Abstract
Medicaid prior authorization (PA) policies for treatment of hepatitis C virus (HCV) with direct‐acting antiviral (DAA) therapy are changing. We aimed to evaluate effects of changes in PA requirements on treatment uptake and to determine the factors associated with DAA treatment among Florida Medicaid beneficiaries with HCV. This is a retrospective cohort analysis of Florida’s Medicaid administrative claims and electronic medical records (2013‐2018). A total of 14,063 newly diagnosed patients with HCV were grouped based on human immunodeficiency virus (HIV) co‐infection and/or a substance use disorder (SUD) (7,735 HCV mono‐infected with a SUD, 5,180 HCV mono‐infected without a SUD, 564 HCV/HIV co‐infected with a SUD, and 584 HCV/HIV co‐infected without a SUD). Although the treatment rate increased three‐fold after June 1, 2016, when a fibrosis‐stage restriction was eliminated, only 8% received DAAs. Compared to HCV mono‐infected without a SUD, HCV mono‐infected with a SUD and HCV/HIV co‐infected with a SUD were 47% (adjusted hazard ratio, 0.53; 95% confidence interval, 0.47‐0.60) and 59% (adjusted hazard ratio, 0.41; 95% confidence interval, 0.28‐0.61) less likely to initiate DAAs. Those with HCV/HIV/SUD did not experience a DAA initiation increase after a fibrosis‐stage restriction was eliminated. Compared with Whites, Blacks were less likely to receive DAAs but were more likely to complete treatment. Use of medication‐assisted therapy was low, despite those on medication‐assisted therapy being 60% more likely to initiate DAA therapy and no more likely to discontinue therapy. Conclusion: Despite changes in Florida’s Medicaid PA requirements for DAA treatment, only 8% received treatment. Disparities in treatment access were found among patients with HIV and a SUD, and who were Black.
In our retrospective cohort study analysis using Florida Medicaid claims data (2013‐2018), we examined the effects of prior authorization policy on access to direct‐acting antiviral (DAA) treatment and identified factors associated with disparities among Florida Medicaid patients with hepatitis C virus (HCV). Our work shows that the number of Florida Medicaid patients treated for HCV has increased, but treatment rates are still very low (8%); compared to HCV mono‐infected patients without substance use disorders (SUD), HCV mono‐infected patients with SUD and HCV/HIV patients co‐infected with SUD were 47% and 59% less likely to initiate DAAs, but no difference was observed in HCV/HIV patients co‐infected without SUD. Disparities in access to treatment continue among patients with SUD, blacks, and pregnant women among Medicaid patients with HCV.

Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- aHR
adjusted hazard ratio
- CI
confidence interval
- DAA
direct‐acting antiviral
- DCC
decompensated cirrhosis
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- HR
hazard ratio
- ICD‐CM
International Classification of Diseases, Clinical Modification
- IDSA
Infectious Diseases Society of America
- MAT
Medication assisted therapy
- PA
prior authorization
- RBV
ribavirin
- SUD
substance use disorder
Hepatitis C virus (HCV) is the most frequently reported bloodborne infection in the United States and a leading cause of liver‐related morbidity, liver transplantation, and mortality.( 1 ) HCV treatment has greatly improved with the introduction of direct‐acting antiviral (DAA) therapy, with therapeutic efficacy in more than 95% of patients across the four major HCV genotypes with limited adverse effects.( 2 , 3 ) Curative DAA treatments for chronic HCV infection have allowed treatment in patients previously considered hard to treat in the interferon era (e.g., human immunodeficiency virus [HIV] co‐infection).( 2 )
However, high drug costs have led private and public insurers, including Medicaid, to restrict access to these medications, requiring that patients meet specific prior authorization (PA) approval.( 4 , 5 , 6 ) In recent years, although many states have eliminated or reduced their restrictions based on patients’ liver disease stage and HIV co‐infection, most of them restrict access to new HCV therapy from illicit drug or alcohol users. In Florida, the state with the third highest number of persons living with HCV infection (about 150,000 persons), Medicaid reduced its restrictions for patients with HCV/HIV co‐infection and eliminated fibrosis‐stage restrictions (Supporting Table S1) in 2016.( 7 ) However, Florida Medicaid requires abstinence from illicit drugs and/or alcohol for 1 month and the prescribing of DAAs by or in consultation with a specialist.( 7 )
Medicaid’s PA policy has generated concern about unintended consequences within the liver‐health community and the public at large, as these restrictions are not grounded in clinical evidence.( 6 ) Furthermore, this policy is inconsistent with HCV treatment guidelines and may create a disparity in access to HCV treatment. For example, the National Institutes of Health HCV guidelines and the American Association for the Study of Liver Diseases (AASLD)/Infectious Diseases Society of America (IDSA) advocate the inclusion of persons with a substance use disorder (SUD) in HCV treatment and recommend that persons with HIV/HCV co‐infection and ongoing risk factors for transmission, including those who inject drugs, be given high priority for treatment because of their high risk for complications.( 8 ) Patients denied access to HCV therapies may progress to hepatic fibrosis and be at risk for the development of end‐stage liver disease and extra hepatic manifestations.( 9 , 10 , 11 ) Indeed, a recent study suggests that deferring HCV therapy reduces treatment effectiveness and increases the risk of liver‐related complications and death.( 12 )
Several studies have reported that nearly half of Medicaid beneficiaries have been denied access to DAA‐based treatments for chronic HCV infection.( 13 , 14 , 15 ) These studies were conducted before the restrictions against initiating DAA therapy for those on Medicaid were changed and updated in many states, and did not provide information about the reasons for these disparities in access to DAA therapy and or about therapy completion rates among Medicaid beneficiaries.
Therefore, we aimed to evaluate the effects of change in Florida Medicaid PA policies on DAA treatment uptake and to identify the population most at risk for not receiving DAA treatment as well as the factors (e.g., race/ethnicity) associated with access to HCV treatment among persons with HCV, to include those co‐infected with HIV as well as those with and without a SUD among Florida Medicaid beneficiaries.
Materials and Methods
Data Source
We conducted a retrospective cohort study using Florida Medicaid administrative claims containing person‐level information of enrollment, medical services, and prescriptions for over 4 million beneficiaries enrolled in the Florida Medicaid program. Of those, approximately 17% of Florida Medicaid beneficiaries’ data were able to be extracted, as they had visited a One Florida health clinic, which uses an electronic medical record system under the OneFlorida Data Trust.( 16 ) OneFlorida is a partnership of 11 health systems and affiliated practices in Florida, several statewide insurance programs, and Florida’s Agency for Health Care Administration, which oversees Florida Medicaid. This study was approved by the institutional review board of the University of Florida.
Study Population
Identification of Newly Diagnosed Patients With Chronic HCV
We used Florida Medicaid administrative claims to establish the new chronic HCV cohort for the study period of January 1, 2013, to December 31, 2018. We included patients aged 18‐64 years, who were not dually eligible for Medicare, whose first chronic HCV diagnosis occurred without any chronic HCV diagnosis in the previous 12 months using the International Classification of Diseases (ICD), Clinical Modification codes (ICD‐9‐CM codes 070.44, 070.54, 070.70, and 070.71, and ICD‐10‐CM codes B18.2, B19.20, B19.21, and Z22.52). A patient was determined to have chronic HCV if they had met a previously validated algorithm of one inpatient or two outpatient diagnoses of chronic HCV on separate days within 1 year.( 17 ) Patients were also required to be continuously enrolled in Florida Medicaid 1 year before and 3 months after their first HCV diagnosis with a 30‐day grace period. Patients were excluded if they had decompensated cirrhosis (DCC), hepatocellular carcinoma (HCC), or liver transplantation during the 1‐year period before their first chronic HCV diagnosis. For this analysis, we also excluded patients with any HCV therapy before the index date.
Presence of a SUD and/or HIV Co‐infection Among Patients With Chronic HCV
We further categorized patients with chronic HCV based on any SUD diagnosis and/or HIV co‐infection recorded from 1 year before through 3 months after their first HCV diagnosis. Patients were grouped into four cohorts: (1) HCV mono‐infected with a SUD; (2) HCV mono‐infected without a SUD; (3) HCV/HIV co‐infected with a SUD; and (4) HCV/HIV co‐infected without a SUD (Fig. 1).
FIG. 1.

Flow chart of the cohort creation.
By using a previously validated algorithm, patients were considered to have a SUD if they had at least one inpatient or one outpatient claim of drug or alcohol use disorders using ICD‐9/ICD‐10 codes (Supporting Table S2) or had a record of methadone, buprenorphine, or naltrexone used for opioid use disorder or alcohol use disorder.( 18 , 19 , 20 ) We used Current Procedural Terminology codes (H0020, J1230) in medical claims to capture methadone dispensed for opioid use disorder. We used National Drug Codes in pharmacy claims to identify buprenorphine and naltrexone. The following SUD diagnoses were examined: opioid use disorder, other drug‐related disorders (e.g., cocaine, heroin, sedatives), and alcohol use disorder. Patients were considered to have HIV co‐infection if they had at least one inpatient or outpatient claim with an HIV diagnosis using ICD‐9/ICD‐10 codes.
DAA Treatment Initiation Rate
We included the following Food and Drug Administration–approved and available all‐oral DAA therapies, or recommended by the AASLD/IDSA for treatment‐naïve patients: [sofosbuvir/ledipasvir, sofosbuvir + simeprevir, sofosbuvir, sofosbuvir + daclatasvir, paritaprevir/ritonavir/ombitasvir ± dasabuvir, elbasvir/grazoprevir, sofosbuvir/velpatasvir, sofosbuvir/velpatasvir/voxilaprevir, and glecaprevir/pibrentasvir] ± ribavirin (RBV). We grouped them into two types of DAA treatment: DAA only or DAA with RBV.
To estimate the initiation rate for DAA therapy, we used pharmacy claims data to determine when the first all‐oral DAA prescription was filled and provided the overall rate as per 1,000 person‐years over the study period. For each individual, the person‐years of follow‐up were calculated from the first chronic HCV diagnosis date to the date of first DAA prescription, end of enrollment, or December 31, 2018, whichever occurred first.
DAA Treatment Discontinuation Rate
For patients with chronic HCV who initiated all‐oral DAA therapy, we compared early discontinuation rates among the four groups. Discontinuation was defined as a gap in therapy of 30 days.( 21 ) Patients were considered to have discontinued early if the observed treatment duration (summing the number of days from the filling of the first prescription to the date of the last prescription claim’s fill date plus the last prescription claim’s days’ supply) plus 30 days was shorter than the expected treatment. The expected treatment duration was based on 2017 AASLD/IDSA treatment guidelines, accounting for baseline cirrhosis diagnosis before the index date.( 22 ) For example, we used 8 weeks for sofosbuvir/ledipasvir among patients without cirrhosis and 12 weeks for the other all‐oral therapies with and without cirrhosis. Because the 8‐week regimen for sofosbuvir/ledipasvir was not recommended by guidelines until 2016, we performed a sensitivity analysis for discontinuation using 12 weeks of sofosbuvir/ledipasvir for 2014‐2015 and 8 weeks for 2016.
Statistical Analysis
Baseline characteristics were compared among the four groups using chi‐square tests for categorical variables. To test trends across years, the Cochrane‐Armitage test was used. To compare treatment initiation rates before and after June 1, 2016, chi‐square tests were used. We used a multivariable Cox proportional hazards regression model to estimate hazard ratios of DAA treatment initiation rates among the four groups, controlling for covariates. Covariates included demographics (i.e., age, sex, race, and ethnicity), comorbidities (i.e., diabetes, mental health disorder, and pregnancy), and other liver diseases (i.e., hepatitis B virus, nonalcoholic fatty liver disease [NAFLD], compensated cirrhosis, DCC, and HCC). It is important to note that although we excluded patients with DCC and HCC before the study initiation date, some patients may have developed it before the initiation of DAAs.
Due to missing values in laboratory data, we were not able to control for laboratory data. We also used a multivariable Cox proportional hazards regression model to estimate hazard ratios of early DAA discontinuation rates among the four groups, controlling for the aforementioned covariates as well as treatment with a DAA alone or DAA + RBV. All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Patient Characteristics
Between January 2013 and December 2018, we identified 14,063 Florida Medicaid patients newly diagnosed with HCV, of which 59% had a SUD diagnosis and 8% had an HIV diagnosis (Table 1). The distribution among the groups was as follows: (1) 7,735 HCV mono‐infected patients with a SUD (55.0%), (2) 5,180 HCV mono‐infected without a SUD (36.8%), (3) 564 HCV/HIV co‐infected patients with a SUD (4.0%), and (4) 584 HCV/HIV co‐infected patients without a SUD (4.2%).
TABLE 1.
Baseline Characteristics of Florida Medicaid Patients With Chronic HCV
| Total (n = 14,063) | HCV Mono‐infected With SUD (n = 7,735) | HCV Mono‐infected Without SUD (n = 5,180) | HCV/HIV Co‐infected With SUD (n = 564) | HCV/HIV Co‐infected Without SUD (n = 584) | P Value | |
|---|---|---|---|---|---|---|
| Age (years) | <0.001 | |||||
| Mean (SD) | 47.0 (12.7) | 44.5 (12.9) | 50.0 (12.4) | 48.4 (10.5) | 51.8 (9.7) | |
| 18‐34 | 3,265 (23.2%) | 2,290 (29.6%) | 860 (16.6%) | 77 (13.7%) | 38 (6.5%) | |
| 35‐44 | 2,175 (15.5%) | 1,396 (18.0%) | 603 (11.6%) | 96 (17.0%) | 80 (13.7%) | |
| 45‐54 | 3,165 (22.5%) | 1,606 (20.8%) | 1,171 (22.6%) | 202 (35.8%) | 186 (31.8%) | |
| 55‐64 | 5,458 (38.8%) | 2,443 (31.6%) | 2,546 (49.2%) | 189 (33.5%) | 280 (47.9%) | |
| Gender | <0.001 | |||||
| Male | 6,334 (45.0%) | 3,425 (44.3%) | 2,228 (43.0%) | 321 (56.9%) | 360 (61.6%) | |
| Female | 7,729 (55.0%) | 4,310 (55.7%) | 2,952 (57.0%) | 243 (43.1%) | 224 (38.4%) | |
| Race | <0.001 | |||||
| White | 9,006 (64.0%) | 5,647 (73.0%) | 2,968 (57.3%) | 216 (38.3%) | 175 (30.0%) | |
| Black | 2,535 (18.0%) | 871 (11.3%) | 1,133 (21.9%) | 247 (43.8%) | 284 (48.6%) | |
| American Indian/Alaska Native/Asian/native Hawaiian/other Pacific Islander | 112 (0.8%) | 40 (0.5%) | 71 (1.4%) | — | — | |
| Other* | 904 (6.4%) | 383 (5.0%) | 423 (8.2%) | 37 (6.6%) | 61 (10.4%) | |
| Unknown | 1,506 (10.7%) | 794 (10.3%) | 585 (11.3%) | 64 (11.3%) | 63 (10.8%) | |
| Ethnicity | <0.001 | |||||
| Hispanic | 1,191 (8.5%) | 486 (6.3%) | 561 (10.8%) | 61 (10.8%) | 83 (14.2%) | |
| Non‐Hispanic | 11,158 (79.3%) | 6,348 (82.1%) | 3,941 (76.1%) | 436 (77.3%) | 433 (74.1%) | |
| Other † | 1,714 (12.2%) | 901 (11.6%) | 678 (13.1%) | 67 (11.9%) | 68 (11.6%) | |
| Comorbidity | ||||||
| Diabetes | 2,783 (19.8%) | 1,264 (16.3%) | 1,263 (24.4%) | 125 (22.2%) | 131 (22.4%) | <0.001 |
| Schizophrenia/bipolar | 2,450 (17.4%) | 1,612 (20.8%) | 617 (11.9%) | 163 (28.9%) | 58 (9.9%) | <0.001 |
| Depression | 5,560 (39.5%) | 3,708 (47.9%) | 1,411 (27.2%) | 302 (53.5%) | 139 (23.8%) | <0.001 |
| Epilepsy | 1,115 (7.9%) | 757 (9.8%) | 265 (5.1%) | 60 (10.6%) | 33 (5.7%) | <0.001 |
| Pregnancy | 1,471 (10.5%) | 1,013 (13.1%) | 422 (8.1%) | 19 (3.4%) | 17 (2.9%) | <0.001 |
| Other liver disease | ||||||
| Nonalcoholic liver disease | 662 (4.7%) | 393 (5.1%) | 221 (4.3%) | 36 (6.4%) | 12 (2.1%) | <0.001 |
| Hepatitis B virus | 591 (4.2%) | 302 (3.9%) | 157 (3.0%) | 68 (12.1%) | 64 (11.0%) | <0.001 |
| Liver severity | ||||||
| Cirrhosis | 682 (4.8%) | 412 (5.3%) | 240 (4.6%) | 11 (2.0%) | 19 (3.3%) | <0.001 |
| SUD | ||||||
| Opioid | 4,016 (28.6%) | 3,842 (49.7%) | NA | 174 (30.9%) | NA | <0.001 |
| Other drug‐related | 5,920 (42.1%) | 5,463 (70.6%) | NA | 457 (81.0%) | NA | <0.001 |
| Cocaine, heroin | 2,647 (18.8%) | 2,379 (30.8%) | NA | 268 (47.5%) | NA | <0.001 |
| Sedatives, hypnotics, anxiolytics, tranquilizers, and barbiturates | 761 (5.4%) | 721 (9.3%) | NA | 40 (7.1%) | NA | <0.001 |
| Stimulants | 748 (5.3%) | 707 (9.1%) | NA | 41 (7.3%) | NA | <0.001 |
| Other (unspecified or specified) drug dependence and drug‐induced mental disorders | 4,039 (28.7%) | 3,757 (48.6%) | NA | 282 (50.0%) | NA | <0.001 |
| Cannabis | 1,851 (13.2%) | 1,684 (21.8%) | NA | 167 (29.6%) | NA | <0.001 |
| Hallucinogens | 85 (0.6%) | 80 (1.0%) | NA | — | NA | <0.001 |
| Alcohol | 3,081 (21.9%) | 2,852 (36.9%) | NA | 229 (40.6%) | NA | <0.001 |
| Medication‐assisted therapy for opioid use disorder | 532 (3.8%) | 516 (6.7%) | NA | 16 (2.8%) | NA | |
| Methadone | 250 (1.8%) | 240 (3.1%) | NA | — | NA | <0.001 |
| Buprenorphine | 220 (1.6%) | 217 (2.8%) | NA | — | NA | <0.001 |
| Naltrexone | 66 (0.5%) | 63 (0.8%) | NA | — | NA | <0.001 |
Data are reported as numbers (percentages) unless otherwise indicated. “—” indicates the numbers of patients with 10 or less observations were not presented.
Multiple races, refused to answer, and other.
No information, other, refused to answer, and unknown.
HCV mono‐infected patients without a SUD were more likely to be female (57.0%) and white (57.3%), whereas HCV/HIV co‐infected patients without a SUD were more likely to be male (61.6%) and Black (48.6%). HCV mono‐infected with a SUD were more likely to be White (73.0%), whereas HCV/HIV co‐infected patients with a SUD were more likely to be Black (43.8%). Among patients with a SUD, almost 50% of HCV mono‐infected patients had a diagnosis of opioid use disorder, whereas HCV/HIV co‐infected patients tended to have a diagnosis of cocaine or heroin use disorder. Only 3.8% of patients with a SUD received medication assisted therapy (MAT) for opioid use disorder. Among the pregnant women, almost 70% also had a SUD.
Trends in Initiation Rate of All‐Oral DAAs Among Florida Medicaid Patients With Chronic HCV During 2013‐2018
Figure 2 displays the trends in the treatment initiation rates between 2014 (November 2013 to December 2014) and 2018, going from 11.4 to 44.1 per 1,000 person‐years, which equates to 26 patients treated in 2014 to 350 patients in 2018. During 2014‐2018, for the HCV mono‐infected patients, DAA initiation rates (per 1,000 person‐years) increased from 8.3 to 31.0 (3.7 times) and from 18.8 to 64.1 (3.4 times) for those with and without a SUD, respectively. A similar trend was observed in HCV/HIV co‐infected patients. For HCV/HIV co‐infected patients with a SUD, DAA initiation rates (per 1,000 person‐years) increased from 7.2 to 29.2 (4.1 times), and for HCV‐HIV co‐infected patients without a SUD, it increased from 0 to 58.8 in 2016 and then decreased to 42.2 in 2018.
FIG. 2.

Trends in initiation rates of all‐oral DAAs per 1,000 person years among Florida Medicaid patients with chronic HCV, stratified by HIV co‐infection and/or SUD, 2014‐2018.
DAA Treatment Initiation Rates Before and After June 1, 2016
Since Florida Medicaid changed its policy on who was able to receive DAA therapy based on staging of liver fibrosis on June 1, 2016, we compared DAA‐initiation person time rates before and after June 1, 2016. Figure 3 displays these changes. There was a significant increase in DAA initiation for all patients from before and after June 1, 2016 (17.9 to 54.5 per 1,000 person‐years, P < 0.01). There also was a significant difference in the rate of initiation between groups in which the HCV mono‐infected group with a SUD had a 3.5‐times increase in their initiation rate, whereas the HCV/HIV with a SUD group had the lowest increase of only 1.3 times. In addition, all groups had significant increases within each group (P < 0.01), except those with HCV/HIV with a SUD (P = 0.08).
FIG. 3.
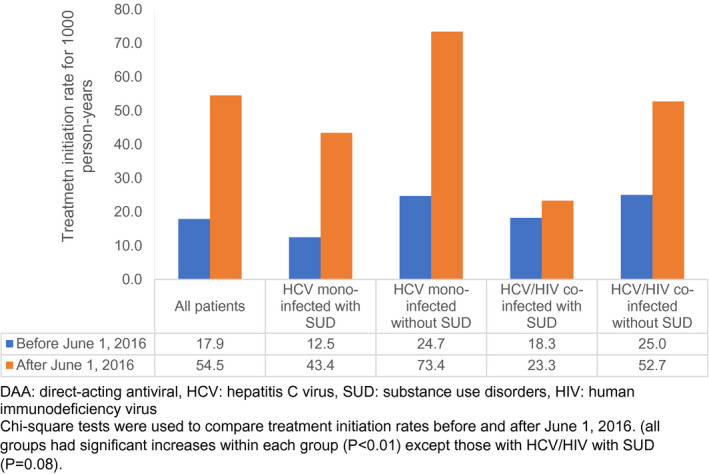
Initiation rates of all‐oral DAA therapy per 1,000 person‐years among Florida Medicaid patients with chronic HCV before and after June 1, 2016, stratified by HIV co‐infection and/or SUD.
Overall DAA Treatment Initiation Rates Among Florida Medicaid Patients
From 2013 to 2018, of the 14,063 Florida Medicaid enrollees with a diagnosis of HCV, only 8% of these patients (n = 1,118) received all‐oral DAA treatment (Table 2), of which 76% received DAA only (n = 852) and 24% received DAA plus RBV (n = 266). The remaining 92% (n = 12,945) did not receive any HCV treatment. Only 5.9% of HCV mono‐infected patients with a SUD and 4.8% of HCV/HIV co‐infected patients with a SUD initiated DAA therapy, which was significantly lower compared with the initiation rate of 11.1% and 9.6% among HCV mono‐infected and HCV/HIV co‐infected patients without a SUD (P < 0.01), respectively. After adjusting for covariates, compared to HCV mono‐infected patients without a SUD, HCV mono‐infected patients with a SUD and HCV/HIV co‐infected patients with a SUD were 47% (adjusted hazard ratio [aHR], 0.53; 95% confidence interval [CI], 0.47‐0.60) and 59% (aHR, 0.41; 95% CI, 0.28‐0.61) less likely to initiate DAAs; however, no significant difference was observed in HCV/HIV co‐infected patients without a SUD (aHR, 0.85; 95% CI, 0.64‐1.13).
TABLE 2.
Unadjusted and Adjusted Initiation Rates of All‐Oral DAA Therapy for Florida Medicaid Patients With Chronic HCV, Stratified by the Presence of HIV Co‐infection and/or SUD (n = 14,063)
| No. of DAA Initiation | % of DAA Initiation | Person‐Years | Crude Incidence /1,000 Person‐Years | Adjusted HR* (95% CI) | |
|---|---|---|---|---|---|
| Total (n = 14,063) | 1,118 | 7.9% | 29,068 | 38.5 | N/A |
| DAA only † | 852 | ||||
| DAA + RBV ‡ | 266 | ||||
| HCV mono‐infected with SUD (n = 7,735) | 459 | 5.9% | 15,479 | 29.7 | 0.53 (0.47‐0.60) |
| DAA only † | 341 | ||||
| DAA + RBV ‡ | 118 | ||||
| HCV mono‐infected without SUD (n = 5,180) | 576 | 11.1% | 10,822 | 53.2 | Reference |
| DAA only † | 435 | ||||
| DAA + RBV ‡ | 141 | ||||
| HCV/HIV co‐infected with SUD (n = 564) | 27 | 4.8% | 1,346 | 20.1 | 0.41 (0.28‐0.61) |
| DAA only † | 25 | ||||
| DAA + RBV ‡ | 2 | ||||
| HCV/HIV co‐infected without SUD (n = 584) | 56 | 9.6% | 1,422 | 39.4 | 0.85 (0.64‐1.13) |
| DAA only † | 51 | ||||
| DAA + RBV ‡ | 5 |
Cox proportional hazards model was used to adjust for age, sex, race, ethnic, diabetes, mental disorder, pregnancy, nonalcoholic liver disease, hepatitis B virus, cirrhosis, DCC, and HCC.
Included sofosbuvir, sofosbuvir + simeprevir, sofosbuvir/ledipasvir, paritaprevir/ritonavir/ombitasvir ± dasabuvir, elbasvir/grazoprevir, sofosbuvir/velpatasvir ± voxilaprevir, daclatasvir, and glecaprevir/pibrentasvir.
Included [sofosbuvir, sofosbuvir + simeprevir, sofosbuvir/ledipasvir, paritaprevir/ritonavir/ombitasvir ± dasabuvir, elbasvir/grazoprevir, sofosbuvir/velpatasvir ± voxilaprevir, daclatasvir, and glecaprevir/pibrentasvir] + RBV.
Factors Related to the Receipt of DAA Therapy
Figure 4 summarizes the other factors associated with DAA initiation among Florida Medicaid patients with chronic HCV. Factors associated with a significantly lower probability of initiating DAAs included being 45‐55 years old (compared with being 55‐64 years old; aHR, 0.81; 95% CI, 0.70‐0.95), being Black (aHR, 0.71; 95% CI, 0.52‐0.98), and being of other race (aHR, 0.59; 95% CI, 0.42‐0.85), compared with being White and pregnant (aHR, 0.59; 95% CI, 0.42‐0.84). In contrast, patients with cirrhosis (aHR, 2.28; 95% CI, 1.92‐2.71) and NAFLD (aHR, 2.09; 95% CI, 1.74‐2.50) were more likely to receive DAAs. Furthermore, as indicated in Supporting Table S3, for those patients with chronic HCV with available laboratory data, the treated patients had more advanced liver disease compared with the untreated patients.
FIG. 4.

Forest plot of the receipt of all‐oral DAAs among Florida Medicaid patients with chronic HCV.
Among patients with chronic HCV with a SUD, patients with alcohol as well as opioid or other drugs (aHR, 0.53; 95% CI, 0.40‐0.70) or opioid with other drugs (aHR, 0.61; 95% CI, 0.45‐0.82) polysubstance use disorders, compared to those with other drugs alone were less likely to receive DAAs. However, patients receiving MAT for a SUD were more likely to receive DAAs (aHR, 1.60; 95% CI, 1.10‐2.33) (Supporting Fig. S1).
DAA Treatment Discontinuation Rate
Because of the small sample size for HCV/HIV co‐infected patients who discontinued DAAs (<5), we regrouped patients into two groups (patients with HCV with a SUD versus patients with HCV without a SUD) to evaluate whether there were differences in DAA discontinuation among patients with HCV with (n = 486) and without (n = 632) a SUD. Overall, approximately 6.8% of patients with HCV who initiated DAA therapy discontinued treatment early (Table 3). The DAA treatment discontinuation rate was slightly higher for patients with a SUD compared to patients without a SUD (6.7 vs. 5.9 per 1,000 person‐weeks). However, after adjusting for covariates, there was no significant difference in the early treatment discontinuation rates between the SUD and non‐SUD groups (aHR, 1.41; 95% CI, 0.87‐2.17).
TABLE 3.
Unadjusted and Adjusted Discontinuation Rates of All‐Oral DAA Therapy for Florida Medicaid Patients With Chronic HCV, Stratified by Presence of SUD (n = 1,118)
| No. of DAA Discontinuations | % of DAA Discontinuations | Person‐Weeks | Crude Incidence/1,000 Person‐Weeks | Adjusted HR* (95% CI) | |
|---|---|---|---|---|---|
| HCV with SUD (n = 486) | 36 | 7.4% | 5,355 | 6.7 | 1.41 (0.87‐2.17) |
| DAA only † | 24 | ||||
| DAA + RBV ‡ | 13 | ||||
| HCV without SUD (n = 632) | 40 | 6.3% | 6,830 | 5.9 | Reference |
| DAA only † | 33 | ||||
| DAA + RBV ‡ | 7 |
Cox proportional hazards model was used to adjust for age, sex, race, ethnic, diabetes, mental disorder, pregnancy, HIV, nonalcoholic liver disease, hepatitis B virus, cirrhosis, and DCC.
Included sofosbuvir, sofosbuvir + simeprevir, sofosbuvir/ledipasvir, paritaprevir/ritonavir/ombitasvir ± dasabuvir, elbasvir/grazoprevir, sofosbuvir/velpatasvir ± voxilaprevir, daclatasvir, and glecaprevir/pibrentasvir.
Included [sofosbuvir, sofosbuvir + simeprevir, sofosbuvir/ledipasvir, paritaprevir/ritonavir/ombitasvir ± dasabuvir, elbasvir/grazoprevir, sofosbuvir/velpatasvir ± voxilaprevir, daclatasvir, and glecaprevir/pibrentasvir] + RBV.
Compared with Whites, Blacks (aHR, 0.14; 95% CI, 0.04‐0.49) and other races (aHR, 0.17; 95% CI, 0.04‐0.66) were less likely to discontinue their DAA treatment early, as were those aged 35‐44 (compared to those aged 55‐64 [aHR, 0.35; 95% CI, 0.16‐0.79]) and those with cirrhosis (aHR, 0.46; 95% CI, 0.23‐0.94) (Supporting Fig. S2). All other factors were not significantly associated with early discontinuation.
Among those with a SUD, only Blacks (aHR, 0.04; 95% CI, 0.00‐0.55) or other races (aHR, 0.03; 95% CI, 0.00‐0.53) when compared with Whites were less likely to discontinue DAA treatment early (Supporting Fig. S3). In a sensitivity analysis using a 12‐week sofosbuvir/ledipasvir treatment during 2014‐2015 and an 8‐week treatment during 2016 for treatment discontinuation, the study results remained consistent (Supporting Table S4).
Discussion
In this study of Florida Medicaid beneficiaries with chronic HCV, we found that from 2013 to 2018, the DAA treatment rate increased 4‐fold with a very low early discontinuation rate (<7%), but DAA treatment overall was extremely low, as only 8% of Florida Medicaid patients with chronic HCV received DAA treatment. The increase in treatment rate can be attributed to the changes of DAA treatment eligibility over the course of the study. Specifically, on June 1, 2016, Florida Medicaid relaxed the criteria necessary for reimbursement of DAA treatment especially for patients with a fibrosis level < F3, recommending treating those co‐infected with HIV as well as reducing the prescribers’ requirement burden. These findings are encouraging in that as barriers are removed for treatment, treatment uptake may continue to increase with very few patients discontinuing treatment.
On the other hand, despite changes in policy, 92% of patients with chronic HCV on Medicaid remained untreated. Although reimbursement restrictions based on fibrosis stage were lifted in 2016, it is important to note that barriers to accessing DAA therapies based on 1 month of sobriety and DAA prescriptions provided by or in consultation with a specialist still remain in Florida, preventing those at the highest risk of complications from receiving HCV treatment. In addition to the barriers due to Medicaid policy, we think there are other multilevel issues related to the provider, patient, and health system structure that have led to low HCV treatment rates.( 23 , 24 ) Many physicians are unwilling or hesitant to treat people actively using drugs with DAA therapies due to concerns about reinfection, adherence, and medication cost.( 25 ) Patient‐level barriers include poor knowledge of HCV, leading to misperceptions about HCV infection and its treatment, ongoing stigma associated with having HCV, mental health issues, and general challenges with accessing the health care system.( 26 ) At the system level, limited infrastructure for providing HCV assessment and treatment, particularly in primary care services and substance treatment centers as well as limited accessibility of testing locations, are also large barriers to care.( 26 , 27 )
In addition, those with a SUD and chronic HCV received treatment at an even lower rate (5.9%), which is substantially lower than those covered by other insurance or indigent care (14%).( 13 ) However, those on MAT were almost 60% more likely to initiate DAA treatment and were no more likely to discontinue treatment than those without MAT. Similar to our findings, a previous study reported that individuals retained in buprenorphine treatment were more likely to initiate HCV treatment, most likely as a result of better engagement with the health care system.( 28 ) However, despite this finding, we found that less than 4% (the national average is 28%) were receiving MAT.( 29 ) The role of MAT in the prescriber’s and patient’s behavior is an area that needs further exploration if the uptake of DAA treatment is to happen; however, enhancing access to MAT treatment should be taken into consideration.( 30 , 31 , 32 )
In addition, the group least likely to be treated with DAAs over this study period and one that saw no change in their uptake of treatment was that with HCV/HIV and a SUD. This finding should come as no surprise, as all three diseases carry a substantial burden of stigmatization resulting in difficulty treating this cohort due to their transience, social isolation, and inadequate medical and substance use disorder treatment services.( 33 ) Therefore, proven harm‐reduction strategies such as the pairing of needle exchange sites with MAT therapy, HCV and HIV testing, as well as investigating ways to decrease the stigma associated with these diseases will be needed to reach the goal of eliminating hepatitis by 2030.( 34 , 35 )
We also found that racial disparities exist in those receiving DAA treatment, as Blacks, who represented about 20% of Florida Medicaid patients with HCV and approximately 50% of the HCV/HIV population, were almost 30% less likely to receive HCV treatment compared with Whites. Such a finding is disquieting for several reasons. First, recent studies have confirmed that DAAs have similar effectiveness in Blacks compared with Whites, so they should be prescribed as indicated. We also found that once Blacks initiated treatment, they were 86% less likely to discontinue treatment after controlling for all other covariates. In addition, because Blacks made up 50% of the HCV/HIV population, this group was at higher risk for disease progression, which would suggest that treatment was necessary, especially as the presence of HIV is known to hasten liver disease progression and Blacks are more likely to experience complications of HCV including HCC, end‐stage liver disease, and death from cirrhosis.( 22 , 29 ) Future studies are needed to advance our understanding about the reasons for this racial disparity.
Not surprisingly, patients with cirrhosis were more likely to receive and continue treatment once initiated. Such a finding is probably a result of more health care encounters due to their advanced liver disease and the fact that Florida Medicaid’s original requirements necessary to receive DAA treatment was the presence of advanced liver disease. However, a somewhat surprising finding was that among the pregnant women with chronic HCV, almost 70% also had a SUD. Because DAA treatment is not yet approved or recommended by AASLD for use during pregnancy, this group will need to be followed very closely so that treatment can be initiated when eligible. Currently, there are ongoing clinical trials investigating treatment in pregnant women with ledipasvir/sofosbuvir.( 36 , 37 , 38 ) Another study on the use of sofosbuvir/velpatasvir during the postpartum period is ongoing, with results due in September 2020.( 39 ) As such, this group should be targeted to better understand their unique treatment needs and evaluate the risks and benefits to mother and fetus, and efforts should be made to treat women of childbearing age before pregnancy.( 40 )
These findings also point to opportunities to examine Medicaid program policies nationally, as Medicaid is the single largest source of coverage for individuals with a SUD and/or HIV in the United States. With a provision of the Patient Protection and Affordable Care Act of 2020, previously uninsured adults become eligible in Medicaid expansion plans. This provision can positively affect those with HCV, as their prevalence is twice as high among the uninsured compared with the rest of the U.S. population. Therefore, we suggest that our findings can help state and national Medicaid policymakers determine how to best expand their plans to capture the most underserved HCV populations to increase the uptake of treatment, especially among those with a SUD and/or HIV and those who are Black. To overcome these barriers, we suggest several strategies that include regular HCV screening to enhance HCV case finding, providing continual updated education to health care professionals and allowing all nonspecialist care providers the ability to prescribe treatment for qualified candidates, and co‐locating screening, diagnosis, and treatment services for HCV at current SUD treatment centers, both inpatient or outpatient.( 24 ) Interventions to enhance HCV treatment uptake include integrated HCV care, MAT use, and psychiatric services delivered by a multidisciplinary team with case management services.( 41 , 42 ) The potential integrated care model in which infectious disease specialists and addiction specialists coordinate SUD and HCV treatment in clinical care has been highlighted in other studies.( 28 , 43 , 44 ) Our findings inform state Medicaid policymakers about the low HCV treatment uptake and low MAT use in Florida, even though some restrictions to HCV treatment have been removed. As such, other states should note that when barriers are in place, HCV treatment uptake may not be optimal. To the best of our knowledge, there has been no formal study among the states that have loosened their criteria for HCV treatment to which we could compare our findings. This is an important point, as it demonstrates a gap in our knowledge about how changes in policies are being reflected in actual real‐world practice. We encourage other states to conduct similar studies to enhance our knowledge about how policy changes are translated into clinical practice and their associated outcomes, so that appropriate plans can be initiated to ensure a successful expansion of Medicaid.
This study has several strengths. Unlike previous studies that used a sample of patients with HCV from one specialty pharmacy or outpatient‐based urban health care settings, our study included all Florida Medicaid patients with HCV and compared their treatment uptake and completion of therapy by the presence of HIV co‐infection and/or SUD. We included prescriptions for HCV treatment, which allowed us to estimate the completion of therapy and early discontinuation. Another strength of this study is the relatively large number of Medicaid patients with HCV, which allowed us the opportunity to conduct several subgroup analyses (e.g., race/ethnicity). This study provides the first real‐world evidence of HCV treatment access and completion of DAA treatment among the vulnerable Medicaid population in the United States, which may help other states with similar PA policies understand the disparities in access among patients with chronic HCV with and without SUDs.
There are also limitations to this study, as this was an analysis of administrative claims data using ICD‐9/ICD‐10 codes. Although a prior study validated the use of billing ICD codes for the diagnosis of a SUD, and this approach is commonly used in retrospective claims data analyses, some individuals with a SUD remain unrecognized and underdiagnosed in routine practice. It is also possible that individuals with chronic HCV were not screened, and if screened, did not follow up with primary care providers or specialists for further diagnostic evaluation and treatment. Thus, we may have underestimated the number of patients with chronic HCV.( 17 , 19 , 45 ) In addition, we used prescription claims, so we do not know the reasons related to patients not receiving treatment. Many patients lacked follow‐up lab work after treatment, so we were unable to determine the overall sustained virologic response (SVR) rate or whether there is a lower SVR in certain populations. Although our results suggest that restrictive PA policies for DAA therapies have resulted in access disparities to HCV therapy among Florida Medicaid patients, other factors including patients’ refusal and physician’s concern about reinfection might contribute to the low treatment rates. In addition, Medicaid restrictions apply to recent substance use; however, we were not able to differentiate between patients with a former and current SUD, which may alter our results, but given the low uptake, our results would unlikely change much, if at all. Although we excluded patients who received prior HCV treatment (e.g., interferon‐based treatment), we recognize that we may have inadvertently included patients who received treatment outside of Florida. Finally, our results might not be generalizable to patients in other states where reimbursement policies differ.
Despite current availability of effective DAA therapies, this study demonstrates that only 8% of Florida Medicaid patients with HCV received DAA treatment during 2013‐2018. Access to HCV treatment improved since Florida Medicaid relaxed the restriction on fibrosis staging in 2016, but Medicaid patients with chronic HCV still lack access to treatment. Disparities in access to care are also seen among those who are Black, who have a SUD, and who have HIV. However, once patients with a SUD and/or HIV co‐infection initiated DAA therapy, they completed therapy at the same rate as those without a SUD or HIV co‐infection. Therefore, to improve access to care, Medicaid programs nationally and Florida’s Medicaid policy makers need to consider offering less‐restrictive requirements for HCV treatment and further investigate the barriers to treatment that still exist for those patients with a SUD and/or HIV, so that access to DAA treatment can increase. The lack of treatment uptake in these populations may hinder the United States in reaching the global health goal of eliminating hepatitis by 2030.
Supporting information
Fig S1
Fig S2
Fig S3
Table S1
Table S2
Table S3
Table S4
Acknowledgment
We thank Debbie L. Wilson, Ph.D. (University of Florida), for providing editorial assistance in the preparation of this manuscript.
Supported by the National Institute on Drug Abuse (K01DA045618) and National Institute on Alcohol Abuse and Alcoholism (U24AA022002).
Potential conflict of interest: Dr. Nelson receives grants from Gilead, AbbVie, and Merck.
References
- 1. Ditah I, Ditah F, Devaki P, Ewelukwa O, Ditah C, Njei B, et al. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol 2014;60:691‐698. [DOI] [PubMed] [Google Scholar]
- 2. Falade‐Nwulia O, Suarez‐Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct‐acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med 2017;166:637‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet 2015;385:1124‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid reimbursement of Sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med 2015;163:215‐223. [DOI] [PubMed] [Google Scholar]
- 5. Do A, Mittal Y, Liapakis A, Cohen E, Chau H, Bertuccio C, et al. Drug authorization for Sofosbuvir/Ledipasvir (Harvoni) for chronic HCV infection in a real‐world cohort: a new barrier in the HCV care cascade. PLoS One 2015;10:e0135645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Canary LA, Klevens RM, Holmberg SD. Limited access to new hepatitis C virus treatment under state Medicaid programs. Ann Intern Med 2015;163:226‐228. [DOI] [PubMed] [Google Scholar]
- 7. National Viral Hepatitis Roundtable (NVHR) and Center for Health Law and Policy Innovation . Hepatitis C: the state of Medicaid access 2017 national summary report. https://nvhr.org/sites/default/files/.users/u33/State%20of%20HepC_2017.pdf. Accessed February 14, 2020. [Google Scholar]
- 8. American Association for the Study of Liver Disease (AASLD)/Infectious Disease Society of America (IDSA)/International Antiviral Society‐USA (IAS‐USA) . HCV guidance: recommendations for testing, managing, and treating hepatitis C 2019. https://www.hcvguidelines.org/evaluate/testing‐and‐linkage. Accessed February 14, 2020. [Google Scholar]
- 9. Lo Re V, 3rd , Gowda C, Urick PN, Halladay JT, Binkley A, Carbonari DM, et al. Disparities in absolute denial of modern hepatitis C therapy by type of insurance. Clin Gastroenterol Hepatol 2016;14:1035‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck‐Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta‐analysis of observational studies. Ann Intern Med 2013;158:329‐337. [DOI] [PubMed] [Google Scholar]
- 11. Veldt BJ, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP, Zeuzem S, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med 2007;147:677‐684. [DOI] [PubMed] [Google Scholar]
- 12. Park H, Wang W, Henry L, Nelson DR. Impact of all‐oral direct‐acting antivirals on clinical and economic outcomes in patients with chronic hepatitis C in the United States. Hepatology 2019;69:1032‐1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jain MK, Thamer M, Therapondos G, Shiffman ML, Kshirsagar O, Clark C, et al. Has access to hepatitis C virus therapy changed for patients with mental health or substance use disorders in the direct‐acting‐antiviral period? Hepatology 2019;69:51‐63. [DOI] [PubMed] [Google Scholar]
- 14. Lo Re V, 3rd , Gowda C, Urick PN, Halladay JT, Binkley A, Carbonari DM, et al. Disparities in absolute denial of modern hepatitis C therapy by type of insurance. Clin Gastroenterol Hepatol 2016;14:1035‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wong RJ, Jain MK, Therapondos G, Shiffman ML, Kshirsagar O, Clark C, et al. Race/ethnicity and insurance status disparities in access to direct acting antivirals for hepatitis C virus treatment. Am J Gastroenterol 2018;113:1329‐1338. [DOI] [PubMed] [Google Scholar]
- 16. Yuan J, Malin B, Modave F, Guo Y, Hogan WR, Shenkman E, et al. Towards a privacy preserving cohort discovery framework for clinical research networks. J Biomed Inform 2017;66:42‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Niu B, Forde KA, Goldberg DS. Coding algorithms for identifying patients with cirrhosis and hepatitis B or C virus using administrative data. Pharmacoepidemiol Drug Saf 2015;24:107‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Durand M, Wang Y, Venne F, Lelorier J, Tremblay CL, Abrahamowicz M. Diagnostic accuracy of algorithms to identify hepatitis C status, AIDS status, alcohol consumption and illicit drug use among patients living with HIV in an administrative healthcare database. Pharmacoepidemiol Drug Saf 2015;24:943‐950. [DOI] [PubMed] [Google Scholar]
- 19. Heslin K, Elixhauser A, Steiner C. Hospitalizations involving mental and substance use disorders among adults, 2012. Statistical Brief #191 (June 2015). https://www.hcup‐us.ahrq.gov/reports/statbriefs/sb191‐Hospitalization‐Mental‐Substance‐Use‐Disorders‐2012.jsp. Accessed February 14, 2020. [PubMed] [Google Scholar]
- 20. Laine C, Hauck WW, Gourevitch MN, Rothman J, Cohen A, Turner BJ. Regular outpatient medical and drug abuse care and subsequent hospitalization of persons who use illicit drugs. JAMA 2001;285:2355‐2362. [DOI] [PubMed] [Google Scholar]
- 21. Puenpatom A, Hull M, McPheeters J, Schwebke K. Disease burden, early discontinuation, and healthcare costs in hepatitis C patients with and without chronic kidney disease treated with interferon‐free direct‐acting antiviral regimens. Clin Drug Investig 2017;37:687‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. American Association for the Study of Liver Disease (AASLD)/Infectious Disease Society of America (IDSA)/International Antiviral Society‐USA (IAS‐USA) . HCV guidance: recommendations for testing, managing, and treating hepatitis C 2017. https://www.hcvguidelines.org. Accessed February 14, 2020. [Google Scholar]
- 23. Malespin M, Harris C, Kanar O, Jackman K, Smotherman C, Johnston A, et al. Barriers to treatment of chronic hepatitis C with direct acting antivirals in an urban clinic. Ann Hepatol 2019;18:304‐309. [DOI] [PubMed] [Google Scholar]
- 24. Grebely J, Bruneau J, Bruggmann P, Harris M, Hickman M, Rhodes T, et al. Elimination of hepatitis C virus infection among PWID: the beginning of a new era of interferon‐free DAA therapy. Int J Drug Policy 2017;47:26‐33. [DOI] [PubMed] [Google Scholar]
- 25. Asher AK, Portillo CJ, Cooper BA, Dawson‐Rose C, Vlahov D, Page KA. Clinicians’ views of hepatitis C virus treatment candidacy with direct‐acting antiviral regimens for people who inject drugs. Subst Use Misuse 2016;51:1218‐1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grebely J, Oser M, Taylor LE, Dore GJ. Breaking down the barriers to hepatitis C virus (HCV) treatment among individuals with HCV/HIV coinfection: action required at the system, provider, and patient levels. J Infect Dis 2013;207:S19‐S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Swan D, Long J, Carr O, Flanagan J, Irish H, Keating S, et al. Barriers to and facilitators of hepatitis C testing, management, and treatment among current and former injecting drug users: a qualitative exploration. Aids Patient Care STDs 2010;24:753‐762. [DOI] [PubMed] [Google Scholar]
- 28. Norton BL, Beitin A, Glenn M, DeLuca J, Litwin AH, Cunningham CO. Retention in buprenorphine treatment is associated with improved HCV care outcomes. J Subst Abuse Treat 2017;75:38‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services . Effective treatments for opioid addiction. https://www.drugabuse.gov/sites/default/files/policybrief‐effectivetreatments.pdf. Accessed September 17, 2020. [Google Scholar]
- 30. Valerio H, McAuley A, Innes H, Palmateer N, Dj G, Munro A, et al. Determinants of hepatitis C antiviral effectiveness awareness among people who inject drugs in the direct‐acting antiviral era. Int J Drug Policy 2018;52:115‐122. [DOI] [PubMed] [Google Scholar]
- 31. Day E, Broder T, Bruneau J, Cruse S, Dickie M, Fish S, et al. Priorities and recommended actions for how researchers, practitioners, policy makers, and the affected community can work together to improve access to hepatitis C care for people who use drugs. Int J Drug Policy 2019;66:87‐93. [DOI] [PubMed] [Google Scholar]
- 32. Platt L, Minozzi S, Reed J, Vickerman P, Hagan H, French C, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev 2017;9:CD012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hernández D, Castellón PC, Fernández Y, Torress‐Cardona FA, Parish C, Gorshein D, et al. When “the cure” is the risk: understanding how substance use affects HIV and HCV in a layered risk environment in San Juan, Puerto Rico. Health Educ Behav 2017;44:748‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fraser H, Zibbell J, Hoerger T, Hariri S, Vellozzi C, Martin NK, et al. Scaling‐up HCV prevention and treatment interventions in rural United States‐model projections for tackling an increasing epidemic. Addiction 2018;113:173‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Buckley GJ, Strom BL. A national strategy for the elimination of viral hepatitis emphasizes prevention, screening, and universal treatment of hepatitis C. Ann Intern Med 2017;166:895‐896. [DOI] [PubMed] [Google Scholar]
- 36. Chappell CA, Krans EE, Bunge K, Macio I, Bogen D, Scarsi KK. A phase 1 study of ledipasvir/sofosbuvir in pregnant women with hepatitis C virus. Conference on Retroviruses and Opportunistic Infections, Seattle, WA, 2019. [Google Scholar]
- 37. Gn Y. Treatment of chronic hepatitis C with ledipasvir/sofosbuvir combination during pregnancy. Hepatol Int 2018;12:S292‐S293. [Google Scholar]
- 38. U.S. National Library of Medicine . Study of hepatitis C treatment during pregnancy. https://clinicaltrials.gov/ct2/history/NCT02683005?V_9=View#StudyPageTop. Accessed February 14, 2020. [Google Scholar]
- 39. U.S. National Library of Medicine . Evaluation of the national history of vertical transmission of chronic hepatitis C virus infection in pregnancy with targeted elimination by postpartum treatment. https://www.clinicaltrials.gov/ct2/show/NCT03570112. Accessed February 14, 2020. [Google Scholar]
- 40. Kushner T, Terrault NA. Hepatitis C in pregnancy: a unique opportunity to improve the hepatitis C cascade of care. Hepatol Commun 2019;3:20‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ho SB, Brau N, Cheung R, Liu L, Sanchez C, Sklar M, et al. Integrated care increases treatment and improves outcomes of patients with chronic hepatitis C virus infection and psychiatric illness or substance abuse. Clin Gastroenterol Hepatol 2015;13:2005‐2014.e2001‐e2003. [DOI] [PubMed] [Google Scholar]
- 42. Moussalli J, Delaquaize H, Boubilley D, Lhomme JP, Merleau Ponty J, Sabot D, et al. Factors to improve the management of hepatitis C in drug users: an observational study in an addiction centre. Gastroenterol Res Pract 2010;2010:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martin SA, Bosse J, Wilson A, Losikoff P, Chiodo L. Under one roof: identification, evaluation, and treatment of chronic hepatitis C in addiction care. Addict Sci Clin Pract 2018;13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Serota DP, Barocas JA, Springer SA. Infectious complications of addiction: a call for a new subspecialty within infectious diseases. Clin Infect Dis 2020;70:968‐972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goldberg DS, Lewis JD, Halpern SD, Weiner MG, Lo Re V, 3rd . Validation of a coding algorithm to identify patients with hepatocellular carcinoma in an administrative database. Pharmacoepidemiol Drug Saf 2013;22:103‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Table S1
Table S2
Table S3
Table S4


