Abstract
Despite scant evidence, current guidelines indicate that esophageal varices are a relative contraindication to transesophageal echocardiography (TEE). The aim of this study is to compare the risk of gastrointestinal bleeding following TEE among cirrhotic patients with and without endoscopically‐documented esophageal varices. This is a retrospective analysis of patients with cirrhosis who underwent upper endoscopy within 4 years of TEE at five institutions between January 2000 and March 2020. Primary outcome was overt gastrointestinal bleeding. Secondary outcomes were hemoglobin decline by at least 2 g/dL or blood transfusion within 48 hours following TEE. Of the 191 patients, 79 (41.4%) had esophageal varices (30.4% large). No patient experienced a primary outcome. Secondary outcomes occurred in 52 (27.2%): 28 (35.4%) with esophageal varices and 24 (21.4%) without varices. After propensity‐score covariate adjustment, the odds ratio for a secondary outcome in patients with esophageal varices was 1.49 (95% confidence interval 0.74‐2.99). Restricting analysis to those who underwent endoscopy within 1 year of TEE did not significantly alter results. The risk of a secondary outcome was identical between patients who had upper endoscopy prior (27.5%) versus subsequent (26.7%; P = 1.00) to TEE. Conclusions: Among patients with cirrhosis, there was no overt gastrointestinal bleeding after TEE. The likelihood of a 2 g/dL decline in hemoglobin or blood transfusion within 48 hours following TEE was not significantly higher in patients with esophageal varices after controlling for confounders. Patients who underwent upper endoscopy before TEE did not manifest a lower risk of secondary outcomes versus those who had endoscopy after TEE, suggesting that routine preprocedural endoscopy is of marginal utility.
Abbreviations
- CI
confidence interval
- INR
international normalized ratio
- MELD‐Na
Model for End‐Stage Liver Disease–Sodium
- OR
odds ratio
- TEE
transesophageal echocardiography
- TIPS
transjugular intrahepatic portosystemic shunt
Current guidelines consider esophageal varices a relative contraindication to transesophageal echocardiography (TEE).( 1 ) For this reason, upper endoscopy is often requested in patients with cirrhosis before performing TEE in order to assess for the presence of varices. However, scant evidence exists to support the utility or efficacy of this practice. Most reports that have attempted to quantify the risk of bleeding in patients with cirrhosis and esophageal varices consist of retrospective case series of TEE performed intra‐operatively during orthotopic liver transplantation,( 2 , 3 , 4 , 5 , 6 ) with meta‐analysis indicating a pooled incidence of postprocedural bleeding of 1.4%.( 7 ) Data regarding the outcomes of patients with cirrhosis and esophageal varices who undergo nonoperative TEE are limited to three small cases series encompassing a total of 58 patients, in whom no bleeding events were noted.( 7 , 8 , 9 )
As patients with cirrhosis are at generally higher risk of adverse procedural outcomes, including hemorrhage,( 10 , 11 ) it is notable that only two studies of variceal bleeding post TEE included a comparator group.( 12 ) A retrospective analysis of intra‐operative TEE at the time of liver transplantation found no difference in blood transfusion requirements between those with or without esophageal varices, and noted a single case of overt bleeding in a patient who had undergone a prior transjugular intrahepatic portosystemic shunt (TIPS) procedure and had no varices on autopsy.( 2 ) Similarly, an analysis of the 2016 Nationwide Readmissions Data reported that, of hospitalized patients discharged with a diagnosis of esophageal varices, the 242 (0.3%) who underwent TEE had similar rates of gastrointestinal bleeding (~1%) as those who did not, although findings were based solely on diagnosis codes.( 12 ) No study has examined the influence of variceal grade on bleeding risk or whether endoscopic screening of patients with cirrhosis prior to performing TEE reduces the risk of postprocedural bleeding. For these reasons, the aim of the present study was to assess whether the presence and grade of endoscopically verified esophageal varices increases the risk of hemorrhage following TEE in patients with cirrhosis, and whether endoscopic screening before performing TEE reduces bleeding risk.
Patients and Methods
Study Design and Patient Selection
This is a multicenter, retrospective study of cirrhotic patients with and without esophageal varices who underwent TEE at one of five medical centers between January 2000 and March 2020. These five centers performed a combined total of 43,899 TEE procedures over this time period, ranging from 18,295 studies at the highest volume center to 3,345 studies at the lowest. Patients were identified using the Partners HealthCare Research Patient Data Registry, which gathers clinical data from all major hospitals within Partners Healthcare. All patients who underwent TEE and an upper endoscopy with the procedure report containing the word “varices” or “varix” during the predefined time period were identified. Patients were excluded if (1) the diagnosis of cirrhosis could not be confirmed on chart review by two independent physician reviewers; (2) the time interval between TEE and upper endoscopy exceeded 4 years (regardless of the order of performance); or (3) the patient experienced a portal vein thrombus, or underwent variceal band ligation, a TIPS procedure, or liver transplantation during the interval between upper endoscopy and TEE. Institutional review board approval was obtained from Partners Healthcare.
Data Collection
The dates of upper endoscopy and TEE were recorded, with the upper endoscopy nearest to the date of the TEE utilized when more than one endoscopy had been performed. The presence and grading of the size of esophageal varices (grade I, II, or III) was extracted from the endoscopy report. If more than one grade was indicated, the largest was used. Esophageal varices were considered “small” if grade I and “large” if grade II or III, in accordance with Baveno and American Association for the Study of Liver Diseases guidance.( 13 , 14 ) Demographic characteristics included age at the time of TEE, sex, and race. Clinical information collected included the etiology of cirrhosis, a history of any decompensating event (e.g., variceal hemorrhage, hepatic encephalopathy, ascites), and whether patients were receiving a nonselective beta‐blocker. The most recent laboratory values immediately before TEE were recorded, including international normalized ratio (INR), bilirubin, creatinine, sodium, platelet count, and hemoglobin. The Model for End‐Stage Liver Disease–Sodium (MELD‐Na) score was calculated from these lab values. Additional clinical parameters included whether patients received an antiplatelet and/or anticoagulation agent within 7 days of TEE, whether patients were on hemodialysis, whether TEE was performed in the inpatient or outpatient setting, and whether the TEE was used to guide a therapeutic intervention (including intra‐operative) or was solely diagnostic.
Outcomes
The primary outcome was overt manifestation of gastrointestinal bleeding within 48 hours following TEE, defined as either (1) gross hematemesis, melena, and/or hematochezia, and/or (2) evidence of active or recent hemorrhage on upper endoscopy. Secondary outcomes occurring within 48 hours after undergoing TEE were (1) a hemoglobin decline of at least 2 g/dL, and/or (2) receipt of a blood transfusion.
Statistical Analyses
Categorical variables are presented as frequencies with percentages and continuous variables as means with standard deviations. Univariate analysis of categorical variables was performed with Fisher’s exact test, while continuous variables were analyzed with Student's t test. Propensity‐score adjustment of the odds ratio (OR) for the study outcome was performed using logistic regression with a priori covariates, including age, platelet count, hemoglobin, MELD‐Na score, use of a nonselective beta‐blocker, receipt of an antiplatelet agent or anticoagulant, and whether the TEE was therapeutic, performed inpatient, or intra‐operatively. Statistical significance was defined as a two‐sided P value less than 0.05. Statistical analyses were conducted using SAS version 9.4 (Cary, NC). Sensitivity analysis restricting the study cohort to patients with shorter intervals between upper endoscopy and TEE was performed, as were subanalyses comparing patients undergoing upper endoscopy before or after TEE, those who had underwent band ligation or TIPS prior to the interval between upper endoscopy and TEE, and those who had TEE performed for liver transplantation and nonoperative indications. Additionally, an exploratory analysis was performed in which the grade of esophageal varices was reclassified based on review of upper endoscopy images by two independent hepatologists blinded to the grading on the original endoscopy report.
Results
Patient Characteristics
A total of 347 patients underwent both TEE and upper endoscopy (with report commenting on the presence or absence of esophageal varices) between January 2000 and March 2020. After excluding those patients who did not have underlying cirrhosis, as determined by chart review (Fig. 1), a total of 191 patients were included in the analyses. Of these, 79 (41.4%) had endoscopically documented esophageal varices, with 55 (69.6%) graded as small and 24 (30.4%) as large. Baseline patient characteristics, stratified by the presence or absence of esophageal varices, are detailed in Table 1. There was a significantly higher prevalence of decompensated cirrhosis and nonselective beta‐blocker use, as well as lower platelet count, lower serum sodium, and higher MELD‐Na score among those with esophageal varices. The indications for TEE were similar between the groups, with the exception that more patients in the variceal group underwent TEE during liver transplantation (Table 2). No TEE procedure was aborted prematurely.
FIG. 1.
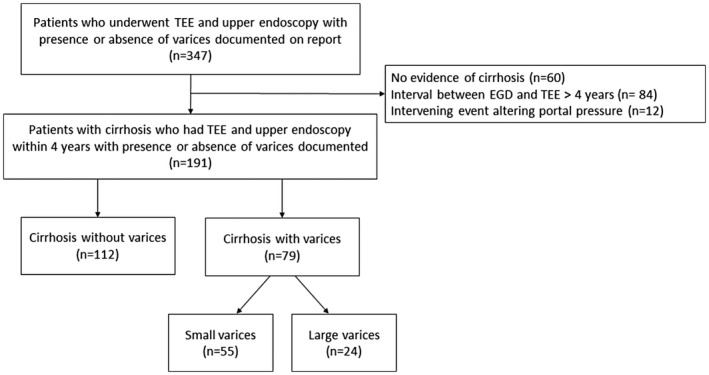
Study criteria and patient breakdown. Abbreviation: EGD, esophagogastroduodenoscopy.
TABLE 1.
Patient Characteristics at Time of TEE
| Parameter | Esophageal Varices (n = 79) | No Esophageal Varices (n = 112) | P Value |
|---|---|---|---|
| Age in years | 61.0 ± 12.0 | 61.8 ± 12.7 | 0.63 |
| Male | 48 (60.8) | 76 (67.9) | 0.36 |
| Race | |||
| Caucasian | 71 (89.9) | 104 (92.9) | 0.60 |
| Black | 5 (6.3) | 4 (3.6) | 0.49 |
| Asian | 1 (1.3) | 2 (1.8) | 1.00 |
| Other | 1 (1.3) | 1 (0.9) | 1.00 |
| Unknown | 1 (1.3) | 1 (0.9) | 1.00 |
| Cirrhosis etiology* | |||
| Alcohol | 25 (31.7) | 47 (42.0) | 0.17 |
| Nonalcoholic steatohepatitis | 26 (32.9) | 23 (20.5) | 0.06 |
| Hepatitis C virus | 25 (31.7) | 25 (22.3) | 0.18 |
| Cardiac | 12 (15.2) | 16 (14.3) | 1.00 |
| Other | 12 (15.2) | 16 (14.3) | 1.00 |
| Any liver decompensation | 42 (53.2) | 31 (27.7) | 0.0005 |
| Hemodialysis | 9 (11.4) | 6 (5.4) | 0.17 |
| Nonselective beta‐blocker therapy | 16 (20.3) | 10 (8.9) | 0.03 |
| Large esophageal varices † | 24 (30.0) | — | |
| Days between upper endoscopy and TEE | 225.7 ± 308.1 | 300.4 ± 337.6 | 0.12 |
| TEE performed as inpatient | 70 (88.6) | 96 (85.7) | 0.67 |
| TEE performed intra‐operatively | 32 (40.5) | 34 (30.4) | 0.17 |
| Therapeutic TEE | 36 (45.6) | 43 (38.4) | 0.37 |
| Antiplatelet agent within 7 days before TEE | 25 (31.7) | 38 (33.9) | 0.76 |
| Anticoagulation within 7 days before TEE | 24 (30.4) | 37 (33.0) | 0.75 |
| Hemoglobin ‡ (g/dL) | 10.4 ± 2.3 | 11.0 ± 2.7 | 0.12 |
| Platelet count ‡ (109/L) | 107.7 ± 50.6 | 152.2 ± 83.0 | <0.0001 |
| INR ‡ | 1.7 ± 0.7 | 1.6 ± 0.6 | 0.12 |
| Serum bilirubin ‡ (mg/dL) | 3.8 ± 7.6 | 2.8 ± 5.6 | 0.30 |
| Serum creatinine ‡ (mg/dL) | 1.7 ± 1.4 | 1.4 ± 1.5 | 0.16 |
| Serum sodium ‡ (mEq/L) | 136.0 ± 4.4 | 138.0 ± 4.6 | 0.004 |
| MELD‐Na score ‡ | 21.3 ± 7.8 | 16.4 ± 7.5 | <0.0001 |
Continuous variables are reported as mean ± SD; categorical variables are reported as n (%).
Total exceeds group size due to presence of concurrent cirrhosis etiologies.
Upper endoscopy performed within 4 years of TEE.
Laboratory variables reflect nearest value prior to TEE.
TABLE 2.
Type and Indication for TEE
| Indication | Esophageal Varices (n = 79) | No Esophageal Varices (n = 112) | P Value |
|---|---|---|---|
| Diagnostic | 43 (54.4) | 69 (61.6) | 0.37 |
| Endocarditis | 23 (29.1) | 36 (32.1) | 0.66 |
| Intracardiac thrombus (before cardioversion) | 11 (13.9) | 18 (16.1) | 0.68 |
| Cardiac valve function | 7 (8.9) | 7 (6.3) | 0.50 |
| Patent foramen ovale | 2 (2.5) | 8 (7.1) | 0.16 |
| Therapeutic, nonsurgical | 4 (5.0) | 9 (8.0) | 0.56 |
| Transcatheter aortic valve replacement | 0 (0.0) | 3 (2.7) | 0.27 |
| Cardiac valve dilatation | 1 (1.3) | 0 (0.0) | 0.41 |
| Cardiac ablation | 0 (0.0) | 1 (0.9) | 1 |
| Watchman placement | 2 (2.5) | 3 (2.7) | 0.95 |
| MitraClip placement | 1 (1.3) | 1 (0.9) | 0.80 |
| Atrial septal defect closure | 0 (0.0) | 1 (0.9) | 1 |
| Therapeutic, intra‐operative | 32 (40.5) | 34 (30.4) | 0.17 |
| Liver transplantation | 21 (26.6) | 14 (12.5) | 0.01 |
| Cardiac transplantation | 1 (1.3) | 0 (0.0) | 0.41 |
| Lung transplantation | 1 (1.3) | 0 (0.0) | 0.41 |
| Combined cardiac and liver transplantation | 0 (0.0) | 1 (0.9) | 1 |
| Coronary artery bypass graft | 4 (5.1) | 8 (7.1) | 0.56 |
| Surgical cardiac valve repair or replacement | 3 (3.8) | 9 (8.0) | 0.23 |
| Pericardiectomy | 0 (0.0) | 1 (0.9) | 1 |
| Pulmonary artery embolectomy | 1 (1.3) | 0 (0.0) | 0.41 |
| Implantable cardioverter‐defibrillator replacement | 1 (1.3) | 0 (0.0) | 0.41 |
| Impella removal | 0 (0.0) | 1 (0.9) | 1 |
Variables are reported as n (%).
Outcomes
Rates of Bleeding Following TEE
In the 48 hours following TEE, no patient developed a primary outcome of overt gastrointestinal bleeding. A total of 52 patients (27.2%) experienced at least one secondary outcome: either a 2 g/dL hemoglobin decline and/or blood transfusion within 48 hours after TEE (Table 3). There were no deaths during the 48‐hour post‐TEE assessment period. A secondary outcome occurred in a significantly higher proportion of patients with esophageal varices (35.4%) as compared with those without varices (21.4%; P = 0.047), as well as in those with large (45.8%) versus small (30.9%; P = 0.21) varices. While patients with esophageal varices were significantly more likely to experience a secondary outcome than those without esophageal varices (OR 2.01, 95% confidence interval [CI] 1.06‐3.84, P = 0.03), after propensity‐score adjustment for a priori covariates (i.e., age, platelet count, hemoglobin, MELD‐Na score, nonselective beta‐blocker, antiplatelet agent, anticoagulant, therapeutic, intra‐operative, or inpatient TEE), this difference was no longer significant (OR 1.49, 95% CI 0.74‐2.99, P = 0.27).
TABLE 3.
Bleeding Outcomes Following TEE
| Outcomes | All Patients (n = 191) | Esophageal Varices* (n = 79) | No Esophageal Varices (n = 112) | P Value |
|---|---|---|---|---|
| Primary outcome | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 |
| Secondary outcomes | ||||
| Any secondary outcome | 52 (27.2) | 28 (35.4) | 24 (21.4) | 0.047 |
| Hemoglobin drop ≥ 2 g/dL | 42 (22.0) | 22 (27.9) | 20 (17.9) | 0.11 |
| Blood transfusion | 34 (17.8) | 21 (26.6) | 13 (11.6) | 0.01 |
Variables are reported as n (%).
Large esophageal varices in 24 (30.4%) patients.
To address potential concerns that inaccurate estimates of the presence and/or size of esophageal varices may occur when a more extended time has elapsed between upper endoscopy and TEE, we examined the effect of restricting the study cohort to patients with shorter intervals (i.e., 3, 2, and 1 year) between upper endoscopy and TEE. As indicated in Table 4, progressive shortening of the interval between endoscopy and TEE had no significant effect on the adjusted OR of a secondary outcome. Similarly, when variceal size was graded by two independent, blinded hepatologists through a review of all available upper endoscopy images, there was no significant change in study findings. It is notable that patients who experienced a secondary outcome were significantly more likely to have undergone an intra‐operative (84.6% vs. 15.8%, P < 0.0001) or therapeutic (88.5% vs. 23.7%, P < 0.0001) TEE, suggesting that procedural blood losses could be a major determinate of hemoglobin decline or need for blood transfusion. Counterintuitively, anticoagulation use within the previous 7 days was significantly lower among those who had a secondary outcome (17.31% vs. 37.41%, P = 0.0087), whereas there was no significant difference in MELD‐Na, platelet count, creatinine, INR, nonselective beta‐blocker use, or antiplatelet use in those who did or did not have a hemoglobin decline or blood transfusion requirement.
TABLE 4.
Odds Ratio for Secondary Outcomes With Decreasing Time Interval Between Upper Endoscopy and TEE
| Interval Between Upper Endoscopy and TEE | Cohort Size, n | Esophageal Varices, n (%) | No Esophageal Varices, n (%) | Unadjusted OR* for Secondary Outcome (95% CI) | P Value | Adjusted OR* for Secondary Outcome (95% CI) | P Value |
|---|---|---|---|---|---|---|---|
| ≤4 years (original) | 191 | 79 (41.4) | 112 (58.6) | 2.01 (1.06‐3.84) | 0.03 | 1.49 (0.74‐2.99) | 0.27 |
| ≤3 years | 186 | 77 (41.4) | 109 (58.6) | 2.26 (1.17‐4.37) | 0.02 | 1.63 (0.80‐3.33) | 0.18 |
| ≤2 years | 168 | 72 (42.9) | 96 (57.1) | 2.43 (1.22‐4.86) | 0.01 | 1.54 (0.72‐3.28) | 0.27 |
| ≤1 year | 137 | 62 (45.3) | 75 (54.7) | 2.40 (1.10‐5.23) | 0.03 | 1.45 (0.62‐3.40) | 0.40 |
Patients with esophageal varices compared to those without esophageal varices.
In a further attempt to assess whether the circumstances under which the TEE procedure was performed alters the risk of a secondary outcome, we analyzed two subsets of patients: (1) those patients who underwent a TEE that was not associated with an operative procedure; and (2) those who underwent TEE at time of liver transplantation. The nonoperative cohort consisted of 125 patients, of whom 47 had esophageal varices (12 large). The unadjusted OR of a secondary outcome was 1.72 (95% CI: 0.41‐7.23). After propensity‐score adjustment, the OR was 1.13 (95% CI: 0.24‐5.29). Of the 35 patients who underwent TEE at the time of liver transplant, 21 had esophageal varices (7 large). The unadjusted OR of a secondary outcome among those with varices was 3.33 (95% CI: 0.65‐17.18). The small number of patients in this latter subset precluded propensity‐score adjustment.
Influence of Upper Endoscopy on TEE Outcomes
To evaluate whether performing an upper endoscopy before TEE alters the risk of bleeding, we compared outcomes for the 131 (68.6%) patients who underwent an upper endoscopy before TEE with the 60 (31.4%) who had the procedure afterwards (Table 5). Although there was a strong trend toward a higher frequency of large esophageal varices in patients who underwent upper endoscopy after versus before TEE (20.0% vs. 9.1%, P = 0.06), there was no difference in the proportion of patients reaching primary (0% vs. 0%, P = 1.00) or secondary (27.5% vs. 26.7%, P = 1.00) outcomes between the groups. There also was no difference in secondary outcomes among the 14 (17.7%) patients who had undergone variceal ligation or TIPS before TEE (35.71% vs. 35.38%, P = 1.00) compared with those who did not.
TABLE 5.
Bleeding Outcomes Based on Timing of Upper Endoscopy With Respect to TEE
| Esophageal Varices and Outcomes | Upper Endoscopy Before TEE, n = 131 | Upper Endoscopy After TEE, n = 60 | P Value |
|---|---|---|---|
| Esophageal varices | |||
| Any esophageal varices identified * | 52 (39.7) | 27 (45.0) | 0.53 |
| Large esophageal varices identified † | 12 (9.1) | 12 (20.0) | 0.06 |
| Outcomes | |||
| Primary outcome | 0 (0.0) | 0 (0.0) | 1 |
| Secondary outcomes | |||
| Any secondary outcome | 36 (27.5) | 16 (26.7) | 1 |
| Hemoglobin drop ≥ 2 g/dL | 28 (21.4) | 14 (23.3) | 0.85 |
| Blood transfusion | 25 (19.1) | 9 (15.0) | 0.55 |
Mean 174.8 ± 243.6 days from upper endoscopy to subsequent TEE; 323.6 ± 391.5 days from TEE to subsequent upper endoscopy.
Mean 144.1 ± 157.8 days from upper endoscopy to subsequent TEE; 361.5 ± 459.4 days from TEE to subsequent upper endoscopy.
Discussion
Although currently published guidelines indicate that esophageal varices represent a relative contraindication to performing transesophageal echocardiography,( 1 ) these recommendations are based almost entirely on expert opinion, as there is scant literature evidence to support this guidance. To date, most investigations regarding the risk of TEE in patients with esophageal varices are retrospective observational studies of overt hemorrhage following intra‐operative( 2 , 3 , 4 , 5 , 6 ) or non‐intraoperative( 7 , 8 , 9 ) TEE involving small cohorts (Table 6). In aggregate, these reports suggest that the incidence of overt postprocedural bleeding is low (0%‐0.4%); however, their small size, heterogeneity, variably defined endpoints, and absence of a comparator cohort makes extrapolation difficult. The only large database study showed no difference in rates of in‐hospital bleeding between patients with esophageal varices who did or did not undergo TEE, with the caveat that patient characteristics and endpoints were based solely on diagnosis codes and were not clinically validated.( 12 )
TABLE 6.
Summary of Studies on Bleeding Outcomes Following TEE in Patients With Esophageal Varices
| Study | Population | Study n (n with EV) | Nature of TEE | Study Type | Bleeding Outcomes | Bleeding Events |
|---|---|---|---|---|---|---|
| Burger‐Klepp et al. 2012( 2 ) | Patients undergoing liver transplantation | 396 (287) | Intra‐operative | Observational, retrospective, comparative (287 EV vs. 109 no EV) | Overt variceal hemorrhage requiring transfusion, endoscopic intervention, balloon tamponade, or surgery | 1 patient (0.3%) with hemorrhage had a TIPS without varices on autopsy; no difference in transfusion requirement between EV and no EV groups |
| Pai et al. 2015( 3 ) | Patients undergoing liver transplantation | 232 (161) | Intra‐operative | Observational, retrospective, noncomparative | Esophageal or gastric mucosal injury or variceal hemorrhage | 1 patient (0.4%) with variceal hemorrhage during anhepatic phase |
| Suriani et al. 1996( 5 ) | Patients undergoing liver transplantation | 100 (23) | Intra‐operative | Observational, retrospective, noncomparative | Esophageal trauma, EV hemorrhage | 1 patient (1.0%) with blood from orogastric tube had no EV |
| Markin et al. 2015( 6 ) | Patients undergoing liver transplantation | 116 (not defined) | Intra‐operative | Observational, retrospective, noncomparative | Esophageal trauma, upper gastrointestinal bleeding by orogastric lavage | 1 patient (0.9%) with hemorrhage had elective variceal ligation one day prior |
| Nigatu et al. 2018( 7 ) | Patients with EV | 20 (20) | Nonoperative | Observational, retrospective, noncomparative | Hematemesis, melena, hematochezia, blood transfusion, or hemoglobin decline by ≥ 2 g/dL within 48 hours | No events |
| Spier et al. 2009( 8 ) | Patients with EV | 14 (14) | Nonoperative | Observational, retrospective, noncomparative | Hemoglobin decline by ≥ 2 g/dL or blood transfusion within 48 hours | No events |
| Pantham et al. 2013( 9 ) | Patients with EV | 24 (24) | Nonoperative | Observational, retrospective, noncomparative | Hemoglobin decline by ≥ 2 g/dL within 48 hours | No events |
| Hudhud et al. 2019( 12 ) | ICD‐10 discharge code for EV | 81,328 (81,328) | Not specified | Observational, retrospective, comparative (242 TEE vs. 81,086 no TEE) | ICD‐10 code for in‐hospital postprocedural complications (any intraprocedural or postprocedural bleeding or hematoma in digestive system, hematemesis, melena, and/or postprocedural anemia) | 0.9% in TEE group; 1.1% in non‐TEE group (NS) |
Abbreviations: EV, esophageal varices; ICD‐10, International Classification of Diseases, 10th Revision; NS, nonsignificant.
In line with prior reports, no patient in our study experienced a primary outcome of overt variceal hemorrhage. This finding is somewhat intuitive, as bleeding from varices generally is a consequence of vessel rupture from elevated portal pressure as opposed to external injury.( 15 )
We also utilized standard outcome definitions of gastrointestinal hemorrhage (i.e., 2 g/dL hemoglobin decline and/or blood transfusion within 48 hours) to further assess postprocedural bleeding risk. As TEE is known to occasionally induce bleeding as a result of direct trauma to the esophageal mucosa,( 16 ) and as patients with cirrhosis have a nontrivial incidence of gastrointestinal hemorrhage in the absence of intervention,( 17 ) we included a comparator group of cirrhotic patients without esophageal varices as a control. While approximately one‐quarter of all patients with cirrhosis experienced a secondary outcome following TEE, after adjusting for confounders, the odds of a bleeding event was not significantly higher in those with esophageal varices. These findings are in concordance with those of Burger‐Klepp et al., who reported no difference in blood transfusion requirements between patients with or without esophageal varices who underwent intra‐operative TEE at the time of liver transplantation.( 2 ) We speculate that the relatively high prevalence of secondary outcomes in the absence of overt gastrointestinal bleeding is primarily the result of non‐gastrointestinal blood loss. This conjecture is supported by our finding that patients who experienced any secondary outcome were substantially more likely to have undergone TEE intra‐operatively (84.6% vs. 15.8%, P < 0.0001) or as part of a therapeutic intervention (88.5% vs. 23.7%, P < 0.0001) than those who did not.
While it has been recommended that patients with cirrhosis who have not previously been evaluated should undergo endoscopic examination before TEE to assess for the presence of esophageal varices,( 8 ) to our knowledge, there have been no studies examining the utility of this guidance. We attempted to address whether a preprocedure endoscopy reduces the risk of postprocedural bleeding by comparing outcomes in patients with cirrhosis who underwent upper endoscopy before versus after TEE. We postulated that the cohort of patients who were screened endoscopically before the TEE would be more likely to have smaller or no varices compared to patients who did not undergo endoscopy before TEE. As anticipated, of the nearly one‐third of patients who underwent a TEE without prior endoscopic investigation, a slightly higher proportion had esophageal varices (45.0% vs. 39.7%; P = 0.53), with a strong trend toward the presence of large esophageal varices (20.0% vs. 9.1%, P = 0.06) observed. Despite this, there was no difference in bleeding outcomes as compared with patients who underwent upper endoscopy before TEE (26.7% vs. 27.5%, P = 1.00). These data suggest that performing routine upper endoscopy prior to TEE to assess for varices is of limited utility.
Strengths of this study include the robust clinical parameters, objective validation of esophageal varices, rigorous outcome measures, and inclusion of a control cohort of patients with cirrhosis as comparators. Limitations of the study include its moderate sample size and retrospective nature, raising the possibility of selection bias or unmeasured confounders that could impact outcomes. We attempted to control for potential inconsistencies in the grading of varices through sensitivity analyses using shorter time intervals between upper endoscopy and TEE, and through adjudication of varices by independent observers, neither of which altered the findings. The relatively modest number of cases identified over an extended 20‐year time frame may be a consequence of current guidelines that discourage the performance of TEE in patients with cirrhosis and esophageal varices. Although patients with large esophageal varices did not exhibit a significantly higher risk of a secondary outcome, the small number of patients makes firm conclusions difficult.
In summary, our study supports a very low risk of overt gastrointestinal bleeding following TEE in patients with cirrhosis and esophageal varices. While the likelihood of a 2‐g/dL decline in hemoglobin and/or need for blood transfusion within 48 hours of TEE approaches 25% to 30%, this was no more likely to occur in patients with varices and most likely reflects the complicated nature of patients with cirrhosis, as well as non‐gastrointestinal blood loss related to the interventions for which TEE was performed (e.g., liver transplant, heart valve repair, hemolysis). The finding that bleeding was no more likely to occur in patients undergoing endoscopy prior to versus subsequent to TEE argues against the utility of routinely performing preprocedure endoscopy in patients with cirrhosis.
Supported by the National Institute of Diabetes and Digestive and Kidney Diseases (Grant/Award No. 5T32DK007533‐35).
Potential conflict of interest: Nothing to report.
References
- 1. Hahn RT, Abraham T, Adams MS, Bruce CJ, Glas KE, Lang RM, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013;26:921‐964. [DOI] [PubMed] [Google Scholar]
- 2. Burger‐Klepp U, Karatosic R, Thum M, Schwarzer R, Fuhrmann V, Hetz H, et al. Transesophageal echocardiography during orthotopic liver transplantation in patients with esophagoastric varices. Transplantation 2012;27:192‐196. [DOI] [PubMed] [Google Scholar]
- 3. Pai SL, Aniskevich S 3rd, Feinglass NG, Ladlie BL, Crawford CC, Peiris P, et al. Complications related to intraoperative transesophageal echocardiography in liver transplantation. SpringerPlus 2015;4:4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Myo Bui CC, Worapot A, Xia W, Delgado L, Steadman RH, Busuttil RW, et al. Gastroesophageal and hemorrhagic complications associated with intraoperative transesophageal echocardiography in patients with model for end‐stage liver disease score 25 or higher. J Cardiothorac Vasc Anesth 2015;29:594‐597. [DOI] [PubMed] [Google Scholar]
- 5. Suriani RJ, Cutrone A, Feierman D, Konstadt S. Intraoperative transesophageal echocardiography during liver transplantation. J Cardiothorac Vasc Anesth 1996;10:699‐707. [DOI] [PubMed] [Google Scholar]
- 6. Markin NW, Sharma A, Grant W, Shillcutt SK. The safety of transesophageal echocardiography in patients undergoing orthotopic liver transplantation. J Cardiothorac Vasc Anesth 2015;29:588‐593. [DOI] [PubMed] [Google Scholar]
- 7. Nigatu A, Yap JE, Lee Chuy K, Go B, Doukky R. Bleeding risk of transesophageal echocardiography in patients with esophageal varices. J Am Soc Echocardiogr 2019;32:674‐676.e2. [DOI] [PubMed] [Google Scholar]
- 8. Spier BJ, Larue SJ, Teelin TC, Leff JA, Swize LR, Borkan SH, et al. Review of complications in a series of patients with known gastro‐esophageal varices undergoing transesophageal echocardiography. J Am Soc Echocardiogr 2009;22:396‐400. [DOI] [PubMed] [Google Scholar]
- 9. Pantham G, Waghray N, Einstadter D, Finkelhor RS, Mullen KD. Bleeding risk in patients with esophageal varices undergoing transesophageal echocardiography. Echocardiography 2013;30:1152‐1155. [DOI] [PubMed] [Google Scholar]
- 10. Northup PG, Friedman LS, Kamath PS. AGA clinical practice update on surgical risk assessment and perioperative management in cirrhosis: expert review. Clin Gastroenterol Hepatol 2019;17:595‐606. [DOI] [PubMed] [Google Scholar]
- 11. Newman KL, Johnson KM, Cornia PB, Wu P, Itani K, Ioannou GN. Perioperative evaluation and management of patients with cirrhosis: risk assessment, surgical outcomes, and future directions. Clin Gastroenterol Hepatol 2020;18:2398‐2414.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hudhud D, Allaham H, Eniezat M, Enezate T. Safety of performing transoesophageal echocardiography in patients with oesophageal varices. Heart Asia 2019;11:e011223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Franchis R, Pascal JP, Ancona E, Burroughs AK, Henderson M, Fleig W, et al. Definitions, methodology and therapeutic strategies in portal hypertension. A Consensus Development Workshop, Baveno, Lake Maggiore, Italy, April 5 and 6, 1990. J Hepatol 1992;15:256‐261. [DOI] [PubMed] [Google Scholar]
- 14. Garcia‐Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology . Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007;46:922‐938. [DOI] [PubMed] [Google Scholar]
- 15. Garcia‐Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2017;65:310‐335. [DOI] [PubMed] [Google Scholar]
- 16. Hilberath JN, Oakes DA, Shernan SK, Bulwer BE, D’Ambra MN, Eltzschig HK. Safety of transesophageal echocardiography. J Am Soc Echocardiogr 2010;23:1115‐1127. [DOI] [PubMed] [Google Scholar]
- 17. Loffredo L, Pastori D, Farcomeni A, Violi F. Effects of anticoagulants in patients with cirrhosis and portal vein thrombosis: a systematic review and meta‐analysis. Gastroenterology 2017;153:480‐487. [DOI] [PubMed] [Google Scholar]


