Abstract
Nonalcoholic fatty liver disease (NAFLD) is a major public health problem worldwide and the most common chronic liver disease. NAFLD currently affects approximately one in every four people in the United States, and its global burden is expected to rise in the next decades. Despite being a prevalent disease in the general population, only a minority of patients with NAFLD will develop nonalcoholic steatohepatitis (NASH) with advanced liver fibrosis (stage 3‐4 fibrosis) and liver‐related complications. Certain populations, such as patients with type 2 diabetes mellitus (T2DM), are recognized to be at the highest risk for developing NASH and advanced fibrosis. Both the American Diabetes Association and the European Association for the Study of Diabetes recommend screening of all T2DM for NAFLD. Incorporating a simple noninvasive algorithm into the existing diabetic care checklists in the primary care practice or diabetologist’s office would efficiently identify patients at high risk who should be referred to specialists. The proposed algorithm involves a first‐step annual fibrosis‐4 score (FIB‐4) followed by vibration‐controlled transient elastography (VCTE) for those with indeterminate or high‐risk score (FIB‐4 ≥1.3). Patients at low‐risk (FIB‐4 <1.3 or VCTE <8 kPa) can be followed up by primary care providers for lifestyle changes and yearly calculation of FIB‐4, while patients at high risk (FIB‐4 ≥1.3 and VCTE ≥8 kPa) should be referred to a liver‐specialized center. Conclusion: Patients with T2DM or prediabetes should be screened for NASH and advanced fibrosis. The proposed simple algorithm can be easily incorporated into the existing workflow in the primary care or diabetology clinic to identify patients at high risk for NASH and advanced fibrosis who should be referred to liver specialists.

Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- ADA
American Diabetes Association
- CVD
cardiovascular disease
- EASL
European Association for the Study of the Liver
- FIB‐4
fibrosis‐4 index
- HCC
hepatocellular carcinoma
- NAFL
nonalcoholic fatty liver
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- PCP
primary care provider
- T2DM
type 2 diabetes mellitus
- VCTE
vibration controlled transient elastography
Over the last decades, the prevalence of nonalcoholic fatty liver disease (NAFLD) has been rising exponentially( 1 , 2 , 3 ) and is currently estimated to affect approximately 80 million, or one in every four, people in the United States,( 4 ) with a projection of more than 100 million individuals affected by 2030.( 5 ) NAFLD can result in cirrhosis, liver failure, and hepatocellular carcinoma (HCC) and has become one of the leading indications for liver transplantation in the United States.( 6 , 7 )
NAFLD is defined as the accumulation of hepatic steatosis in ≥5% of hepatocytes in the absence of excessive alcohol consumption (<20 g/day for women and <30 g/day for men). It spans a wide spectrum of liver disease, ranging between two different histologic entities: nonalcoholic fatty liver (NAFL), a relatively benign disease, and nonalcoholic steatohepatitis (NASH), a more serious process. NAFL is defined by hepatic steatosis without evidence of hepatocellular injury, whereas NASH is defined by steatosis accompanied by lobular inflammation, hepatocyte ballooning (cell death), with or without fibrosis.( 8 , 9 ) NAFL has a low risk of liver‐related complications, whereas NASH has a potentially progressive course that can lead to liver fibrosis, cirrhosis, end‐stage liver disease, HCC, and/or liver transplantation as well as extrahepatic complications, notably cardiovascular disease (CVD), extrahepatic malignancy, and chronic kidney disease.( 10 )
Out of 80 million Americans currently diagnosed with NAFLD, approximately 25 million (up to 30%) have NASH and 5 million among them (up to 20%) have developed or will develop advanced fibrosis (stage 3‐4 fibrosis) from NASH.( 5 , 11 ) Once patients develop advanced liver fibrosis, the risk of liver‐related morbidity and mortality is largely increased. Therefore, the challenge for primary care providers (PCPs) is to identify early ‐ in their daily practice ‐ patients at high risk of NASH with advanced fibrosis who will need to be referred to liver specialists for monitoring and treatment of liver complications, potential upcoming treatments, and in case of end‐stage liver disease, assessment of indications for liver transplantation.
However, NAFLD is frequently underdiagnosed, and patients are often presented to specialty clinics at advanced stages when therapeutic options are limited. In a recent study, cirrhosis was diagnosed incidentally in 2 out of 3 NAFLD patients with cirrhosis.( 12 ) Furthermore, in a survey conducted among PCPs, 85% underestimated the prevalence of NAFLD,( 13 ) while at the same time, 78% of PCPs did not consider themselves well prepared to manage patients with NAFLD/NASH.( 14 )
The paradox of NAFLD as a highly prevalent disease with only a small proportion progressing to severe disease has led to NAFLD currently being one of the most challenging public health problems worldwide. Population‐wide policies to effectively identify, refer, and manage those patients are needed.( 15 ) A successful strategy would include simple cost‐effective tools and algorithms for PCPs to screen, diagnose, and refer patients at high risk of developing liver complications to liver specialists for further work‐up and management while those at low risk of developing liver complications could be managed by PCPs. Effective risk stratification of patients would both increase the referral of patients at high risk and decrease the referral of those at low risk to liver specialists, thereby improving health care access and resource allocation to those who need it the most.
Common Risk Factors for NAFLD
NAFLD is closely associated with insulin resistance and is often considered the hepatic manifestation of metabolic syndrome.( 16 ) In a 2016 meta‐analysis on patients with NAFLD, the rates of obesity, type 2 diabetes mellitus (T2DM), dyslipidemia, and hypertension were 51%, 23%, 69%, and 39%, respectively.( 4 )
T2DM is one of the strongest risk factors for the development of NASH, advanced fibrosis/cirrhosis,( 17 , 18 ) HCC,( 19 ) and mortality.( 18 , 20 ) Moreover, the underlying association between NAFLD and T2DM is two way, suggesting that NAFLD may precede and/or enhance the development of T2DM and promote diabetes‐associated adverse outcomes.( 21 ) T2DM affects 10.5% of Americans (≈34 million), and approximately 1.5 million new cases are diagnosed every year. It is estimated that 40%‐70% of patients with T2DM have underlying NAFLD,( 22 , 23 , 24 ) and among those, 37% have NASH and 17% will develop advanced fibrosis.( 24 ) Extrapolated in the United States population, this means that up to 24 million patients with T2DM have NAFLD, while 9 million have NASH and 4 million are at risk for advanced fibrosis (Fig. 1).
FIG. 1.
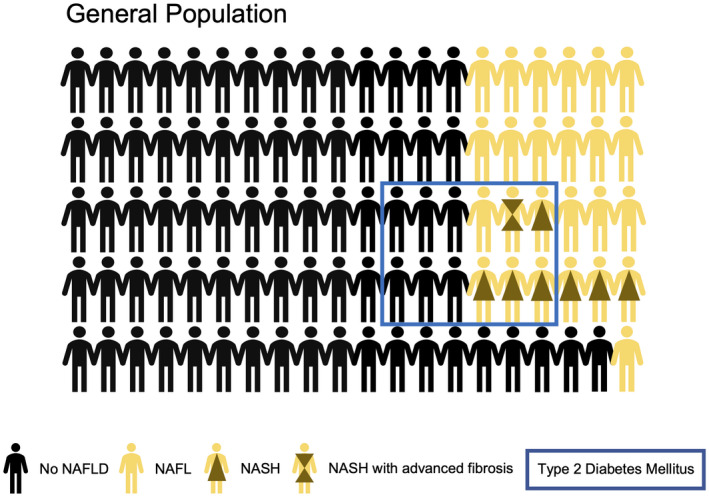
Schematic representation of the proportions of patients with NAFL, NASH, and advanced fibrosis in the general population and among patients with T2DM. In the general population, approximately 25% of patients have NAFLD; among those, up to 30% have NASH, of whom up to 20% have developed or will develop advanced liver fibrosis (stage 3‐4 fibrosis). T2DM represents approximately 10% of the U.S. population. It is estimated that 40%‐70% of patients with T2DM have underlying NAFLD, and among those ≈37% have NASH and ≈17% will develop advanced fibrosis.
Similarly to T2DM, obesity is prevalent in the general population. In the United States, according to the National Health and Nutrition Examination Survey, 42.9% of adults are currently obese and 9.2% are extremely obese (defined by a body mass index ≥40 kg/m2). Obesity is one of the most important risk factors for NAFLD and has been linked with the presence and severity of liver fibrosis. A prospective cohort( 25 ) of 40,700 patients with NAFLD showed that obesity and weight gain were independent predictors of the presence of liver fibrosis.
Genetic and epigenetic determinants have also been found to play a role in the natural history of patients with NAFLD,( 26 ) particularly because family members of patients with NASH are reported to have a more severe disease.( 27 , 28 ) Genome‐wide association studies have revealed links between specific single nucleotide polymorphisms and the course of the disease, including the patatin‐like phospholipase domain‐containing 3 (PNPLA3), transmembrane 6 superfamily member 2 (TM6SF2),( 29 ) and more recently 17‐beta hydroxysteroid dehydrogenase 13 (HSD17B13),( 30 ) membrane‐bound O‐acyltransferase domain‐containing 7 (MBOAT7), and transmembrane channel‐like 4 (TMC4)( 31 ) variants.
Clinical Disease Progression and Liver‐Related Complications
The natural history and different rates of disease progression and clinical manifestations can be attributed to multiples factors, such as metabolic comorbidities, microbiome, environmental, and genetic/epigenetic factors.( 32 )
NASH, and most importantly the presence of advanced liver fibrosis, are critical determinants of long‐term prognosis and are associated with a higher rate of overall mortality,( 33 ) liver‐related mortality,( 34 , 35 ) and CVD.( 36 , 37 ) Thus, identifying patients at high risk earlier in the disease course is vital to prevent and monitor for the risk of liver‐related complications, such as liver failure and HCC, the most common primary liver cancer.
In parallel with the observed rise in prevalence of NAFLD, the burden of NAFLD‐related HCC is also increasing.( 38 ) Cirrhosis from all etiologies is a risk factor for HCC. NASH is one of the leading etiologies of HCC, with projection models showing the NASH contribution to HCC overtaking chronic hepatitis C by 2025.( 39 ) In general, the 5‐year survival rate for HCC from all etiologies is low (approximately 15%) due to diagnosis at a late stage.( 40 ) Patients with cirrhosis should be screened every 6 months with liver imaging so that they can be diagnosed early at a curable stage. In particular, patients with NASH cirrhosis are less likely to be screened for HCC than patients with cirrhosis from other etiologies, leading to diagnosis at more advanced stages when curative therapies are no longer possible and hence an even poorer prognosis,( 41 , 42 , 43 ) with an overall survival of approximately 11 months (vs. 15.5 months for patients with non‐NASH HCC).( 44 )
Liver transplantation is the only curative treatment for HCC and liver failure. Due to the rising incidence of liver failure and HCC from NAFLD, NALFD has become one of the leading indications for liver transplantation in the United States. Data from the Scientific Registry of Transplant Recipients between 2002 and 2016 show that the proportion of patients with NASH and HCC increased almost 8‐fold (2.1% to 16.2%, P < 0.001) while the proportion of patients with NASH on the liver transplant waiting list increased more than 10‐fold.( 45 ) Unfortunately, despite the increased need, available organs remain a limited resource.
Extrahepatic Complications
As NAFLD is considered a hepatic manifestation of the metabolic syndrome, patients with NAFLD are at increased risk for complications associated with the metabolic syndrome, such as CVD, cancer, and chronic kidney disease.( 46 ) CVD is the most common cause of mortality in patients with NAFLD. The same risk factors for more severe NASH are also risk factors for CVD, including male sex, age, insulin resistance and T2DM, abdominal obesity, hypertension, dyslipidemia, and increased carotid‐artery intimal medial thickness.( 47 ) A 2016 meta‐analysis( 48 ) of 16 cohort studies over a median follow‐up of 7 years showed that NAFLD is associated with a higher risk of fatal and nonfatal cardiovascular events, including myocardial infarction stroke, unstable angina, and coronary revascularization.
A strong association between NAFLD and chronic kidney disease has been largely described in the literature.( 49 ) NASH is associated with a 2‐fold increase risk of chronic kidney disease, and patients with advanced liver fibrosis are at a 5‐fold higher risk of chronic kidney disease compared to patients without fibrosis, independently of the presence of diabetes.( 50 )
Finally, NAFLD as well as metabolic syndrome are also linked to other extrahepatic diseases, such as colorectal cancers, osteoporosis, psoriasis, and various endocrinopathies (e.g., polycystic ovary syndrome, thyroid dysfunction).( 9 )
Risk Stratification by PCP
NAFLD is an increasing global entity, and PCPs have a crucial role in the screening, stratification, management, and referral of these patients. However, a large number of high‐risk cases remain undiagnosed, and low risk patients are unnecessarily referred to specialists.( 51 , 52 )
Despite NAFLD being a prevalent disease in the general population (and thus in the primary care clinic), only a minority of patients with NAFLD have NASH and advanced liver fibrosis and are at high risk of developing liver‐related complications. It is this significant minority who need to be evaluated and managed by specialists to prevent and monitor for liver‐related complications while the remaining patients need primary care management of their cardiovascular risk as well as their metabolic syndrome. Risk stratification is therefore crucial for the appropriate referral of the high‐risk minority to specialists.
For such a prevalent disease with a minority of high risk patients, universal screening would not be efficient or cost effective.( 53 ) Further, triggers for evaluation, such as increased liver tests or abdominal ultrasound findings, are insensitive for detecting advanced fibrosis.( 54 ) Therefore, a targeted approach to screen the population with the highest risk for advanced fibrosis, such as those with T2DM and obesity, would be most efficient and effective at identifying that significant minority of patients with advanced fibrosis from NASH. In this manuscript, we focus on patients with T2DM.
PCPs and diabetologists represent the most important link in the chain of management of these patients because they are the first medical point of contact for this population. A simple, noninvasive, stepwise algorithm that is incorporated in the existing workflow and care systems of providers would ensure a higher screening rate.
Who to Screen? T2DM and Prediabetes Patients
Selective screening on high‐risk populations, such as patients with T2DM, will increase the yield as these patients have a high pretest probability and therefore a higher positive predictive value. Because patients with T2DM and prediabetes are at the highest risk for advanced fibrosis, we advocate for widespread screening of these patients in the primary care setting.
While there is variability in society guidelines on how and who to screen, there is universal recognition that patients with T2DM are at high risk for NAFLD, NASH, and advanced fibrosis. Although the European Association for the Study of the Liver (EASL) 2016 guidelines and the 2020 American Diabetes Association (ADA) guidelines both propose screening of all patients with T2DM, the American Association for the Study of Liver Diseases (AASLD) 2018 guidelines are more nuanced. The 2016 EASL guidelines( 9 ) proposed screening by means of liver enzymes and/or ultrasound assessment. All patients with steatosis, independently of liver enzymes, or individuals with persistently elevated liver enzymes should be further evaluated. The most recent 2020 guidelines published by the ADA( 55 ) also recommend that all patients with T2DM and prediabetes be evaluated for NAFLD. They recommend evaluation for NAFLD by measuring baseline and yearly liver enzymes and referral to a specialized center for persistently elevated or worsening transaminases. The AASLD guidelines( 8 ) state that “there should be a high index of suspicion for NAFLD and NASH in patients with T2DM,” but did not recommend systematic screening for NAFLD. They recommend the use of noninvasive measures of fibrosis, such as the NAFLD fibrosis score, fibrosis‐4 index (FIB‐4), or vibration controlled transient elastography (VCTE) to identify those at low or high risk for advanced fibrosis.
However, there is no clear consensus about how to implement screening and which patients should be referred to specialized centers. Moreover, these guidelines add one more task to PCPs and diabetologists to have to evaluate and consider. A recent article published by the U.S. Members of the Global NASH Council recommends risk stratifying patients according to metabolic risk factors, including T2DM, using FIB‐4 as the first initial assessment. Patients with a FIB‐4 score ≥1.3 should undergo further evaluation by a liver specialist.( 56 )
What to Screen for? NASH With Advanced Fibrosis
NASH, and most importantly liver fibrosis, are critical determinants of long‐term prognosis of patients with NAFLD. Liver biopsy is the gold standard for the assessment of NASH and fibrosis; however, its invasive nature, high cost, sampling variability, and interobserver and intraobserver variability make it less suitable for screening and disease monitoring in clinical practice and unattractive to clinicians and patients.( 57 ) Many biomarkers have been investigated for the diagnosis of NASH and fibrosis. While we do not yet have a sufficiently accurate test to diagnose NASH available to be used in clinical practice, we do have a variety of biomarkers to estimate the stage of liver fibrosis.( 58 , 59 ) These biomarkers include clinical scoring systems (NAFLD fibrosis score, FIB‐4, aspartate aminotransferase/platelet ratio index, BARD score), commercially available assays (enhanced liver fibrosis panel, Fibro Test [FibroSURE], HepatoScore, and FibroMeter), and physical measurements, such as liver stiffness (measured by VCTE, acoustic radiation force impulse, shear wave elastography, and magnetic resonance elastography). These noninvasive tools do not completely eliminate the need for liver biopsy but they drastically reduce the number of patients who need a liver biopsy.
As recently published by Armstrong and Marchesini,( 60 ) the use of a noninvasive scoring system, such as FIB‐4 or NAFLD fibrosis score, is the simplest and most accurate strategy to identify patients at high risk of advanced fibrosis. It is widely known that serum transaminases, which were extensively used in the past, are not a good indicator of the presence or severity of disease because many patients with NAFLD have normal serum transaminases, even in the presence of cirrhosis. In a recent study,( 61 ) the use of VCTE in a high‐risk population (hazardous alcohol and/or T2DM) in primary care resulted in the diagnosis of cirrhosis in 3% of this population. Interestingly, 60% of these patients were obese or presented with T2DM.
Hence, risk assessment of NAFLD may be performed in primary care clinics using noninvasive testing in order to avoid unnecessary referrals.( 62 , 63 , 64 ) We recommend the use of FIB‐4 and/or VCTE according to local resources, availability, and clinical context. The use of the FIB‐4 score is attractive in the primary care setting because it is based on common clinical parameters (age, aspartate aminotransferase, alanine aminotransferase, and platelets) that are widely available and can be easily calculated during routine visits. Moreover, when compared to other noninvasive tests, the FIB‐4 score has been shown to have the best diagnostic accuracy and a high negative predictive value (≥90%) for advanced fibrosis when using the lower cutoff (1.3).( 65 , 66 , 67 , 68 ) Noninvasive scores, such as FIB‐4, are best used to rule out rather than to rule in advanced fibrosis due to their higher specificity and negative predictive value, which argues in favor of our strategy to screen all patients with prediabetes and T2DM.
Liver stiffness as measured by VCTE is the best evaluated point‐of‐care technique, with a high negative predictive value and low operative cost. A recent study( 69 ) using data from 261 patients biopsy‐proven from the European NAFLD registry showed that VCTE had better diagnostic performance than a general clinical score in assessing fibrosis. However, VCTE requires availability of the machine, a trained technician, and interpretation of the results and therefore is usually less accessible to PCPs.
How to Screen? Integration of Screening for NASH With Advanced Fibrosis Into an Existing Care Model for Primary Care or Diabetology Clinics
The optimal screening program needs to be a simple algorithm that can be seamlessly integrated into an existing workflow. Patients with T2DM are complex, with multiple comorbidities that can be rapidly time consuming in primary care clinics that are already overburdened. The average length of visits in a primary care office is estimated to be 17 minutes,( 70 ) which can be very limiting when multiple medical problems need to be managed and lifestyle measures should be explained and adapted to each patient’s reality. Interestingly, a study( 71 ) showed that if PCPs do the screening, counseling, immunization, drug prescription, routine chronic care, and treatment of acute conditions, they could in reality accommodate care for less than half of their practice. Strategies employed currently by physicians to meet the recommended standard of care issued by the ADA include a checklist system that mirrors the guidelines as well as patient navigators.
Most physicians have a checklist system that mirrors the guidelines. The most recent guidelines recommend screening patients with prediabetes or T2DM for NAFLD. A simple approach is to incorporate the calculation of FIB‐4 into the existing checklists used in the diabetic population in primary care clinics. The 2020 guidelines published by the ADA( 55 ) include a checklist that includes baseline and yearly transaminases. The addition of platelet count to this checklist would easily allow for the calculation of the FIB‐4 score to identify patients at high risk of advanced fibrosis. An indeterminate or high‐risk score would then prompt additional evaluation with VCTE. This kind of approach would benefit from the introduction of a patient navigator that could manage the checklist and collaborate directly with PCPs and would allow integrating the screening of patients with advanced liver fibrosis into an already well‐validated model of care. A recent study showed that FIB‐4 followed by VCTE is likely the most cost‐effective strategy for screening or detecting cirrhosis among patients with NAFLD in primary care clinics when compared to FIB‐4 followed by magnetic resonance elastography or liver biopsy, proving that a sequential strategy with FIB‐4 and VCTE may be a valid option to risk stratify these patients.( 72 )
The introduction of a patient navigator into the care system of patients with chronic liver diseases( 73 ) or patients with diabetes( 74 , 75 ) has shown an improvement in care and glycemic control and better patient engagement. We believe that the integration of a patient navigator who comanages the checklist with the PCP would also greatly improve the screening rates as well as the rates of patients following up with subsequent testing and liver specialist services if needed. In the absence of a patient navigator, the use of electronic medical record reminders/flags could also be used. This strategy has been shown to improve the management of patients with diabetes and could be extended to the NAFLD population.( 76 , 77 )
In summary, we propose the following stepwise approach for screening and management of patients with NAFLD (Fig. 2):
-
Incorporation of the FIB‐4 score into the care checklist and care pathway to identify patients at high risk of NASH with advanced fibrosis.
Addition of a platelet count and FIB‐4 calculator to the care checklist of the patient with diabetesor prediabetes.( 55 ) The formula for FIB‐4 is readily available online.
Involvement of a patient navigator to (i) flag patients who need laboratory measurements for the calculation of FIB‐4; (ii) identify patients with indeterminate or high‐risk FIB‐4 scores who need referral to a specialized liver center and/or referral for VCTE; (iii) follow‐up to ensure that the patient underwent VCTE or the specialist appointment.
-
Referral for VCTE
FIB‐4 <1.3: Low risk patients (patients are unlikely to have advanced fibrosis). Follow‐up with PCPs for appropriate preventive interventions of lifestyle changes and a yearly calculation of FIB‐4.
-
FIB‐4 ≥1.3: Refer the patient for VCTE
(i) if liver stiffness measure is <8 kPa: follow up with PCP and repeat FIB‐4 and VCTE in 1 year; (ii) if liver stiffness measure is ≥8 kPa: Refer the patient to a liver specialist.
(Note, in case of VCTE failure, an alternative, such as shear wave elastography/acoustic radiation force imaging, magnetic resonance elastography [particularly when body mass index is >35 kg/m2] may be considered according to local availability).
Referral to specialized liver centers for further assessment of all patients with FIB‐4 ≥1.3 and VCTE ≥8 kPa.
FIG. 2.
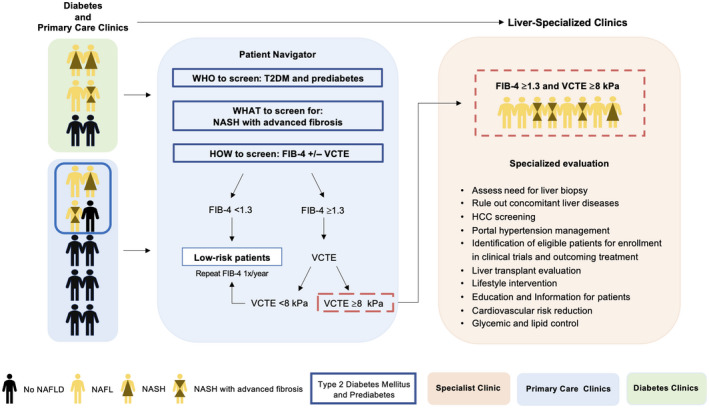
Stepwise approach for screening, risk stratification, and referral of patients with NAFLD between primary care and diabetic clinics and liver specialists. A pragmatic risk stratification algorithm is crucial to identify patients at high risk of advanced liver fibrosis. In this algorithm, we propose screening all patients with T2DM or prediabetes for advanced liver fibrosis by using FIB‐4. The proposed algorithm involves a first‐step annual FIB‐4 score followed by VCTE for those with indeterminate or a high‐risk score (FIB‐4 ≥1.3). Patients at low risk (FIB‐4 <1.3 or VCTE <8 kPa) can be followed up by PCPs for lifestyle changes and yearly calculation of FIB‐4, while patients at high risk (FIB‐4 ≥1.3 and VCTE ≥8 kPa) should be referred to liver‐specialized clinics for further assessment and evaluation.
Regarding extrahepatic complications, patients with NAFLD have a significantly increased risk of cardiovascular mortality, which is independent of the stage of liver disease. We propose that all patients with NAFLD would benefit from a careful assessment of their 10‐year cardiovascular risk using the atherosclerotic CVD risk calculator. Moreover, according to ADA 2020 guidelines( 78 ) and in the absence of contraindications, T2DM patients should benefit from statin therapy in primary prevention in the following cases: (1) all patients between 40 and 75 years without atherosclerotic CVD; (2) all patients between 20 and 39 years with additional atherosclerotic CVD risk factors; (3) all patients with a 10‐year atherosclerotic CVD risk ≥20%. Regarding the increased risk of chronic kidney disease, we propose a close surveillance of serum creatinine, estimated glomerular filtration rate, and albumin‐to‐creatinine ratio on urinary spot.
Conclusion
NALFD is now the leading cause of chronic liver disease in the United States and Europe, and its global burden is expected to rise in the next decades, carrying clinical, economic, and social implications. Despite affecting approximately one quarter of the worldwide population, only a minority of these patients will develop liver‐related morbidity and mortality. There is growing recognition that certain populations, such as patients with T2DM, are at particularly high risk. More specific guidelines are needed in order to help physicians identify patients with NASH at high risk of liver‐related complications. A successful strategy would include incorporation of a simple cost‐effective algorithm into an existing diabetes care system, such as the use of checklists and patient navigators. This algorithm would allow the PCPs to screen, stratify, and refer patients with high risk of NASH and advanced fibrosis to liver specialists for further work‐up and management. Utility studies have already shown that the use of noninvasive screening strategies, particularly in patients at high risk, can be cost effective.
Supported by the University Hospital of Lausanne (to J.V.B.) and the Novartis Foundation for Medical‐Biological Research (to J.V.B.).
Potential conflict of interest: None.
References
Author names in bold designate shared co‐first authorship.
- 1. Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2019;69:2672‐2682. [DOI] [PubMed] [Google Scholar]
- 2. Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology 2020;158:1851‐1864. [DOI] [PubMed] [Google Scholar]
- 3. Younossi ZM, Stepanova M, Younossi Y, Golabi P, Mishra A, Rafiq N, et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2020;69:564‐568. [DOI] [PubMed] [Google Scholar]
- 4. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐Meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 5. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol 2018;113:1649‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547‐555. [DOI] [PubMed] [Google Scholar]
- 8. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328‐357. [DOI] [PubMed] [Google Scholar]
- 9. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) . EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol 2016;64:1388‐1402. [DOI] [PubMed] [Google Scholar]
- 10. Rinella ME, Sanyal AJ. Management of NAFLD: a stage‐based approach. Nat Rev Gastroenterol Hepatol 2016;13:196‐205. [DOI] [PubMed] [Google Scholar]
- 11. Younossi ZM. The epidemiology of nonalcoholic steatohepatitis. Clin Liver Dis (Hoboken) 2018;11:92‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blais P, Husain N, Kramer JR, Kowalkowski M, El‐Serag H, Kanwal F. Nonalcoholic fatty liver disease is underrecognized in the primary care setting. Am J Gastroenterol 2015;110:10‐14. [DOI] [PubMed] [Google Scholar]
- 13. Said A, Gagovic V, Malecki K, Givens ML, Nieto FJ. Primary care practitioners survey of non‐alcoholic fatty liver disease. Ann Hepatol 2013;12:758‐765. [PubMed] [Google Scholar]
- 14. Mari A, Omari S, Abu Baker F, Abu Much S, Said Ahmad H, Khoury T, et al. Non‐alcoholic fatty liver disease: a survey of involvement of primary care physicians. Minerva Gastroenterol Dietol 2019;65:255‐258. [DOI] [PubMed] [Google Scholar]
- 15. Lazarus JV, Ekstedt M, Marchesini G, Mullen J, Novak K, Pericas JM, et al.; EASL International Liver Foundation NAFLD Policy Review Collaborators . A cross‐sectional study of the public health response to non‐alcoholic fatty liver disease in Europe. J Hepatol 2020;72:14‐24. [DOI] [PubMed] [Google Scholar]
- 16. Bril F, Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care 2017;40:419‐430. [DOI] [PubMed] [Google Scholar]
- 17. Hossain N, Afendy A, Stepanova M, Nader F, Srishord M, Rafiq N, et al. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1224‐1229, 1229.e1‐2. [DOI] [PubMed] [Google Scholar]
- 18. Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol 2004;2:262‐265. [DOI] [PubMed] [Google Scholar]
- 19. Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015;62:1723‐1730. [DOI] [PubMed] [Google Scholar]
- 20. Stepanova M, Rafiq N, Makhlouf H, Agrawal R, Kaur I, Younoszai Z, et al. Predictors of all‐cause mortality and liver‐related mortality in patients with non‐alcoholic fatty liver disease (NAFLD). Dig Dis Sci 2013;58:3017‐3023. [DOI] [PubMed] [Google Scholar]
- 21. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10:330‐344. [DOI] [PubMed] [Google Scholar]
- 22. Williamson RM, Price JF, Glancy S, Perry E, Nee LD, Hayes PC, et al.; Edinburgh Type 2 Diabetes Study Investigators . Prevalence of and risk factors for hepatic steatosis and nonalcoholic fatty liver disease in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care 2011;34:1139‐1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 2007;30:1212‐1218. [DOI] [PubMed] [Google Scholar]
- 24. Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta‐analysis. J Hepatol 2019;71:793‐801. [DOI] [PubMed] [Google Scholar]
- 25. Kim Y, Chang Y, Cho YK, Ahn J, Shin H, Ryu S. Obesity and weight gain are associated with progression of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2019;17:543‐550.e542. [DOI] [PubMed] [Google Scholar]
- 26. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013;10:686‐690. [DOI] [PubMed] [Google Scholar]
- 27. Caussy C, Soni M, Cui J, Bettencourt R, Schork N, Chen CH, et al.; Familial NAFLD Cirrhosis Research Consortium . Nonalcoholic fatty liver disease with cirrhosis increases familial risk for advanced fibrosis. J Clin Invest 2017;127:2697‐2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loomba R, Schork N, Chen CH, Bettencourt R, Bhatt A, Ang B, et al.; Genetics of NAFLD in Twins Consortium . Heritability of hepatic fibrosis and steatosis based on a prospective twin study. Gastroenterology 2015;149:1784‐1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trepo E, Valenti L. Update on NAFLD genetics: from new variants to the clinic. J Hepatol 2020;72:1196‐1209. [DOI] [PubMed] [Google Scholar]
- 30. Ma Y, Belyaeva OV, Brown PM, Fujita K, Valles K, Karki S, et al. 17‐beta hydroxysteroid dehydrogenase 13 is a hepatic retinol dehydrogenase associated with histological features of nonalcoholic fatty liver disease. Hepatology 2019;69:1504‐1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mancina RM, Dongiovanni P, Petta S, Pingitore P, Meroni M, Rametta R, et al. The MBOAT7‐TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology 2016;150:1219‐1230.e1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Friedman SL, Neuschwander‐Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med 2018;24:908‐922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Musso G, Gambino R, Cassader M, Pagano G. Meta‐analysis: natural history of non‐alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non‐invasive tests for liver disease severity. Ann Med 2011;43:617‐649. [DOI] [PubMed] [Google Scholar]
- 34. Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease‐specific mortality in NAFLD after up to 33 years of follow‐up. Hepatology 2015;61:1547‐1554. [DOI] [PubMed] [Google Scholar]
- 35. Taylor RS, Taylor RJ, Bayliss S, Hagstrom H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Gastroenterology 2020;158:1611‐1625.e12. [DOI] [PubMed] [Google Scholar]
- 36. Francque SM, van der Graaff D, Kwanten WJ. Non‐alcoholic fatty liver disease and cardiovascular risk: pathophysiological mechanisms and implications. J Hepatol 2016;65:425‐443. [DOI] [PubMed] [Google Scholar]
- 37. Henson JB, Simon TG, Kaplan A, Osganian S, Masia R, Corey KE. Advanced fibrosis is associated with incident cardiovascular disease in patients with non‐alcoholic fatty liver disease. Aliment Pharmacol Ther 2020;51:728‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, et al. Risk of hepatocellular cancer in patients with non‐alcoholic fatty liver disease. Gastroenterology 2018;155:1828‐1837.e1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ahmed O, Liu L, Gayed A, Baadh A, Patel M, Tasse J, et al. The changing face of hepatocellular carcinoma: forecasting prevalence of nonalcoholic steatohepatitis and hepatitis C cirrhosis. J Clin Exp Hepatol 2019;9:50‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis 2015;19:223‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Patwardhan V, Paul S, Corey KE, Mazhar SM, Richter JM, Thiim M, et al. Hepatocellular carcinoma screening rates vary by etiology of cirrhosis and involvement of gastrointestinal sub‐specialists. Dig Dis Sci 2011;56:3316‐3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aby E, Phan J, Truong E, Grotts J, Saab S. Inadequate hepatocellular carcinoma screening in patients with nonalcoholic steatohepatitis cirrhosis. J Clin Gastroenterol 2019;53:142‐146. [DOI] [PubMed] [Google Scholar]
- 43. Loomba R, Lim JK, Patton H, El‐Serag HB. AGA clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: expert review. Gastroenterology 2020;158:1822‐1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weinmann A, Alt Y, Koch S, Nelles C, Duber C, Lang H, et al. Treatment and survival of non‐alcoholic steatohepatitis associated hepatocellular carcinoma. BMC Cancer 2015;15:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, et al.; Global Nonalcoholic Steatohepatitis Council . Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol 2019;17:748‐755.e743. [DOI] [PubMed] [Google Scholar]
- 46. Adams LA, Anstee QM, Tilg H, Targher G. Non‐alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017;66:1138‐1153. [DOI] [PubMed] [Google Scholar]
- 47. Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010;363:1341‐1350. [DOI] [PubMed] [Google Scholar]
- 48. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non‐alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta‐analysis. J Hepatol 2016;65:589‐600. [DOI] [PubMed] [Google Scholar]
- 49. Targher G, Byrne CD. Non‐alcoholic fatty liver disease: an emerging driving force in chronic kidney disease. Nat Rev Nephrol 2017;13:297‐310. [DOI] [PubMed] [Google Scholar]
- 50. Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, Hamaguchi M, et al. Association of non‐alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta‐analysis. PLoS Med 2014;11:e1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Puri P, Fuchs M. Population management of nonalcoholic fatty liver disease. Fed Pract 2019;36:72‐82. [PMC free article] [PubMed] [Google Scholar]
- 52. Shaheen AA, Riazi K, Medellin A, Bhayana D, Kaplan GG, Jiang J, et al. Risk stratification of patients with nonalcoholic fatty liver disease using a case identification pathway in primary care: a cross‐sectional study. CMAJ Open 2020;8:E370‐E376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Allen AM, Van Houten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare cost and utilization in nonalcoholic fatty liver disease: real‐world data from a large U.S. claims database. Hepatology 2018;68:2230‐2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tapper EB, Lok ASF. Use of liver imaging and biopsy in clinical practice. N Engl J Med 2017;377:756‐768. [DOI] [PubMed] [Google Scholar]
- 55. American Diabetes Association . 4. Comprehensive medical evaluation and assessment of comorbidities: Standards of Medical Care in Diabetes‐2020. Diabetes Care 2020;43(Suppl. 1):S37‐S47. [DOI] [PubMed] [Google Scholar]
- 56. Younossi ZM, Corey KE, Alkhouri N, Noureddin M, Jacobson I, Lam B, et al.; US Members of the Global Nash Council . Clinical assessment for high‐risk patients with non‐alcoholic fatty liver disease in primary care and diabetology practices. Aliment Pharmacol Ther 2020;52:513‐526. [DOI] [PubMed] [Google Scholar]
- 57. Harris R , Harman DJ, Card TR, Aithal GP, Guha IN. Prevalence of clinically significant liver disease within the general population, as defined by non‐invasive markers of liver fibrosis: a systematic review. Lancet Gastroenterol Hepatol 2017;2:288‐297. [DOI] [PubMed] [Google Scholar]
- 58. Vilar‐Gomez E, Chalasani N. Non‐invasive assessment of non‐alcoholic fatty liver disease: clinical prediction rules and blood‐based biomarkers. J Hepatol 2018;68:305‐315. [DOI] [PubMed] [Google Scholar]
- 59. Castera L, Friedrich‐Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 2019;156:1264‐1281.e1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Armstrong MJ, Marchesini G. Referral pathways for NAFLD fibrosis in primary care—no longer a 'needle in a haystack'. J Hepatol 2019;71:246‐248. [DOI] [PubMed] [Google Scholar]
- 61. Harman DJ, Ryder SD, James MW, Wilkes EA, Card TR, Aithal GP, et al. Obesity and type 2 diabetes are important risk factors underlying previously undiagnosed cirrhosis in general practice: a cross‐sectional study using transient elastography. Aliment Pharmacol Ther 2018;47:504‐515. [DOI] [PubMed] [Google Scholar]
- 62. Davyduke T, Tandon P, Al‐Karaghouli M, Abraldes JG, Ma MM. Impact of implementing a "FIB‐4 First" strategy on a pathway for patients with NAFLD referred from primary care. Hepatol Commun 2019;3:1322‐1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Boursier J, Guillaume M, Leroy V, Irles M, Roux M, Lannes A, et al. New sequential combinations of non‐invasive fibrosis tests provide an accurate diagnosis of advanced fibrosis in NAFLD. J Hepatol 2019;71:389‐396. [DOI] [PubMed] [Google Scholar]
- 64. Srivastava A, Gailer R, Tanwar S, Trembling P, Parkes J, Rodger A, et al. Prospective evaluation of a primary care referral pathway for patients with non‐alcoholic fatty liver disease. J Hepatol 2019;71:371‐378. [DOI] [PubMed] [Google Scholar]
- 65. McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non‐invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non‐alcoholic fatty liver disease. Gut 2010;59:1265‐1269. [DOI] [PubMed] [Google Scholar]
- 66. Sun W, Cui H, Li N, Wei Y, Lai S, Yang Y, et al. Comparison of FIB‐4 index, NAFLD fibrosis score and BARD score for prediction of advanced fibrosis in adult patients with non‐alcoholic fatty liver disease: a meta‐analysis study. Hepatol Res 2016;46:862‐870. [DOI] [PubMed] [Google Scholar]
- 67. Alkayyali T, Qutranji L, Kaya E, Bakir A, Yilmaz Y. Clinical utility of noninvasive scores in assessing advanced hepatic fibrosis in patients with type 2 diabetes mellitus: a study in biopsy‐proven non‐alcoholic fatty liver disease. Acta Diabetol 2020;57:613‐618. [DOI] [PubMed] [Google Scholar]
- 68. Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network . Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Labenz C, Huber Y, Kalliga E, Nagel M, Ruckes C, Straub BK, et al. Predictors of advanced fibrosis in non‐cirrhotic non‐alcoholic fatty liver disease in Germany. Aliment Pharmacol Ther 2018;48:1109‐1116. [DOI] [PubMed] [Google Scholar]
- 70. Tai‐Seale M, McGuire TG, Zhang W. Time allocation in primary care office visits. Health Serv Res 2007;42:1871‐1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Altschuler J, Margolius D, Bodenheimer T, Grumbach K. Estimating a reasonable patient panel size for primary care physicians with team‐based task delegation. Ann Fam Med 2012;10:396‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vilar‐Gomez E, Lou Z, Kong N, Vuppalanchi R, Imperiale TF, Chalasani N. Cost effectiveness of different strategies for detecting cirrhosis in patients with nonalcoholic fatty liver disease based on United States health care system. Clin Gastroenterol Hepatol 2020;18:2305‐2314.e2312. [DOI] [PubMed] [Google Scholar]
- 73. McBrien KA, Ivers N, Barnieh L, Bailey JJ, Lorenzetti DL, Nicholas D, et al. Patient navigators for people with chronic disease: a systematic review. PLoS One 2018;13:e0191980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Horny M, Glover W, Gupte G, Saraswat A, Vimalananda V, Rosenzweig J. Patient navigation to improve diabetes outpatient care at a safety‐net hospital: a retrospective cohort study. BMC Health Serv Res 2017;17:759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. English TM, Masom D, Whitman MV. The impact of patient navigation on diabetes. J Healthc Manag 2018;63:e32‐e41. [DOI] [PubMed] [Google Scholar]
- 76. Varroud‐Vial M. Improving diabetes management with electronic medical records. Diabetes Metab 2011;37(Suppl. 4):S48‐S52. [DOI] [PubMed] [Google Scholar]
- 77. Hechter RC, Qian L, Luo Y, Ling Grant DS, Baxter R, Klein NP, et al. Impact of an electronic medical record reminder on hepatitis B vaccine initiation and completion rates among insured adults with diabetes mellitus. Vaccine 2019;37:195‐201. [DOI] [PubMed] [Google Scholar]
- 78. American Diabetes Association . 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes‐2020. Diabetes Care 2020;43(Suppl. 1):S111‐S134. [DOI] [PubMed] [Google Scholar]


