantitative susceptibility mapping) and β-amyloid plaque load (PET distribution volume ratio) in all participants of the PET group (n = 97) with family-wise error−corrected cluster level significance of P value less than .05 (combined with an uncorrected voxel-level significance P < .001) and a cluster size threshold of 200 voxels on example axial slices of anatomic MRI (top two rows) and sagittal and coronal slices showing the hippocampus (bottom row). B, Scatterplot of the adjusted response data and adjusted response function of the multiple linear regression model between global cognitive composite score and susceptibility values in two clusters (marked by arrows in A), in which the cognitive composite score negatively correlates with tissue susceptibility values. Similarly, negative associations were observed between tissue susceptibility values in the frontal cortex cluster and, C, episodic memory scores and, D, language scores. All cognitive scores are continuous variables and have z-score per unit. The reported β values are standardized coefficients.
Associations between Structure Volume and QSM or Distribution Volume Ratio
Further analyses confirmed no significant correlations between structure volume and iron (QSM) or β-amyloid (distribution volume ratio) in all selected ROIs (Appendix E10 [online]).
Discussion
Previous studies suggested that elevated brain iron in the presence of β-amyloid is associated with lower cognitive performance among individuals with mild cognitive impairment or dementia, but it is unclear whether this is also the case for cognitively normal older adults. In our study, by using quantitative susceptibility mapping in MRI and PET imaging of β-amyloid in cognitively normal older adults, we demonstrated that elevated brain iron content especially in the hippocampus is associated with lower performance on tests of global cognition (β = −0.24; P = .005), episodic memory (β = −0.24; P = .004), and visuospatial function (β = −0.34; P = .003), independent of β-amyloid burden.
Possible interactions between brain iron and the characteristic AD pathology of β-amyloid and τ have been suggested in postmortem histologic studies that have found amyloid plaques and neurofibrillary tangles to be enriched with iron (14,29). Versus the interaction with β-amyloid, our results suggest that among older individuals with normal cognition, iron appears to be more consistently related to cognitive performance than β-amyloid. Brain iron level in the hippocampus better predicted global cognitive composite scores than volumetric measures, whereas β-amyloid measures were unrelated to cognitive performance. Our results are consistent with a previous meta-analysis (30) that reported that increased amyloid burden is only weakly associated with episodic memory and measures of global cognition among individuals who are cognitively normal. In addition, our results demonstrated that elevated brain iron correlated with lower cognitive performance with larger coefficients and smaller P values in participants negative for β-amyloid. This may be partly because of the increased detrimental effect on cognition from other disease processes including β-amyloid, τ, and neurodegeneration within the participants positive for β-amyloid.
APOE ε4 is the major known genetic risk factor for late-onset AD (22). However, studies examining cross-sectional associations between APOE ε4 status and cognition among cognitively normal older adults have been mixed (31). This likely reflects cohort differences in the age of participants, cognitive measures used, and levels of preclinical AD pathology. Consistent with present results, studies that carefully screened participants for cognitive deficits at enrollment or subsequently excluded participants with incipient cognitive impairment tended to find no differences in cognitive test performance between ε4 carriers and noncarriers (32). Possible interactions from APOE to brain iron and β-amyloid have been suggested in MCI (19) but may not be evident in the preclinical phase of AD (20,21).
Concerns regarding whether lower scores on cognitive measures predict subsequent cognitive decline were addressed by demonstrated associations between baseline global cognitive scores and the rate of cognitive decline in our participants, which indicated that lower baseline cognitive scores are indeed associated with a higher risk of progression to MCI and dementia (Appendix E12 [online]).
We speculate that the damaging effect of iron on cognitive function among cognitively normal older adults reflects β-amyloid-independent mechanisms, such as iron-related oxidative stress and iron-dependent cell death (ie, ferroptosis) (33). Such mechanisms may also include possible interactions between iron and τ tangles (11), which usually start to accumulate in the hippocampus (34). The associations we found between elevated hippocampal iron levels and episodic memory and visuospatial processing are consistent with the role of the hippocampus in both cognitive domains. The association between hippocampal iron levels and the global cognitive score is likely because the global score contains two episodic memory tests. Other brain regions including the frontal cortex, temporal cortex, and entorhinal cortex may have similar relevance to the memory and global cognition. In addition, elevated iron content in the putamen and globus pallidus have been observed during aging and many neurodegenerative diseases likely suggesting an altered systematic brain iron homeostasis. Our results also suggest that elevated brain iron content in these regions are related to lower performance in global cognition, executive, visuospatial, and language functions.
The limitations of our study included its cross-sectional design and the separate acquisition of PET and MRI images. The false discovery rate of 0.25 used in the Benjamini-Hochberg correction was set to increase statistical power. Therefore, several reported correlations with marginal significance (eg, P = .04) may lose significance if stricter multiple comparison corrections are used. Spatial variations and different statistical powers may explain some observed discrepancy between the ROI-based and voxel-based analyses (eg, the lack of associations of brain iron in the hippocampus clusters with cognition in the whole group) (Fig 5) versus the ROI-based analysis.
In conclusion, our results suggest that cerebral iron elevation is related to lower cognitive function among older adults with normal cognition, independent of β-amyloid load. Further investigations regarding the interaction between iron and τ are warranted, along with longitudinal studies to determine whether brain iron levels predict variability in cognitive trajectories among individuals at different stages along the Alzheimer disease (AD) continuum. With no current effective therapies or preventive strategies for AD, understanding the role of brain iron in AD is vital because it could be a new target of treatment (35).
APPENDIX
SUPPLEMENTAL FIGURES
Disclosures of Conflicts of Interest: L.C. disclosed no relevant relationships. A.S. disclosed no relevant relationships. K.O. disclosed no relevant relationships. A.F. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money paid to author for consultancy from Anatomy Works; money to author for royalties from Elsevier. Other relationships: disclosed no relevant relationships. Y.Z. disclosed no relevant relationships. M.A. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money paid to author for consultancy from Biogen and Eli Lilly. Other relationships: disclosed no relevant relationships. P.C.M.v.Z. Activities related to the present article: disclosed grant from Philips Healthcare; disclosed travel support from Philips Healthcare. Activities not related to the present article: disclosed payment for lectures including service on speakers bureaus from Philips Healthcare; disclosed technology licensed to Philips Healthcare; disclosed royalties from Philips Healthcare. Other relationships: disclosed no relevant relationships. X.L. disclosed no relevant relationships.
Study supported by the National Center for Research Resources and National Institute of Biomedical Imaging and Bioengineering (P41EB015909), National Institute on Aging (R03AG065527, U19AG033655), Office of the Director (S10OD021648) of the National Institutes of Health.
Abbreviations:
- AD
- Alzheimer disease
- APOE ε4
- apolipoprotein E ε4
- MCI
- mild cognitive impairment
- QSM
- quantitative susceptibility mapping
- ROI
- region of interest
References
- 1.Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 2015;313(19):1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts BR, Lind M, Wagen AZ, et al. Biochemically-defined pools of amyloid-β in sporadic Alzheimer’s disease: correlation with amyloid PET. Brain 2017;140(5):1486–1498. [DOI] [PubMed] [Google Scholar]
- 3.Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol 2013;12(4):357–367. [DOI] [PubMed] [Google Scholar]
- 4.Lim YY, Maruff P, Pietrzak RH, et al. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain 2014;137(Pt 1):221–231. [DOI] [PubMed] [Google Scholar]
- 5.Sevigny J, Chiao P, Bussière T, et al. Addendum: The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2017;546(7659):564. [DOI] [PubMed] [Google Scholar]
- 6.Honig LS, Vellas B, Woodward M, et al. Trial of Solanezumab for Mild Dementia Due to Alzheimer’s Disease. N Engl J Med 2018;378(4):321–330. [DOI] [PubMed] [Google Scholar]
- 7.Ayton S, Faux NG, Bush AI; Alzheimer’s Disease Neuroimaging Initiative . Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by APOE. Nat Commun 2015;6(1):6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu WZ, Zhong WD, Wang W, et al. Quantitative MR phase-corrected imaging to investigate increased brain iron deposition of patients with Alzheimer disease. Radiology 2009;253(2):497–504. [DOI] [PubMed] [Google Scholar]
- 9.Ghadery C, Pirpamer L, Hofer E, et al. R2* mapping for brain iron: associations with cognition in normal aging. Neurobiol Aging 2015;36(2):925–932. [DOI] [PubMed] [Google Scholar]
- 10.Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 2014;13(10):1045–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lane DJR, Ayton S, Bush AI. Iron and Alzheimer’s Disease: An Update on Emerging Mechanisms. J Alzheimers Dis 2018;64(s1):S379–S395. [DOI] [PubMed] [Google Scholar]
- 12.Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem 1958;3(1):41–51. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Wu B, Batrachenko A, et al. Differential developmental trajectories of magnetic susceptibility in human brain gray and white matter over the lifespan. Hum Brain Mapp 2014;35(6):2698–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith MA, Harris PLR, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci U S A 1997;94(18):9866–9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Spincemaille P, Liu Z, et al. Clinical quantitative susceptibility mapping (QSM): Biometal imaging and its emerging roles in patient care. J Magn Reson Imaging 2017;46(4):951–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acosta-Cabronero J, Williams GB, Cardenas-Blanco A, Arnold RJ, Lupson V, Nestor PJ. In vivo quantitative susceptibility mapping (QSM) in Alzheimer’s disease. PLoS One 2013;8(11):e81093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moon Y, Han SH, Moon WJ. Patterns of Brain Iron Accumulation in Vascular Dementia and Alzheimer’s Dementia Using Quantitative Susceptibility Mapping Imaging. J Alzheimers Dis 2016;51(3):737–745. [DOI] [PubMed] [Google Scholar]
- 18.Kim HG, Park S, Rhee HY, et al. Quantitative susceptibility mapping to evaluate the early stage of Alzheimer’s disease. Neuroimage Clin 2017;16:429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Bergen JM, Li X, Hua J, et al. Colocalization of cerebral iron with Amyloid beta in Mild Cognitive Impairment. Sci Rep 2016;6(1):35514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayton S, Fazlollahi A, Bourgeat P, et al. Cerebral quantitative susceptibility mapping predicts amyloid-β-related cognitive decline. Brain 2017;140(8):2112–2119. [DOI] [PubMed] [Google Scholar]
- 21.van Bergen JMG, Li X, Quevenco FC, et al. Simultaneous quantitative susceptibility mapping and Flutemetamol-PET suggests local correlation of iron and β-amyloid as an indicator of cognitive performance at high age. Neuroimage 2018;174:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albert M, Soldan A, Gottesman R, et al. Cognitive changes preceding clinical symptom onset of mild cognitive impairment and relationship to ApoE genotype. Curr Alzheimer Res 2014;11(8):773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Z, Sur S, Soldan A, et al. Brain oxygen extraction by using MRI in older individuals: relationship to apolipoprotein E genotype and amyloid burden. Radiology 2019;292(1):140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soldan A, Pettigrew C, Cai Q, et al. Cognitive reserve and long-term change in cognition in aging and preclinical Alzheimer’s disease. Neurobiol Aging 2017;60:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Chen L, Kutten K, et al. Multi-atlas tool for automated segmentation of brain gray matter nuclei and quantification of their magnetic susceptibility. Neuroimage 2019;191:337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y, Resnick SM, Ye W, et al. Using a reference tissue model with spatial constraint to quantify [11C]Pittsburgh compound B PET for early diagnosis of Alzheimer’s disease. Neuroimage 2007;36(2):298–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bilgel M, An Y, Zhou Y, et al. Individual estimates of age at detectable amyloid onset for risk factor assessment. Alzheimers Dement 2016;12(4):373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casanova R, Srikanth R, Baer A, et al. Biological parametric mapping: A statistical toolbox for multimodality brain image analysis. Neuroimage 2007;34(1):137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto A, Shin RW, Hasegawa K, et al. Iron (III) induces aggregation of hyperphosphorylated tau and its reduction to iron (II) reverses the aggregation: implications in the formation of neurofibrillary tangles of Alzheimer’s disease. J Neurochem 2002;82(5):1137–1147. [DOI] [PubMed] [Google Scholar]
- 30.Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology 2013;80(14):1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Donoghue MC, Murphy SE, Zamboni G, Nobre AC, Mackay CE. APOE genotype and cognition in healthy individuals at risk of Alzheimer’s disease: A review. Cortex 2018;104:103–123. [DOI] [PubMed] [Google Scholar]
- 32.Knight RG, Tsui HS, Abraham WC, Skeaff CM, McMahon JA, Cutfield NJ. Lack of effect of the apolipoprotein E ε4 genotype on cognition during healthy aging. J Clin Exp Neuropsychol 2014;36(7):742–750. [DOI] [PubMed] [Google Scholar]
- 33.Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 2012;149(5):1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jack CR Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013;12(2):207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nikseresht S, Bush AI, Ayton S. Treating Alzheimer’s disease by targeting iron. Br J Pharmacol 2019;176(18):3622–3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.