ABSTRACT
Naloxone-induced noncardiogenic pulmonary edema is a rare but reported entity that can occur following naloxone use in the reversal of opioid overdose. Proposed mechanisms include an adrenergic crisis secondary to catecholamine surge which causes more volume shift to pulmonary vasculature, subsequently leading to pulmonary edema. It appears to be more common when higher doses of naloxone are used. We present a case of a patient with opioid overdose came with altered mental status developed early features of pulmonary edema following the administration of multiple doses of naloxone. She responded well with the administration of diuretics and oxygen supplementation. Her oxygen requirements improved and didn’t require mechanical ventilation.
KEYWORDS: Naloxone, pulmonary edema, opioids
1. Introduction
Naloxone is a competitive opioid receptor antagonist that is used for the reversal of opioid effects. The side effects of it include seizures, hypertension, arrhythmias, respiratory depression, and rarely pulmonary edema. Naloxone-induced pulmonary edema (NPE) is a rare but lethal complication of naloxone and has been reported scarcely. The pathophysiology of NPE is secondary to unrestricted catecholamine surge following opioid reversal, constriction of pulmonary vasculature due to central neurogenic mechanism, and development of negative pulmonary edema resulting from breathing against a closed glottis. Here we report a case of NPE in a patient who came to the ED with altered mental status due to opioid overdose.
2. Case presentation
We present a 62-year-old female with a past medical history of hypertension, bipolar disorder, chronic obstructive pulmonary disease, and opioid use disorder. She was hospitalized at an inpatient psychiatric facility in 2017. She was started on valproate 500 mg twice daily and duloxetine 60 mg. The patient has been following with outpatient psychiatric clinic, who changed valproate to risperidone 2 mg twice daily. Her current home medications are oxycodone 15 mg 4 times a day, trazadone 100 mg daily at night, duloxetine 60 mg daily, risperidone 2 mg twice daily, and metoprolol tartrate 25 mg daily.
She was brought to the emergency department by EMS after she was found unresponsive by her family. When the EMS arrived at her home, she was given 4 mg naloxone intranasally, which resulted in the improvement of her mental status.
In the Emergency Department, vital signs on initial arrival were as follows: Temperature: 99.0 F, Blood Pressure: 115/74, Heart Rate: 64, Respiratory Rate: 15, Pulse Oximetry: 97% on room air. On physical examination, the patient was sleepy but arousable by verbal stimulation. She had small pupils bilaterally. She was oriented to place and person but wasn’t answering questions or following commands properly. Lung and heart exam revealed a normal bilateral vesicular breathing sounds with no wheezes or crackles. She had a regular rate and normal rhythm S1, S2 sounds no murmurs or gallops. She had no lower limb edema bilaterally. She was not in respiratory distress. She received additional three doses of IV 0.4 mg of naloxone as she was drowsy. Laboratory was remarkable for elevated creatinine of 1.74. A urine drug screen was positive for opiates, methadone, and benzodiazepines. The patient later in the emergency department was noted to become short of breath, with oxygen saturation decreasing to 80%. She was also tachycardic to 120 s. A Non-rebreathing mask was applied and resulted in an improvement of oxygen saturation to 95%.
Upon auscultation of the chest, she was noted to have bilateral crackles. A portable chest x-ray was obtained that showed findings highly suggestive of interstitial edema, pulmonary vascular congestion, and early pulmonary edema (Figure 1).
Figure 1.
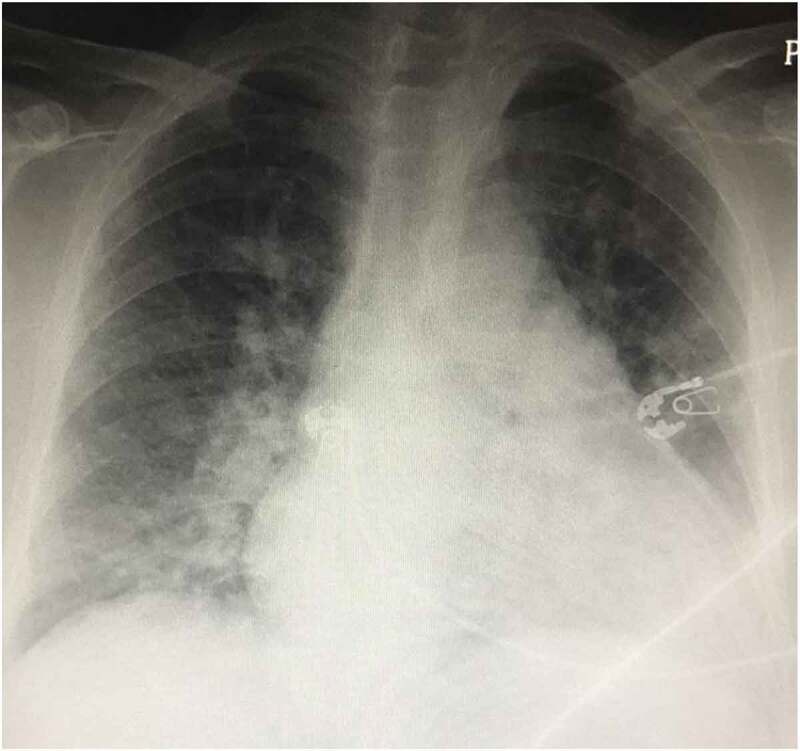
Arterial blood gases were obtained while the patient was on 2 L nasal cannula (28% oxygen) PH 7.37, PO2 64, PCO2 55, HCO3 28.5, O2 saturation of 94% consistent with hypoxemia and chronic respiratory acidosis.
COVID 19 PCR was negative. WBC 12.7 k/ul [Normal range 4.8–10.8], Hemoglobin 13.9 gm/dl, platelets 160 [130–400 k]. AST 16[15–41 u/l], bilirubin total 0.8 mg/dl [0.2–2 mg/dl],
Alk 62 u/l [Normal range 38–126], ALT 14 u/l [Normal range 14–54 u/l], BUN 28 [8–20 mg/dl], Cr 1.76 mg/dl [Normal range 0.4–1 mg/dl], glucose 238, calcium 8.9, sodium 131 mmol/l, potassium 5 mmol/l, CPK 38 u/l, blood alcohol level <10 mg/dl, troponin I 0.04 ng/ml, acetaminophen level 5 ul/ml, salicylate level <4, BNP 111. CT scan of the head without contrast showed no evidence of an acute intracranial abnormality. EKG showed normal sinus rhythm, left ventricular hypertrophy with repolarization abnormalities. She was given furosemide 40 mg intravenously and was admitted to the intensive care unit.
Initially, the patient remained sleepy but easily arousable. She was maintaining an oxygen saturation of 92 on 4 L nasal cannula. She never required intubation, a trial of noninvasive mechanical ventilation was not attempted because of fluctuating mental status. She was kept in the ICU for monitoring and started on IV furosemide 40 mg daily. Repeat ABG in the morning was still showing hypoxemia and chronic compensated respiratory acidosis with a PH of 7.41, PCO2 53, PO2 54, HCO3-30, oxygen saturation of 90% on 4 L nasal cannula. She was more awake the next day. The patient reports that she might have taken multiple oxycodone at one time. An echocardiogram was obtained, which showed normal global left ventricular systolic function with left ventricular ejection fraction is 55% to 60%, moderate concentric left ventricular hypertrophy, and a mildly dilated left atrium (4 cm). Diastolic grading could not be performed on the echo due to fusion of E/A waves (however other parameters were normal; E/e’ Ratio: 8.3, TR max velocity 2.8 cm). Suspicion for heart failure as a cause of pulmonary edema was low given the absence of significant findings on echo. Pulmonology consultation was obtained; the patient was started on incentive spirometry and acapella. On the third day, she was maintaining saturation of 100% on room air; however, she remained in sinus tachycardia up to 120 s, serum creatinine improved to 1.4 mg/dl. A repeat chest X-ray showed improvement of mild interstitial prominence (Figure 2).
Figure 2.
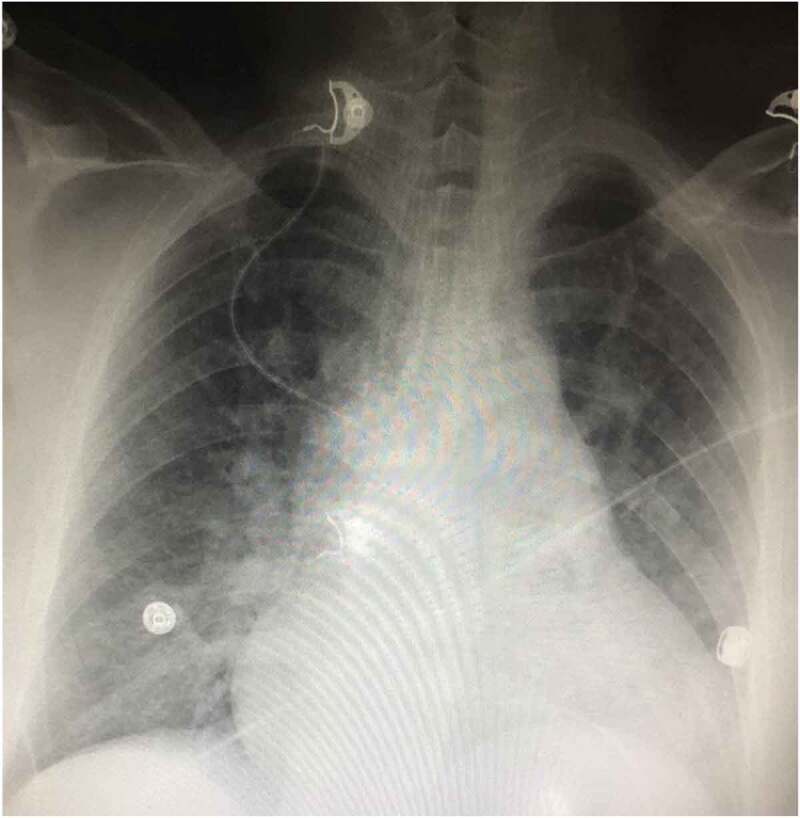
Psychiatry was consulted and restarted the patient on her psychiatric medications and determined that the patient had the capacity to make her decisions. The patient signed out against medical advice later that day.
3. Discussion
Naloxone, sold under the brand name Narcan has gained widespread popularity since its FDA approval in the 1970s. Through its intrinsic opiate antagonist property, it reverses respiratory depression induced by large doses of narcotics. Hence it is very useful in surgeries for postop anesthetic reversal and in opioid overdose. Although having many advantages, it is not without complications. Reports show hypotension, arrhythmias, seizures, and noncardiogenic pulmonary edema associated with naloxone use.
The incidence of naloxone-induced pulmonary edema has been rarely described. It has been reported mostly in patients with co-existing heart disease, obstructive sleep apnea, and even in young adults who received a regular dose of naloxone. This calls for extreme caution in the use of naloxone for narcotic reversal [1].
The proposed mechanism is that naloxone triggers a centrally medicated catecholamine surge, which results in an adrenergic crisis [1,2]. This adrenergic surge causes shifts in blood volume from the systemic or high-pressure bed to the pulmonary or lower pressure bed, which increases permeability and subsequently, pulmonary edema [3].
Research studies report that is a temporal relationship between the dose of naloxone administered and an increase in the risk of noncardiogenic pulmonary edema. Ideally, the appropriate dose to be used should be based on an agonist: antagonist ratio. These ratios were predetermined in vitro under equilibrium conditions [3,4]. In clinical practice, however, equilibrium conditions do not hold true. Various factors affect how patients respond to naloxone. First, patients have different sensitivity levels to narcotic use. The rate of narcotic elimination differs from person to person [3,4]. This is further complicated by the use of multiple narcotics and the inability to determine the dose of narcotics in patients with a substance abuse history.
This makes the application of agonist: antagonist ratio more challenging. The currently accepted dosing for opioid overdose is 0.4–2 mg, with repeated doses given until a meaningful response is achieved from the patient [5–7]. This definitely creates room for increased risk of complications.
In the case of our patient, we ruled out an allergic reaction due to the absence of tongue swelling, laryngeal edema, and skin rash. It was not cardiogenic pulmonary edema since the patient had no history of cardiac disease, and the echocardiogram was normal. Although the patient had no significant hemodynamic changes following naloxone administration, naloxone induced noncardiogenic pulmonary edema could not be excluded as studies have shown pulmonary edema is possible even in low doses [1,8–10].
The most important question that arises is what dose of naloxone is safe. The answer so far is that it varies amongst patients and different clinical scenarios. The advantages of naloxone in respiratory depression cannot be underrated. However, because of this potentially life-threatening complication, a secure airway is critical when administering naloxone. Also, the use of the lowest, most effective dose necessary to elicit reversal cannot be overemphasized.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Horng H, Ho M, Huang C, et al. Negative pressure pulmonary edema following naloxone administration in a patient with fentanyl-induced respiratory depression. Acta Anaesthesiologica Taiwanica. 2010;48(3):155–157. [DOI] [PubMed] [Google Scholar]
- [2].Jiwa N, Sheth H, Silverman R.. Naloxone-induced non-cardiogenic pulmonary edema: a case report. Drug Saf Case Rep. 2018;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Flacke JW, Flacke WE, Williams GD. Acute pulmonary edema following naloxone reversal of high-dose morphine anesthesia. Anesthesiology. 1977;47(4):376–377. [DOI] [PubMed] [Google Scholar]
- [4].Kosterlitz HK, Watt AG. Kinetic parameters of narcotic agonists and antagonists with particular reference to N-allyl noroxymorphone (naloxone). Br J Pharmacol. 1968;33:266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. https://www.uptodate.com/contents/naloxone-drug-information?sectionName=Adult
- [6].Farkas A, Lynch MJ, Westover R, et al. Pulmonary complications of opioid overdose treated with naloxone. Ann Emerg Med. 2020;75(1):39–48. [DOI] [PubMed] [Google Scholar]
- [7].Clarke SF. Naloxone in opioid poisoning: walking the tightrope. Emer Med J. 2005;22(9):612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Partridge BL, Ward CF. Pulmonary edema following low-dose naloxone administration. Anesthesiology. 1986;65:709–710. [DOI] [PubMed] [Google Scholar]
- [9].Schwartz JA, Koenigsberg MD. Naloxone-induced pulmonary edema. Ann Emerg Med. 1987;16(11):1294–1296. [DOI] [PubMed] [Google Scholar]
- [10].Reed CR, Glauser FL. Drug-induced noncardiogenic pulmonary edema. Chest. 1991;100(4):1120–1124. [DOI] [PubMed] [Google Scholar]


