Abstract
Background
US contrast agents are gas-filled microbubbles (MBs) that can be locally destroyed by using external US. Among other bioeffects, US-triggered MB destruction, also known as UTMD, has been shown to sensitize solid tumors to radiation in preclinical models through localized insult to the vascular endothelial cells.
Purpose
To evaluate the safety and preliminary efficacy of combining US-triggered MB destruction and transarterial radioembolization (TARE) in participants with hepatocellular carcinoma (HCC).
Materials and Methods
In this pilot clinical trial, participants with HCC scheduled for sublobar TARE were randomized to undergo either TARE or TARE with US-triggered MB destruction 1–4 hours and approximately 1 and 2 weeks after TARE. Enrollment took place between July 2017 and February 2020. Safety of US-triggered MB destruction was evaluated by physiologic monitoring, changes in liver function tests, adverse events, and radiopharmaceutical distribution. Treatment efficacy was evaluated by using modified Response Evaluation Criteria in Solid Tumors (mRECIST) on cross-sectional images, time to required next treatment, transplant rates, and overall survival. Differences across mRECIST reads were compared by using a Mann-Whitney U test, and the difference in prevalence of tumor response was evaluated by Fisher exact test, whereas differences in time to required next treatment and overall survival curves were compared by using a log-rank (Mantel-Cox) test.
Results
Safety results from 28 participants (mean age, 70 years ± 10 [standard deviation]; 17 men) demonstrated no significant changes in temperature (P = .31), heart rate (P = .92), diastolic pressure (P = .31), or systolic pressure (P = .06) before and after US-triggered MB destruction. No changes in liver function tests between treatment arms were observed 1 month after TARE (P > .15). Preliminary efficacy results showed a greater prevalence of tumor response (14 of 15 [93%; 95% CI: 68, 100] vs five of 10 [50%; 95% CI: 19, 81]; P = .02) in participants who underwent both US-triggered MB destruction and TARE (P = .02).
Conclusion
The combination of US-triggered microbubble destruction and transarterial radioembolization is feasible with an excellent safety profile in this patient population and appears to result in improved hepatocellular carcinoma treatment response.
© RSNA, 2020
Summary
Combined US-triggered microbubble destruction and hepatocellular carcinoma radioembolization showed improved treatment response compared with radioembolization alone and no changes in vital signs or liver function.
Key Results
■ US-triggered microbubble (MB) destruction combined with transarterial radioembolization for treating hepatocellular carcinoma demonstrated no significant changes in physiologic participant parameters.
■ Liver function testing at 1 month showed similar changes between participants undergoing radioembolization versus radioembolization with MB therapy (eg, change in albumin, −0.41 g/dL vs −0.13 g/dL, respectively [P = .16]; change in alanine aminotransferase, −2.7 IU/L vs −3.5 IU/L, respectively [P = .98]).
■ Preliminary efficacy results showed that MB destruction may improve radioembolization outcomes with a greater prevalence of tumor response (14 of 15 [93%; 95% CI: 68, 100] vs five of 10 [50%; 95% CI: 19, 81]; P = .02).
Introduction
Transarterial embolization is recommended for the 15%–25% of patients with hepatocellular carcinoma (HCC) who present with Barcelona Clinic Liver Cancer stage B or more advanced disease (1–3). Within the umbrella of embolic therapies, transarterial radioembolization (TARE) with yttrium 90 (90Y) is gaining in popularity as a treatment option in this patient population. It relies on catheter-directed delivery of radioactive microspheres, consisting of 20- to 60-µm glass beads containing 90Y into the tumor arterial blood supply with maximum tissue penetration in the liver of 10 mm (4,5). Thus, although delivered dosages are high (110–150 Gy), radiation exposure in malignant tissue is highly dependent on source location, which contributes to the less-than-ideal response rates (4,6).
Contrast-enhanced US uses gas microbubbles (MBs) that are encapsulated by an outer protein or lipid shell for stability. When insonated at sufficient acoustic pressures (>100 kPa), the MBs generate nonlinear but stable oscillations in response to an oscillating pressure wave (7,8). At higher acoustic pressures, the MBs undergo destruction via gas diffusion and inertial cavitation (7). Both stable MB oscillations and inertial MB cavitation induce bioeffects in living tissue that can be used for therapeutic purposes (9,10). Among these bioeffects, inertial MB cavitation (generally induced at mechanical indexes > 0.2) can sensitize solid tumors to radiation, as described by Czarnota et al (11). Their group showed a nearly 10-fold improvement in radiosensitivity in a murine subcutaneous tumor model of prostate cancer because of temporary vascular disruption, and they demonstrated that this behavior was dependent on inertial MB cavitation (11). Subsequent work validated these findings in similar models of bladder, breast, and colon cancers (12–14). This synergistic response is dependent on ceramide production, which acts as an apoptotic signaling molecule in endothelial cells and can reduce the radiation dosages required to permanently disrupt the tumor vasculature (15). Later mechanistic studies showed tumor sensitization from MB cavitation arises from mechanical insult to endothelial cells within the tumor vasculature, which results in both activation of the acid sphingomyelinase-ceramide pathway (16) and upregulation of uridylyltransferase glycosyltransferase 8, which then accelerates the transfer of galactose to ceramide (17). This work has been replicated in an orthotopic model of human HCC, showing a 170% reduction in tumor growth and roughly a 320% improvement in animal survival when US-triggered MB destruction, also known as UTMD, was combined with radiation therapy (18). We present, to our knowledge, the first-in-humans randomized clinical trial combining US-triggered MB destruction with HCC radioembolization.
Materials and Methods
Study Participants
The US scanner was provided by Siemens Healthineers (Mountain View, Calif), but authors had sole control of the data. This protocol (clinicaltrials.gov NCT 03199274, FDA IND 126768) was approved by the institutional review board of Thomas Jefferson University. All participants provided written informed consent. Whereas the target sample size for the full efficacy portion of the trial is 104 participants (on the basis of 85% power to detect a 0.6 effect size on tumor response) with an eventual one-to-one randomization allocation, an early analysis was performed in 28 patients to demonstrate feasibility and report interim findings. Participants in the control arm received standard-of-care TARE, whereas participants in the experimental arm underwent TARE with three US-triggered MB destruction sessions: 1–4 hours after radioembolization and at approximately 1 and 2 weeks after treatment. Eligible patients were consecutively identified from our center’s multidisciplinary liver tumor board with exclusion and inclusion criteria provided in Table 1. Treatment assignment was on the basis of a randomization schedule created by the project statistician (S.W.K.).
Table 1:
Study Inclusion and Exclusion Criteria

Radioembolization
At our institution, radioembolization is used to downstage tumors to within Milan criteria (19) for transplant, bridge to transplant, or to palliate disease in patients who are ineligible for transplant. Patients referred for HCC TARE had Liver Imaging Reporting and Data System LR-5 masses or LR-4/M masses (20) with subsequent HCC tissue diagnosis. Patients undergo planning arteriograms with intra-arterial administration of technetium 99m macroaggregated albumin to ensure radioembolization is feasible and safe. This is used to calculate the lung shunt fraction, which must be less than 30 Gy during a single treatment. The target volume is calculated from cone-beam CT and dosage is determined using the medical internal radiation dose model.
Therapeutic Protocol
US imaging was performed by using a S3000 HELX scanner with a 6C1 probe (Siemens Healthineers) by a sonographer (C.E.W., with 6 years of contrast-enhanced US experience). In participants with multiple tumors that were treated in the same interventional session by using TARE (one patient), the largest tumor was assigned for US-triggered MB destruction and the secondary tumor was used as a control tumor when evaluating treatment response. Five milliliters of Optison (GE Healthcare, Princeton, NJ) were suspended in a 50-mL bag of saline manually reagitated every 2–3 minutes and infused at a rate of 120 mL per hour (monitored by K.B., a research nurse with 14 years of experience). After confirmation of contrast enhancement, a series of US-triggered MB destruction replenishment sequences was initiated.
Participants temporarily halted respiration while a 4-second US-triggered MB destruction sequence (mechanical index, 1.13 at 1.5 MHz, transmitting 2.3-µsec pulses at a pulse repetition frequency of 100 Hz) was transmitted, followed by nonlinear lower intensity imaging of contrast replenishment (mechanical index, 0.06) for 10 seconds. This sequence was repeated three to five times at the tumor midline then modified for the remainder of the contrast material infusion (∼10 minutes) to consist of sweeps through the entire tumor.
Safety Monitoring
Temperature, heart rate, and blood pressure were obtained before and after US-triggered MB destruction by a research nurse (K.B.). Immediate adverse event monitoring was performed for 30 minutes after US-triggered MB destruction while delayed adverse events were monitored for 30 days by using National Institutes of Health criteria (21). Blood sampling for liver function test monitoring was performed before radioembolization and approximately 1 month after radioembolization as part of the participant’s standard of care. A subset of six participants (three participants who underwent TARE and three who underwent TARE with US-triggered MB destruction) underwent SPECT imaging with additional planar imaging of the lung bases 1–2 hours after radioembolization and US-triggered MB destruction (where applicable). These data were obtained sequentially in a subset of participants because of the logistical difficulties of performing US-triggered MB destruction before SPECT. The radiopharmaceutical distribution was assessed as clinical standard of care by a radiologist (C.I., with 34 years of experience) blinded to the participant’s treatment arm.
Therapeutic Efficacy Monitoring
Two board-certified radiologists (A.L. and P.O., with 15 and 23 years of experience in body imaging) who were blinded to the treatment arm assignment evaluated each participant’s contrast-enhanced MRI before embolization and 4–6 months after radioembolization and provided a consensus read by using the modified Response Evaluation Criteria in Solid Tumors, or mRECIST (22). In participants who underwent further tumor intervention or died before the 4-month imaging window (two participants in each arm), a 1- to 3-month follow-up examination was used. Other than US-triggered MB destruction, all participants were provided identical standard of care, with re-treatment decisions decided by a multidisciplinary tumor board blinded to assigned study arm. For each participant, time to required next treatment for the specific tumor, overall survival, and transplant status were monitored. A hepatologist (J.C.) and transplant surgeon (W.M.) blinded to the participant’s treatment arm excluded participants who were not candidates for transplant for reasons other than tumor burden. Rates of transplant were then compared in the liver transplant-eligible subset.
Statistical Analysis
Statistical analyses were performed by using software (SAS V9.4; SAS Institute, Carey, NC). Differences across mRECIST distributions were compared by using a Mann-Whitney U test and the difference in prevalence of tumor response by Fisher exact test, whereas differences in time to required next treatment and overall survival curves were compared by using a log-rank (Mantel-Cox) test. Changes in vital signs were compared by using least-squares mean changes with robust 95% CIs estimated by repeated measures generalized estimating equations regression modeling adjusted for time. For this analysis, P values less than .05 were considered to indicate statistical significance.
Results
Participant Characteristics and US Findings
As of our study’s submission, 108 patients were screened, 44 patients were deemed eligible, and 28 participants were enrolled and assigned to one of the two study arms (Figs 1, 2). All enrolled participants had at least 6 months of follow-up available. Enrolled participant demographics are provided in Table 2; 17 of 28 participants were men (mean age, 70 years ± 10). Other than body mass index (P = .02), participant demographics and tumor characteristics did not differ substantially between the two study arms.
Figure 1:
Flowchart of patient enrollment for analysis of protocol safety and interim efficacy analysis. TARE = transarterial radioembolization, UTMD = US-triggered microbubble destruction.
Figure 2:
Summary of participant involvement over the course of the study. TARE = transarterial radioembolization, Tx = treatment, UTMD = US-triggered microbubble destruction.
Table 2:
Summary of Participant Demographics and Safety Data
US contrast enhancement and MB destruction were observed in all examinations. Figure 3 illustrates this US-triggered MB destruction sequence in a tumor that later showed complete response 4 months after treatment. Despite treatment with local-regional embolization therapy, active blood flow still appeared within the tumor immediately following treatment because the glass microspheres (20–30 µm) do not fully occlude blood supply to the tumor (Fig 3, A). MBs were readily destroyed during US-triggered MB destruction (Fig 3, B) and reperfused back into the tumor after US-triggered MB destruction (Fig 3, C, D). Quarterly dosimetry reports from badges worn by the performing sonographer were all less than 0.08 mSv, indicating no noticeable radiation exposure during the US-triggered MB destruction examinations.
Figure 3:
Example contrast-enhanced US series show the sequence of US-triggered microbubble (MB) destruction in an 80-year-old male participant 2 hours after radioembolization. Imaging was performed in dual mode with the nonlinear contrast mode (left side of A–D) images showing MB signal and B mode (right side of A–D) used for anatomic guidance. A, Marked MB enhancement within the tumor (arrows) was observed 2 hours after radioembolization. B, At the initiation of the destructive pulse, a higher mechanical index pulse was generated, causing US-triggered MB destruction. Immediately following the 4-second destructive pulse, US-triggered MB destruction was confirmed by, C, an absence of contrast signal in the tumor and surrounding liver followed by, D, gradual MB reperfusion (required for repeat US-triggered MB destruction) back into the tumor.
Safety Assessments
US-triggered MB destruction after HCC radioembolization appears to be well tolerated. Within the TARE-alone group, one participant reported fatigue after treatment and one participant experienced an ST-elevation myocardial infarct immediately following the procedure. Within the TARE with US-triggered MB destruction group, one participant developed an upper torso rash 24 hours after treatment. This rash resolved within 2 days without intervention and did not reappear after the second or third Optison (GE Healthcare) infusions. In participants who underwent US-triggered MB destruction, there were no noticeable changes in body temperatures (mean change in temperature, 0.1°F; 95% CI: −0.1, 0.4; P = .31), heart rate (mean change, −0.1 beats per minute; 95% CI: −3.2, 2.9; P = .92), systolic pressure (mean change, −4 mm Hg; 95% CI: −8, 0; P = .06), or diastolic pressure (mean change, 1 mm Hg; 95% CI: −1, 3; P = .31) before and after US-triggered MB destruction.
SPECT of the abdomen with additional planar imaging of the chest was performed in six participants (three participants who underwent TARE alone and three participants who underwent TARE with US-triggered MB destruction). Successful localization of delivered radiopharmaceutical within the targeted liver area was confirmed in all participants irrespective of US-triggered MB destruction augmentation. No radiopharmaceutical activity was detected outside the liver, confirming absence of agent dislodging in participants who underwent US-triggered MB destruction. In addition, a review of all available CT examinations, chest radiography, MRI examinations, and clinical records within 4 months following treatment showed no evidence of the adverse effects that would mostly likely be expected with 90Y bead repositioning because of US-triggered MB destruction, such as pulmonary fibrosis, radiation pneumonitis, and gastroduodenal injury.
One-month follow-up liver function tests were available in 26 participants (two patients died or were referred to hospice before follow-up laboratory collection). Importantly, similar changes in liver function values were observed between patients undergoing radioembolization and radioembolization with MB therapy (change in albumin, −0.4 g/dL ± 0.6 vs −0.1 g/dL ± 0.4 [P = .16]; change in alanine aminotransferase, −2.7 IU/L ± 7.9 vs −3.5 IU/L ± 9.2 [P = .98]; change in aspartate transaminase, 0 IU/L ± 6.6 vs 3.0 IU/L ± 10.9 [P = .72]; change in bilirubin, 0.1 mg/dL ± 0.5 vs 0.1 mg/dL ± 0.4 [P = .92]; change in hemoglobin, −0.5 g/dL ± 2.8 vs 0.5 g/dL ± 2.8 [P = .80]; change in white blood cell count, −0.2 109/L ± 1.0 vs −0.1 109/L ± 2.1 [P = .99]).
Preliminary Efficacy
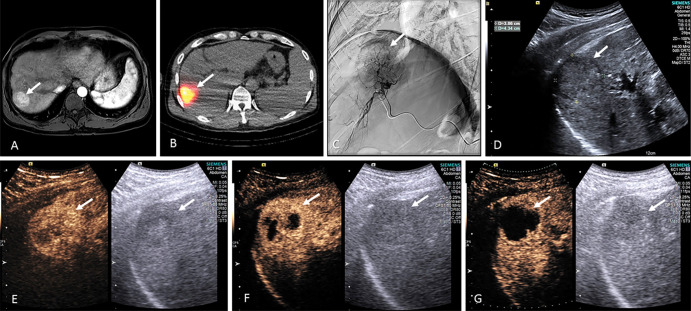
Early results indicate that US-triggered MB destruction sensitizes tumors to radioembolization and may improve both tumoral response and overall participant survival. An example series from a participant deemed to have complete response at 4-month MRI follow-up is provided in Figure 4. This participant was subsequently listed for orthotopic liver transplantation. Notice the observed progression in contrast-enhanced US–derived vascularity reduction within the tumor (Fig 4, E–G).
Figure 4:
Example series of from a 54-year-old male participant undergoing hepatocellular carcinoma radioembolization with US-triggered microbubble (MB) destruction. The series shows baseline contrast-enhanced MRI scans in, A, transverse plane, B, technetium 99m macroaggregated albumin SPECT-CT in the transverse plane during treatment planning, C, angiography during yttrium 90, D, B-mode US immediately after radioembolization, and peak contrast-enhanced US enhancement during US-triggered MB destruction, E, 2 hours, F, 1 week, and, G, 2 weeks after radioembolization.
Blinded tumor response evaluations were completed by two radiologists in consensus for 10 tumors randomized to TARE alone and 15 tumors assigned to TARE combined with US-triggered MB destruction (with follow-up imaging pending in the remaining participants). Four- to 6-month contrast-enhanced MRI was used for follow-up in 21 of these tumors, whereas earlier clinical intervention or death necessitated the use of earlier MRI studies (1–3 months) in four participants (two in each arm). The tumor response evaluation results, summarized in Figure 5, showed an improved distribution of responses but these were not statistically significant (P = .06). Participants who underwent US-triggered MB destruction with TARE showed a greater prevalence of partial or complete tumor response (14 of 15 [93%; 95% CI: 68, 100] vs five of 10 [50%; 95% CI: 19, 81], respectively; P = .02).
Figure 5:
Summary of blinded consensus tumor response evaluations from 25 tumors (10 randomized to transarterial radioembolization [TARE] alone, 15 assigned to TARE combined with US-triggered microbubble destruction [UTMD]). Responses ranged from stable disease to complete response, with a potentially greater prevalence of tumor response in participants undergoing US-triggered microbubble destruction (P = .02).
Consensus interpretation by a hepatologist and transplant surgeon of transplant eligibility aside from tumor burden deemed four participants in the TARE-alone group and 10 participants in the TARE with US-triggered MB destruction group eligible for orthotopic liver transplant. Of these participants, two (50% of eligible participants) in the TARE-alone group and seven (70% of eligible participants) in the TARE with US-triggered MB destruction group were listed to undergo liver transplant. Fifty-five percent of participants in the radioembolization-alone arm required tumor retreatment with a median time to required next treatment of 148 days, whereas only 35% in the US-triggered MB destruction group required retreatment with a time to required next treatment yet to be identified (median not yet reached). These results are encouraging but not significant (P = .24). Three participants in the TARE-alone arm died (all from disease progression), whereas only one participant who underwent both TARE and US-triggered MB destruction died (from complications from hip fracture thought to be unrelated to underlying HCC). Early results that compared overall survival curves were promising but not significant, with a median survival of 550 days in participants undergoing TARE alone, whereas the median survival of TARE with US-triggered MB destruction participants was not yet identified (P = .07).
Discussion
In this first-in-humans trial, we describe the feasibility and early safety and efficacy findings of combining US-triggered microbubble (MB) destruction with transarterial radioembolization (TARE) to augment the treatment of hepatocellular carcinoma. Results from the first 28 participants in this ongoing trial demonstrate the feasibility of this approach, with MB infusion and destruction successfully observed at three points following radioembolization. Physiologic monitoring during MB therapy showed no significant changes in physiologic patient parameters before and after US-triggered MB destruction (P > .06). When comparing changes in liver function between groups undergoing TARE alone compared with TARE with US-triggered MB destruction, liver function was not compromised by US-triggered MB destruction in these participants (change in albumin, −0.4 g/dL ± 0.6 vs −0.1 g/dL ± 0.4 [P = .16]; change in alanine aminotransferase, −2.7 IU/L ± 7.9 vs −3.5 IU/L ± 9.2 [P = .98]; change in aspartate transaminase, 0 IU/L ± 6.6 vs 3.0 IU/L ± 10.9 [P = .72]; change in bilirubin, 0.1 mg/dL ± 0.5 vs 0.1 mg/dL ± 0.4 [P = .92]; change in hemoglobin, −0.5 g/dL ± 2.8 vs 0.5 g/dL ± 2.8 [P = .80]; and change in white blood cell count, −0.2 109/L ± 1.0 vs −0.1 109/L ± 2.1 [P = .99]). Importantly, while monitoring of longer term response continues, participants who underwent US-triggered MB destruction with TARE also demonstrated an improved rate of tumoral response on modified Response Evaluation Criteria in Solid Tumors (14 of 15 [93%; 95% CI: 68, 100] vs five of 10 [50%; 95% CI: 19, 81], respectively; P = .02). These findings are consistent with prior preclinical findings (11–18).
The ability to monitor tumor vascularity within the first 2 weeks of treatment is also noted. Tumor vascularity is retained immediately following TARE, as noted by persistent enhancement at the initial US-triggered MB destruction examination. This persistent blood flow following radioembolization contrasts with the blood flow response to transarterial chemoembolization, where complete avascularity on contrast-enhanced US indicates complete treatment (23). Additionally, reductions in tumor vascularity were noted over the course of therapy, which may be useful for predicting tumoral response earlier than the current clinical standard.
There are numerous factors that can affect the efficacy of TARE. These include the desired dose as determined by the interventional radiologist, the activity administration model used, the radiosensitivity of the tumor and normal liver, and the flow dynamics within the tumor bed and feeding vessels. The desired dose depends on treatment intent, the volume of liver to be treated, liver function, patient performance status, and the ability to modify perfusion within a treatment volume so that blood flow can be redistributed to the tumor. Radiation segmentectomy (superselective embolization [≤ two segments] with > 190 Gy) was adopted by some institutions as definitive treatment of small tumors (24,25). Whereas this technique is occasionally used at our institution in patients with earlier stage disease, percutaneous microwave ablation remains the clinical standard of care for Barcelona Clinic Liver Cancer stage A disease.
Both stable and inertial MB cavitation have well-documented bioeffects that can be harnessed for improving therapy (9,10). These approaches have begun to be translated to clinical trials, including the use of MB cavitation for enhancing systemic chemotherapy in patients with pancreatic cancer (26) and for restoring epicardial and microvascular flow in patients with myocardial infarction (27). Our study continues this trend of clinical translation by demonstrating the applicability and safety of previously described cavitation-based bioeffects (11–17) in participants undergoing radiation therapy in HCC. Although our pilot study focuses on a relatively narrow clinical application, we anticipate similar augmentation in a wide variety of radiotherapies.
Our study had limitations. The sample size from our ongoing study was small and treatment efficacy cannot yet be conclusively defined. Participants in the treatment arm showed a lower body mass index, although we do not expect this to influence tumor outcomes in light of similar Child-Pugh points, Eastern Cooperative Oncology Group Performance Score, Barcelona Clinic Liver Cancer stage, and ascites prevalence between the two groups. Although we assume that inertial cavitation took place with our current US-triggered MB destruction protocol, findings are limited to empirical observation and should be studied further moving forward. Our study used Optison (GE Healthcare) because of our previous preclinical results (18). However, to our knowledge, no work to date has directly compared commercial agents for US-triggered MB destruction radiosensitization, which may lead to selection of an optimal US contrast agent with improved therapeutic efficacy. SPECT to evaluate 90Y redistribution after US-triggered MB destruction was only performed in a subset of six sequential participants because of logistics and, therefore, conclusions from these data were limited. However, no participants in this trial developed symptoms that would be expected from bead repositioning. Finally, findings were from a single-center nonblinded study. Multicenter trials that also include patient blinding (with either sham US-triggered MB destruction or contrast-enhanced US without MB destruction to monitor response without inducing bioeffects) are likely needed to gain clinical approval.
In conclusion, the mechanisms and potential therapeutic advantages of combining US-triggered microbubble (MB) destruction with radiation therapy have been well documented in a variety of preclinical models. Our findings demonstrated the feasibility of combining US-triggered MB destruction with hepatocellular carcinoma radioembolization and the early safety and efficacy in this population of participants.
Acknowledgments
Acknowledgments
Equipment was provided by Siemens Healthineers. We thank Nancy Pedano, CCRC, Marsha Robinson, MS, and Diana Zaccagnini, BS, for coordinator support and Jennifer Wilson, MS, for assistance with manuscript editing.
Disclosures of Conflicts of Interest: J.R.E. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money to author’s institution for grant, equipment, and contrast agent support from GE Healthcare; money to author for lectures from Lantheus Medical Imaging; contrast agent support from Lantheus Medical Imaging; equipment support from Cannon. Other relationships: disclosed no relevant relationships. F.F. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed board membership from American Institute of US in Medicine; money paid to author for consultancy from Samumed; money to author’s institution for numerous NIH grants; money paid to author for lectures from Lantheus Medical Imaging; disclosed several patents, but none licensed and no income; disclosed money paid to author for grant reviewer position from the DOD and NIH, the American Society of Echocardiography, and the RSNA Research and Education Foundation; GE Healthcare supplied US scanners and contrast agents for research projects; Canon Medical Systems supplied US scanners for research projects; Lantheus Medical Imaging and Bracco supplied US contrast agents for research projects. Other relationships: disclosed no relevant relationships. C.E.W. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed payment to author for lectures from Bracco Imaging. Other relationships: disclosed no relevant relationships. L.J.D. disclosed no relevant relationships. K.B. disclosed no relevant relationships. S.G. disclosed no relevant relationships. M.T. disclosed no relevant relationships. A.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money to author for board membership from Bracco Diagnostics; consultancy from GE Healthcare, Bioclinica, WCC; grants from Bracco Diagnostics, GE Healthcare, Canon Medical, and Siemens Healthcare; payment for lectures from GE Healthcare; royalties from Elsevier. Other relationships: disclosed no relevant relationships. P.O. disclosed no relevant relationships. J.B.L. disclosed no relevant relationships. C.I. disclosed no relevant relationships. J.C. disclosed no relevant relationships. W.M. disclosed no relevant relationships. S.W.K. disclosed no relevant relationships. K.A. disclosed no relevant relationships. A.T. disclosed no relevant relationships. A.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed stock in HistoSonics. Other relationships: disclosed no relevant relationships. S.S.N. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money to author for lectures from Boston Scientific and Sirtex Medical. Other relationships: disclosed no relevant relationships. C.M.S. disclosed no relevant relationships.
Study supported by the National Institutes of Health (R01 CA238241).
Abbreviations:
- HCC
- hepatocellular carcinoma
- MB
- microbubble
- TARE
- transarterial radioembolization
References
- 1.Ahmed O, Liu L, Gayed A, et al. The changing face of hepatocellular carcinoma: forecasting prevalence of nonalcoholic steatohepatitis and hepatitis C cirrhosis. J Clin Exp Hepatol 2019;9(1):50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M; American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma: an update. Hepatology 2011;53(3):1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saraswat VA, Pandey G, Shetty S. Treatment algorithms for managing hepatocellular carcinoma. J Clin Exp Hepatol 2014;4(Suppl 3):S80–S89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreana L, Isgrò G, Marelli L, et al. Treatment of hepatocellular carcinoma (HCC) by intra-arterial infusion of radio-emitter compounds: trans-arterial radio-embolisation of HCC. Cancer Treat Rev 2012;38(6):641–649. [DOI] [PubMed] [Google Scholar]
- 5.Paul SB, Sharma H. Role of Transcatheter Intra-arterial Therapies for Hepatocellular Carcinoma. J Clin Exp Hepatol 2014;4(Suppl 3):S112–S121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirchner T, Marquardt S, Werncke T, et al. Comparison of health-related quality of life after transarterial chemoembolization and transarterial radioembolization in patients with unresectable hepatocellular carcinoma. Abdom Radiol (NY) 2019;44(4):1554–1561. [DOI] [PubMed] [Google Scholar]
- 7.Lyshchik A, ed. Fundamentals of CEUS. Philadelphia, Pa: Elsevier, 2019. [Google Scholar]
- 8.Eisenbrey JR, Forsberg F. Contrast-enhanced ultrasound for molecular imaging of angiogenesis. Eur J Nucl Med Mol Imaging 2010;37(Suppl 1):S138–S146. [DOI] [PubMed] [Google Scholar]
- 9.Kooiman K, Roovers S, Langeveld SAG, et al. Ultrasound-Responsive Cavitation Nuclei for Therapy and Drug Delivery. Ultrasound Med Biol 2020;46(6):1296–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Souza JC, Sultan LR, Hunt SJ, et al. Microbubble-enhanced ultrasound for the antivascular treatment and monitoring of hepatocellular carcinoma. Nanotheranostics 2019;3(4):331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czarnota GJ, Karshafian R, Burns PN, et al. Tumor radiation response enhancement by acoustical stimulation of the vasculature. Proc Natl Acad Sci U S A 2012;109(30):E2033–E2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran WT, Iradji S, Sofroni E, Giles A, Eddy D, Czarnota GJ. Microbubble and ultrasound radioenhancement of bladder cancer. Br J Cancer 2012;107(3):469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Kaffas A, Nofiele J, Giles A, Cho S, Liu SK, Czarnota GJ. Dll4-notch signalling blockade synergizes combined ultrasound-stimulated microbubble and radiation therapy in human colon cancer xenografts. PLoS One 2014;9(4):e93888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai P, Tarapacki C, Tran WT, et al. Breast tumor response to ultrasound mediated excitation of microbubbles and radiation therapy in vivo. Oncoscience 2016;3(3-4):98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nofiele JT, Karshafian R, Furukawa M, et al. Ultrasound-activated microbubble cancer therapy: ceramide production leading to enhanced radiation effect in vitro. Technol Cancer Res Treat 2013;12(1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Kaffas A, Al-Mahrouki A, Hashim A, Law N, Giles A, Czarnota GJ. Role of acid sphingomyelinase and ceramide in mechano-acoustic enhancement of tumor radiation responses. J Natl Cancer Inst 2018;110(9):1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Mahrouki A, Giles A, Hashim A, et al. Microbubble-based enhancement of radiation effect: Role of cell membrane ceramide metabolism. PLoS One 2017;12(7):e0181951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daecher A, Stanczak M, Liu JB, et al. Localized microbubble cavitation-based antivascular therapy for improving HCC treatment response to radiotherapy. Cancer Lett 2017;411:100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334(11):693–699. [DOI] [PubMed] [Google Scholar]
- 20.Wilson SR, Lyshchik A, Piscaglia F, et al. CEUS LI-RADS: algorithm, implementation, and key differences from CT/MRI. Abdom Radiol (NY) 2018;43(1):127–142. [DOI] [PubMed] [Google Scholar]
- 21.Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Cancer Therapy Evaluation Program (CTEP), National Cancer Institute. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. Accessed April 3, 2020. [Google Scholar]
- 22.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw CM, Eisenbrey JR, Lyshchik A, et al. Contrast-enhanced ultrasound evaluation of residual blood flow to hepatocellular carcinoma after treatment with transarterial chemoembolization using drug-eluting beads: a prospective study. J Ultrasound Med 2015;34(5):859–867. [DOI] [PubMed] [Google Scholar]
- 24.Lewandowski RJ, Gabr A, Abouchaleh N, et al. Radiation Segmentectomy: Potential Curative Therapy for Early Hepatocellular Carcinoma. Radiology 2018;287(3):1050–1058. [DOI] [PubMed] [Google Scholar]
- 25.Salem R, Gabr A, Riaz A, et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology 2018;68(4):1429–1440. [DOI] [PubMed] [Google Scholar]
- 26.Dimcevski G, Kotopoulis S, Bjånes T, et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J Control Release 2016;243:172–181. [DOI] [PubMed] [Google Scholar]
- 27.Mathias W Jr, Tsutsui JM, Tavares BG, et al. Sonothrombolysis in ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol 2019;73(22):2832–2842. [DOI] [PubMed] [Google Scholar]








![Summary of blinded consensus tumor response evaluations from 25 tumors (10 randomized to transarterial radioembolization [TARE] alone, 15 assigned to TARE combined with US-triggered microbubble destruction [UTMD]). Responses ranged from stable disease to complete response, with a potentially greater prevalence of tumor response in participants undergoing US-triggered microbubble destruction (P = .02).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7b2b/7850263/50c2e92f4942/radiol.2020202321.fig5.jpg)