Finite therapy of HBV/HDV co‐infection with REP 2139‐Ca and pegIFN has good long term safety and is accompanied by high rates of functional cure of HDV, normalization of liver function and additional functional cure of HBV. HBV functional cure is accompanied inactivation / clearance of cccDNA and clearance of integrated HBV DNA.

Abstract
The nucleic acid polymer REP 2139 inhibits assembly/secretion of hepatitis B virus (HBV) subviral particles. Previously, REP 2139‐Ca and pegylated interferon (pegIFN) in HBV/hepatitis delta virus (HDV) coinfection achieved high rates of HDV RNA and hepatitis B surface antigen (HBsAg) loss/seroconversion in the REP 301 study (NCT02233075). The REP 301‐LTF study (NCT02876419) examined safety and efficacy during 3.5 years of follow‐up. In the current study, participants completing therapy in the REP 301 study were followed for 3.5 years. Primary outcomes were safety and tolerability, and secondary outcomes were HDV functional cure (HDV RNA target not detected [TND], normal alanine aminotransferase [ALT]), HBV virologic control (HBV DNA ≤2,000 IU/mL, normal ALT), HBV functional cure (HBV DNA TND; HBsAg <0.05 IU/mL, normal ALT), and HBsAg seroconversion. Supplemental analysis included high‐sensitivity HBsAg (Abbott ARCHITECT HBsAg NEXT), HBV pregenomic RNA (pgRNA), HBsAg/hepatitis B surface antibody (anti‐HBs) immune complexes (HBsAg ICs), and hepatitis B core‐related antigen (HBcrAg). Asymptomatic grade 1‐2 ALT elevations occurred in 2 participants accompanying viral rebound; no other safety or tolerability issues were observed. During therapy and follow‐up, HBsAg reductions to <0.05 IU/mL were also <0.005 IU/mL. HBsAg ICs declined in 7 of 11 participants during REP 2139‐Ca monotherapy and in 10 of 11 participants during follow‐up. HDV functional cure persisted in 7 of 11 participants; HBV virologic control persisted in 3 and functional cure (with HBsAg seroconversion) persisted in 4 of these participants. Functional cure of HBV was accompanied by HBV pgRNA TND and HBcrAg <lower limit of quantitation. Conclusion: REP 2139‐Ca + pegIFN is not associated with long‐term safety or tolerability issues. The establishment of HDV functional cure and HBV virologic control/functional cure and HBsAg seroconversion are durable over 3.5 years and may reflect removal of integrated HBV DNA from the liver. Further investigation is warranted in larger studies.
Abbreviations
- ALT
alanine aminotransferase
- anti‐HBs
hepatitis B surface antigen antibody
- cccDNA
covalently closed circular DNA
- EOT
end of therapy
- FW
follow‐up week
- HBcrAg
hepatitis B core‐related antigen
- HBeAg
hepatitis B e antigen
- HBsAg
hepatitis B virus surface antigen
- HBV
hepatitis B virus
- HDAg
hepatitis delta antigen
- HDV
hepatitis delta virus
- INR
international normalized ratio
- LLOQ
lower limit of quantitation
- LMS
liver median stiffness
- NAP
nucleic acid polymer
- NUC
nucleos(t)ide inhibitor
- PCR
polymerase chain reaction
- pegIFN
pegylated interferon
- pgRNA
pregenomic RNA
- qHBsAg
quantitative hepatitis B virus surface antigen
- QW
once weekly
- RUO
Research Use Only
- SVP
subviral particle
- TND
target not detected
- Y
year
Chronic hepatitis delta virus (HDV) infection is one of the most aggressive forms of viral hepatitis,( 1 , 2 ) affecting up to 40 million individuals worldwide.( 3 , 4 ) HDV is an obligate satellite infection of chronic hepatitis B virus (HBV) infection, requiring the HBV surface antigen (HBsAg) protein for its envelopment( 2 ) through the same assembly and secretion pathway as HBV subviral particles (SVPs).( 5 ) Clearance of HBsAg from the circulation during therapy of HBV/HDV coinfection is considered to be essential for the functional cure of both HDV( 6 , 7 ) (HDV RNA target not detected [TND] with normal alanine aminotransferase [ALT] in the absence of therapy) and HBV( 8 , 9 ) (HBV DNA TND; HBsAg <0.05 IU/mL with normal ALT in the absence of therapy). Attempting to control HDV infection with nucleos(t)ide analog HBV reverse‐transcriptase inhibitors (NUCs) is largely ineffective because HDV replication can be supported by HBsAg derived only from integrated HBV DNA,( 10 , 11 , 12 ) which is not affected by NUCs. Pegylated interferon (pegIFN) can achieve clearance of HDV RNA but with little effect on HBsAg, even when combined with NUCs,( 12 , 13 ) and rebound of HDV RNA occurs in a significant proportion of subjects after removal of therapy.( 13 , 14 )
Nucleic acid polymers (NAPs) are oligonucleotides with broad spectrum antiviral activity that increases with length and is saturated at 40 nucleotides.( 15 , 16 ) This activity is dependent on phosphorothioate modification of internucleotide linkages but is independent of sequence composition or sugar modifications.( 15 , 16 ) This broad spectrum antiviral activity is driven by interaction with structurally similar exposed hydrophobic surfaces of amphipathic alpha helices, which have been identified in many confirmed targets for NAPs in viral and nonviral infectious agents.( 16 ) REP 2139 is a NAP optimized for tolerability and formulation by the adoption of a poly adenosine–cytidine sequence and complete 2′ O‐methylation of the ribose sugar, which does not have any impact on antiviral activity( 17 , 18 ) but removes off‐target effects and maximizes drug solubility.( 16 ) REP 2139 selectively blocks the assembly of SVPs derived from covalently closed circular DNA (cccDNA) or integrated HBV DNA.( 19 , 20 , 21 ) This effect is driven by the interaction with an as yet unidentified host protein( 19 , 22 ) with an exposed hydrophobic surface( 20 ) similar to target domains for NAPs confirmed in other viral and nonviral infectious systems.( 16 ) The inhibition of SVP assembly by REP 2139 simultaneously blocks the release of HBsAg and lowers intracellular HBsAg( 20 ) without affecting the production of viral proteins or the secretion of hepatitis B e antigen (HBeAg) or Dane particles.( 21 ) REP 2139 has been shown to directly interact with the large and small forms of the hepatitis delta antigen (HDAg),( 22 ) which is consistent with the nonspecific interaction of other oligonucleotides with HDAg.( 23 , 24 )
Preclinical studies with a variety of NAPs, including REP 2055 and REP 2139,( 15 , 16 ) have verified the sequence and sugar modification‐independent effects on HBV infection, including the clearance of HBV DNA and HBsAg from both the circulation and the liver.( 18 , 25 , 26 ) Further, multilog reduction of cccDNA in the liver persists after removal of therapy.( 25 , 26 ) Clinical studies with the NAPs REP 2055 and REP 2139( 17 , 19 , 27 ) in monotherapy have confirmed these results in vivo, including HBsAg loss and seroconversion, HBeAg seroconversion (in subjects who are HBeAg positive), HBV DNA, HBV RNA, and HDV RNA loss (in patients coinfected with HDV). When combined with immunotherapies, such as thymosin α1 and pegIFN, REP 2139‐based combination therapy was accompanied by rapid HBsAg clearance and seroconversion; high rates of therapeutic transaminase flares; HBV DNA, HBV RNA, hepatitis B core‐related antigen (HBcrAg), and HDV RNA clearance during therapy( 19 ); and high rates of functional cure of HBV and HDV.( 19 , 28 )
The REP 301‐LTF study (NCT02876419) investigated the long‐term safety and efficacy following therapy with REP 2139‐Ca and pegIFN in the REP 301 study (NCT02233075).( 19 ) In the current study, long‐term evaluation extended the total follow‐up after removal of all therapy to 3.5 years and demonstrated the long‐term stability of functional cure of HBV and HDV with no safety issues related to this therapy. Several experimental virologic parameters were additionally analyzed during therapy in the REP 301 and REP 301‐LTF studies. The extent of HBsAg reduction during the REP 301 and REP 301‐LTF studies was examined using a high‐sensitivity HBsAg test capable of detecting HBsAg concentrations in serum as low as 0.005 IU/mL.( 29 ) The role of hepatitis B surface antibody (anti‐HBs) in clearing HBsAg was examined using a research assay for HBsAg/anti‐HBs immune complexes (ICs). Additionally, changes in serum HBV pregenomic RNA (pgRNA) and HBcrAg were evaluated.
Materials and Methods
Study Design
The REP 301 study was an open‐label, nonrandomized study of the safety and efficacy of REP 2139‐Ca in monotherapy, transitioning to combination therapy with pegIFN followed by monotherapy with pegIFN in 12 participants with treatment‐naive chronic HBV/HDV coinfection.( 19 ) REP 301‐LTF was a long‐term follow‐up study in which 11 of 12 participants completing therapy were evaluated every 6 months for 3 years (for a total of 3.5 years of follow‐up). The first visit in REP 301‐LTF (6 months from the final 24‐week follow‐up in the REP 301 study) has been published.( 19 ) The REP 301 protocol has been published,( 19 ) and the REP 301‐LTF protocol can be found at http://replicor.com/wp‐content/uploads/2020/09/REP301.pdf.
Primary outcomes were the safety and tolerability of REP 2139‐Ca + pegIFN therapy over the 3 years of follow‐up. Secondary outcomes were maintenance of virologic control of HBV (HBV DNA ≤2,000 IU/mL with normal ALT), functional cure of HBV (HBV DNA TND; HBsAg <0.05 IU/mL), HBsAg seroconversion, and functional cure of HDV (HDV RNA TND with normal ALT).
The REP 301‐LTF study was conducted at the Toma Ciorbă Infectious Diseases Hospital, and complete data were available in November 2019. The study was compliant with current Good Clinical Practice and the Declaration of Helsinki and approved by the National Ethics Committee and National Medicines Agency of the Republic of Moldova. Protocol deviations were permitted to allow a single missed follow‐up visit in all participants (Tables 1 and 2) due to financial constraints from the sponsor.
TABLE 1.
Liver Function in the REP 301‐LTF Study
| Participant | Parameter | Baseline* | EOT* | FW24* | F 1Y* | F1.5Y | F2Y | F2.5Y | F3Y | F3.5Y |
|---|---|---|---|---|---|---|---|---|---|---|
| 001‐01 | ALT (U/L) | 188 | 80 | 33 | 37 | 27 | 29 | 24 | NA | 24 |
| Total bilirubin (μmol/L) | 6.2 | 5.6 | 3.5 | 3.9 | 7.3 | 5.4 | 8.9 | NA | 6.3 | |
| Albumin (g/L) | 42.9 | 39 | 42.7 | 42.6 | 44.5 | 43.7 | 45.1 | NA | 45.9 | |
| INR | 0.97 | 0.98 | 1.06 | 0.96 | 1.09 | 1.03 | 0.97 | NA | 0.97 | |
| Platelets (109/L) | 191 | 84 | 134 | 86 | 135 | 143 | 158 | NA | 160 | |
| LMS (kPa) | 8.4 | 17.1 | 12 | 10.9 | 9.3 | 7.9 | 5.8 | 7 | 7.5 | |
| 001‐02 | ALT (U/L) | 98 | 53 | 21 | 24 | 25 | 24 | 29 | NA | 26 |
| Total bilirubin (μmol/L) | 6.5 | 6 | 5.7 | 7.2 | 10.9 | 5.5 | 6.3 | NA | 7.6 | |
| Albumin (g/L) | 42.1 | 44.7 | 44.5 | 44.9 | 46.8 | 46.4 | 45.9 | NA | 45.2 | |
| INR | 0.99 | 1.06 | 0.99 | 0.98 | 1.07 | 1 | 0.99 | NA | 1.03 | |
| Platelets (109/L) | 234 | 106 | 183 | 222 | 245 | 255 | 247 | NA | 248 | |
| LMS (kPa) | 7.7 | 9.9 | 7.3 | 6.1 | 5.4 | 5.7 | 6.1 | 6.6 | 5.1 | |
| 001‐03 | ALT (U/L) | 53 | 191 | 20 | 25 | 18 | 21 | 25 | NA | 25 |
| Total bilirubin (μmol/L) | 6.1 | 12.2 | 11 | 9.7 | 14.8 | 8.5 | 15.5 | NA | 9.8 | |
| Albumin (g/L) | 44.9 | 48.4 | 44.7 | 46.9 | 51 | 45.6 | 48 | NA | 47.8 | |
| INR | 1.02 | 1.03 | 1.01 | 1.02 | 1.09 | 1.03 | 0.98 | NA | 1.05 | |
| Platelets (109/L) | 134 | 80 | 127 | 142 | 188 | 151 | 154 | NA | 157 | |
| LMS (kPa) | 14.8 | 17.1 | 14.6 | 12 | 9.5 | 9.5 | 8.8 | 9.9 | 10.6 | |
| 001‐06 | ALT (U/L) | 95 | 53 | 17 | 21 | 21 | 18 | 18 | NA | 14 |
| Total bilirubin (μmol/L) | 9.9 | 7 | 5.5 | 8.4 | 10.7 | 7 | 7.9 | NA | 3.6 | |
| Albumin (g/L) | 44.7 | 48.9 | 45.2 | 45.8 | 50.2 | 46 | 46 | NA | 48.7 | |
| INR | 1.06 | 1.07 | 1.06 | 1.02 | 1.11 | 1.01 | 1.0 | NA | 1.06 | |
| Platelets (109/L) | 276 | 197 | 278 | 301 | 298 | 321 | 312 | NA | 432 | |
| LMS (kPa) | 6.8 | 7.1 | 8.1 | 6.3 | 4.8 | 6.7 | 7.7 | ND | 6.1 | |
| 01‐009 | ALT (U/L) | 85 | 34 | 56 | 71 | 54 | 76 | 69 | NA | 75 |
| Total bilirubin (μmol/L) | 9.9 | 5.7 | 5.7 | 6.6 | 7.1 | 11.9 | 9.1 | NA | 12.1 | |
| Albumin (g/L) | 43.9 | 45.5 | 43.8 | 44.2 | 37.6 | 49.4 | 48.6 | NA | 48.1 | |
| INR | 1.05 | 1.06 | 1.02 | 0.98 | 1.17 | 1.06 | 1.03 | NA | 1.05 | |
| Platelets (109/L) | 130 | 77 | 123 | 110 | 200 | 153 | 156 | NA | 155 | |
| LMS (kPa) | 12 | 12 | 10.2 | 19.8 | 18.4 | 14.3 | 13.8 | 17.3 | 15.4 | |
| 001‐11 | ALT (U/L) | 200 | 133 | 57 | 29 | 30 | 24 | 27 | NA | 27 |
| Total bilirubin (μmol/L) | 5.6 | 3.6 | 4.6 | 9.4 | 7.8 | 5.3 | 8.7 | NA | 5.7 | |
| Albumin (g/L) | 41.9 | 38.1 | 42.4 | 42.8 | 48.7 | 47 | 44.3 | NA | 45.3 | |
| INR | 1.1 | 1.03 | 1.1 | 1.02 | 1.08 | 1.05 | 1.04 | NA | 1.06 | |
| Platelets (109/L) | 179 | 122 | 210 | 212 | 195 | 210 | 215 | NA | 198 | |
| LMS (kPa) | 9.6 | 10.3 | 6.9 | 6.6 | 5.4 | 7.1 | 6 | 5.8 | 6.6 | |
| 001‐17 | ALT (U/L) | 62 | 46 | 29 | 42 | 35 | 43 | NA | 36 | 42 |
| Total bilirubin (μmol/L) | 13.1 | 8.6 | 16 | 12.4 | 10.4 | 14.4 | NA | 13.7 | 10.8 | |
| Albumin (g/L) | 47.4 | 47.2 | 47.6 | 47.8 | 47.5 | 53 | NA | 48.6 | 47.8 | |
| INR | 1.09 | 0.98 | 1.08 | 1.06 | 1.1 | 1.12 | NA | 1.14 | 1.02 | |
| Platelets (109/L) | 175 | 84 | 181 | 172 | 197 | 210 | NA | 246 | 208 | |
| LMS (kPa) | 9.5 | 9.8 | 7.8 | 8.4 | 5.1 | 6.2 | 5.4 | 5.8 | 6.3 | |
| 001‐20 | ALT (U/L) | 29 | 47 | 53 | 37 | 64 | 69 | NA | 45 | 45 |
| Total bilirubin (μmol/L) | 6 | 5.9 | 5.8 | 7.9 | 9.6 | 5.7 | NA | 7.5 | 10.3 | |
| Albumin (g/L) | 39.5 | 44.4 | 44.3 | 43.4 | 44.6 | 46.5 | NA | 46.2 | 45.7 | |
| INR | 1.02 | 0.89 | 1.02 | 1.04 | 1.04 | 1.06 | N/A | 1.06 | 0.98 | |
| Platelets (109/L) | 251 | 163 | 268 | 242 | 303 | 262 | NA | 235 | 258 | |
| LMS (kPa) | 8.8 | 10.2 | 15 | 9 | 9.9 | 9.9 | 15.6 | 14.3 | 9 | |
| 001‐22 | ALT (U/L) | 101 | 58 | 33 | 29 | 43 | 38 | NA | 69 | 95 |
| Total bilirubin (μmol/L) | 14.2 | 11.2 | 9.2 | 13.4 | 17.2 | 17.4 | NA | 14.1 | 17 | |
| Albumin (g/L) | 44.1 | 42.9 | 44.5 | 50.3 | 51.7 | 53.3 | NA | 47.8 | 46.7 | |
| INR | 1.16 | 1.12 | 1.11 | 1.11 | 1.2 | 1.2 | NA | 1.17 | 1.06 | |
| Platelets (109/L) | 107 | 59 | 94 | 102 | 96 | 133 | NA | 132 | 116 | |
| LMS (kPa) | 11.9 | 11.8 | 8.9 | 11.8 | 9.4 | 6.8 | 6.9 | 8.7 | 7.6 | |
| 001‐24 | ALT (U/L) | 160 | 97 | 191 | 153 | 153 | 180 | NA | 263 | 193 |
| Total bilirubin (μmol/L) | 21 | 8.5 | 12.2 | 11.2 | 13.8 | 13.2 | NA | 17.9 | 11.3 | |
| Albumin (g/L) | 46.9 | 42.2 | 46.6 | 45.5 | 45.9 | 47.7 | NA | 45.6 | 39.6 | |
| INR | 1.08 | 0.94 | 1.02 | 1.03 | 1.08 | 1.11 | NA | 1.13 | 1.06 | |
| Platelets (109/L) | 194 | 93 | 170 | 207 | 146 | 178 | NA | 170 | 121 | |
| LMS (kPa) | 7.8 | 8.2 | 10.4 | 10.8 | 9.3 | 9.9 | 11.9 | 11.8 | 10.6 | |
| 001‐26 | ALT (U/L) | 85 | 51 | 46 | 39 | 57 | 47 | NA | 38 | 46 |
| Total bilirubin (μmol/L) | 22 | 11 | 19.3 | 17.1 | 13.8 | 10.1 | NA | 16.7 | 20.9 | |
| Albumin (g/L) | 45.9 | 37.9 | 46.3 | 49.2 | 49.7 | 49.8 | NA | 48 | 51.9 | |
| INR | 1.17 | 1.12 | 1.09 | 1.13 | 1.14 | 1.15 | NA | 1.13 | 1.08 | |
| Platelets (109/L) | 96 | 49 | 75 | 75 | 73 | 70 | NA | 88 | 83 | |
| LMS (kPa) | 30.7 | 27 | 34.3 | 33.3 | 14.6 | 21.8 | 22.5 | 24.3 | 27.7 |
Previously published (in graphic format).( 19 )
Abbreviation: NA, not assessed (see Materials and Methods).
TABLE 2.
Virologic Response in the REP 301 and REP 301‐LTF Studies
| Participant | Parameter | Baseline* | EOT* | FW24* | F 1Y* | F1.5Y | F2Y | F2.5Y | F3Y | F3.5Y |
|---|---|---|---|---|---|---|---|---|---|---|
| 001‐01 | HBsAg (IU/mL) | 13,988 | <0.005 | <0.005 | <0.005 | <0.005 | 0.05 | 0.15 | NA | 0.83 |
| anti‐HBs (mIU/mL) | 1.03 | 6,633 | 7,585 | 5,073 | 820 | 318 | 151 | NA | 86 | |
| HBV DNA (IU/mL) | <LLOQ | <LLOQ | TND | <LLOQ | TND | TND | <LLOQ | NA | TND | |
| HDV RNA (IU/mL) | 394,000 | TND | TND | TND | TND | TND | TND | NA | TND | |
| HBV RNA (log10 copies/mL) | TND | TND | TND | ND | ND | ND | ND | NA | ND | |
| HBV pgRNA (log10 U/mL) | ND | ND | ND | TND | TND | <LLOQ | TND | NA | 1.86 | |
| HBcrAg (log10 U/mL) | <LLOQ | <LLOQ | <LLOQ | <LLOQ | <LLOQ | <LLOQ | <LLOQ | NA | <LLOQ | |
| 001‐02 | HBsAg (IU/mL) | 27,264 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | NA | <0.005 |
| anti‐HBs (mIU/mL) | 1.29 | 51,970 | 13,540 | 1,873 | 639 | 345 | 320 | NA | 172 | |
| HBV DNA (IU/mL) | <LLOQ | TND | ND † | TND | TND | TND | TND | NA | TND | |
| HDV RNA (IU/mL) | 47,100,000 | TND | TND | TND | TND | TND | TND | NA | TND | |
| HBV RNA (log10 copies/mL) | TND | TND | TND | ND | ND | ND | ND | NA | ND | |
| HBV pgRNA (log10 U/mL) | ND | ND | ND | TND | TND | TND | TND | NA | TND | |
| HBcrAg (log10 U/mL) | <LLOQ | <LLOQ | <LLOQ | <LLOQ | <LLOQ | <LLOQ | <LLOQ | NA | <LLOQ | |
| 001‐03 | HBsAg (IU/mL) | 28,261 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | NA | <0.005 |
| anti‐HBs (mIU/mL) | <0.1 | 86,532 | 13,566 | 5,079 | 2,603 | 1,261 | 590 | NA | 612 | |
| HBV DNA (IU/mL) | <LLOQ | TND | TND | TND | 179 | TND | TND | NA | TND | |
| HDV RNA (IU/mL) | 697,000 | TND | TND | TND | TND | TND | TND | NA | TND | |
| HBV RNA (log10 copies/mL) | TND | TND | TND | ND | ND | ND | ND | NA | ND | |
| HBV pgRNA (log10 U/mL) | ND | ND | ND | TND | TND | TND | TND | NA | TND | |
| HBcrAg (log10 U/mL) | 4.1 | <LLOQ | <LLOQ | <LLOQ | <LLOQ | <LLOQ | <LLOQ | NA | <LLOQ | |
| 001‐06 | HBsAg (IU/mL) | 17,511 | 29.7 | 239 | 146 | 103 | 19.6 | 0.29 | NA | <0.005 |
| anti‐HBs (mIU/mL) | 2.1 | 1.13 | 1.07 | 0.1 | 0.56 | 0.7 | 1.21 | NA | 7.6 | |
| HBV DNA (IU/mL) | 726 | <LLOQ | 13 | 21 | 122 | 183 | TND | NA | TND | |
| HDV RNA (IU/mL) | 5,490,000 | TND | TND | TND | TND | TND | TND | NA | TND | |
| HBV RNA (log10 copies/mL) | 1.73 | TND | TND | ND | ND | ND | ND | NA | ND | |
| HBV pgRNA (log10 U/mL) | ND | ND | ND | TND | <LLOQ | <LLOQ | TND | NA | <LLOQ | |
| HBcrAg (log10 U/mL) | 4.1 | 2.8 | <LLOQ | <LLOQ | <LLOQ | <LLOQ | <LLOQ | NA | <LLOQ | |
| 001‐09 | HBsAg (IU/mL) | 16,426 | 399 | 2,646 | 6,621 | 10,627 | 10,772 | 9,080 | NA | 8,686 |
| anti‐HBs (mIU/mL) | <0.1 | 7.76 | 1.42 | 0.78 | <0.1 | <0.1 | <0.1 | NA | <0.1 | |
| HBV DNA (IU/mL) | 104 | 31 | 10 | 1,696 | 6,386 | 297 | 148 | NA | 546 | |
| HDV RNA (IU/mL) | 211,000 | TND | 1,390 | 4,438 | 7,890 | 4,253 | TND | NA | 1,580 | |
| HBV RNA (log10 copies/mL) | TND | TND | TND | ND | ND | ND | ND | NA | ND | |
| HBV pgRNA (log10 U/mL) | ND | ND | ND | 2.66 | 2.65 | <LLOQ | 1.72 | NA | 2.53 | |
| HBcrAg (log10 U/mL) | 3.2 | 4.5 | 4.4 | 4.40 | 4.60 | 4.50 | 4.30 | NA | 4.10 | |
| 001‐11 | HBsAg (IU/mL) | 12,382 | <0.005 | <0.05 | <0.05 | 15.7 | 71.4 | 461 | NA | 1,596 |
| anti‐HBs (mIU/mL) | 0.55 | 34,749 | 1,772 | 231 | 4.32 | 1.39 | 0.76 | NA | 0.38 | |
| HBV DNA (IU/mL) | <LLOQ | <LLOQ | TND | TND | <LLOQ | TND | TND | NA | 13 | |
| HDV RNA (IU/mL) | 12,100,000 | TND | TND | TND | TND | TND | TND | NA | TND | |
| HBV RNA (log10 copies/mL) | TND | TND | TND | ND | ND | ND | ND | NA | ND | |
| HBV pgRNA (log10 U/mL) | ND | ND | ND | TND | TND | TND | TND | NA | TND | |
| HBcrAg (log10 U/mL) | <LLOQ | <LLOQ | <LLOQ | <LLOQ | <LLOQ | <LLOQ | <LLOQ | NA | <LLOQ | |
| 001‐17 | HBsAg (IU/mL) | 8,314 | 0.26 | 214 | 4,754 | 6,250 | 5,479 | NA | 5,693 | 5,221 |
| anti‐HBs (mIU/mL) | <0.1 | 28 | <0.1 | <0.1 | 0.11 | <0.1 | NA | 0.29 | 0.22 | |
| HBV DNA (IU/mL) | 350 | <LLOQ | 10 | 1,992 | 1,386 | 1,894 | NA | 105 | 569 | |
| HDV RNA (IU/mL) | 1,690,000 | TND | TND | TND | TND | TND | NA | TND | TND | |
| HBV RNA (log10 copies/mL) | TND | TND | TND | ND | ND | ND | NA | ND | ND | |
| HBV pgRNA (log10 U/mL) | ND | ND | ND | 2.56 | 2.12 | 2.19 | NA | 2.19 | 2.17 | |
| HBcrAg (log10 U/mL) | <LLOQ | 2.9 | 2.3 | <LLOQ | <LLOQ | <LLOQ | NA | <LLOQ | <LLOQ | |
| 001‐20 | HBsAg (IU/mL) | 13,430 | 9,964 | 14,943 | 14,917 | 18,955 | 18,162 | NA | 21,993 | 22,503 |
| anti‐HBs (mIU/mL) | <0.1 | <0.1 | 0.19 | <0.1 | <0.1 | <0.1 | NA | <0.1 | <0.1 | |
| HBV DNA (IU/mL) | <LLOQ | 25 | 28 | 10 | 37 | 76 | NA | TND | TND | |
| HDV RNA (IU/mL) | 27,400 | 4,920 ‡ | 2,250 | 485 | 2,440 | 9,300 | NA | 7,880 | 11,800 | |
| HBV RNA (log10 copies/mL) | TND | TND | TND | ND | ND | ND | NA | ND | ND | |
| HBV pgRNA (log10 U/mL) | ND | ND | ND | <LLOQ | <LLOQ | <LLOQ | NA | TND | <LLOQ | |
| HBcrAg (log10 U/mL) | 4.5 | 4.6 | 4.3 | 4.60 | 4.60 | 4.60 | NA | 4.60 | 4.70 | |
| 001‐22 | HBsAg (IU/mL) | 7,836 | 917 | 1,225 | 1,281 | 1,508 | 1,386 | NA | 1,362 | 1,341 |
| anti‐HBs (mIU/mL) | <0.1 | <0.1 | 0.3 | 0.11 | <0.1 | <0.1 | NA | <0.1 | <0.1 | |
| HBV DNA (IU/mL) | 16 | <LLOQ | 23 | 10 | 18 | <LLOQ | NA | 160 | <LLOQ | |
| HDV RNA (IU/mL) | 1,090,000 | TND | 17 | 93.8 | 101 | 1,340 | NA | 1,090 | 2,310 | |
| HBV RNA (log10 copies/mL) | 2.22 | TND | TND | ND | ND | ND | NA | ND | ND | |
| HBV pgRNA (log10 U/mL) | ND | ND | ND | TND | TND | TND | NA | <LLOQ | <LLOQ | |
| HBcrAg (log10 U/mL) | 5 | 4.6 | 4.3 | 4.00 | 3.90 | 3.70 | NA | 3.40 | 3.60 | |
| 001‐24 | HBsAg (IU/mL) | 20,473 | 34,201 | 29,724 | 32,519 | 26,814 | 28,317 | NA | 29,012 | 25,184 |
| anti‐HBs (mIU/mL) | <0.1 | <0.1 | <0.1 | <0.1 | <0.82 | 0.1 | NA | 0.31 | <0.1 | |
| HBV DNA (IU/mL) | <LLOQ | <LLOQ | 10 | 35 | 13 | 1 | NA | 65 | 37 | |
| HDV RNA (IU/mL) | 1,890,000 | 1,090,000 § | 3,280,000 | 2,074,000 | 955,000 | 1,490,000 | NA | 1,190,000 | 906,000 | |
| HBV RNA (log10 copies/mL) | TND | TND | TND | ND | ND | ND | NA | ND | ND | |
| HBV pgRNA (log10 U/mL) | ND | ND | ND | <LLOQ | 1.93 | 2.12 | NA | TND | <LLOQ | |
| HBcrAg (log10 U/mL) | 2.8 | <LLOQ | <LLOQ | 3.10 | 3.10 | 3.00 | NA | 3.20 | 3.10 | |
| 001‐26 | HBsAg (IU/mL) | 5,854 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | NA | <0.005 | <0.005 |
| anti‐HBs (mIU/mL) | 0.25 | 11,291 | 32,543 | 30,018 | 15,920 | 7,773 | NA | 3,034 | 677 | |
| HBV DNA (IU/mL) | 256 | <LLOQ | TND | TND | TND | TND | NA | TND | TND | |
| HDV RNA (IU/mL) | 3,760,000 | TND | TND | TND | TND | TND | NA | TND | TND | |
| HBV RNA (log10 copies/mL) | TND | TND | TND | ND | ND | ND | NA | ND | ND | |
| HBV pgRNA (log10 U/mL) | ND | ND | ND | TND | TND | TND | NA | TND | TND | |
| HBcrAg (log10 U/mL) | 4.5 | 3.1 | <LLOQ | <LLOQ | <LLOQ | <LLOQ | NA | <LLOQ | <LLOQ |
Previously published (in graphic format)19 except HBV pgRNA.
PCR inhibition prevented HBV DNA determination.
HDV RNA rebound occurred after removal of REP 2139‐Ca.
Abbreviations: NA, no serum taken for assessment during this visit (see Materials and Methods); ND, not determined.
Participants
Baseline parameters of the participants have been published.( 19 ) Briefly, participants were Caucasian, noncirrhotic, 18‐55 years old, and with chronic HBV/HDV coinfection and ALT <10 times upper limit of normal. Before therapy, all participants were HBeAg negative, anti‐HDV antigen (anti‐HDAg) and HDV RNA positive, and with HBsAg >1,000 IU/mL at baseline. All participants in the REP 301‐LTF study completed 15 weeks of REP 2139‐Ca (500 mg once weekly [QW]) followed by 15 weeks of REP 2139‐Ca (250 mg QW) and pegIFN (180 µg QW), followed by 33 weeks of pegIFN (180 µg QW). A single participant in the REP 301 study (participant 001‐14) experienced pegIFN‐induced liver injury early after the introduction of pegIFN. This participant subsequently recovered after removal of therapy, had rebound of the HDV infection,( 19 ) and was not included in the REP 301‐LTF study.
Safety and Efficacy Assessments
At each visit, physical evaluation and FibroScan were accompanied by evaluation of liver, kidney, and hematologic and lipid function at Good Clinical Laboratory Practice (2011) and International Organization for Standardization (15189:2007) certified test laboratories used in the REP 301 study.( 19 ) Virologic responses assessed included quantitative HBsAg (qHBsAg; lower limit of quantitation [LLOQ] = 0.05 IU/mL) and anti‐HBs, qualitative HBeAg and anti‐HBe (all Abbott ARCHITECT), HBV DNA (Abbott real‐time polymerase chain reaction [RT‐PCR] assay; LLOQ = 10 IU/mL), and HDV RNA (Robogene Mark II; LLOQ = 400 IU/mL). Sample dilution was performed when HBsAg was >250 IU/mL and anti‐HBs >1,000 mIU/mL. During therapy and the first 24 weeks of follow‐up in the REP 301 study, total HBV RNA was determined by RT‐qPCR, as reported,( 19 ) with an LLOQ of 309 copies/mL. During the REP 301‐LTF study, HBV pgRNA was detected using the Abbott RealTime 0.2 mL HBV RNA Research Use Only (RUO).( 30 ) This dual‐target assay is designed to quantify HBV RNA from highly conserved targets in the core and X genes located at the 5′ and 3′ ends of the full‐length pgRNA, respectively. Assay values are reported as log U/mL as determined by calibration of the assay with a DNA secondary standard (Abbott Molecular, Des Plaines, IL) that is traceable to the World Health Organization (WHO) HBV DNA standard, where 1 U of reported HBV RNA is equal to 1 IU of HBV DNA. LLOQs were calculated to be 1.81 log10 U/mL and 1.65 log10 U/mL for core and X targets, respectively. This LLOQ translates to 152 copies/mL using the conversion factor from 1 IU of HBV DNA to 3.41 copies/mL HBV DNA using the WHO standard. The prototype HBsAg IC assay is an automated two‐step chemiluminescent microparticle immunoassay (CMIA) that uses mouse monoclonal antibodies to specifically capture HBsAg from the serum. After a wash step to remove unbound material, such as free immunoglobulin G (IgG), an acridinium‐labeled anti‐human IgG antibody is added to the reaction. Following a wash cycle, pretrigger and trigger solutions are added, and the resulting chemiluminescent reaction is measured as a relative light unit. The ARCHTECT HBsAg NEXT Qualitative assay, as described,( 29 ) is a CMIA for the qualitative detection of HBsAg in human serum and plasma. Analytic sensitivity at the assay cutoff is 0.005 IU/mL. HBcrAg is a Fujirebio RUO chemiluminescent immunoassay for use on the automated Lumipulse instrument; quantitation range is 3‐7 log U/mL. HBsAg, anti‐HBs, HBV DNA, and HDV RNA assessments were supervised by C.E., A.K., and U.D. HBsAg NEXT, HBsAg IC, HBV pgRNA, and HBcrAg assessments were supervised by M.A., J.G., V.H., M.C.K., and G.C. HBV total RNA and HBcrAg (Fujirebio) for baseline, end of therapy (EOT), and follow‐up week (FW)24 in these participants have been published.( 19 ) All plotted virology data are provided in raw form in Table 1.
Statistical Analysis
The REP 301‐LTF study was a small uncontrolled exploratory study limited to the enrollment of 11 participants from the REP 301 study( 19 ); therefore, no formal sample size calculation or statistical evaluation was done. For purposes of plotting, HDV RNA TND was right censored to 1 IU/mL; qHBsAg nonreactive was right censored to 0.01 IU/mL; anti‐HBs <0.1 mIU/mL was right censored to 0.1 mIU/mL; HBV DNA <LLOQ was right censored to 10 IU/mL; HBV DNA TND was right censored to 1 IU/mL; HBV RNA TND was right censored to 1 log10 copies/mL; HBV pgRNA TND was right censored to or 1 log10 U/mL; HBV pgRNA <LLOQ was right censored to 1.5 log10 U/mL; and HBcrAg <LLOQ was right censored to 2.5 log10 U/mL.
Results
As reported in the REP 301 study,( 19 ) 12 participants received 15 weeks of REP 2139‐Ca monotherapy (500 mg QW), followed by 15 weeks of REP 2139‐Ca (250 mg QW) and 180 µg of pegIFN, followed by 33 weeks of pegIFN monotherapy (180 µg QW). The REP 301 study included 24 weeks of follow‐up. One participant in the REP 301 study did not complete therapy due to pegIFN‐induced liver decompensation (see below), which resolved after removal of therapy, and was not eligible for enrollment in the REP 301‐LTF study.
Adverse Events
Enrollment in the REP 301‐LTF began in August 2016, with the first visit occurring for each participant 6 months after the last follow‐up visit in the REP 301 study. Including the 24‐week initial follow‐up in the REP 301 study and the extended 3‐year follow‐up in the REP 301‐LTF study, 3.5 years of follow‐up were completed by all 11 participants in November 2019. No serious adverse events (AEs) were observed during the study. Three participants experienced AEs considered unrelated to therapy. Participant 001‐09 experienced grade one thrombocytopenia and ALT elevation at FW 48 (FW 1 year [Y]) coincident with a rebound in HBV DNA and HDV RNA (Table 1). The thrombocytopenia in this participant self‐resolved without supportive therapy at FW 1.5Y, but grade one ALT elevation was persistent until the end of follow‐up. Liver function (bilirubin, albumin, and international normalized ratio [INR]) was normal throughout follow‐up (Table 1). Grade two ALT elevations in participant 001‐24 emerged after removal of therapy and were accompanied by HDV RNA and HBsAg rebound to baseline during pegIFN monotherapy, as reported.( 19 ) The ALT elevations persisted to the end of follow‐up in this participant with no alteration in liver function (Table 1). Participant 001‐26 had grade two thrombocytopenia at baseline that persisted throughout follow‐up (Table 1). Grade two thrombocytopenia attributed to pegIFN resolved in participant 001‐22 by FW 2Y (Table 1). As described during therapy in the REP 301 study,( 19 ) the introduction of pegIFN in participant 001‐14 was rapidly accompanied (within 2 weeks) by a biochemical flare (maximum ALT [ALTmax], 786 U/L; aspartate aminotransferase [AST]max, 592 U/L; bilirubinmax, 34.3 μmol/mL) with no alteration in albumin or decline in platelets beyond the normal thrombocytopenia accompanying pegIFN and similar to that observed in all other participants.( 19 ) Jaundice, ascites, and encephalopathy were absent during this decompensation, which recovered following the early removal of all therapy. HDV RNA continually declined during this biochemical flare (to 3 U/L), but this participant experienced virologic rebound of HBV and HDV during follow‐up.
Safety
No abnormal alteration in liver (bilirubin, albumin, INR), kidney, hematologic (other than described above), or lipid function was observed throughout follow‐up in any participant (Table 1, and data not shown).
Liver median stiffness (LMS) declined or became normal during follow‐up in 5 participants (all with virologic control or functional cure of HBV and HDV), was stable in 3 participants, and was elevated relative to baseline but stable in 3 participants (all with rebound of HBV and HDV) (Table 1).
Efficacy
At the end of therapy in the REP 301 study, HDV RNA was not detectable in 9 participants and was maintained in 7 of these participants at 24 weeks of follow‐up.( 19 ) HDV RNA was maintained TND throughout the 3.5‐year extended follow‐up in these 7 participants (Fig. 1A; Tables 2 and 3). HBsAg reduction to <0.05 IU/mL was achieved in 5 participants during therapy, persisted in 3 participants throughout follow‐up, and was achieved in a fourth participant during follow‐up (Fig. 1B). Samples with HBsAg reductions to <0.05 IU/mL during the REP 301 and REP 301‐LTF studies (Fig. 1B) were further subjected to analysis by the high‐sensitivity HBsAg NEXT assay (analytic sensitivity at the cutoff, 0.005 IU/mL) (Fig. 1C). This analysis confirmed that HBsAg during therapy in 5 participants continued below 0.05 IU/mL and following initial HBsAg NEXT reactivity at the first time points assessed in each of these 5 participants (Fig. 1B, left) was <0.005 IU/mL for the remainder of therapy. During extended follow‐up, 3 participants maintained HBsAg <0.005 IU/mL throughout 3.5 years of follow‐up, and an additional participant (01‐006) achieved HBsAg <0.005 IU/mL at the end of follow‐up (Fig. 1C; Tables 2 and 3).
FIG. 1.
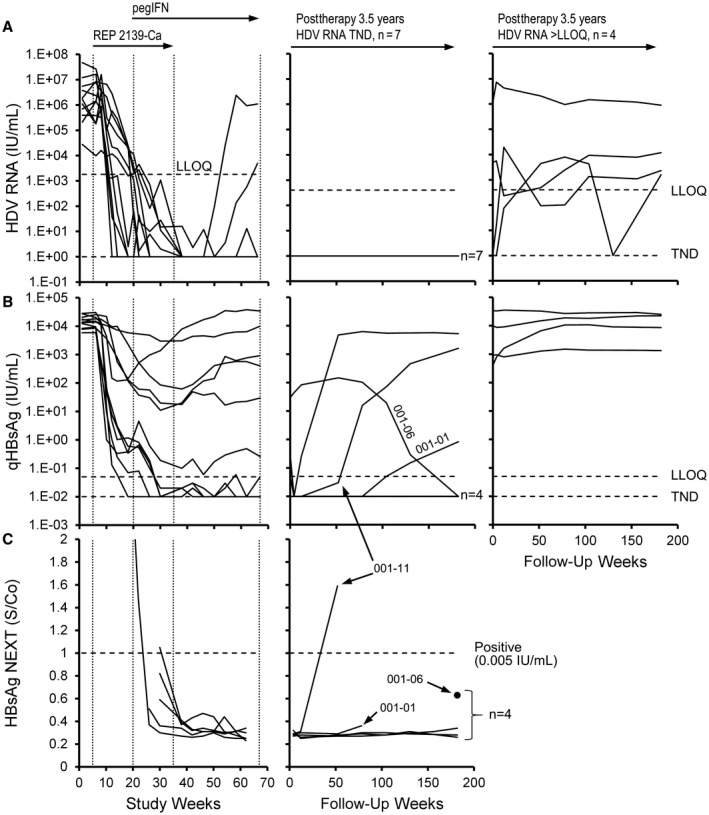
HDV RNA and HBsAg response during therapy in the REP 301 study and during the extended follow‐up in the REP 301‐LTF study. (A) Individual HDV RNA dynamics during therapy (left) and extended follow‐up in participants with HDV RNA either TND (middle) or >LLOQ (right) at the end of follow‐up are presented. (B,C) Reductions in qHBsAg below 0.05 IU/mL were evaluated by the high‐sensitivity HBsAg NEXT assay. Four participants had HBsAg reported at <0.05 IU/mL at the end of follow‐up by qHBsAg assay; all were confirmed to have HBsAg <0.005 IU/mL at the end of follow‐up by the HBsAg NEXT assay. HDV RNA and qHBsAg values from baseline to F1Y (FW52) have been published.( 19 )Abbreviation: S/Co, signal/cut‐off for positivity.
TABLE 3.
Summary of Outcomes in the REP 301‐LTF Study
| Follow‐Up Completed | Participants at 1 year* | Participants at 3.5 years | |
|---|---|---|---|
| Clinical response | Normal ALT | 8/11 (73%) | 8/11 (73%) |
| Normalization † of liver stiffness | 3/11 (27%) | 4/11 (36%) | |
| Continually declining ‡ liver stiffness | 2/11 (18%) | 2/11 (18%) | |
| HBsAg response | <100 IU/mL | 5/11 (45%) | 5/11 (45%) |
| <10 IU/mL | 5/11 (45%) | 5/11 (45%) | |
| <1 IU/mL | 5/11 (45%) | 5/11 (45%) | |
| <0.05 IU/mL | 5/11 (45%) | 4/11 (36%) | |
| ≤0.005 IU/mL | 4/11 (36%) | 4/11 (36%) | |
| Seroconversion | 5/11 (42%) | 4/11 (36%) | |
| HDV RNA response | >2 log10 reduction from baseline | 9/11 (82%) | 9/11 (82%) |
| TND | 7/11 (65%) | 7/11 (64%) | |
Data show number (percentage) of participants out of total.
Previously published.( 19 )
Liver median stiffness ≤7 kpa.
Liver median stiffness <baseline and continually declining during follow‐up.
Rapid increases occurred in anti‐HBs in 5 participants following the introduction of pegIFN (Fig. 2A, left in bold); this slowly declined in these participants during extended follow‐up in 4 participants, with HBsAg seroconversion persistent until the end of the extended follow‐up (Fig. 2A, middle; Table 2). HBsAg IC present at baseline declined in 9 of 11 participants during REP 2139‐Ca monotherapy, falling generally with the reduction in HBsAg, and stayed constant or only slowly increased during the rapid increases observed in anti‐HBs during the 15 weeks of combination therapy with pegIFN (Fig. 2B, left in bold). Following removal of REP 2139‐Ca, a rebound in HBsAg IC was observed in 3 participants (001‐01, 001‐06, and 001‐24) during pegIFN monotherapy, 1 of whom (001‐01) experienced HBsAg seroconversion during therapy (Fig. 2A,B). HBsAg IC levels declined in 10 of 11 participants during REP 301‐LTF follow‐up (Fig. 2B). HBsAg IC declines or increases were not correlated with ALT flares observed during therapy (Fig. 2C).
FIG. 2.
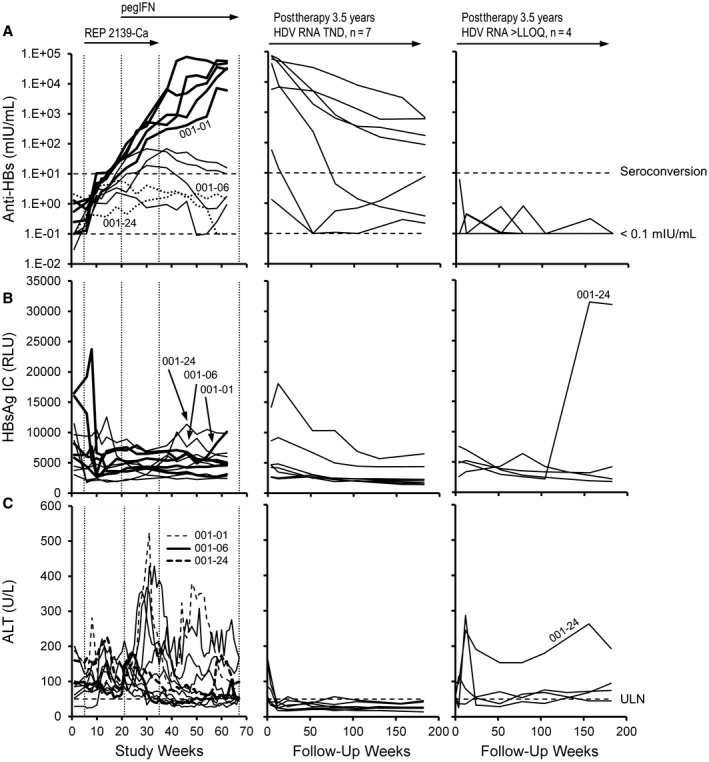
Changes in response in the REP 301 and REP 301‐LTF studies. (A) Anti‐HBs, (B) HBsAg IC, and (C) ALT during therapy (left) and in participants maintaining HDV RNA TND (middle) or >LLOQ (right) at the end of the extended follow‐up. Lines in bold during therapy in (A) and (B) indicate the 5 participants who experienced rapid elevations in anti‐HBs following addition of pegIFN. Anti‐HBs and ALT in 3 participants with mild rebounds in HBsAg IC during therapy in (B) are identified in (A) and (C). ALT and anti‐HBs values from baseline to F1Y (FW52) have been published.( 19 )Abbreviation: RLU, relative light unit.
Of these 7 participants who maintained HDV RNA TND throughout the extended follow‐up, virologic control of HBV was present in 3 participants and functional cure of HBV was present in 4 participants (Tables 2 and 4). HBV RNA TND and HBcrAg remained <LLOQ throughout the extended follow‐up in these 4 participants (Fig. 3B,C; Tables 2 and 4). HBV DNA TND and HBsAg <1 IU/mL were persistent in 5 of 7 of these participants (Fig. 3A; Tables 2 and 4), and HBcrAg was >LLOQ in all 7 participants (Fig. 3C; Tables 2 and 3C).
TABLE 4.
Summary of Outcomes in Patients With Functional Cure of HDV*
| Follow‐Up Completed | Participants at 1 year † | Participants at 3.5 years | |
|---|---|---|---|
| Functional cure of HDV during follow‐up | 7 | 7 | |
| HBV DNA response | ≤2,000 IU/mL | 7/7 (100%) | 7/7 (100%) |
| TND | 4/7 (57%) | 5/7 (71%) | |
| HBV virologic response | VC of HBV ‡ | 3/7 (43%) | 3/7 (43%) |
| FC of HBV § | 4/7 (57%) | 4/7 (57%) | |
| VC + FC: no therapy required low risk of progression, reduced risk of HCC | 7/7 (100%) | 7/7 (100%) | |
| HBV RNA | <LLOQ (1.65 log10 U/mL) | 7/7 (100%) || | 6/7 (86%) ¶ |
| TND | 7/7 (100%) || | 4/7 (57%) ¶ , # | |
| HBcrAg | <LLOQ (3 log10 U/mL) | 7/7 (100%) | 7/7 (100%) |
Data show number (percentage) of participants out of total.
HDV RNA TND with normal ALT.
Previously published.( 19 )
HBV DNA <2,000 IU/mL with normal ALT.
HBV DNA TND, HBsAg <0.05 IU/mL with normal ALT.
Total HBV RNA (includes pgRNA; LLOQ = 309 copies/mL).
HBV pgRNA (estimated LLOQ = 152 copies/mL).
All with HBsAg <0.005 IU/mL.
Abbreviations: FC, functional cure; HCC, hepatocellular carcinoma; VC, virologic control.
FIG. 3.
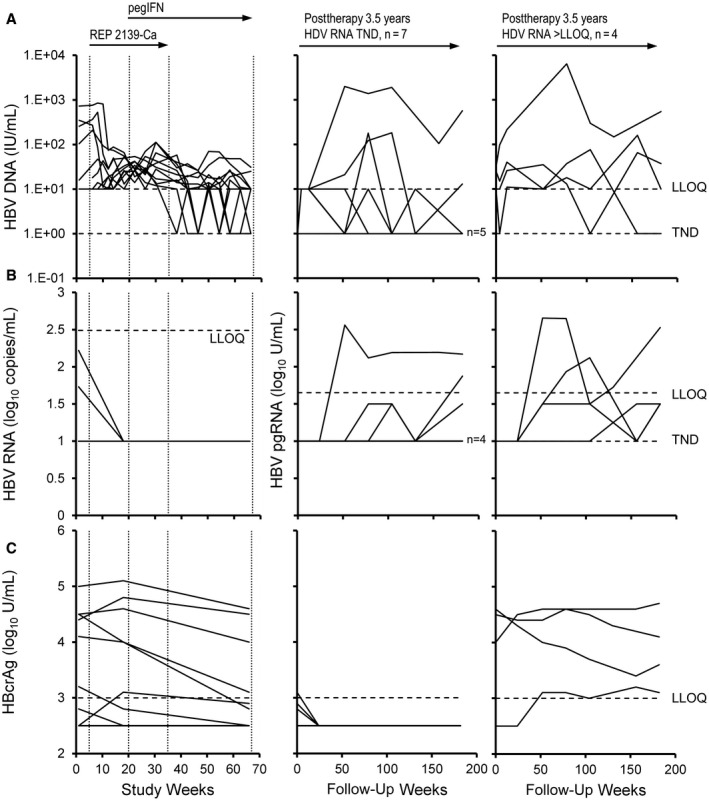
Changes in response in the REP 301 and REP 301‐LTF studies. (A) HBV DNA, (B) HBV RNA, and (C) HBcrAg during therapy (left) and in participants maintaining HDV RNA TND (middle) or >LLOQ (right) at the end of the extended follow‐up. In (B), total HBV RNA is presented during therapy and HBV pgRNA is presented during follow‐up (middle and right). HBV DNA, total HBV RNA, and HBcrAg from baseline to F1Y (FW52) were previously published.( 19 )
Discussion
The long‐term safety of combined treatment with REP 2139 and pegIFN has been demonstrated with a 2‐year follow‐up in HBeAg‐positive chronic HBV infection( 17 ) and a 1‐year follow‐up in HBeAg‐negative chronic HBV infection.( 28 ) The REP 301‐LTF study extends this safety envelope to 3.5 years and additionally confirms the long‐term stability of both virologic control and functional cure of HBV infection as well as HBsAg seroconversion and functional cure of HDV infection that have been reported.( 17 , 19 , 28 )
During the follow‐up, the only safety‐related observations were mild ALT elevations and thrombocytopenia subsequent to rebound in HBV or HDV infection and were not considered REP 2139 related. However a further improved safety profile with comparable efficacy might be realized if pegIFN were substituted with other immunotherapies, such as thymosin α1, which when added to REP 2139 yielded at least comparably improved antiviral responses relative to the addition of pegIFN to REP 2139 therapy in HBeAg‐positive chronic HBV infection.( 17 ) This highlights the importance of improving the response rate with other NAP‐based combination approaches.
LMS declines/normalization that were observed in 5 of 7 patients with functional cure of HDV in the current study are consistent with similar declines in LMS observed following REP 2139‐based combination therapy in HBV monoinfection.( 28 ) With the extended follow‐up significantly removing the confounding impact of liver inflammation on liver stiffness, these declines suggest reversal not only of liver inflammation but also fibrosis, but this remains to be confirmed in additional larger studies and by liver biopsy.
HBsAg levels in participants, previously reported as 0.00 IU/mL (nonreactive) during therapy (in 5 of 11) and at the end of follow‐up (in 4 of 11), by qHBsAg assay were shown to continue decreasing below 0.005 IU/mL using the more sensitive HBsAg NEXT assay. These data indicate that HBsAg clearance achieved during and persisting after therapy with REP 2139‐Ca + pegIFN was more profound than previously reported. Interestingly, HBsAg IC declined alongside HBsAg during REP 2139‐Ca monotherapy and either did not rebound or only increased slowly following the rapid increase in anti‐HBs and therapeutic transaminase elevations following the introduction of pegIFN. Moreover, HBsAg IC decline continued during the 3.5‐year follow‐up in 10 of 11 participants. These observations suggest that the clearance of HBsAg during therapy and persisting after therapy is not primarily achieved by clearance of HBsAg by anti‐HBs but may reflect elimination of HBsAg production in the liver by reduction or transcriptional inactivation of cccDNA, which is observed with NAPs in vivo,( 25 , 26 ) and/or removal of hepatocytes with integrated HBV DNA.( 10 ) Further studies will be required to investigate this hypothesis. The decline in anti‐HBs observed during follow‐up in the 4 participants with functional cure still resulted in significant anti‐HBs titers at the end of follow‐up (86‐677 mIU/mL), and these declines may be a result of the lack of HBsAg present to stimulate continual production of anti‐HBs as observed following resolution of HBV infection( 31 ) or several years after vaccination.( 32 ) Control of HBV DNA during therapy and follow‐up in participants maintaining HDV RNA TND was consistent with declines in and/or absence of detectable HBV RNA and HBcrAg, suggesting persistent inactivation or reduction of cccDNA in patients with virologic control or functional cure of HBV. These observations further validate the HBsAg clearance observed in these participants.
HBsAg response during therapy in the REP 301 study was not significantly different in the 4 patients with HBcrAg <LLOQ at baseline versus other patients with detectable HBcrAg at baseline.( 19 ) At the end of follow‐up in the REP 301‐LTF study, HBsAg was 0.83 IU/mL and <0.005 IU/mL in 2 of these participants but 1,596 IU/mL and 5,221 IU/mL in the other 2 participants; also, HBsAg <0.005 IU/mL was maintained at the end of follow‐up in 3 participants with significant (>4 log10 U/mL) HBcrAg present at baseline. These observations suggest that HBsAg clearance and the establishment of functional cure are not related to cccDNA inactivation/loss but to clearance of integrated HBV DNA. Larger studies will be required to further investigate this hypothesis.
The results from this extended follow‐up are promising and suggest that the immunologic control of HBV and HDV infection established by REP 2139‐based combination therapy is durable over the long term. Additional, larger, randomized controlled studies are warranted and should include participants with cirrhosis, which form a larger percentage of the HBV/HDV‐coinfected population than in HBV monoinfection.( 2 , 33 , 34 ) The regimen of 48 weeks of simultaneous therapy with tenofovir disoproxil fumarate (TDF), the magnesium chelate complex of REP 2139 (REP 2139‐Mg), and pegIFN has achieved the highest rates of functional cure of chronic HBV infection with NAP therapy to date,( 28 ) and this triple combination regimen will be evaluated in future controlled trials in HBV/HDV coinfection.
Similar analysis of high‐sensitivity HBsAg NEXT, HBsAg IC, HBV RNA, and HBcrAg are underway in the REP 401 study in participants who are HBeAg negative with HBV monoinfection and should shed additional light on the reproducibility of the responses observed in HBV/HDV coinfection. Additional important future clinical milestones will be the transition of REP 2139‐Mg to subcutaneous (SC) therapy. Injection site reactions (ISRs) are common with phosphorothioate oligonucleotides( 35 ) and are mirrored by analogous intravenous (IV) tolerability issues in early clinical studies with NAPs in the absence of chelate complex formulation.( 17 , 19 ) The recent demonstration of asymptomatic IV infusion of REP 2139‐Mg in the presence of TDF and pegIFN( 28 ) strongly suggests that ISRs with SC administration of REP 2139‐Mg will be minimal.
In conclusion, we demonstrated that combination therapy of chronic HBV/HDV coinfection with REP 2139‐Ca + pegIFN is associated with long‐term safety, persistent virologic control, and functional cure of both HBV and HDV infection.
Supported by Replicor Inc. (REP 301 and REP 301‐LTF studies) and Abbott Diagnostics (experimental virologic testing).
Registration on ClinicalTrials.gov: NCT02233075, NCT02876419.
Potential conflicts of interest: Dr. Anderson, Dr. Cloherty, Dr. Gersch, Dr. Holzmeyer, and Dr. Kohns own stock in and are employed by Abbott. Dr. Bazinet owns stock in and is employed by Replicor. Dr. Vaillant owns stock in and is employed by Replicor. The other authors have nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1. Gish RG, Yi DH, Kane S, Clark M, Mangahas M, Baqai S, et al. Coinfection with hepatitis B and D: epidemiology, prevalence and disease in patients in Northern California. J Gastroenterol Hepatol 2013;28:1521‐1525. [DOI] [PubMed] [Google Scholar]
- 2. Sureau C, Negro F. The hepatitis delta virus: replication and pathogenesis. J Hepatol 2016;64(Suppl.):S102‐S116. [DOI] [PubMed] [Google Scholar]
- 3. Wedemeyer H, Manns MP. Epidemiology, pathogenesis and management of hepatitis D: update and challenges ahead. Nat Rev Gastroenterol Hepatol 2010;7:31‐40. [DOI] [PubMed] [Google Scholar]
- 4. Chen HY, Shen DT, Ji DZ, Han PC, Zhang WM, Ma JF, et al. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta‐analysis. Gut 2019;68:512‐521. [DOI] [PubMed] [Google Scholar]
- 5. Bonino F, Heermann KH, Rizzetto M, Gerlich WH. Hepatitis delta virus: protein composition of delta antigen and its hepatitis B virus‐derived envelope. J Virol 1986;58:945‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Petersen J, Thompson AJ, Levrero M. Aiming for cure in HBV and HDV infection. J Hepatol 2016;65:835‐848. [DOI] [PubMed] [Google Scholar]
- 7. Farci P, Niro GA. Current and future management of chronic hepatitis D. Gastroenterol Hepatol (N Y) 2018;14:342‐351. [PMC free article] [PubMed] [Google Scholar]
- 8. Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. European Association for the Study of the Liver . EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370‐398. [DOI] [PubMed] [Google Scholar]
- 10. Freitas N, Cunha C, Menne S, Gudima SO. Envelope proteins derived from naturally integrated hepatitis B virus DNA support assembly and release of infectious hepatitis delta virus particles. J Virol 2014;88:5742‐5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abbas Z, Memon MS, Umer MA, Abbas M, Shazi L. Co‐treatment with pegylated interferon alfa‐2a and entecavir for hepatitis D: a randomized trial. World J Hepatol 2016;8:625‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wedemeyer H, Yurdaydin C, Dalekos GN, Erhardt A, Çakaloğlu Y, Değertekin H, et al.; HIDIT Study Group . Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med 2011;364:322‐331. [DOI] [PubMed] [Google Scholar]
- 13. Wedemeyer H, Yurdaydin C, Hardtke S, Caruntu FA, Curescu MG, Yalcin K, et al.; HIDIT‐II study team . Peginterferon alfa‐2a plus tenofovir disoproxil fumarate for hepatitis D (HIDIT‐II): a randomised, placebo controlled, phase 2 trial. Lancet Infect Dis 2019;19:275‐286. [DOI] [PubMed] [Google Scholar]
- 14. Heidrich B, Yurdaydin C, Kabacam G, Ratsch BA, Zachou K, Bremer B, et al.; HIDIT‐1 Study Group . Late HDV RNA relapse after peginterferon alpha‐based therapy of chronic hepatitis delta. Hepatology 2014;60:87‐97. [DOI] [PubMed] [Google Scholar]
- 15. Vaillant A. Nucleic acid polymers: Broad spectrum antiviral activity, antiviral mechanisms and optimization for the treatment of hepatitis B and hepatitis D infection. Antiviral Res 2016;133:32‐40. [DOI] [PubMed] [Google Scholar]
- 16. Vaillant A. REP 2139: antiviral mechanisms and applications in achieving functional control of HBV and HDV infection. ACS Infect Dis 2019;5:675‐687. [DOI] [PubMed] [Google Scholar]
- 17. Al‐Mahtab M, Bazinet M, Vaillant A. Safety and efficacy of nucleic acid polymers in monotherapy and combined with immunotherapy in treatment‐naive Bangladeshi patients with HBeAg+ chronic hepatitis B infection. PLoS One 2016;11:e0156667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roehl I, Seiffert S, Brikh C, Quinet J, Jamard C, Dorfler N, et al. Nucleic acid polymers with accelerated plasma and tissue clearance for chronic hepatitis B therapy. Mol Ther Nucleic Acids 2017;8:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bazinet M, Pantea V, Cebotarescu V, Cojuhari L, Jimbei P, Albrecht J, et al. Safety and efficacy of REP 2139 and pegylated interferon alfa‐2a for treatment‐naive patients with chronic hepatitis B virus and hepatitis D virus co‐infection (REP 301 and REP 301‐LTF): a non‐randomised, open‐label, phase 2 trial. Lancet Gastroenterol Hepatol 2017;2:877‐889. [DOI] [PubMed] [Google Scholar]
- 20. Blanchet M, Sinnathamby V, Vaillant A, Labonté P. Inhibition of HBsAg secretion by nucleic acid polymers in HepG2.2.15 cells. Antiviral Res 2019;164:97‐105. [DOI] [PubMed] [Google Scholar]
- 21. Boulon R, Blanchet M, Lemasson M, Vaillant A, Labonté P. Characterization of the antiviral effects of REP 2139 on the HBV lifecycle in vitro. Antiviral Res 2020;183:104853. [DOI] [PubMed] [Google Scholar]
- 22. Shamur MM, Peri‐Naor R, Mayer R, Vaillant A. Interaction of nucleic acid polymers with the large and small forms of hepatitis delta antigen protein [Abstract 942]. Hepatology 2017;66:504A. [Google Scholar]
- 23. Alves C, Cheng H, Tavanez JP, Casaca A, Gudima S, Roder H, et al. Structural and nucleic acid binding properties of hepatitis delta virus small antigen. World J Virol 2017;6:26‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang CC, Chang TC, Lin CW, Tsui HL, Chu PB, Chen BS, et al. Nucleic acid binding properties of the nucleic acid chaperone domain of hepatitis delta antigen. Nucleic Acids Res 2003;31:6481‐6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Noordeen F, Scougall CA, Grosse A, Qiao Q, Ajilian BB, Reaiche‐Miller G, et al. Therapeutic antiviral effect of the nucleic acid polymer REP 2055 against persistent duck hepatitis B virus infection. PLoS One 2015;10:e0140909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quinet J, Jamard C, Burtin M, Lemasson M, Guerret S, Sureau C, et al. Nucleic acid polymer REP 2139 and nucleos(T)ide analogues act synergistically against chronic hepadnaviral infection in vivo in Pekin ducks. Hepatology 2018;67:2127‐2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jansen L, Vaillant A, Stelma F, Koostra NA, Bazinet M, Al‐Mahtab M, et al. Serum HBV‐RNA levels decline significantly in chronic hepatitis B patients dosed with the nucleic acid polymer REP 2139‐Ca. J Hepatol 2015;62:S250. [Google Scholar]
- 28. Bazinet M, Pantea V, Placinta G, Moscalu I, Cebotarescu V, Cojuhari L, et al. Safety and efficacy of 48 weeks REP 2139 or REP 2165, tenofovir disoproxil, and pegylated interferon alfa‐2a in patients with chronic HBV infection naive to nucleos(t)ide therapy. Gastroenterology 2020;158:2180‐2194. [DOI] [PubMed] [Google Scholar]
- 29. Kuhns MC, Holzmayer V, McNamara AL, Sickinger E, Schultess J, Cloherty GA. Improved detection of early acute, late acute, and occult hepatitis B infections by an increased sensitivity HBsAg assay. J Clin Virol 2019;118:41‐45. [DOI] [PubMed] [Google Scholar]
- 30. Butler EK, Gersch J, McNamara A, Luk K‐C, Holzmayer V, de Medina M, et al. Hepatitis B virus serum DNA and RNA levels in nucleos(t)ide analog‐treated or untreated patients during chronic and acute infection. Hepatology 2018;68:2106‐2117. [DOI] [PubMed] [Google Scholar]
- 31. Greub G, Frei PC. Presence of low levels of anti‐HBs antibody in so‐called 'anti‐HBc alone' subjects. Liver 2001;21:380‐383. [DOI] [PubMed] [Google Scholar]
- 32. Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev 2006;28:112‐125. [DOI] [PubMed] [Google Scholar]
- 33. Buti M, Homs M, Rodriguez‐Frias F, Funalleras G, Jardí R, Sauleda S, et al. Clinical outcome of acute and chronic hepatitis delta over time: a long‐term follow‐up study. J Viral Hepat 2011;18:434‐442. [DOI] [PubMed] [Google Scholar]
- 34. Bockmann JH, Grube M, Hamed V, von Felden J, Landahl J, Wehmeyer M, et al. High rates of cirrhosis and severe clinical events in patients with HBV/HDV co‐infection: longitudinal analysis of a German cohort. BMC Gastroenterol 2020;20:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Meer L, Moerland M, Gallagher J, van Doorn MBA, Prens EP, Cohen AF, et al. Injection site reactions after subcutaneous oligonucleotide therapy. Br J Clin Pharmacol 2016;82:340‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]


