Abstract
Acquired hepatocerebral degeneration refers to a neurological syndrome consisting of various movement disorders and cognitive impairment in advanced liver cirrhosis or portosystemic shunt. Neurological signs and symptoms may be attributed to the accumulation of toxic substances in the brain. The most common neurological presentation of this is parkinsonism. Our prospective study aimed to investigate the prevalence of parkinsonism in patients with cirrhosis who were evaluated for liver transplant and to identify any correlation between findings on brain magnetic resonance imaging (MRI) and severity of parkinsonism. Of the 120 enrolled participants with liver cirrhosis, 62 (52%) exhibited signs of parkinsonism and all had MRI basal ganglia hyperintensity. Eighteen patients from this group were transplanted and showed statistically significant improvements in their Unified Parkinson's Disease Rating Scale (UPDRS) scores. Conclusion: The data suggest the reversibility of the neurological impairment seen in cirrhosis, and therefore the effectiveness of transplantation in improving parkinsonian symptoms. There was no correlation between severity of MRI findings and clinical motor UPDRS part III. Laboratory findings showed no correlation among the abnormal levels, MRI brain signal abnormality, or UPDRS scores.
Abbreviations
- AHD
acquired hepatocerebral degeneration
- L/Thal
left thalamus
- L/WH
left white matter
- MELD
Model for End‐Stage Liver Disease
- MMSE
Mini Mental Status Examination
- MRI
magnetic resonance imaging
- NASH
nonalcoholic steatohepatitis
- PD
Parkinson’s disease
- R/Thal
right thalamus
- R/WH
right white matter
- ROI
region of interest
- T1WI
T1‐weighted imaging
- UPDRS
Unified Parkinson’s Disease Rating Scale
Acquired hepatocerebral degeneration (AHD) is seen in patients with advanced hepatobiliary disease and presents with a variety of neurological syndromes, parkinsonism being the most common. AHD is considered to be chronic and progressive in nature in contrast to hepatic encephalopathy, which is an acute and reversible condition, often preceding AHD.( 1 ) Portosystemic shunting is an important predisposing factor in the development of AHD, as toxic substances can enter the brain via the systemic circulation.( 2 ) Mechanism of AHD is believed to be due to accumulation of those toxic substances, such as ammonia or manganese, in vulnerable areas of the brain, leading to neuroinflammation and subsequently to neurodegeneration.( 3 , 4 ) As basal ganglia appear to be susceptible to accumulation of the toxic materials in AHD, parkinsonism is the most common clinical syndrome seen. Parkinsonism and other movement disorders (primarily ataxia) have been seen in AHD, and it is estimated that about 60% of patients will be affected.( 5 , 6 ) Prevalence of parkinsonism in AHD varies in the literature, and ranges from 3.5%‐4.2%,( 7 , 8 ) to up to 21.6%.( 5 )
Parkinsonism in AHD is described as symmetrical parkinsonism with predominant action and postural tremor rather than typical resting and asymmetrical tremor in idiopathic Parkinson’s disease (PD).( 5 , 9 ) Parkinsonism in AHD has variable response to levodopa.( 5 ) Clinically, symptoms tend to gradually progress unless liver transplant is performed. With advances in technology, we have more neuroimaging data on patients with AHD. Typically there is a symmetric, high signal intensity on T1‐weighted imaging (T1WI) in the globus pallidus, sometimes extending to the putamen, caudate nucleus and midbrain, and rarely to the cerebellum.( 2 , 10 , 11 , 12 , 13 ) The high signal intensity on T1WI is thought to be due to manganese accumulation.( 14 , 15 ) Patients who have lesions with high signal intensity on T1W1 do not necessarily develop symptoms of AHD. In the past few years, dopaminergic scans are suggesting presynaptic dopaminergic neurodegeneration in patients with AHD. Both 18F‐DOPA positron emission tomography and DaTscan showed presynaptic dopaminergic neurodegeneration.( 2 , 16 , 17 )
We present an observational prospective study looking at individuals with liver disease and related parkinsonism as well as the effect of transplantation, magnetic resonance imaging (MRI) signal abnormalities, and laboratory findings in this patient population. The primary study aim was to study the frequency of parkinsonism in patients with cirrhosis at Lahey Hospital and Medical Center. A secondary aim was to explore the effect of liver transplantation on the timing of clinical and radiographic improvements following transplantation. Additional aims included exploring the frequency and type of MRI abnormalities associated with parkinsonism, and to see whether there is correlation with abnormal laboratory findings.
Methods
Study Design
Setting
This prospective, single‐center observational study was executed by the Department of Neurology in collaboration with the Department of Liver Transplantation and Hepatobiliary Diseases at the Lahey Hospital and Medical Center in Burlington, Massachusetts, and was approved by the local institutional review board. During a 5‐year period (2005‐2010), a total of 120 participants were enrolled, and participants were followed for up to 3 years.
Participants
All individuals who were diagnosed with liver disease and undergoing liver transplant evaluation were eligible to participate. Only those being evaluated for liver transplant were included due to increased likelihood of transplantation occurring within the study timeframe of 3 years. Liver disease was defined as the presence of one or more of the following diagnoses: cryptogenic cirrhosis, alcoholic cirrhosis, nonalcoholic steatohepatitis (NASH), viral hepatitis (hepatitis B, hepatitis C), Laennec’s cirrhosis, autoimmune‐associated (primary biliary cirrhosis, primary sclerosing cholangitis), Budd–Chiari syndrome, genetic (alpha‐1 antitrypsin deficiency), and liver cancer (hepatocellular carcinoma). Participants with an inability to provide consent, diagnosed with Wilson Disease, having a history of prior parkinsonism, and/or having drug‐induced parkinsonism, were ineligible. Enrolled participants not cleared for liver transplant, not listed, or who were later removed from the liver transplant waiting list were withdrawn and did not undergo further study evaluation.
Variables
Standard liver evaluation: Participants underwent extensive evaluation standard to liver transplantation, including, but not limited to, Child‐Pugh score, standard blood work (urinalysis, complete blood count, blood chemistries, and liver function tests), chest x‐ray, electrocardiography, computed tomography/MRI, endoscopy, liver ultrasound, and pre‐operative evaluations. The Model for End‐Stage Liver Disease (MELD) score was used as a determinant of liver disease severity. MELD is a scoring system for assessing the severity of chronic liver disease and is used by the United Network for Organ Sharing for prioritizing allocation of liver transplants.( 18 )
Baseline Visit
Written, informed consent was obtained and participants were examined in the neurology department as part of their routine pre‐transplantation workup. Baseline assessment included eligibility review and neurological evaluation, including Mini Mental Status Examination (MMSE) to evaluate cognitive status and Unified Parkinson’s Disease Rating Scale (UPDRS) evaluation for the presence of parkinsonism. The UPDRS was developed to evaluate various aspects of PD, including nonmotor and motor experiences of daily living and motor complications. The UPDRS scale consists of the following four segments: mentation, behavior, and mood (part I), activities of daily living (part II), motor sections (part III), and complications of therapy.( 19 ) All patients who were on medications that could induce parkinsonism were excluded from the study.
Parkinsonism Evaluation
Parkinsonism was defined as an abnormal UPDRS motor part III score of greater than or equal to 1. The UPDRS is the most frequently used rating scale for signs and symptoms of parkinsonism and has been shown to have excellent test‐retest reliability. Each question is rated from 0 (normal) to 4 (severe). Therefore, the higher the UPDRS score, the greater the disability from PD. Participants were evaluated by a trained and experienced master’s level research assistant who videotaped the UPDRS examination, which was later reviewed for confirmation by a blinded movement disorder specialist.
Stratification
After initial evaluations, participants were stratified according to UPDRS score and entered into 1 of 2 groups. Group 1 (parkinsonian) consisted of participants who demonstrated parkinsonism or extrapyramidal symptoms (e.g., tremor, rigidity, bradykinesia, postural instability, gait impairment) with an abnormal UPDRS score of >1 on the motor function score of the UPDRS (UIII) or per evaluator discretion. All patients, in addition to having UPDRS, were videotaped and reviewed by the movement disorders expert who also made the decision in ambiguous patients.
Group 2 (non‐parkinsonian) consisted of participants who exhibited no signs of parkinsonism or a score of <1 on the UIII, or did not meet group 1 criteria due to the evaluator’s discretion. Discretion was used if an abnormality was present but there were insufficient signs to fulfill the criteria, or if a comorbidity or underlying issue contributed to abnormal scoring, such as another neurological disease, recent hand/leg surgery, or a pre‐existing condition that would exclude proper or complete evaluation.
Laboratory Testing
Participants in the parkinsonism group underwent additional testing for serum copper, ammonia, and iron levels before transplantation, to rule out other possible diagnoses. Participants were ruled out for Wilson Disease by copper level. Ammonia level was tested to assess correlation with cirrhosis. Testing for ammonia, copper, and serum manganese levels occurred at the time of baseline UPDRS or within 60 days of the UPDRS administration. Although there are data suggesting that level of manganese in blood does not predict neurological impairment in AHD, and that cerebrospinal fluid level might be a more sensitive indicator, we decided not to include this, as many patients might not participate in the study due to more invasive nature of lumbar puncture.
Brain Imaging
The parkinsonism group also underwent brain MRI to detect brain abnormalities. Baseline MRI occurred within 60 days of initial UPDRS assessment. MRI was performed on 1.5T magnetic resonance scanners (GE Healthcare, Milwaukee, WI) using an eight‐channel head coil. Typical axial T1WI parameters include field of view = 24 cm, slice profile = 5 × 6.5 mm, flip angle = 90°, echo time = 8 ms, repetition time = 500 ms, matrix = 320 × 224, and number of excitations = 2. The ratio of signal intensity within symmetrically placed (25 mm2) regions of interest (ROIs) within the medial globus pallidus/thalamus and medial globus pallidus/forceps minor white matter were acquired in an effort to normalize for minor variations in the T1 pulse sequence, equipment software enhancements, and head positioning during the 6‐year prospective time interval. All ROIs were placed by the same neuroradiologist with 30 years of neuroimaging experience. The neuroradiologist who interpreted the images was blinded to the UPDRS and other findings (e.g., transplant status, biological findings). The ROIs for the medial globus pallidus were symmetrically positioned bilaterally, just posterior to the anterior commissure and 10 mm off midline. In a similar fashion, the thalamic ROIs were placed symmetrically within the inferomedial thalamus. Symmetric bilateral ROIs of the forceps minor were acquired within deep frontal white matter at the level of the corpus callosum genu. Ratios of medial globus pallidus/thalamic and medial globus pallidus/white matter were calculated and subsequently correlated with clinical metrics. Signal intensity within the adenohypophysis/span on sagittal T1WI was considered elevated if its signal intensity was equal to or greater than that of the corpus callosum. As dedicated thin‐section pituitary MRI was not acquired, formal pituitary/corpus callosum signal intensity ratios were not performed. Brain MRIs of patients with PD and no history of cirrhosis were used as a control. As these patients experienced similar motor and neurological symptoms, this was a historical cohort. Because we were not able to scan the non‐parkinsonism group, and it is not known how many participants would have had abnormal images, we used a control group of PD patients who already had brain imaging and compared those to pre‐transplantation and post‐transplantation imaging. Those patients with PD were the historical cohort with similar UPDRS motor scores based on a chart review and were followed and rated by the same blinded movement disorders specialist.
Follow‐up
Participants in the parkinsonian group underwent three follow‐up visits for UPDRS evaluation at 6 weeks, 3 months, and 1 year following transplantation. Additionally, at 1 year following transplantation, each patient underwent a repeat brain MRI. Participants with abnormal baseline UPDRS scores not transplanted within a year’s time underwent UPDRS re‐evaluation 1 year from initial evaluation. Participants in the non‐parkinsonian group underwent yearly repeat UPDRS testing. This was done to evaluate for change in score over time, given continued liver disease. If a change in UPDRS was noted and indicative of parkinsonism, these participants were switched to the parkinsonism group and followed accordingly; however, this only occurred for 1 patient. Participants in both groups were seen for UPDRS evaluation yearly for up to 3 years until transplantation occurred. Once transplanted, participants in both groups were returned to their corresponding 6‐week, 3‐month, and 1‐year follow‐up schedule. If transplantation had not occurred within the 3‐year follow‐up period, participants were withdrawn from the study, ending study participation.
Bias
To control for observer variability, an experienced trained UPDRS evaluator was used throughout the study duration. To reduce rater bias, a movement disorder specialist blinded to participant stratification and study status reviewed UPDRS evaluations. MRI results were bunched and evaluated at three time points and included pre‐transplantation and post‐transplantation participant images, compared with MRI scans of age‐matched individuals with PD. Selection bias was reduced by allowing any individual who was being evaluated for liver transplant to consent for participation. In an effort to sustain patient retention, study visits occurred via standard of care and were conducted on days when patients were visiting the clinic for another appointment or at mutually convenient times. Participants were also compensated $50 for travel and inconvenience for study participation.
Study Size
Sample size was based on availability at the time of study initiation, the number of projected yearly transplants, and a project feasibility.
Statistical Methods
For statistical methods we used an alpha level of 0.05 for all statistical tests. Two‐sample t tests, chi square, Fischer’s exact test, correlation coefficients, and McNemar’s test of matched pairs were used, as appropriate, to compare study groups. We combined the groups with PD and non‐PD, then examined the association between type of liver disease and PD symptoms (yes/no) in a two‐way table using Fisher’s exact test. A two‐sample t test assuming equal variances was used to compare liver disease duration by PD symptom groups (yes/no). A two‐sample t test assuming equal variances was also used to compare liver‐disease level (MELD) by PD symptom groups. UIII levels varied across the type of liver disease, stratified by PD symptoms (yes/no). To examine whether there is a statistically significant association between UIII and type of liver disease, we used an analysis of variance test. We used a two‐sample t test to compare the level of parkinsonism (sUIII) by mortality groups (yes/no), among patients with PD symptoms. When looking at levels of ammonia, manganese and iron, and correlation with parkinsonism, we used the following statistical tests: A scatterplot was created to examine the correlation between ammonia levels and UII levels. A two‐sample t test assuming unequal variances was then used to compare parkinsonism (UIII) by ammonia groups (elevated vs. normal). Tables of ammonia group (high vs. normal) and each type of parkinsonism were examined, and chi‐square P values or Fisher’s exact P values were calculated. To determine whether ammonia level had any effect on MRI findings, correlations between elevated ammonia at screening and MRI scores were calculated with 95% confidence. Correlations between elevated manganese levels at screening, MRI scores, and UIII scores were calculated using McNemar’s test of matched pairs with 95% confidence. To determine whether liver diagnosis affects whether a patient has parkinsonism, groups with PD were combined with groups with no PD, and we examined the association between type of liver disease and PD symptoms (yes/no) in a two‐way table. A two‐sample t test was used to examine potential association between manganese level and liver shunt presence. To determine whether the level of liver disease is related to death incidence and whether there was a difference between the two groups, Kaplan Meier was used to compare liver disease level (MELD) by mortality groups (yes/no), stratified by PD symptom groups. The association between type of liver disease and PD symptoms (yes/no) was examined in a two‐way table. To determine whether liver disease duration was related to death incidence and whether there was a difference between the two groups, a t test was done to compare liver disease duration by mortality groups (yes/no), stratified by PD symptom groups. Furthermore, to assess the effect of liver disease type, disease duration, and level of liver disease or parkinsonism on mortality, a t test was completed to compare liver disease level (MELD) by PD symptom groups (yes/no). To determine whether patients who died in the first year had a higher degree of parkinsonism, a t test was used again to compare the level of parkinsonism (sUIII) by mortality groups (yes/no), among patients with PD symptoms. All analyses were performed using Microsoft Excel for Windows (2013).
Results
Participants
Over 200 individuals with liver disease were scheduled with Neurology and approached for possible inclusion in this trial. Of those approached, 120 patients consented, were examined, and included in this study. In a cohort of 120 patients, 62 patients were stratified into group 1 (parkinsonism) and 58 into group 2 (non‐parkinsonism) (Fig. 1.)
FIG. 1.
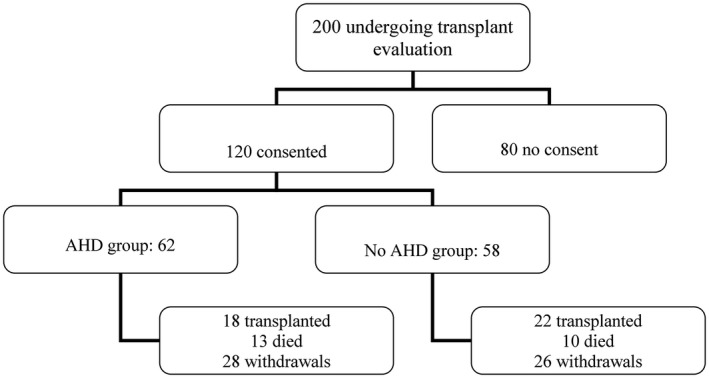
Schematics.
Descriptive Data
Of the 120 enrolled participants, 62 (or 52%) exhibited signs of parkinsonism and were assigned to the parkinsonism group. Fifty eight participants (or 48%) showed no signs of parkinsonism and were stratified to the non‐parkinsonism group. Liver disease was characterized by type and included mixed (multiple diagnoses), alcoholic cirrhosis, viral (B, C), cryptogenic, NASH, and other (autoimmune, genetic, cancer, Laennec’s cirrhosis). The clinical characteristics of enrolled participants are presented in Table 1.
TABLE 1.
Demographics
| Parkinsonism Group | Non‐parkinsonism Group | |
|---|---|---|
| N = 62 | N = 58 | |
| Gender | 37 M, 25 F | 46 M, 12 F |
| Mean age, years | 55.5 (range 38‐68) | 54.2 (range 20‐69) |
| Race | 54 Caucasian | 50 Caucasian |
| 5 Hispanic | 7 Hispanic | |
| 3 African American | 2 African American | |
| Disease category | 27 Mixed | 26 Mixed |
| 15 Alcohol | 12 Other | |
| 10 Viral | 11 Viral | |
| 10 Other | 9 Alcohol | |
| Disease duration, years | 8.4 (range 0.6‐30) | 6.0 (range 0.1‐20) |
| Mean MELD | 14.4 ± 4.5 | 14.0 ± 5.7 |
| Manganese, μg/L | 18.55 ± 12.2 | 16.98 + 8.0 |
| Ammonia, mol/L | 59.7 ± 36.2 | Not done |
| Copper, μg/dL | 95.7 ± 25.9 | Not done |
| Iron, μg/dL | 118.4 ± 57.7 | 103.2 ± 56.0 |
| UI total | 3.9 + 2.4 | 1.8 + 1.8 |
| UII total | 7.6 + 5.9 | 2.1 + 2.1 |
| UIII (motor) total | 15.2 + 8.3 | 0 |
| UIV total | 1.9 + 0.09 | 1.0 + 1.0 |
| UPDRS total | 28.6 | 10 |
| ADLs | 84.5 ± 17.3 | 96 ± 6.5 |
| MMSE | 27.3 + 2.9 | Not done |
Abbreviations: ADLs, activities of daily living; F, female; M, male.
Outcome Data
In the parkinsonism group, of the 62 participants with parkinsonism, liver disease types included 27 mixed, 15 alcoholic, 10 viral, 5 NASH, and 5 other (the mean disease duration was 8.4 years; MELD was 14.4 [range 7‐27]; manganese was 18.55 μg/L [range 6.9‐64.1]; ammonia was elevated at 59.7 μmol/L [>35]; copper was 95.7 μg/DL [range 39‐152]; and iron was 118.4 μg/dL [range 17‐236]. The mean MMSE was 27 (range 18‐30), mean UIII was 15.2, and overall UPDRS score was 28.6. None were treated with drugs known to induce neurological symptoms or MRI abnormalities or were receiving treatment for parkinsonism.
In the non‐parkinsonism group, of the 58 participants with no extrapyramidal or clinical signs of parkinsonism, liver disease included 26 mixed, 11 viral, 9 alcoholic, 6 NASH, and 6 other (mean disease duration was 6 years [range 0.16‐20.00 years]; MELD was 14.0 [range 6‐25]; manganese was 16.98 μg/L [range 6.0‐34.2]; and iron was 103.2 μg/dL [range 17‐225]). The mean UIII was 0, and the overall UPDRS score was 10 (+4.76).
Main Results
Parkinsonism
The mean baseline UIII for the parkinsonism group (n = 62) was 15.24, suggesting mild parkinsonism. The predominant symptoms included body bradykinesia, postural instability, action, and postural tremor and gait abnormality. Paired t tests were used to compare score summaries from baseline to follow‐up visits (P < 0.05). In the 18 transplanted participants, the mean baseline UIII was 15.9 (Table 2, Fig. 2). Statistically significant reduction of UIII was seen starting 3 months following transplant. UIII improved to 10.1 at 3 months (P = 0.02) and 7.1 at 12 months (P < 0.0001). Most improvement was seen in bradykinesia and gait. We had seen the same statistically significant reduction in total UPDRS from baseline (30.6 to 20.4 [P = 0.03] at 3 months) and 16.6 (P = 0.0002) at 1 year.
TABLE 2.
Changes in Mean Scores Over Time for the Parkinsonism Group Transplanted Patients
| Pretransplant Baseline (n = 18) | After Transplant 6 weeks (n = 18) | After Transplant 3 months (n = 18) | After Transplant 1 year (n = 14) | |
|---|---|---|---|---|
| UI total | 4.2 + 2.7 | 3.2 + 2.0 | 2.6 + 2.3* | 2.9 + 2.1 |
| UII total | 8.2 + 6.4 | 8.8 + 6.2 | 6.1 + 5.0 | 5.5 + 6.4 |
| UIII (motor) Total | 15.9 + 7.5 | 15.8 + 8.2 | 10.1 + 8.6* | 7.1 + 9.5 † |
| UIV total | 2.2 + 0.7 | 1.5 + 0.9* | 1.6 + 0.9* | 1.1 + 1.0 † |
| UPDRS total | 30.6 + 13.3 | 28.9 + 13.7 | 20.4 + 14.7* | 16.6 + 18.0 † |
| Bradykinesia | 7.9 ± 3.6 | 5.5 ± 4.6 | 3.9 ± 4.4 † | 3.1 ± 5.2 † |
| Tremor | 1.6 ± 1.8 | 3.4 ± 3.9 | 1.5 ± 2.0 | 0.7 ± 1.6 |
| Rigidity | 1.7 ± 2.2 | 2.2 ± 2.2 | 1.4 ± 2.3 | 0.8 ± 2.0 |
| Gait | 5.3 ± 2.9 | 4.5 ± 2.5 | 3.1 ± 2.5* | 2.6 ± 3.6 † |
P ≤ 0.05.
P ≤ 0.005.
FIG. 2.
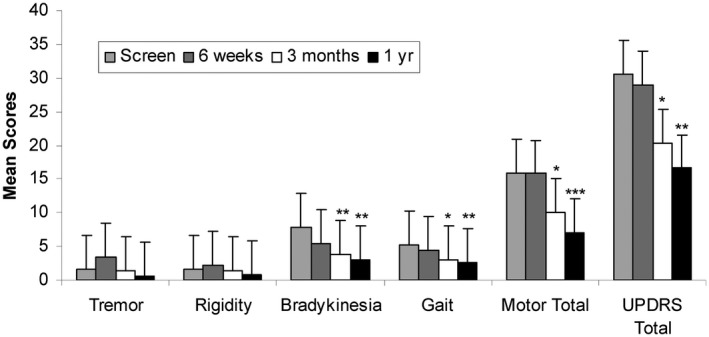
Changes in motor mean scores from baseline to after transplantation. *P ≤ 0.05; **P ≤ 0.005; ***P ≤ 0.001.
Other Neurological
We saw significant improvement in UI from baseline to month 3 , which continued to show a nonsignificant trend of improvement. UPDRS IV showed statistically significant improvement at week 6, month 3, and month 12. UPDRS II showed nonsignificant improvement.
Biological
Ammonia
Baseline ammonia levels were elevated in 37 (60%) parkinsonian group patients (15.8 + 9.5 μmol/dL) and were within normal range in 24 patients (40%) (13.9 + 6.02 μmol/dL). Ammonia levels were not statistically associated with motor UPDRS score (P = 0.036). There was no association between ammonia and tremor (P = 0.61) or rigidity (P = 0.11) and no association with bradykinesia (P = 0.64) or gait (P = 1.00). There was no correlation between ammonia and MRI parameter coefficients (P > 0.05).
Manganese
No statistical or clinically significant differences were seen between the two groups (parkinsonism [n = 54] vs. non‐parkinsonism [n = 52]) in baseline manganese (18.55 μg/L vs. 16.98 μg/L; P = 0.39). Manganese was elevated in 42 (78%) parkinsonism group patients with a baseline of 17.46 μg/L (+10.98 μg/L). Manganese levels were not statistically associated with UIII (P = 0.97) or MRI ratio parameters coefficients (P > 0.05). Our study found that high manganese levels were not associated with shunt presence (P = 0.50).
Iron
The mean iron level was found to be higher in the parkinsonism group (n = 60) than in the no AHD (n = 53) group, representing a slight trend (118.4 μg/dL vs. 103.2 μg/dL; P = 0.10), although not clinically significant.
Liver Disease
In the parkinsonism group (n = 62), there was no associations between liver severity MELD (n = 58) and UPDRS motor score (P = 0.71), ammonia (P = 0.86), manganese (P = 0.90), or iron levels (P = 0.30). Also there was no association between UIII levels and liver disease type.
Neuroradiological
Basal ganglia hyperintensity was seen in all pre‐transplant images, resolving 1 year following transplant (n = 14). UIII was not statistically correlated (P > 0.05) with any of the four MRI parameters before or 1 year after transplant; however, all correlations were positive. Larger drops in MRI ratios were associated with larger drops in UIII. The absence of significance is suspected due to the rather small sample size. We explored the differences between the MR images of the parkinsonism group to the PD control group. There were statistical differences seen between the pre‐transplant parkinsonism group (n = 47) and the PD group (n = 32) in the following: Right thalamus (R/Thal) (P < 0.0001); Left thalamus (L/Thal) (P < 0.0001); Right white matter (R/WH) (P = 0.05), and Left white matter (L/WH) (P = 0.004). A difference was found in transplanted parkinsonism participants 1 year following transplantation (n = 14) versus the PD group (n = 32) in the L/Thal (P = 0.007). Significant improvements were also seen in the MRI ratios from pre‐transplant to 1 year following transplant in the 14 participants with transplanted AHD (R/Thal P ≤ 0.001; L/Thal P = 0.0007; R/WH P = 0.008; L/WH P = 0.02).
Other Associations
Mortality Rates
Mortality rates did not differ between the two groups based on those alive at 1 year from baseline (parkinsonism group, n = 50; no parkinsonism, n = 47) with an odds ratio of 1.03 (P = 0.96). We explored liver disease severity using MELD scores and its relationship with death incidence. There was no statistically significant association between MELD severity and mortality in either group (P > 0.5). We also examined the association between liver disease type and mortality. No significant associations between liver disease type and mortality in patients with parkinsonism (P = 0.23) or without parkinsonism (P = 0.42) were found. There was no statistically significant associations between liver disease duration and mortality in patients with parkinsonism (P = 0.22) or without parkinsonism (P = 0.60) We explored the relationship between liver type, disease duration, and liver disease severity with parkinsonism and their relation to mortality. Associations between single predictor variables and mortality indicated no statistically significant associations (P > 0.05). Twelve of the 61 parkinsonism group patients died within the first year of the trial. We wanted to explore parkinsonism severity with mortality and found no statistically significant association between level of parkinsonism and mortality (P = 0.82).
Discussion
Key Results
We report detailed prospective clinical, neuroradiological, and biological data from a large group of patients with liver disease and parkinsonism. In our study we found a greater prevalence of parkinsonism in patients with liver disease than estimated in other studies. In our study, parkinsonism affected over half the patients with liver disease and affected both sexes, appearing in middle ages and in all races. Type of liver disease was not a determinant, although a mix of liver diseases (i.e., alcoholic cirrhosis and hepatitis C) was the most frequent etiology followed by alcoholic and viral cirrhosis. Disease duration, MELD score, and manganese levels were not determinants either, with no difference compared to the non‐parkinsonism group. Parkinsonian syndrome improved following transplantation, with the greatest effect at 1 year following transplantation. Bradykinesia was the predominant baseline symptom followed by postural instability, tremor, gait problems, and rigidity. At 6 weeks following transplantation we did not find any statistically significant improvements; however, bradykinesia and gait showed a trend of improvement. By 3 months after transplantation, we saw statistically significant improvements in total motor UPDRS and in bradykinesia, gait and total UPDRS, but no clinical or statistically significant improvements in tremor or rigidity. By 1 year we found significant improvements in all baseline symptoms, with statistical significance in total motor UPDRS, bradykinesia, gait and total UPDRS, and clinical improvements in tremor and rigidity. Mentation, behavior, and mood showed improvements at 3 months following transplantation and a trend at 1 year, whereas other complications on the UPDRS showed statistical significance at all follow‐up time points. Mortality rates were similar in each group, with no statistically significant associations among MELD, liver disease type, liver disease duration, or presence of parkinsonism symptoms. Basal ganglia hyperintensities were found in all parkinsonism participants before transplant, but showed resolution 1 year after transplantation. We also found increased pituitary signal abnormality in over half of those patients with parkinsonism before transplant compared to only 7% in the control PD group, with full resolution 1 year following transplant. MRI ratio parameters showed statistically significant improvements in all four ratios following transplantation as well as compared to the PD group before transplant. MRI abnormalities were not statistically significant with motor UPDRS scores; however, correlations were positive, with larger drops in MRI associated with larger drops in UIII. The lack of statistical significance is suspected due to the small sample size of 14.
Limitations
There are limitations to the current study. Participants were only followed 1 year after transplantation. Although we were able to determine that neurological symptoms appear to resolve following transplantation based on physical examination and imaging, we do not know whether these results are durable. The longitudinal effects were not examined, and it is not known whether these results would be consistent or found years later. Attrition rate was low, but was similar across both groups. In addition, it is common for patients in Massachusetts to be evaluated at one clinic but seek care elsewhere once accepted, to improve their odds of receiving a liver transplant. Imaging evidence showed increased basal ganglia T1WI abnormality in the parkinsonism group; however, we were not able to scan the non‐parkinsonism group due to lack of resources, and it is not known how many participants would have abnormal images. Therefore, we were unable to show any definitive difference in terms of imaging between the two groups. In an effort to control for this limitation, we used a control group of patients with PD who already had brain imaging and compared those to before and after transplantation imaging. Further research should establish whether there are longitudinal benefits to transplantation. We also did not review post‐transplantation complications and possible effect on 6‐week and 3‐month follow‐up data, or the role of liver disease severity and compliance with treatment (i.e., lactulose). Another limitation was in patients with ambiguous exams, as they were only seen in person by one examiner (trained research assistant). To improve the rating status, we had a second, blinded movement disorders specialist who reviewed all patients’ UPDRS scores as well as video from patients. This was useful for interrater agreement as well as patients with ambiguous exam. The movement disorders specialist’s data were used if there was interrater disagreement.
Interpretation
AHD, in contrast to reversible hepatic encephalopathy, is characterized by a chronic, progressive, and irreversible course without spontaneous recovery, with the exception of cases that recover after liver transplantation. The exact prevalence and incidence of AHD are unknown, because its epidemiology has rarely been reported. A variety of movement disorders, primarily parkinsonism and cerebellar ataxia, have been found to occur in about 60% of patients with AHD.( 6 , 20 ) Parkinsonism is present in up to 25% of patients with AHD.( 5 , 6 , 20 ) The putative pathomechanism in AHD remains unclear and includes complex actions among toxic substance accumulation, neuroinflammation, oxidative stress, and inducible nitric oxide stress.( 1 ) Accumulation of toxic substances, especially manganese, has gained more support over the past 2 decades. The manganese theory was supported by MRI findings including high signal intensities in the bilateral globus pallidus and adjacent areas on T1WI. Manganese may also be responsible for parkinsonism in AHD by significantly altering the dopaminergic neurotransmission.( 21 ) Our study showed that 52% of patients with AHD developed parkinsonism, which is a higher number than previous studies. Several prospective studies showed a different prevalence of parkinsonism in the range of 0.8% up to 21.6%. Methawasin et al.( 6 ) included 143 patients, but categorized patients into groups of movement disorder such as an intentional tremor seen at 37.1%, which was followed by bradykinesia, parkinsonism, and postural tremors at 29.4%, 10.5%, and 6.3%, respectively. A prospective study by Burkhard et al.( 5 ) was a small study with only 51 patients, and 21.6% exhibited moderate to severe parkinsonism. In this study, patients were rated by movement disorder specialists. The study by Tryc et al. included 214 patients, of whom 4.2% developed parkinsonism,( 8 ) and Kang at al.( 9 ) included 1,361 patients, of whom 1.7% developed parkinsonism. A large retrospective study by Fernandez‐ Rodriquez et al. included 1,000 patients and showed a prevalence of AHD to be 0.8%, and all patients had parkinsonism or cerebellar syndrome.( 20 ) We suspect that the higher number in our study was influenced by several factors: It was a prospective study and included a large number of patients, the rating was performed by a movement disorders specialist, and patients who had a combination of bradykinesia plus tremor, bradykinesia and postural abnormalities, or bradykinesia and rigidity were defined as parkinsonism. We suspect the frequency of parkinsonism related to liver disease is largely underestimated and that the prevalence may be much higher if actively screened as shown in our cohort. Although liver transplant is an ultimate treatment for liver cirrhosis, only some studies showed a positive outcome in AHD and specifically parkinsonism.( 22 ) In contrast, other studies showed a less favorable outcome, including only transient improvement, recurrence or newly developed AHD, primarily due to failure of the transplanted liver.( 21 , 23 , 24 ) Eighteen patients from our group were transplanted and showed statistically significant improvements in their UPDRS scores 12 months after liver transplant; resolution of basal ganglia hyperintensities was seen in the parkinsonism group before transplant. The outcomes of our patients with parkinsonism appear to suggest the reversibility of the neurological impairment seen in liver cirrhosis, and therefore the effectiveness of transplantation; however, those patients were only followed for 1 year after transplant, so it is unknown whether the benefit will be sustained for longer period of time. Our MRI findings were not associated with severity of liver disease or ammonia or manganese level. MRI was not performed in the non‐parkinsonism group, and manganese levels were not retested following transplantation, so we were not able to analyze this association after transplantation. Our study showed no difference in mortality rates between the parkinsonism and non‐parkinsonism groups. However, we only report 1‐year follow‐up data. Further years of follow‐up data are needed to assess mortality‐rate differences between groups.
In conclusion, most of our patients demonstrated both resolution and improvement of neurological symptoms from MRI findings of the parkinsonism group after orthotropic liver transplantation. There was no correlation between severity of MRI findings and clinical motor UPDRS part III. Laboratory findings showed no correlation among the abnormal levels, MRI brain signal abnormality, or UPDRS scores. The MRI finding in AHD with the high signal intensity on T1WI are believed to be due to accumulation of manganese, secondary to defective liver clearance mechanisms. Recent evidence has led to the hypothesis that manganese in AHD may be associated with presynaptic dopaminergic degeneration and altering dopaminergic neurotransmission, thus causing parkinsonism.( 25 ) With liver cirrhosis, an improvement in the clearance mechanism of the liver and decreased toxic levels of manganese could be one of the culprits for improvement of parkinsonism in the posttransplant patients.
Supported by a grant from the Robert E. Wise Research & Education Institute. This is a Lahey sponsored grant, but no members played a role in the conceptualization, design, analysis, or interpretation of results.
Potential conflict of interest: Nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1. Shin HW, Park HK. Recent updates on acquired hepatocerebral degeneration. Tremor Other Hyperkinet Mov (N Y) 2017;7:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferrara J, Jankovic J. Acquired hepatocerebral degeneration. J Neurol 2009;256:320‐332. [DOI] [PubMed] [Google Scholar]
- 3. Jankovic J. Searching for a relationship between manganese and welding and Parkinson’s disease. Neurology 2005;64:2021‐2028. [DOI] [PubMed] [Google Scholar]
- 4. Powers KM, Smith‐Weller T, Franklin GM, Longstreth WT Jr., Swanson PD, Checkoway H. Parkinson’s disease risks associated with dietary iron, manganese, and other nutrient intakes. Neurology 2003;60:1761‐1766. [DOI] [PubMed] [Google Scholar]
- 5. Burkhard PR, Delavelle J, Du Pasquier R, Spahr L. Chronic parkinsonism associated with cirrhosis: a distinct subset of acquired hepatocerebral degeneration. Arch Neurol 2003;60:521‐528. [DOI] [PubMed] [Google Scholar]
- 6. Methawasin K, Chonmaitree P, Wongjitrat C, Rattanamongkolgul S, Asawavichienjinda T. Movement disorders in non‐Wilsonian cirrhotic patients: a report of the prevalence and risk factors from a study done in a medical school in an agricultural‐based community. J Mov Disord 2016;9:28‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tryc AB, Goldbecker A, Berding G, Ru¨mke S, Afshar K, Shahrezaei GH, et al. Cirrhosis‐related parkinsonism: prevalence, mechanisms and response to treatments. J Hepatol 2013;58:698‐705. [DOI] [PubMed] [Google Scholar]
- 8. Kang JH, Tsai MC, Lin CC, Lin HL, Lin HC. Increased risk of parkinsonism among patients with cirrhosis: a 7‐year follow‐up study. Liver Int 2011;31:685‐691. [DOI] [PubMed] [Google Scholar]
- 9. Klos KJ, Ahlskog JE, Josephs KA, Fealey RD, Cowl CT, Kumar N. Neurologic spectrum of chronic liver failure and basal ganglia T1 hyperintensity on magnetic resonance imaging: probable manganese neurotoxicity. Arch Neurol 2005;62:1385‐1390. [DOI] [PubMed] [Google Scholar]
- 10. Maffeo E, Montuschi A, Stura G, Giordana MT. Chronic acquired hepatocerebral degeneration, pallidal T1 MRI hyperintensity and manganese in a series of cirrhotic patients. Neurol Sci 2014;35:523‐530. [DOI] [PubMed] [Google Scholar]
- 11. Renjen PN, Khanna L, Rastogi R, Khan NI. Acquired hepatocerebral degeneration. BMJ Case Rep 2013;2013:bcr2013009387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Damasio J, Ramos C, Miranda H, Magalhaes M. Acquired hepatocerebral degeneration and Wilson’s disease: differential diagnosis. Movement Disord 2009;24:S400. [Google Scholar]
- 13. Pujol A, Graus F, Peri J, Mercader JM, Rimola A. Hyperintensity in the globus pallidus on T1‐weighted and inversion‐recovery MRI: a possible marker of advanced liver disease. Neurology 1991;41:1526‐1527. [DOI] [PubMed] [Google Scholar]
- 14. Pomier‐Layrargues G, Spahr L, Butterworth RF. Increased manganese concentrations in pallidum of cirrhotic patients. Lancet 1995;345:735. [DOI] [PubMed] [Google Scholar]
- 15. Nagappa M, Sinha S, Saini JS, Kallolimath P, Singh N, Kumar A, et al. Non‐Wilsonian hepatolenticular degeneration: clinical and MRI observations in four families from south India. J Clin Neurosci 2016;27:91‐94. [DOI] [PubMed] [Google Scholar]
- 16. Park HK, Kim SM, Choi CG, Lee MC, Chung SJ. Effect of trientine on manganese intoxication in a patient with acquired hepatocerebral degeneration. Mov Disord 2008;23:768‐770. [DOI] [PubMed] [Google Scholar]
- 17. Kim J, Kim JM, Kim YK, Shin JW, Choi SH, Kim SE, et al. Dopamine transporter SPECT of a liver cirrhotic with atypical parkinsonism. Ind Health 2007;45:497‐500. [DOI] [PubMed] [Google Scholar]
- 18. Kamath PS, Kim WR. The model for end‐stage liver disease (MELD). Advanced Liver Disease Study Group. Hepatology 2007;45:797‐805. [DOI] [PubMed] [Google Scholar]
- 19. Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez‐Martin P, et al. Movement Disorder Society UPDRS Revision Task Force Movement Disorder Society–sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Movement Disorders 2008;23:2129‐2170. [DOI] [PubMed] [Google Scholar]
- 20. Fernandez‐ Rodriquez R, Conteras A, De Viloria JG, Grandas F. Acquired hepatocerebral degeneration: clinical characteristics and MRI findings. Eur J Neurol 2010;17;1463‐1470. [DOI] [PubMed] [Google Scholar]
- 21. Servin‐Abad L, Tzakis A, Schiff ER, Regev A. Acquired hepatocerebral degeneration in a patient with HCV cirrhosis: complete resolution with subsequent recurrence after liver transplantation. Liver Transpl 2006;12:1161‐1165. [DOI] [PubMed] [Google Scholar]
- 22. Stracciari A, Baldin E, Cretella L, Delaj L, D'Alessandro R, Guarino M. Chronic acquired hepatocerebral degeneration: effects of liver transplantation on neurological manifestations. Neurol Sci 2011;32:411‐415. [DOI] [PubMed] [Google Scholar]
- 23. Chen Y, Haque M, Yoshida EM. Transient improvement of acquired hepatocerebral degeneration with parkinsonian symptoms after failed liver transplant: case report and literature review. Exp Clin Transplant 2011;9:363‐369. [PubMed] [Google Scholar]
- 24. Papapetropoulos S, Singer C. Management of the extrapyramidal syndrome in chronic acquired hepatocerebral degeneration (CAHD). Mov Disord 2005;20:10881089. [DOI] [PubMed] [Google Scholar]
- 25. Kim Y, Kim JM, Kim JW, Yoo CI, Lee CR, Lee JH, et al. Dopamine transporter density is decreased in parkinsonian patients with a history of manganese exposure: what does it mean? Mov Disord 2002;17:568‐575. [DOI] [PubMed] [Google Scholar]


