Abstract
Background: Methicillin-resistant Staphylococcus aureus (MRSA) is the most common pathogen isolated from hand abscesses. The purpose of this study was to understand trends and changes in longitudinal antibiotic resistance profiles and risk factors for these infections to better guide empiric treatment of hand infections. Methods: We performed a retrospective review of culture-positive hand infections over a 10-year period at an urban academic institution from 2005 to 2014. A subset of MRSA hand infections from 2013 to 2014 was then subanalyzed for risk factors for antibiotic resistance for antibiotics with increasing antibiotic resistance during this period. Results: MRSA grew in 46% of hand infections, with a decreasing incidence over the 10-year study period. However, in the same time period clindamycin and levofloxacin resistance increased from 7% to 31% and 12% to 56%, respectively. Risk factors for clindamycin resistance included nosocomial infections and a history of intravenous drug use and hepatitis C. Risk factors for levofloxacin resistance included a history of diabetes and a fever upon initial presentation. Conclusions: The incidence of multidrug resistance remains high, with growing resistance to clindamycin and levofloxacin. There remains a trend for increased clindamycin resistance for patients with history of intravenous drug use and nosocomial infections. Our findings indicate that clindamycin and levofloxacin should be avoided for empiric treatment for hand infections in patients with these risk factors.
Keywords: MRSA, infection, diagnosis, resistance, hand, anatomy, treatment, research & health outcomes
Introduction
Acute infections of the hand are common problems but can potentially lead to severe consequences.1 Early treatment with antibiotics and surgical drainage is indicated to help decrease hospital stays and prevent complications such as stiffness, fibrosis, sepsis, and amputations.2,3 Furthermore, abscesses due to antibiotic-resistant organisms such as methicillin-resistant Staphylococcus aureus (MRSA) are associated with increased costs as well as morbidity and mortality.3-5 Several factors that influence the outcome of hand infections have been identified and include location, causative organism, timing of treatment, adequacy of surgical drainage, efficacy of antibiotic therapy, and the health status of the host.6 Specifically, proper antibiotic selection is one factor that can help mitigate complications of hand infections.7,8 There has been a growing trend of hand infections caused by MRSA across the United States, and empiric antibiotic selection typically bypasses traditional beta-lactam antibiotics for alternatives such as vancomycin and clindamycin due to this high prevalence of MRSA.9-15
Prior study at our urban academic hospital suggested resistance to clindamycin and levofloxacin in MRSA has risen significantly over recent years.16 The purpose of our study was to update our current understanding of the trends in antibiotic-resistant MRSA and identify risk factors for multidrug resistance to better guide empiric antibiotic treatment of hand infections.
Material and Methods
After obtaining institutional review board approval, a retrospective study was performed over a 10-year period at an urban academic medical center from January 1, 2005, through December 31, 2014, of all culture-positive hand infections. All subjects for the study were identified using the International Classification of Disease, Ninth Revision (ICD-9) codes relevant to hand infections. Diagnoses included cellulitis, abscess, tenosynovitis, and open wounds of the fingers and hands (681.00, 681.01, 681.02, 682.4, 727.05, 727.9, 882.00, 882.01, 883.00, 883.1). All patients aged 18 to 89 with a history of a culture-positive hand infection were included in the study. All demographic data and laboratory data were recorded from review of the medical record.
We classified infections as nosocomial if the medical record review indicated a history of a surgical procedure, dialysis treatment, catheterizations, hospitalizations, or nursing home stays within the year prior to having a culture-positive hand infection. All medical conditions were recorded including a history of human immunodeficiency virus (HIV), hepatitis C virus (HCV), and diabetes. Patients were considered to be febrile if the body temperature was recorded to be over 38.0°C prior to antibiotic administration and/or surgical intervention of the hand infection. A cohort of patients from January 1, 2013, through December 31, 2014, that grew MRSA on culture was additionally analyzed. This timeframe was chosen due to the high rate of MRSA resistance to clindamycin and levofloxacin compared with prior years at this same institution.16 We performed a univariate analysis with odds ratios (ORs) to determine risk factors associated with both single-drug–resistant and multidrug-resistant MRSA infections.
Results
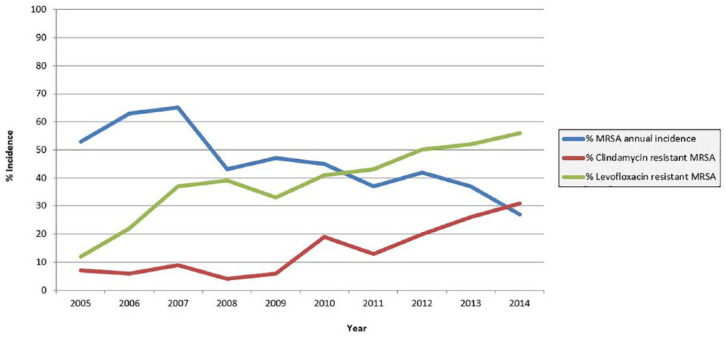
Over the 10-year study, a total of 815 culture-positive hand infections were identified. The most common pathogen isolated on culture was MRSA, which grew in 46% of cases. There was a steady decrease in overall MRSA incidence over the course of the study. Polymicrobial infections accounted for 22% of culture-positive hand infections and showed a steady increase over the 10-year timeframe of the study. A list of pathogens isolated from 2013 through 2014 is compiled in Table 1 for reference, along with the number of times isolates grew in isolation versus in the setting of polymicrobial infection. Despite the decreasing incidence of MRSA, there was a paradoxical increase in both clindamycin and levofloxacin resistance to MRSA hand infections (Figure 1). From 2013 to 2014, levofloxacin was found to be resistant to MRSA in 53.5% of cases and clindamycin was resistant to MRSA in 27.9% of cases. This represents an increase from a previously reported rate of clindamycin resistance of 16% from 2010 to 2012.1
Table 1.
Organisms Isolated From Culture-Positive Hand Infections Between 2013 and 2014..
| Organism | Monomicrobial incidence | Polymicrobial incidence |
|---|---|---|
| Acinetobacter calco-baumannii | 1 | |
| Aeromonas hydrophila | 1 | |
| Citrobacter ssp | ||
| Citrobacter freundii | 1 | 3 |
| Citrobacter koseri | 1 | |
| Diphtheroid bacilli | 1 | 1 |
| Eikenella corrodens | 1 | 3 |
| Enterococcus faecalis | 4 | |
| Enterobacter cloacae | 1 | |
| Escherichia coli | 4 | |
| Finegoldia magna | 1 | |
| Fusobacterium | 1 | 1 |
| Haemophilus parainfluenzae | 1 | 1 |
| Klebsiella ssp | ||
| Klebsiella oxytoca | 2 | |
| Klebsiella pneumoniae | 5 | |
| Lactobacillus | 1 | |
| Pasteurella multocida | 3 | 1 |
| Peptostreptococcus asaccharolyticus | 1 | |
| Prevotella ssp | ||
| Prevotella disiens | 1 | |
| Prevotella bivia | 4 | |
| Proteus mirabilis | 1 | 2 |
| Pseudomonas aeruginosa | 2 | |
| Serratia marcescens | 1 | |
| Serratia liquefaciens | 1 | |
| Staphylococcus ssp | ||
| Coagulase-negative staphylococci | 6 | 2 |
| MSSA | 20 | 13 |
| MRSA | 30 | 12 |
| Streptococcus ssp | ||
| Alpha hemolytic streptococcus | 6 | 7 |
| Group A streptococcus | 6 | 5 |
| Group B streptococcus | 2 | 10 |
| Group C streptococcus | 3 | 4 |
| Group F streptococcus | 2 | |
| Group G streptococcus | 1 | |
| Microaerophilic streptococcus | 1 | |
| Streptococcus anginosus | 2 | 1 |
| Streptococcus constellatus | 1 | 1 |
| Streptococcus intermedius | 2 | |
| Streptococcus mitis | 1 | |
| Tissierella praeacuta | 1 |
Note. Most commonly isolated were methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible S aureus (MSSA). The number of times an organism appeared in isolation and in the setting of polymicrobial infections is recorded in columns 2 and 3 of the table, respectively.
Figure 1.
Annual antibiotic resistance. Annual methicillin-resistant Staphylococcus aureus (MRSA) incidence decreased from 53% to 27% from 2005 to 2014, with an increase in clindamycin and levofloxacin resistance from 7% to 31% and 12% to 56%, respectively, during the same time period.
Notable factors with increased OR for clindamycin resistance to MRSA by univariate analysis were intravenous drug use (IVDU) (OR = 2.2), HIV (OR = 6.0), HCV (OR = 2.3), and nosocomial infection (OR = 2.8); however, none of these factors were statistically significant (Table 2). Factors with increased OR for levofloxacin resistance to MRSA by univariate analysis were diabetes (OR = 2.5) and a fever upon initial presentation (OR = 2.0); however, neither of these reached statistical significance. Factors with increased OR for MRSA resistance to both antibiotics included IVDU (OR = 2.5), fever upon initial presentation (OR = 2.90), HCV (OR = 4.7), HIV (OR = 11.3), and nosocomial infection (OR = 4.0). Table 3 shows the analysis of patient age, white blood cell count (WBC), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) as risk factors for clindamycin and levofloxacin resistance, with only ESR showing a statistically significant difference between levofloxacin sensitive (mean ESR = 37.4 mm/h) and levofloxacin resistant (mean ESR = 58.6 mm/h) isolates; however, both groups were above the threshold for normal ESR of 30 mm/h.
Table 2.
Univariate Analysis of Categorical Risk Factors Associated With Clindamycin and Levofloxacin Resistance to Methicillin-Resistant Staphylococcus aureus..
| Clindamycin |
Levofloxacin |
|||
|---|---|---|---|---|
| Patient Variables | Odds ratio | P | Odds ratio | P |
| Nosocomial source | 2.77 | .146 | 1.33 | .639 |
| Diabetes mellitus | 0.58 | .698 | 2.48 | .294 |
| Hepatitis C | 2.25 | .378 | 0.60 | .687 |
| Intravenous drug abuse | 2.22 | .245 | 0.64 | .474 |
| Human immunodeficiency virus | 6.0 | .184 | 1.81 | 1.00 |
| Fever | 1.39 | .692 | 2.00 | .467 |
| Mental illness | 0.85 | 1.0 | 2.85 | .610 |
| Hypertension | 0.87 | .836 | 1.38 | .606 |
| Cancer | 0 | .548 | 1.81 | 1.00 |
Table 3.
Univariate Analysis of Continuous Risk Factors Associated With Clindamycin and Levofloxacin Resistance to Methicillin-Resistant Staphylococcus aureus.
| Clindamycin |
Levofloxacin |
|||||
|---|---|---|---|---|---|---|
| Patient Variables | Resistant | Susceptible | P | Resistant | Susceptible | P |
| Mean age (years) | 45.8 | 50.5 | .340 | 47.9 | 50.7 | .534 |
| White blood cell count (K/mm3) | 10.3 | 10.4 | .946 | 10.2 | 10.5 | .844 |
| (RR = 4-11) | ||||||
| Erythrocyte sedimentation rate (mm/h) | 50.9 | 47.0 | .728 | 58.6 | 37.4 | .024 |
| (RR = 0-30) | ||||||
| CRP (mg/L) | 40.9 | 51.5 | .551 | 46.0 | 51.9 | .696 |
| (RR = 0-4) | ||||||
Note. RR = normal reference range; CRP = C-reactive protein.
Patients with a history of IVDU, HCV, or nosocomial infections were all twice as likely to have cases of MRSA that were clindamycin resistant, whereas those with a history of HIV were 6 times more likely to have cases of MRSA that were clindamycin resistant. Patients with a history of diabetes were 2.5 times more likely to have cases of MRSA with levofloxacin resistances, and those who presented with a fever (temperature greater than 38.0°C) at presentation were twice as likely to have MRSA that was levofloxacin resistant. Of those patients who had MRSA that was clindamycin resistant, 58% had a history of IVDU compared with only 39% of patients who had MRSA that was levofloxacin resistant. Of the patients who had clindamycin-resistant MRSA, 67% had a history of nosocomial contact compared with 42% of patients with clindamycin-sensitive MRSA.
Discussion
MRSA was first reported in 1961 after the introduction of methicillin.5 By the year 2004, MRSA accounted for 63% of S aureus isolates in US hospitals’ intensive care units as nosocomial infections. Community-acquired MRSA was not reported until the early 1990s, and genetic studies at that time revealed that this was from a novel clone rather than from the spread of nosocomial MRSA.5,17 The mecA gene located on the staphylococcal cassette chromosome mec (SCCmec) encodes for penicillin binding protein 2A, which is responsible for methicillin resistance. Community-acquired MRSA has a smaller, more mobile cassette, which may be responsible for faster spread of community-acquired MRSA compared with hospital-acquired MRSA. Also unlike hospital-acquired MRSA, community-acquired MRSA was initially susceptible to readily available non-beta-lactam antibiotics such as trimethoprim-sulfamethoxazole, clindamycin, and fluoroquinolones, but antibiotic resistance to these agents has increased in recent years.16
Our study showed a recent decrease in the incidence of MRSA (27% in 2014) in culture-positive hand infections compared with previous studies documenting MRSA incidence between 34% and 73% of culture-positive hand infections.1,5,13-16 Despite this decreasing trend, MRSA remained the most common pathogen isolated from culture-positive hand infections in our study.
During the same time period where MRSA incidence was found to have decreased, MRSA resistance to clindamycin and levofloxacin increased from 7% to 31% and 12% to 56%, respectively. An analysis of risk factors for MRSA infections in the same patient population was performed from 2010 through 2012 and found that intravenous drug users and patients with nosocomial infections were 11 and 5 times more likely to have clindamycin-resistant MRSA infections, respectively.1 The conclusion from these data was that patients with a history of IVDU or recent contact with health care facilities appear to be a potential reservoir for emerging multidrug-resistant MRSA.
Our study also found that clindamycin and levofloxacin resistance levels on MRSA infections remain high (27.9% and 53.5%, respectively). The decreasing incidence of overall MRSA infections left a smaller study group for evaluation of risk factors for drug resistance. Factors that showed increased rates of clindamycin resistance were history of IVDU, HIV, HCV, or nosocomial infections, but these were not statistically significant. Factors with increased OR for levofloxacin resistance were history of diabetes and fever on initial presentation, but these also failed to reach statistical significance. Factors with increased OR for both clindamycin and levofloxacin resistance were IVDU, fever upon initial presentation, HCV, HIV, and nosocomial infection.
This current study has a number of limitations. The retrospective nature of the study relies on documentation of risk factors like IVDU, which patients may not always disclose. Similarly, if a patient was previously hospitalized at another institution and did not disclose this prior to treatment for their MRSA-positive hand infection, they would have inappropriately been labeled as community-acquired MRSA. In addition, the incidence of MRSA and clindamycin-resistant MRSA in our urban study population may not be generalizable to community hospitals or different regions. Furthermore, our study had a limited number of patients from 2013 to 2014 for analysis given the lower incidence of MRSA compared with preceding years, which may limit the detection of statistically significant risk factors.
Despite these limitations, our study does confirm a high incidence of clindamycin- and levofloxacin-resistant MRSA infections of the hand, surpassing the suggested threshold of 10% to 15% rates of resistance that would prompt avoiding them as empiric agents.9 The results from this study show that clindamycin and levofloxacin should not be used unless subsequent culture data can confirm sensitivity. For this reason, vancomycin remains first line for empiric treatment and piperacillin-tazobactam may be added for more severe infections or those with a history suggestive of gram-negative involvement (aquatic, fecal, or soil contamination) or ciprofloxacin for penicillin-allergic patients.14 Trimethoprim-sulfamethoxazole and doxycycline are options when transitioning to outpatient therapy.15 There was only one infection in this study that occurred due to a bite wound, but in these cases, we recommend empiric therapy with intravenous ampicillin-sulbactam or amoxicillin-clavulanic acid.
Conclusions
Despite a decrease in the incidence of MRSA in culture-positive hand infections from 2005 through 2014, the percent of clindamycin- and levofloxacin-resistant isolates has increased. Because of this, clindamycin and levofloxacin should not be used as empiric antibiotics for hand infections. There remains a trend for increased clindamycin and levofloxacin resistance for patients with history of IVDU and nosocomial infections. Future studies remain important to continue to monitor these trends.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: No human or animal rights were violated during the conduction of this study as it was performed as a retrospective chart review.
Statement of Informed Consent: No consent was required as this was a retrospective study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Frederick V. Ramsey  https://orcid.org/0000-0001-9952-8654
https://orcid.org/0000-0001-9952-8654
References
- 1. Tosti R, Trionfo A, Gaughan J, et al. Risk factors associated with clindamycin-resistant, methicillin-resistant Staphylococcus aureus in hand abscesses. J Hand Surg Am. 2015;40(4):673-676. doi: 10.1016/j.jhsa.2014.12.044. [DOI] [PubMed] [Google Scholar]
- 2. Houshian S, Seyedipour S, Wedderkopp N. Epidemiology of bacterial hand infections. Int J Infect Dis. 2006;10(4):315-319. doi: 10.1016/j.ijid.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 3. Shorr AF. Epidemiology of staphylococcal resistance. Clin Infect Dis. 2007;45:S171-S176. [DOI] [PubMed] [Google Scholar]
- 4. Shorr AF. Epidemiology and economic impact of methicillin-resistant Staphylococcus aureus: review and analysis of the literature. Pharmacoeconomics. 2007;25(9):751-768. doi: 10.2165/00019053-200725090-00004. [DOI] [PubMed] [Google Scholar]
- 5. Calfee DP. Trends in community versus health care-acquired methicillin-resistant Staphylococcus aureus infections. Curr Infect Dis Rep. 2017;19(12):48. doi: 10.1007/s11908-017-0605-6. [DOI] [PubMed] [Google Scholar]
- 6. Glass KD. Factors related to the resolution of treated hand infections. J Hand Surg Am. 1982;7(4):388-394. [DOI] [PubMed] [Google Scholar]
- 7. O’Malley M, Fowler J, Ilyas AM. Community-acquired methicillin-resistant Staphylococcus aureus infections of the hand: prevalence and timeliness of treatment. J Hand Surg Am. 2009;34(3):504-508. doi: 10.1016/j.jhsa.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 8. Fowler JR, Greenhill D, Schaffer AA, et al. Evolving incidence of MRSA in urban hand infections. Orthopedics. 2013;36(6):796-800. doi: 10.3928/01477447-20130523-27. [DOI] [PubMed] [Google Scholar]
- 9. Kaplan SL. Treatment of community-associated methicillin-resistant Staphylococcus aureus infections. Pediatr Infect Dis J. 2005;24:457-458. [DOI] [PubMed] [Google Scholar]
- 10. Downs DJ, Wongworawat MD, Gregorius SF. Timeliness of appropriate antibiotics in hand infections. Clin Orthop Relat Res. 2007;461:17-19. doi: 10.1097/BLO.0b013e3180986729. [DOI] [PubMed] [Google Scholar]
- 11. Kiran RV, McCampbell B, Angeles AP, et al. Increased prevalence of community-acquired methicillin-resistant Staphylococcus aureus in hand infections at an urban medical center. Plast Reconstr Surg. 2006;118(1):161-166; discussion 167-169. doi: 10.1097/01.prs.0000221078.63879.66. [DOI] [PubMed] [Google Scholar]
- 12. Wilson PC, Rinker B. The incidence of methicillin-resistant Staphylococcus aureus in community-acquired hand infections. Ann Plast Surg. 2009;62(5):513-516. doi: 10.1097/SAP.0b013e31818a6665. [DOI] [PubMed] [Google Scholar]
- 13. Bach HG, Steffin B, Chhadia AM, et al. Community-associated methicillin-resistant Staphylococcus aureus hand infections in an urban setting. J Hand Surg Am. 2007;32(3):380-383. doi: 10.1016/j.jhsa.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 14. Fowler JR, Ilyas AM. Epidemiology of adult acute hand infections at an urban medical center. J Hand Surg Am. 2013;38(6):1189-1193. doi: 10.1016/j.jhsa.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 15. Tosti R, Samuelsen BT, Bender S, et al. Emerging multidrug resistance of methicillin-resistant Staphylococcus aureus in hand infections. J Bone Joint Surg Am. 2014;96(18):1535-1540. doi: 10.2106/JBJS.M.01159. [DOI] [PubMed] [Google Scholar]
- 16. Kistler JM, Thoder JJ, Ilyas AM. MRSA Incidence and Antibiotic Trends in Urban Hand Infections: A 10-Year Longitudinal Study. HAND. 2018. doi: 10.1177/1558944717750921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tosti R, Ilyas AM. Empiric antibiotics for acute infections of the hand. J Hand Surg Am. 2010;35(1):125-128. doi: 10.1016/j.jhsa.2009.10.024. [DOI] [PubMed] [Google Scholar]