Abstract
In patients with decompensated cirrhosis, procedure‐related bleeding is a potentially lethal complication. Routine coagulation tests such as international normalized ratio and platelet count do not predict bleeding risk. We investigated whether thromboelastography (TEG) can identify patients with cirrhosis who are at risk of procedure‐related bleeding. As a part of a prospective study on hemostasis in decompensated cirrhosis, patients had TEG performed on admission and were followed prospectively during hospitalization for the development of procedure‐related bleeding. Eighty patients with cirrhosis were included. Among the 72 who had procedures performed, 7 had procedure‐related bleeding, which was major in three cases (two following paracentesis and one following thoracentesis). Conventional coagulation tests were comparable between bleeding and nonbleeding patients, whereas TEG parameters of k‐time (4.5 minutes vs. 2.2 minutes; P = 0.02), α‐angle (34° vs. 59°; P = 0.003), and maximum amplitude (37 mm vs. 50 mm; P = 0.004) were significantly different (all indicative of hypocoagulability). TEG maximum amplitude (MA), a marker of overall clot stability, accurately discriminated between patients who had major, life‐threatening bleeding (all with MA < 30 mm) and those who had mild or no bleeding (all with MA > 30 mm), whereas a platelet count < 50 × 109/L could not discriminate between bleeding (minor or major) and nonbleeding patients. Conclusion: In a prospective cohort of hospitalized patients with decompensated cirrhosis, TEG parameters associated with hypocoagulability appeared to predict procedure‐related bleeding, particularly a TEG MA < 30 mm. If results are validated in a larger cohort, this could be a threshold to identify patients with decompensated cirrhosis at higher risk for procedure‐related bleeding, in whom to consider preprocedural prophylaxis.
Abbreviations
- ACLF
acute‐on‐chronic liver failure
- AKI
acute kidney injury
- INR
international normalized ratio
- IQR
interquartile range
- MA
maximum amplitude
- MELD
Model for End‐Stage Liver Disease
- TEG
thromboelastography
- VTE
venous thromboembolism
In hospitalized patients with decompensated cirrhosis, procedure‐related bleeding is a rare but potentially lethal complication,( 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 ) as it has been associated with a mortality of up to 78%.( 4 )
Although historically considered to be at high risk of bleeding, patients with cirrhosis, particularly those who are decompensated, actually have a rebalanced hemostatic status that is not appropriately assessed by conventional coagulation parameters, specifically international normalized ratio (INR) and platelet count.( 9 , 10 , 11 , 12 , 13 )
In fact, patients with decompensated cirrhosis, particularly those with acute kidney injury (AKI), have a fragile hemostatic status that is both hypocoagulable and hypercoagulable.( 14 ) It has been shown that INR and platelet count do not predict bleeding risk in patients with cirrhosis,( 5 , 15 , 16 ) and therefore should not be used to guide preprocedural blood product use.( 17 , 18 )
As knowledge on coagulation alterations that occur in patients with cirrhosis has increased, there has been an increased interest in functional hemostatic assays such as thromboelastography (TEG).( 19 ) By measuring the viscoelastic changes of thrombus formation that occur during the hemostatic process, TEG allows for a more global evaluation of hemostasis and real‐time quantification of clot formation and stability.( 20 )
In patients with cirrhosis who undergo invasive procedures, the use of TEG has been recently associated with a significant reduction of blood product requirements( 21 , 22 , 23 , 24 , 25 ); however, the utility of TEG to estimate the risk of procedure‐related bleeding remains unclear.( 26 , 27 , 28 )
In patients with decompensated cirrhosis, it is unclear whether alterations in lab tests assessing hemostasis are truly associated with increased risk of bleeding or simply reflect the underlying severity of liver dysfunction.( 9 ) Similarly, it is also unknown whether the correction of such hemostatic alterations directly mitigates the risk of procedure‐related bleeding.( 9 ) In fact, in a multicenter retrospective cohort of 536 patients with cirrhosis who underwent 1,472 sessions of variceal ligation, hemostatic alterations at baseline (INR > 1.5 and platelet count < 50 × 109/L) and preprocedural administration of fresh frozen plasma and platelets were not predictive of postprocedural bleeding.( 29 )
Because there is a strong and unmet need for guidance on preprocedural estimation of bleeding risk in these patients, we prospectively investigated whether TEG can identify patients with decompensated cirrhosis at risk of procedure‐related bleeding.
Patients and Methods
Patient Selection
Adult patients with decompensated cirrhosis admitted to the inpatient services of Yale New Haven Hospital between January 1 and September 1, 2019, were prospectively evaluated to determine eligibility to participate in a study evaluating coagulation pathways in patients with decompensated cirrhosis with or without AKI.( 14 ) Decompensation was defined by the presence or history of clinically evident decompensating events (ascites, variceal hemorrhage, and hepatic encephalopathy).( 30 ) AKI was defined as an increase in serum creatinine of greater than or equal to 0.3 mg/dL within 48 hours or a 50% increase within 7 days from baseline serum creatinine.( 31 )
Patients admitted for variceal hemorrhage and/or who had variceal hemorrhage and/or any other major bleeding( 32 ) in the 30 days before hospitalization, patients with acute on chronic liver failure (ACLF),( 33 ) and patients who were admitted to the intensive care units were ineligible. Patients with ACLF were excluded because ACLF is a specific syndrome in which inflammation plays a predominant role and is associated with distinct coagulation features.( 34 , 35 ) Patients admitted to the intensive care unit were excluded, as they are more severe and unstable and more likely to have ACLF compared with patients admitted to the medical floor, and because they are more frequently treated with drugs that interfere with hemostasis and coagulation.
At screening, patient medical records, past medical history, and laboratory results were reviewed for the following exclusion criteria: chronic kidney disease; presence and/or history of thromboembolic complications; presence of nonhepatic tumors or any primary hematologic disease; recent surgery; human immunodeficiency virus infection, and history of organ transplantation, including liver. Patients on therapeutic anticoagulation and/or antithrombotic and/or antifibrinolytic therapy were also excluded. All procedures were performed following guidelines by or under the supervision of experienced physicians. All paracentesis/thoracentesis were performed under ultrasound guidance with needles not larger than 15 gauge.
Age and sex‐matched healthy subjects were recruited as controls for TEG parameters. This group constituted 30 healthy controls with no history of acute or chronic disease. None of the controls were taking antithrombotic, anticoagulant, antibiotic, or hormonal therapy.
Study Design
This was a prospective, single‐center, cohort study, approved by the Yale Human Investigation Committee (#2000024288).
The study was conducted in compliance with the Declaration of Helsinki, and all patients gave written, informed consent before enrollment.
As a part of a prospective study on hemostasis in patients with decompensated cirrhosis,( 14 ) patients had TEG performed on admission and were followed prospectively during hospitalization for the development of procedure‐related bleeding.
TEG was performed only for research purposes, and results were not shared with the clinical team caring for the patients.
Thromboelastography
Peripheral blood was collected via venipuncture in citrate‐containing vacutainer tubes using 21 g needles and tourniquet. Citrated kaolin TEG (Thromboelastograph TEG 5000; Haemonetics Corp., Haemoscope Division, Niles, IL) was performed at the Hematology Laboratory of Yale New Haven Hospital within 1 hour after blood collection, by one trained member of the research team (A.Z.), and according to the manufacturer’s instructions.( 36 ) TEG was allowed to run for at least 30 minutes after maximum amplitude on the TEG tracing was achieved.
TEG is a validated test that measures the coagulation state and clot kinetics of whole blood, which is clinically used to assess hemostasis.
TEG measures the properties of whole blood clot formation using torsion as a measure of clot strength (Fig. 1A). The graphical representation of whole‐blood hemostasis using TEG is demonstrated in Fig , 1B.
FIG. 1.
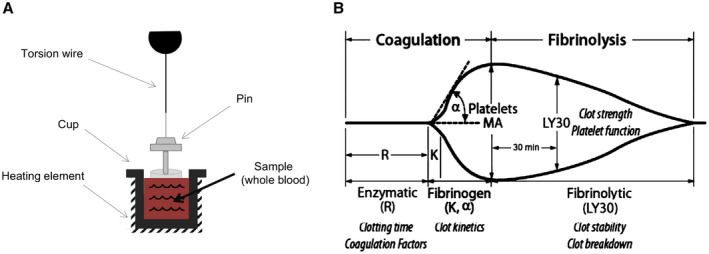
Thromboelastography. (A) TEG measures the properties of clot formation using a small cup that holds the blood sample and slowly oscillates. A pin held by a thin torsion wire is suspended in the blood; as clot forms, it binds the cup and pin together. The torsion on the pin is measured and converted to an electrical signal. Clot strength is directly proportional to torsion on the pin. (B) Graphical presentation of the TEG hemostasis profile for clot formation and lysis, with MA reflecting overall clot stability.
In this study, the following parameters of TEG were recorded:
“r‐time,” defined as the time from the start of the TEG tracing until the trace amplitude reaches 2 mm (normal range: 5‐10 minutes), represents the rate of initial clot formation (initiation phase), and is primarily related to the activity of procoagulant and anticoagulant clotting factors. Prolongation of the r‐time is the result of coagulation factor deficiencies or severe hypofibrinogenemia.
“k‐time,” defined as the time from r‐time until the trace amplitude reaches 20 mm (normal range: 1‐3 minutes), represents dynamic formation of the clot (amplification phase) and is related to the activity of clotting factors, fibrinogen, and platelets.
“α,” which is the angle of the line tangential to the developing trace (normal range: 53°‐72°), measures the speed at which fibrin cross‐linking occurs (thrombin‐burst/propagation phase), and is a function of platelets and plasma factors on the platelet surface.
“MA,” the maximum amplitude of the TEG tracing (normal range: 50‐70 mm), reflects the ultimate strength of the clot (overall stability of the clot) and directly reflects the interaction of platelet function and plasmatic clotting factors.
“LY30,” which is the percentage decrease in amplitude at 30 minutes after MA (normal range: 0‐8%), reflects initial dissolution of the clot (fibrinolysis).
Data Collection
Data collected from the medical record included causes for admission, patient demographics, laboratory data (including conventional coagulation parameters), presence of concomitant complications such as bacterial infection and AKI, use of blood products, and postprocedural bleeding complications. Model for End‐Stage Liver Disease (MELD) score (and not MELD‐Na) was calculated based on biochemical values from the day of enrollment.
Thrombocytopenia was defined by a platelet count ≤ 150 × 109/L and subclassified as mild (100 × 109/L to 150 ×.109/L), moderate (50 × 109/L to 100 × 109/L), or severe (<50 × 109/L).( 37 )
According to the International Society of Thrombosis and Hemostasis guidelines for nonsurgical patients, major bleeding was defined as follows: fatal bleeding and/or symptomatic bleeding in a critical area or organ and/or bleeding causing a fall in hemoglobin level of 2.0 g/L or more, or leading to transfusion of 2 or more units of whole blood or red cells.( 32 )
Postparacentesis hemoperitoneum was defined as the presence of hemoperitoneum based on abdominal computed tomography scan in addition to recent paracentesis, no other potential etiology for bleeding, and significant suspicion by the medical team that bleeding was secondary to paracentesis. The same criteria were used for postthoracentesis hemorrhage.
Statistical Analysis
The primary objective of this study was to determine whether TEG can identify patients with cirrhosis at risk of procedure‐related bleeding.
The number of patients included in this ancillary study was based on the sample‐size calculation for the main trial.( 14 )
Qualitative data are described using frequency and percentage. Quantitative data are described using median with 25% and 75% quartile ranges. Comparisons between independent groups were performed using the Mann‐Whitney U test and Student t test for continuous variables, and chi‐square test of Fisher’s exact test (when the cell value was small, ≤5) for categorical variables. Statistical significance was set at P ≤ 0.05. All analyses were completed using SPSS version 26.
Results
Of the 136 patients with decompensated cirrhosis screened for recruitment (Fig. 2), 80 were included (50 males, 30 females; median age of 57 years; 84% with ascites; median Pugh and MELD scores of 10 and 22, respectively) (Table 1). Median time from hospitalization to recruitment was 2 days (interquartile range [IQR] 1‐3).
FIG. 2.
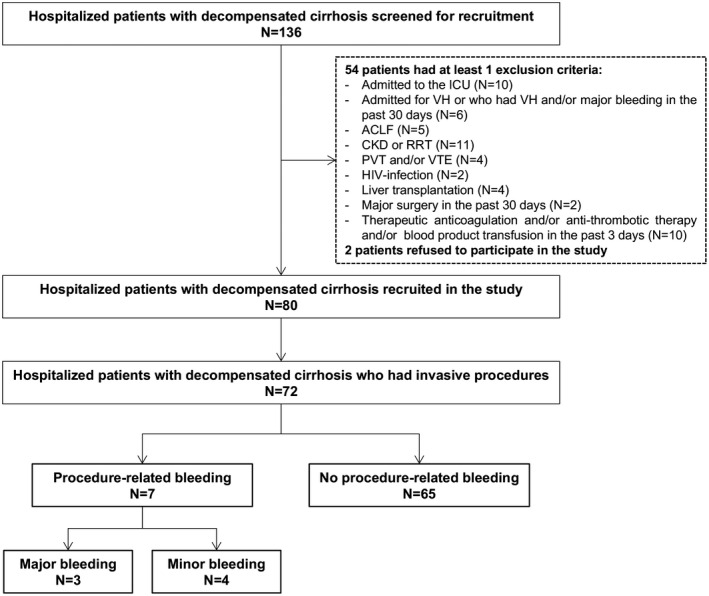
Flow chart of the study. None of the patients received platelet transfusion before baseline sample collection. Five patients received fresh frozen plasma (3 days before enrollment in 3 patients and 5 days before enrollment in 2 patients). Abbreviations: CKD, chronic kidney disease; ICU, intensive care unit; PVT, portal vein thrombosis; RRT, renal replacement therapy; VH, variceal hemorrhage; VTE, venous thromboembolism.
TABLE 1.
Baseline Characteristics of the Study Cohort
| Patients (n = 80) | |
|---|---|
| Age (years) | 57 (52‐65) |
| Male gender (%) | 62 |
| Etiology of cirrhosis (%) | |
| Alcohol | 55 |
| Alcohol + HCV | 10 |
| NASH | 14 |
| HCV | 8 |
| Other | 13 |
| Child‐Pugh score* | 10 (7‐13) |
| MELD score | 22 (17‐27) |
| Ascites (%) | 84 |
| Reason for admission (%) | |
| Abdominal pain/suspected infection | 22 |
| Ascites | 26 |
| AMS or HE | 25 |
| AKI | 6 |
| Trauma | 8 |
| Other | 13 |
| Bacterial infection † (%) | 35 |
| AKI ‡ (%) | 50 |
| VTE prophylaxis (%) | 46 |
| Hepatocellular carcinoma (%) | 11 |
| Total bilirubin, mg/dL | 2.8 (1.6‐5.4) |
| INR | 1.6 (1.3‐1.8) |
| Albumin, g/dL | 3 (2.6‐3.4) |
| Hemoglobin, g/dL | 8.4 (7.5‐10) |
| Platelet count, 109/L | 77 (48‐100) |
| Creatinine, mg/dL | 1.3 (0.8‐1.8) |
| Sodium, mmol/L | 135 (130‐140) |
| Potassium, mmol/L | 4 (3.7‐4.4) |
| AST, U/L | 45 (31‐63) |
| ALT, U/L | 26 (18‐41) |
Median values are reported with 25th and 75th percentile values in parenthesis.
Median (range).
Spontaneous bacterial peritonitis was the most common type of infection.
Etiology of AKI was as follows: prerenal (60%), hepatorenal syndrome (20%), and acute tubular necrosis (20%).
Abbreviations: AMS, altered mental status; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HCV, hepatitis C virus; HE, hepatic encephalopathy; NASH, nonalcoholic steatohepatitis.
Nearly all patients were thrombocytopenic with 21%, 44%, and 30% having mild, moderate, and severe thrombocytopenia, respectively. Median INR was 1.6 (IQR: 1.3‐1.8) (Table 1).
Compared with healthy controls, patients with cirrhosis had a longer r‐time (7.4 minutes [6.2‐8.8] vs. 5.6 minutes [4.6‐7.2]; P = 0.002), a longer k‐time (2.2 minutes [1.8‐4.3] vs. 1.8 minutes [1.2‐2.4]; P = 0.001), a smaller α‐angle (59° [40‐65] vs. 66° [58‐72]; P = 0.0001), and a lower maximum amplitude (50 mm [40‐56] vs. 64 [62‐69]; P = 0.0001) by TEG, all indicative of hypocoagulability. Conversely, LY30 was significantly lower in patients with cirrhosis versus healthy subjects (0% [0‐0.1] vs. 0.2% [0‐0.5]; P = 0.004).
Median length of hospitalization was 11 days (IQR = 7‐16). A total of 153 invasive procedures were performed in 72 patients (76 large volume paracentesis; 18 diagnostic paracentesis; 15 central venous catheter; 11 esophagogastroduodenoscopy with biopsy; 7 coronary angiography and right heart catheterization; 6 permacath catheter for dialysis; 6 transjugular portosystemic shunt; 6 colonoscopy; 4 therapeutic thoracentesis; 2 esophageal varices ligation; 1 diagnostic thoracentesis; and 1 esophageal stricture dilation).
Seven patients experienced procedure‐related bleeding: 4 after large‐volume paracentesis, 2 after therapeutic thoracentesis, and 1 after placement of permacath catheter for dialysis (Table 2). Median time from patient’s recruitment to occurrence of bleeding was 5 days (range: 2‐7).
TABLE 2.
Characteristics of Patients With Decompensated Cirrhosis Who Had Procedure‐Related Bleeding
| Gender | Years | Etiology | Pugh | MELD | Infection | AKI | Creatinine (mg/dL) | INR | Platelet Count (× 109/L) | Prophylaxis | Type of Procedure | Time From Admission to Bleeding (days) | Time From Procedure to Diagnosis of Bleeding | Type of Bleeding | Major* Bleeding |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | 42 | Alcohol | 7 | 21 | No | Yes | 3.1 | 1.3 | 69 | No | Permacath | 3 | 1 day after | Local hematoma | No |
| M | 53 | HCV | 7 | 21 | Yes | Yes | 1.8 | 2.2 | 40 | FFP | LVP | 7 | Same day | Local hematoma | No |
| M | 65 | NASH | 11 | 29 | Yes | Yes | 1.5 | 1.8 | 31 | PLT; FFP | Thoracentesis | 2 | 1 day after | Local hematoma | No |
| M | 78 | HCV | 8 | 10 | No | No | 0.6 | 1.2 | 61 | No | LVP | 6 | Same day | Local hematoma | No |
| F | 53 | PBC | 10 | 26 | Yes | Yes | 1.7 | 2.3 | 70 | FFP | LVP | 6 | Same day | PPH | Yes |
| M | 61 | Alcohol | 11 | 31 | No | Yes | 3.4 | 1.8 | 55 | FFP | LVP | 5 | 1 day after | PPH | Yes |
| M | 52 | Alcohol | 8 | 18 | No | No | 0.7 | 1.5 | 68 | No | Thoracentesis | 4 | Same day | Postthoracentesis hemorrhage | Yes |
Fatal bleeding and/or symptomatic bleeding in a critical area or organ and/or bleeding causing a fall in hemoglobin level of 20 g L−1 or more, or leading to transfusion of 2 or more units of whole blood or red cells.
Abbreviations: F, female; FFP, fresh frozen plasma; HCV, hepatitis C virus; LVP, large‐volume paracentesis; M, male; NASH, nonalcoholic steatohepatitis; PBC, primary biliary cholangitis; PLT, platelets; PPH, postparacentesis hemoperitoneum.
Bleeding was major in three cases (Table 2). Two patients experienced massive postparacentesis hemoperitoneum, and both died after transition to comfort measures. One additional patient had postthoracentesis bleeding requiring three red blood cell transfusions; this patient recovered and was discharged. None of the patients developed portal vein thrombosis or any other thrombotic complication during hospitalization.
Table 3 provides the comparison between patients who had a procedure‐related bleeding and those who did not.
TABLE 3.
Comparison Between Bleeders and Nonbleeders Among Patients Who Had a Procedure Performed During Admission (n = 72)
| Bleeders (n = 7) | Nonbleeders (n = 65) | P Value | |
|---|---|---|---|
| Clinical and laboratory data | |||
| Age, years | 54 (52‐65) | 57 (51‐65) | 0.9 |
| MELD | 26 (18‐31) | 22 (14‐27) | 0.2 |
| Pugh score * | 10 (7‐12) | 10 (7‐13) | 0.9 |
| Infection, % | 43 | 37 | 1 |
| AKI, % | 71 | 46 | 0.3 |
| VTE prophylaxis, % | 71 | 48 | 0.4 |
| Albumin, g/dL | 2.8 (2.5‐3.4) | 3 (2.6‐5.5) | 0.4 |
| Bilirubin, mg/dL | 3.7 (1.4‐9) | 2.6 (1.6‐5.5) | 0.8 |
| Creatinine, mg/dL | 1.7 (0.7‐3.1) | 1 (0.8‐1.8) | 0.3 |
| Platelet count, × 109/L | 61 (40‐69) | 80 (48‐120) | 0.05 |
| Platelet count < 50 × 109/L, % | 29 | 28 | 0.5 |
| INR | 1.8 (1.3‐2.2) | 1.6 (1.3‐1.8) | 0.4 |
| Preprocedural prophylaxis, % | 57 | 25 | 0.06 |
| TEG Parameters | |||
| r‐time, minutes | 8.1 (7.5‐25) | 7.4 (6.2‐8.8) | 0.1 |
| k‐time, minutes | 4.5 (2.7‐9.2) | 2.2 (1.8‐4.2) | 0.02 |
| α‐angle, degrees | 34 (30‐48) | 59 (43‐65) | 0.003 |
| MA, mm | 37 (25‐43) | 50 (41‐57) | 0.004 |
| LY30, % | 0 (0‐0.1) | 0 (0‐0.1) | 0.8 |
Median values are reported with 25th and 75th percentile values in parenthesis.
Median (range).
Severity of cirrhosis, presence of bacterial infection, and AKI were not predictive of bleeding (Table 3). Among conventional coagulation parameters, INR, platelet count, and severity of thrombocytopenia were comparable between the two groups (Table 3).
Conversely, k‐time, α‐angle, and maximum amplitude by TEG were significantly different between patients who had a procedure‐related bleeding and those who did not (all indicative of hypocoagulability in patients who had a procedure‐related bleeding) (Table 3). Interestingly, TEG maximum amplitude accurately discriminated between patients who had major, life‐threatening bleeding (all with MA < 30 mm) and those who had mild or no bleeding (all with MA > 30 mm), whereas a platelet count < 50 × 109/L could not discriminate between patients who had a procedure‐related bleeding (minor or major) and those who did not (Fig. 3).
FIG. 3.
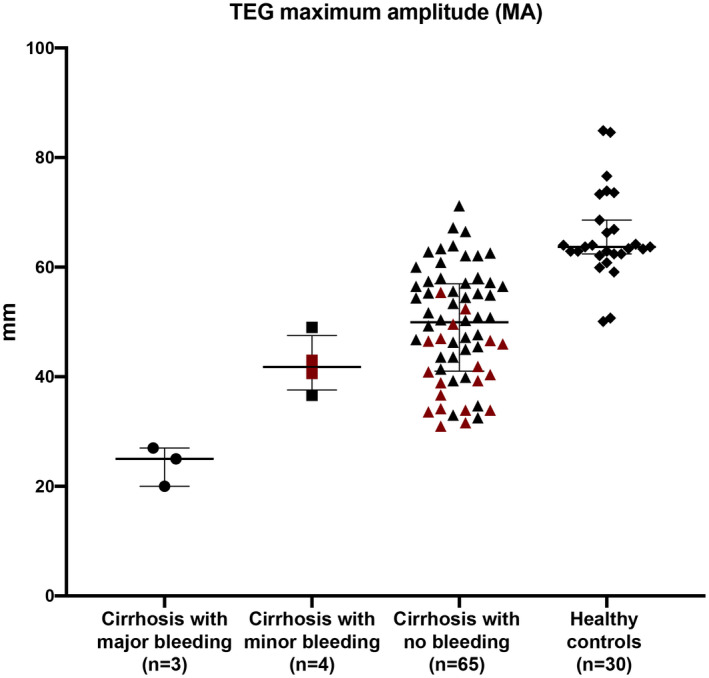
TEG maximum amplitude accurately discriminates between patients who had major, life‐threatening bleeding and those who had minor or no bleeding. Overall clot stability, as assessed by TEG maximum amplitude, is significantly diminished (greater bleeding tendency) in patients with cirrhosis who had major bleeding (all with MA < 30 mm) compared to patients with cirrhosis with minor bleeding, patients with cirrhosis with no bleeding, and healthy controls (all with MA > 30). On the other hand, a platelet count < 50 × 109/L (in red) could not discriminate between patients who had a procedure‐related bleeding (minor or major) and those who did not.
Discussion
Our study shows, in a prospective cohort of hospitalized patients with decompensated cirrhosis, that TEG parameters associated with hypocoagulability appear to predict procedure‐related bleeding. Interestingly, the correlation between TEG parameters and risk of postprocedural bleeding demonstrates that TEG maximum amplitude, a marker of overall clot stability, accurately discriminated between patients who had major, life‐threatening bleeding (all with amplitude < 30 mm) and those who had mild or no bleeding (all with amplitude > 30 mm). If these results are validated in a larger cohort, MA < 30 mm could become the threshold to identify patients with decompensated cirrhosis at higher risk for procedure‐related bleeding, in whom to consider blood product use (fibrinogen supplementation and platelet transfusion) before invasive procedures.
TEG is a point‐of‐care, global, hemostatic device that evaluates the dynamics of clot formation.( 20 ) It provides real‐time data on clot strength and stability as a result of the interplay between soluble clotting factors and inhibitors and platelets. In the setting of cirrhosis, where a multitude of known and unknown hemostatic alterations exist,( 12 ) this functional test appears to be more informative than the quantitative determination of one or more components of the hemostatic cascade.( 18 )
In our study, TEG was significantly more altered in patients who had a procedure‐related bleeding versus those who did not, indicative of a relatively more severe hypocoagulable state. The most profound differences between the groups were in α‐angle and MA (being lower in patients who had a procedure‐related bleeding). Both parameters reflect the functional interplay of procoagulant and anticoagulant factors and platelets. By contrast, LY30, a marker of fibrinolysis, was comparable between patients who had a procedure‐related bleeding and those who did not. These data, in line with previous findings,( 38 ) would suggest that defects in platelets and/or coagulation are most likely involved in the pathophysiology of procedure‐related bleeding in decompensated cirrhosis, whereas fibrinolysis is not substantively affected and may have no role in the occurrence of procedure‐related bleeding. That said, TEG may not be as sensitive to fibrinolysis as it is to the function of platelet and coagulation factors( 39 ); thus, this hypothesis needs to be validated by more specific fibrinolysis testing.
On the other hand, despite TEG values being significantly altered in patients with cirrhosis compared to healthy subjects (indicating impaired clot formation and stability), most patients with cirrhosis did not bleed after a procedure. As noted previously by De Pietri et al.,( 21 ) our findings confirm that the reference values of TEG for healthy individuals are probably too conservative for patients with cirrhosis, and therefore may not be useful to identify those at risk of procedure‐related bleeding.
Interestingly, the lower LY30, a marker of fibrinolysis, in patients with decompensated cirrhosis compared with healthy controls would suggest that there is a relatively hypofibrinolytic state in these patients. This is in line with previous findings that showed no evidence of hyperfibrinolysis in large cohorts of patients with compensated cirrhosis.( 26 , 40 ) However, because TEG is relatively insensitive to fibrinolysis, these results need confirmation through the use of more specific fibrinolysis assays.
Many clinicians still consider patients with cirrhosis to be at high risk of bleeding simply because they have prolongation of the INR or low platelet count. In fact, an indiscriminate use of blood products directed to correct standard coagulation tests is frequently seen in these patients.( 41 ) This behavior is not only associated with increased costs and unnecessary transfusions, but can also potentially harm patients.( 42 ) In our study, in line with recent data from a European survey on blood product use in patients with cirrhosis,( 41 ) approximately 1 of 3 patients undergoing procedures received preprocedural prophylaxis.
However, it is now clear that patients with cirrhosis, particularly those who are decompensated, have a rebalanced hemostatic system that is not appropriately assessed by conventional coagulation parameters.( 9 , 10 , 13 ) Consistent with previous data, in our cohort, INR and platelet count were comparable between patients who had a procedure‐related bleeding and those who did not, none of the 3 patients with major bleeding had a severe coagulation derangement as defined by standard parameters (i.e., INR ≥ 2.5 or platelet count ≤ 50 × 109/L), and a platelet count < 50 × 109/L (severe thrombocytopenia) could not discriminate between patients who had a procedure‐related bleeding (minor or major) and those who did not. This further confirms, in patients with cirrhosis, that alterations in INR and/or platelet count alone do not predict bleeding risk and should not dictate whether a procedure can be safely performed or whether blood product prophylaxis is indicated.
Previous estimates of postparacentesis bleeding risk in patients with cirrhosis have ranged from 0% to 3%.( 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 ) Bleeding risk appears to be higher in more advanced patients (Child C vs. Child A; hospitalized vs. outpatient)( 1 , 4 , 8 ) and in those with renal dysfunction at time of paracentesis.( 4 , 43 , 44 ). In patients who experience intraperitoneal bleeding, mortality ranges between 0%( 7 ) and 83%,( 4 ) likely due to different severity of bleeding complications and/or underlying liver disease.
In our cohort of hospitalized patients with decompensated cirrhosis and multiple risk factors for bleeding, including bacterial infections( 45 ) and AKI,( 4 , 43 , 44 ) 2 of 72 patients experienced a fatal bleed following a procedure. Both patients had a procedure defined as being “low‐risk” (large volume paracentesis under ultrasound guidance), and both had received preprocedural prophylaxis with fresh frozen plasma. This again shows that administration of plasma before procedures in patients with cirrhosis does not prevent bleeding, and is further consistent with studies demonstrating that plasma improves neither thrombin‐generating capacity( 46 ) nor stability of the clot.( 47 ) This also suggests that the need and type of preprocedural prophylaxis depends not only on procedure risk (high vs. low risk procedures), but also on individual patient risk factors such as decompensation, infection, and AKI,( 4 , 43 , 44 , 45 , 48 ) which would warrant further testing such as TEG.
The main limitation of our study is the small number of patients who developed significant postprocedural bleeding. The strength of our protocol is that patients were carefully recruited and prospectively followed for outcomes. Thus, if validated in larger cohorts, our finding of TEG maximum amplitude < 30 mm as a discriminator between major and minor/no bleeding in patients with decompensated cirrhosis could be useful to identify those in whom preprocedure transfusion of platelets or fibrinogen would be warranted. However, before this can be recommended, large prospective studies with bleeding as the clinical endpoint are required to establish whether correction of hemostatic abnormalities actually reduces bleeding risk in patients with decompensated cirrhosis.( 9 ) Another study caveat is that, although TEG provides robust data on clot formation and stability, it is relatively insensitive to hyperfibrinolysis and to increased levels of von Willebrand factor, and does not assess the protein C pathway. Still, we specifically used TEG because of its very easy, real‐time use as well as its standardized interpretation, as noted in studies in which TEG guides blood product use.( 21 , 22 )
In conclusion, in a prospective study of hospitalized patients with decompensated cirrhosis, we show that TEG parameters associated with hypocoagulability appear to predict procedure‐related bleeding, particularly a TEG maximum amplitude < 30 mm.
If these results are validated in a larger cohort, this could become a threshold to identify patients with decompensated cirrhosis at higher risk for procedure‐related bleeding in whom to consider blood product transfusions (fibrinogen and platelets) before invasive procedures. Large prospective studies are warranted to evaluate whether the improvement (or normalization) of TEG maximum amplitude correlates with reducing the risk of bleeding in patients with decompensated cirrhosis undergoing invasive procedures.
Supported by the American Association for the Study of the Liver Foundation Clinical & Translation Research Fellowship Award, and Yale Liver Center National Institutes of Health (P30 DK34989).
Potential conflict of interest: Nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1. De Gottardi A, Thévenot T, Spahr L, Morard I, Bresson–Hadni S, Torres F, et al. Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clin Gastroenterol Hepatol 2009;7:906‐909. [DOI] [PubMed] [Google Scholar]
- 2. Lin SU, Wang M, Zhu Y, Dong J, Weng Z, Shao L, et al. Hemorrhagic complications following abdominal paracentesis in acute on chronic liver failure: a propensity score analysis. Medicine (Baltimore) 2015;94:e2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mallory A, Schaefer JW. Complications of diagnostic paracentesis in patients with liver disease. JAMA 1978;239:628‐630. [PubMed] [Google Scholar]
- 4. Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther 2005;21:525‐529. [DOI] [PubMed] [Google Scholar]
- 5. Rowley MW, Agarwal S, Seetharam AB, Hirsch KS. Real‐time ultrasound‐guided paracentesis by radiologists: near zero risk of hemorrhage without correction of coagulopathy. J Vasc Interv Radiol 2019;30:259‐264. [DOI] [PubMed] [Google Scholar]
- 6. Runyon BA. Paracentesis of ascitic fluid. A safe procedure. Arch Intern Med 1986;146:2259‐2261. [PubMed] [Google Scholar]
- 7. Webster ST, Brown KL, Lucey MR, et al. Hemorrhagic complications of large volume abdominal paracentesis. Am J Gastroenterol 1996;91:366‐368. [PubMed] [Google Scholar]
- 8. Grabau CM, Crago SF, Hoff LK, Simon JA, Melton CA, Ott BJ, et al. Performance standards for therapeutic abdominal paracentesis. Hepatology 2004;40:484‐488. [DOI] [PubMed] [Google Scholar]
- 9. Intagliata NM, Argo CK, Stine JG, Lisman T, Caldwell S h, Violi F. Concepts and controversies in haemostasis and thrombosis associated with liver disease: Proceedings of the 7th International Coagulation in Liver Disease Conference. Thromb Haemost 2018;118:1491‐1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lisman T, Caldwell SH, Burroughs AK, Northup PG, Senzolo M, Stravitz RT, et al. Hemostasis and thrombosis in patients with liver disease: the ups and downs. J Hepatol 2010;53:362‐371. [DOI] [PubMed] [Google Scholar]
- 11. Russo FP, Zanetto A, Campello E, Bulato C, Shalaby S, Spiezia L, et al. Reversal of hypercoagulability in patients with HCV‐related cirrhosis after treatment with direct‐acting antivirals. Liver Int 2018;38:2210‐2218. [DOI] [PubMed] [Google Scholar]
- 12. Zermatten MG, Fraga M, Moradpour D, Bertaggia Calderara D, Aliotta A, Stirnimann G, et al. Hemostatic alterations in patients with cirrhosis: from primary hemostasis to fibrinolysis. Hepatology 2020;71:2135‐2148. [DOI] [PubMed] [Google Scholar]
- 13. Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med 2011;365:147‐156. [DOI] [PubMed] [Google Scholar]
- 14. Zanetto A, Rinder HM, Campello E, et al. Acute kidney injury in decompensated cirrhosis is associated with both hypo‐ and hyper‐coagulable features. Hepatology 2020. Jul 2. 10.1002/hep.31443. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Basili S, Raparelli V, Napoleone L, Talerico G, Corazza GR, Perticone F, et al. Platelet count does not predict bleeding in cirrhotic patients: results from the PRO‐LIVER study. Am J Gastroenterol 2018;113:368‐375. [DOI] [PubMed] [Google Scholar]
- 16. Tripodi A, Caldwell SH, Hoffman M, Trotter JF, Sanyal AJ, et al. Review article: the prothrombin time test as a measure of bleeding risk and prognosis in liver disease. Aliment Pharmacol Ther 2007;26:141‐148. [DOI] [PubMed] [Google Scholar]
- 17. Under the auspices of the Italian Association for the Study of Liver D, the Italian Society of Internal M . Hemostatic balance in patients with liver cirrhosis: report of a consensus conference. Dig Liver 2016;48:455‐467. [DOI] [PubMed] [Google Scholar]
- 18. Simonetto DA, Singal AK, Garcia‐Tsao G, Caldwell SH, Ahn J, Kamath PS, et al. ACG Clinical Guideline: disorders of the hepatic and mesenteric circulation. Am J Gastroenterol 2020;115:18‐40. [DOI] [PubMed] [Google Scholar]
- 19. Zanetto A, Senzolo M, Ferrarese A, Simioni P, Burra P, Rodríguez‐Castro KI, et al. Assessment of bleeding risk in patients with cirrhosis. Curr Hepatol Rep 2015;14:9‐18. [Google Scholar]
- 20. Salooja N, Perry DJ. Thrombelastography. Blood Coagul Fibrinolysis 2001;12:327‐337. [DOI] [PubMed] [Google Scholar]
- 21. De Pietri L, Bianchini M, Montalti R, De Maria N, Di Maira T, Begliomini B, et al. Thrombelastography‐guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology 2016;63:566‐573. [DOI] [PubMed] [Google Scholar]
- 22. Kumar M, Ahmad J, Maiwall R, Choudhury A, Bajpai M, Mitra LG, et al. Thromboelastography‐guided blood component use in patients with cirrhosis with nonvariceal bleeding: a randomized controlled trial. Hepatology 2020;71:235‐246. [DOI] [PubMed] [Google Scholar]
- 23. Rout G, Shalimar X, Gunjan D, Mahapatra SJ, Kedia S, Garg PK, et al. Thromboelastography‐guided blood product transfusion in cirrhosis patients with variceal bleeding: a randomized controlled trial. J Clin Gastroenterol 2020;54:255‐262. [DOI] [PubMed] [Google Scholar]
- 24. Vuyyuru SK, Singh AD, Gamanagatti SR, Rout G, Gunjan D, Shalimar X, et al. A randomized control trial of thromboelastography‐guided transfusion in cirrhosis for high‐risk invasive liver‐related procedures. Dig Dis Sci 2020;65:2104‐2111. [DOI] [PubMed] [Google Scholar]
- 25. Zanetto A, Senzolo M, Blasi A. Perioperative management of antithrombotic treatment. Best Pract Res Clin Anaesthesiol 2020;34:35‐50. [DOI] [PubMed] [Google Scholar]
- 26. Hugenholtz GCG, Lisman T, Stravitz RT. Thromboelastography does not predict outcome in different etiologies of cirrhosis. Res Pract Thromb Haemost 2017;1:275‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shamseddeen H, Patidar KR, Ghabril M, Desai AP, Nephew L, Kuehl S, et al. Features of blood clotting on thromboelastography in hospitalized patients with cirrhosis. Am J Med 2020. May 29. 10.1016/j.amjmed.2020.04.029. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Somani V, Amarapurkar D, Shah A. Thromboelastography for assessing the risk of bleeding in patients with cirrhosis‐moving closer. J Clin Exp Hepatol 2017;7:284‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blasi A, Machlab S, Risco R, Costa‐Freixas JP, Cely GH, Horta D, et al. A multicenter analysis of the role of prophylactic transfusion of blood products in patients with cirrhosis and esophageal varices undergoing endoscopic band ligation. J Hepatol 2020;73:S58‐S98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garcia‐Tsao G, Abraldes JG, Berzigotti A, Bosch J, et al. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017;65:310‐335. [DOI] [PubMed] [Google Scholar]
- 31. Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut 2015;64:531‐537. [DOI] [PubMed] [Google Scholar]
- 32. Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scieentific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost 2005;3:692‐694. [DOI] [PubMed] [Google Scholar]
- 33. Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute‐on‐chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426‐1437. [DOI] [PubMed] [Google Scholar]
- 34. Premkumar M, Saxena P, Rangegowda D, Baweja S, Mirza R, Jain P, et al. Coagulation failure is associated with bleeding events and clinical outcome during systemic inflammatory response and sepsis in acute‐on‐chronic liver failure: an observational cohort study. Liver Int 2019;39:694‐704. [DOI] [PubMed] [Google Scholar]
- 35. Blasi A, Patel VC, Adelmeijer J, Azarian S, Hernandez Tejero M, Calvo A, et al. Mixed fibrinolytic phenotypes in decompensated cirrhosis and acute‐on‐chronic liver failure with hypofibrinolysis in those with complications and poor survival. Hepatology 2020;71:1381‐1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zambruni A, Thalheimer U, Leandro G, Perry D, Burroughs AK, et al. Thromboelastography with citrated blood: comparability with native blood, stability of citrate storage and effect of repeated sampling. Blood Coagul Fibrinolysis 2004;15:103‐107. [DOI] [PubMed] [Google Scholar]
- 37. Peck‐Radosavljevic M. Thrombocytopenia in chronic liver disease. Liver Int 2017;37:778‐793. [DOI] [PubMed] [Google Scholar]
- 38. Lloyd‐Donald P, Vasudevan A, Angus P, Gow P, Mårtensson J, Glassford N, et al. Coagulation in acutely ill patients with severe chronic liver disease: insights from thromboelastography. J Crit Care 2017;38:215‐224. [DOI] [PubMed] [Google Scholar]
- 39. Longstaff C. Measuring fibrinolysis: from research to routine diagnostic assays. J Thromb Haemost 2018;16:652‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bos S, van den Boom B, Kamphuisen P, Adelmeijer J, Blokzijl H, Schreuder T, et al. Haemostatic profiles are similar across all aetiologies of cirrhosis. Thromb Haemost 2019;119:246‐253. [DOI] [PubMed] [Google Scholar]
- 41. Desborough MJR, Hockley B, Sekhar M, Burroughs AK, Stanworth SJ, Jairath V, et al. Patterns of blood component use in cirrhosis: a nationwide study. Liver Int 2016;36:522‐529. [DOI] [PubMed] [Google Scholar]
- 42. Zimmon DS, Kessler RE. The portal pressure‐blood volume relationship in cirrhosis. Gut 1974;15:99‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hung A, Garcia‐Tsao G. Acute kidney injury, but not sepsis, is associated with higher procedure‐related bleeding in patients with decompensated cirrhosis. Liver Int 2018;38:1437‐1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion 1991;31:164‐171. [DOI] [PubMed] [Google Scholar]
- 45. Montalto P, Vlachogiannakos J, Cox DJ, Pastacaldi S, Patch D, Burroughs AK, et al. Bacterial infection in cirrhosis impairs coagulation by a heparin effect: a prospective study. J Hepatol 2002;37:463‐470. [DOI] [PubMed] [Google Scholar]
- 46. Rassi AB, d'Amico EA, Tripodi A, da Rocha TRF, Migita BY, Ferreira CM, et al. Fresh frozen plasma transfusion in patients with cirrhosis and coagulopathy: effect on conventional coagulation tests and thrombomodulin‐modified thrombin generation. J Hepatol 2020;72:85‐94. [DOI] [PubMed] [Google Scholar]
- 47. Müller MCA, Straat M, Meijers JCM, Klinkspoor JH, de Jonge E, Arbous MS, et al. Fresh frozen plasma transfusion fails to influence the hemostatic balance in critically ill patients with a coagulopathy. J Thromb Haemost 2015;13:989‐997. [DOI] [PubMed] [Google Scholar]
- 48. Giannini EG, Bodini G, Furnari M, Marabotto E, et al. Bleeding after paracentesis in patients with decompensated cirrhosis and acute kidney injury: the perfect storm. Liver Int 2018;38:2101. [DOI] [PubMed] [Google Scholar]


