Abstract
Head and neck cancer (HNC) is among the most common malignancy that has a profound impact on human health and life quality. The treatment for HNC, especially for the advanced cancer is stage-dependent and in need of combined therapies. Various forms of adjuvant treatments such as chemotherapy, phototherapy, hyperthermia, gene therapy have been included in the HNC therapy. However, there are still restrictions with traditional administration such as limited in situ therapeutic effect, systemic toxicity, drug resistance, etc. In recent years, stimuli-responsive drug delivery systems (DDSs) have attracted the great attention in HNC therapy. These intelligent DDSs could respond to unique tumor microenvironment, external triggers or dual/multi stimulus with more specific drug delivery and release, leading to enhanced treatment efficiency and less reduced side effects. In this article, recent studies on stimuli-responsive DDSs for HNC therapy were summarized, which could respond to endogenous and exogenous triggers including pH, matrix metalloproteinases (MMPs), reactive oxygen species (ROS), redox condition, light, magnetic field and multi stimuli. Their therapeutic remarks, current limits and future prospect for these intelligent DDSs were discussed. Furthermore, multifunctional stimuli-responsive DDSs have also been reviewed. With the modification of drug carriers or co-loading with therapeutic agents. Those intelligent DDSs showed more biofunctions such as combined therapeutic effects or integration of diagnosis and treatment for HNC. It is believed that stimuli-responsive drug delivery systems showed great potential for future clinic translation and application for the treatment of HNC.
Keywords: Stimuli-responsive drug delivery systems, head and neck cancer, intelligent biomaterials, anticancer therapy, tumor microenvironment
Introduction
Head and neck cancer (HNC) refers to epithelial malignancies of the paranasal sinuses, nasal cavity, oral cavity, pharynx, and larynx. The high incidence and prevalence of HNC profoundly influence human health and reduce the quality of life (Mehanna et al., 2011). In the 2017 global burden of disease study, around 678,900 incident cases were reported. According to the report from GLOBOCAN, around 450,000 estimated new cases of oral cavity and pharyngeal cancer and 23,000 estimated deaths were reported worldwide in 2018 (Fitzmaurice, 2018). Squamous cell carcinoma (SCC) is the most frequently diagnosed HNC. Previous researches have shown that the risk factors of HNC include tobacco intake, heavy alcohol consumption, human papillomavirus (HPV), betel quid, and additional factors such as certain microorganisms and environmental pollutants (Marur & Forastiere, 2016). The current management of HNC is stage-dependent and based on the multidisciplinary teamwork. Especially, Stage III or IV HNCs need the combination of surgical treatment and other forms of adjuvant treatment such as chemotherapy, phototherapy, hyperthermia, gene therapy, etc. (Feller & Lemmer, 2012; Chi et al., 2015). However, there are still some problems remains unsolved, including low tissue selectivity, poor drug solubility, unfavorable bioavailability, chemical instability and systemic adverse side effects of traditional drug administration (Koo et al., 2005; Godin et al., 2011; Epstein et al., 2012; Gong et al., 2012). Besides, drug resistance and cancer recurrence have become great challenges during the clinical application. Even worse, multidrug resistance (MDR) has been reported in many cases of HNC, which leads to the recurrence and progression of HNC (Pérez-Sayáns et al., 2010).
Drug delivery systems (DDSs) that could transport and release therapeutic agents to the tumor site have provide a promising strategy for the HNC therapy, which are usually consist of carriers and associated therapeutics for improved drug stability, local drug administration and controlled drug release (Davoodi et al., 2018). Previous studies have reported that drug could be transported by DDSs with the enhanced permeability and retention (EPR) effect or active targeting properties. They have been widely explored in the treatment of cancer for the higher curative efficiency and less side effect (Matsumura & Maeda, 1986; Greish, 2010; Wu & Zhou, 2015). In recent years, with the prosperous development of intelligent biomaterials, novel generation of DDSs with stimuli-responsive properties have drawn great attention in the HNC therapy (Kalaydina et al., 2018; Ketabat et al., 2019). Such intelligent DDSs could respond to chemical, physical or biological stimuli like pH, enzymes, light, magnetic field and so on, leading to more precise drug delivery, enhanced tumor penetration, better biocompatibility and more controllable drug release (Zhou et al., 2018; Qiao et al., 2019). Various kinds of stimuli-responsive DDSs such as micelles (Zhou et al., 2018), liposomes (Olusanya et al., 2018), hydrogels (Hajebi et al., 2019) and nanoparticles (Hossen et al., 2019) have been fabricated based on the biocompatible materials that could undergo structural changes including the protonation of specific groups, the cleavage of chemical bonds, the molecular conformational change, etc. in response to endogenous or exogenous stimuli, resulting in controlled drug delivery. With the development of the HNC studies, researchers have found that there occurs a unique tumor microenvironment (TME) during the progression of cancer including low pH, overexpressed specific enzymes, high levels of reactive oxygen species (ROS), upregulation of antioxidant and so on, which provide the opportunity for the application of internal stimuli responsive DDSs for more specific drug delivery and release (Koontongkaew, 2013; Weinberg et al., 2019). The stimuli-responsive DDSs are commonly researched for chemotherapy in the HNC research. Many types of chemotherapeutic drugs have been reported loaded on the intelligent DDSs for improved antitumor effect in recent years (Lee, Lee, Kim, et al., 2014; He et al., 2015; Wei et al., 2018). Gene therapy is another promising therapeutic modality for HNC treatment (Bali et al., 2013). In the DDSs research, gene materials could be transferred to tumor cells leading to cell death and inhibition of tumor growth (Liu, Xue, et al., 2014; Zhang, 2015; Lin et al., 2017). In recent years, more and more researches fabricated novel stimuli-responsive DDSs for gene delivery, particularly co-delivery with other antitumor agents (Ma, 2014; Wang et al., 2016). Moreover, during some other kinds of HNC therapy with exogenous stimulus, physical external stimuli responsive DDSs have also been utilized. In the phototherapy research for HNC, light-responsive therapies such as photodynamic therapy (PDT) and photothermal therapy (PTT) have been widely reported with significant in situ therapeutic effect (Fan, Zhu, et al., 2020). In other therapies like hyperthermia for HNC, stimuli-responsive DDSs have also drawn increasing attention. For instance, some magnetic field responsive DDSs have been reported to exhibit in situ heat generation and antitumor effect (Su et al., 2019).
Furthermore, the stimuli-responsive DDSs could also be modified, yielding multifunctional DDSs, for active drug delivery, integration of diagnosis and treatment, combined therapy and so on. For instance, for enhanced delivery efficient and specificity, increasing number of researchers developed DDSs with active targeting properties rather than that with EPR effect. Ligand/receptor-mediated endocytosis is among the most used strategies. The DDSs carriers could be linked with various kinds of ligands which could recognize specific receptors on tumor cells, thus leading to improved internalization to tumor cells and cell killing effect (Liu, Gao, et al., 2014; Li, Wen, You, et al., 2016; Nam et al., 2018). Some magnet-based DDSs or DDSs co-loading with fluorescent materials have been designed for theranostic applications which could combine cancer imaging with treatment (Kim et al., 2013; Bhana et al., 2015; Haedicke et al., 2015). Besides, co-delivery of antitumor agents such as chemotherapeutic drugs, photosensitizers (PSs) and gene materials could demonstrate synergistic therapeutic effect and reduced side effect, which showed great research potential recently (Ma, 2014; Miao et al., 2014; Wang et al., 2016).
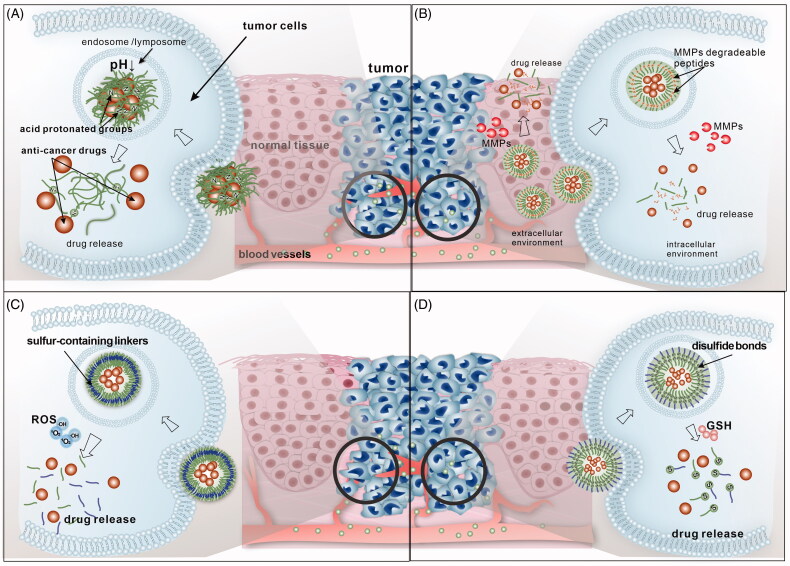
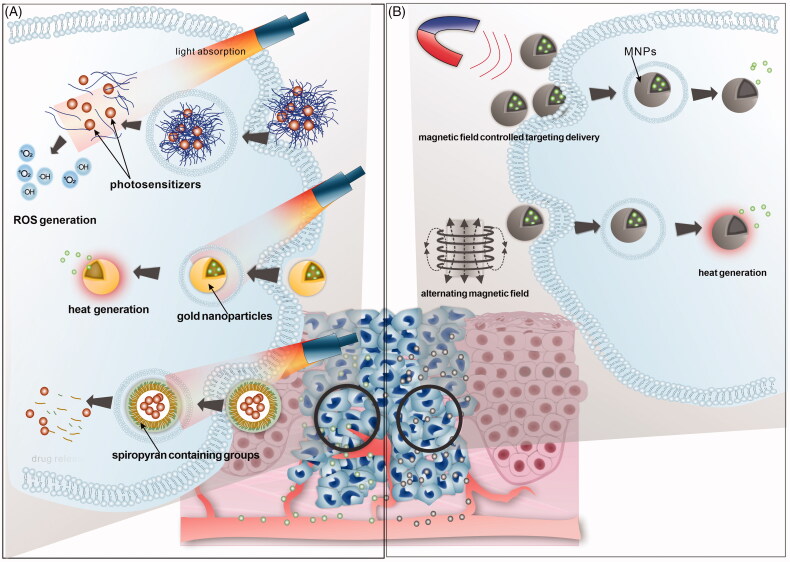
Herein, we reviewed the recent researches on stimuli-responsive DDSs for treatment of head and neck cancer. The DDSs could respond to the tumor TME of HNC such as acidic pH, ROS and matrix metalloproteinases (MMPs), reducing environment (Figure 1). Also, the DDSs that could be triggered by external physical stimuli have been reviewed (Figure 2). Other recent advances on the multistimuli responsive DDSs with more biofunctions have been summarized and prospected as well.
Figure 1.
Illustration of internal stimuli responsive DDSs commonly applied for HNC therapy in recent research. (A) PH-responsive DDSs; (B) MMPs-responsive DDSs; (C) ROS-responsive DDSs; (D) redox-responsive DDSs.
Figure 2.
Illustration of external stimuli responsive DDSs commonly applied for HNC therapy in recent research. (A) Light stimuli responsive DDSs; including smart DDSs for PDT, PTT and light-triggered release DDSs, (B) magnetic field responsive DDSs; including MNPs for magnetic field controlled targeting and MNPs for hyperthermia.
Internal-stimuli responsive DDSs
pH-responsive DDSs
pH-responsive DDSs are among the most reported intelligent DDSs in HNC therapy considering the acidic tumor microenvironment (TME) distinguishing from normal tissue (Figure 1(A)). Hypoxia condition and high rate of anaerobic glycolysis in the TME could cause increasing production of acidic metabolites by tumor cells in the extracellular matrix and develop a mildly acidic microenvironment, the pH of which drop to around 6.5, while the pH of normal tissues is about 7.4. The pH of cytoplasm or organelles are found even lower, such as endosomes (pH 5–6) and lysosomes (pH 4–5) (Matsumura & Maeda, 1986; Kanamala et al., 2016). The acidic TME could promote the extracellular-matrix remodeling and stimulates acid-activated proteases for increased local invasion, metastasis and resistance to treatments (Gillies et al., 2004; Rofstad et al., 2006). Yet, the pH discrepancy could also provide a platform for pH responsive DDSs. In recent years, increasing number of relative researches has been reported for the treatment of HNC to improve the efficiency and specificity of drug delivery (Karimi et al., 2016). Table 1 lists recent reports of pH-triggered DDSs for HNC. Various kinds of carriers including nanoparticles, micelles, hydrogels and so on have been applied and functionalized for pH-responsive drug delivery.
Table 1.
pH-responsive DDSs for HNC therapy.
| Effective pH | Carriersa | Drugsa | Tested cellb/model | Ligand/receptor | Remarks | Ref |
|---|---|---|---|---|---|---|
| 6.8 | OA-b-poly(Lys-g-DMA)-CD Nano-vehicle | DOX | KB | Increased doxorubicin uptake and higher tumor cytotoxicity | Lee, Lee, Kim, et al. (2014) | |
| 6.0 | Starch–(GC–DEAP) Nanogel | D-(KLAKLAK)2 peptide | KB | Increased tumor cell ablation | Kim et al. (2017) | |
| 5.0 | GQDs | CDDP | HSC3, SCC4, and CAL-27 | Combating hypoxia-induced chemoresistance and decreased toxicity of cddp | Wei et al. (2018) | |
| 4.0/5.0/6.0 | PAA-ACL-MSN | DOX | HNE-1 | Great biocompatibility, better therapeutic efficacy and low toxicity | Chen et al. (2014) | |
| 5.0 | MG⊂ Liposome |
DOX | KB | FA receptor | Enhanced specificity of drug release and less side effect | Liu, Gao, et al. (2014) |
| 5.5 | MSN-phSA | Am D | KB | FA receptor | Enhanced cellular drug uptake and selective drug release | Datz et al. (2016) |
| 5.0 | HMSN-GM-CS-FA | PA and DOX | KB | FA receptor | Synergistic antitumor efficacy of photothermal-, photodynamic- and chemotherapy | Yan et al. (2020) |
| 5/6.5 | UCNP-Al-NH-PEG-NH-FA | DOX | KB | FA receptor | A promising NIR imaging agent and an efficient anticancer drug | Tawfik et al. (2018) |
| 5.3 | TW-80-SeNPs | 5Fu and Cet | CNE | EGFR | Excellent MR imaging and tumor inhibition | Huang, Huang, et al. (2019) |
| 5.5/6.5 | bis-amino-terminated PEG | DOX | CAL-27 and SCC-25 | HN-1 peptide | Higher selective cytotoxicity and enhanced tumor inhibition | Wang, Wan, et al. (2017) |
| 5.0 | HPAH micelle | DOX and autophagy inhibitor (LY294002) | HN-6 and CAL-27 | Increased tumor sensitivity to DOX and higher proliferation inhibition of tumor cell | Saiyin et al. (2014) | |
| 5.0 | HPAE NPs | NaAsO2 and MTH1 inhibitor (TH287) | CAL-27 | More effective inhibition of tumor cell proliferation | Li et al. (2017) | |
| 5.0 | PEOz-PLA | DOX and P-gp inhibitor (TPGS1000) | KB and KBv | Desired tumor targeting, enhanced uptake and promising vehicle for overcoming MDR | Zhao et al. (2015) | |
| 6.5 | MOFs@gel | DOX and Cel | KB and SCC-9 | Inhibiting the growth of tumor | Tan et al. (2020) | |
| 6.5 | acetalated dextran polymer | Oxygen Nano-bubbles | CNE2 | Combating the hypoxia-induced resistance | Song et al. (2019) | |
| 5.5 | PEGylated LPH | DTX and P-gp inhibitor (TPGS) | SCC-7 | Inducing tumor cell apoptosis and reducing the tumor size | Kim et al. (2015) |
aThe carriers and drugs are shown in following abbreviations: OA: oleic acid; poly(Lys): Poly(L-lysine); DMA: 2,3-dimethylmaleic acid; CD: β-cyclodextrin; DOX: doxorubicin; GC: glycol chitosan; DEAP: 3-diethylaminopropylamine; GQDs: graphene quantum dots; CDDP: cisplatin; FA: folic acid; hyd: hydrazine; PAA: poly(acrylic acid) homopolymer; ACL: acid cleavable linker; MSN: mesoporous silica nanoparticle; MG: Malachite green carbinol base; phSA: benzene sulfonamide group; Am D: Actinomycin D; HMSN: hollow mesoporous silica nanoparticle; CS: chitosan; PA: pheophorbide a; UCNP: upconversion nanoparticles; Al: Alginate; PEG: Poly(ethylene glycol); TW-80: Tween-80; Se: selenium; NPs: nanoparticles; 5Fu: 5-fluorouracil; Cet: cetuximab; HPAH: hyperbranched polyacylhydrazone; HPAE: hyperbranched poly(amine-ester); MTH1: MutT homolog 1; PEOz: poly(2-ethyl-2-oxazoline); PLA: poly(D,L-lactide); P-gp, P-glycoprotein; MOFs: metal-organic frameworks; Cel: celecoxib; LPH: lipid polymer hybrid; DTX: docetaxel.
bCell lines: KB cell, HNE-1, CNE, CNE2, human nasopharyngeal carcinoma cell lines;HSC3, HN-6, SCC-4, SCC-7, SCC-9, SCC-25, CAL-27, Human oral squamous cell carcinoma cell lines; KBv, KB’s multidrug resistance counterpart.
Two types of pH-sensitive DDSs applied in HNC therapy (Taghizadeh et al., 2015; Li, Yang, et al., 2019). One is that the polymeric systems with ionizable groups undergo conformational or dissolution properties changes in response to altered pH values, thus leading to controlled drug release, which is more commonly applied in pH-sensitive DDSs for HNC. For example, Lee, Lee, Kim, et al. (2014) reported a kind of pH-responsive nano-vehicles with dimethylmaleic acid moieties which could be protonated and degraded at a slightly acidic pH. The DDS showed accelerated release of doxorubicin in pH 6.8 and significantly inhibit the viability of KB cells. Kim et al. (Kim et al., 2017) developed polysaccharidic nanogels with pH-responsive 3-diethylaminopropylamine for the delivery of proapoptotic d-(KLAKLAK)2 peptide. The DDS showed significant a significant increase in peptide release at acidic pH and KB cell ablation. Wei et al. (2018) synthesized a polyethylene glycol (PEG) modified graphene quantum dots-based nanocomposite loaded with Pt, which demonstrated pH-activated drug release and great tumor accumulation.
The other type of pH-responsive DDSs is that the acid-sensitive bonds attached to the polymer backbone may disintegrate in acidic TME, which could trigger the release of anticancer drug molecules. Chen et al. (2014) constructed a mesoporous silica nano-container with an acid cleavable linker for intracellular controlled release. With the degradation of the linker at lysosomal pH, the DDS showed controlled release of doxorubicin, leading to significant cytotoxicity on the HNE-1 cells. Saiyin et al. (2014) developed a pH-responsive nanomicelle for the treatment of oral squamous cell carcinoma (OSCC). The chemotherapy drug doxorubicin was conjugated onto the carriers by acylhydrazone linkages which were cleavable in acidic condition, leading to drug release leading to a significant proliferation inhibition of tumor cells.
Furthermore, positive targeting pH-responsive DDSs have also been fabricated in many studies. Various kinds of ligands have been conjugated DDSs have already been reported. Folic acid (FA) is widely utilized to functionalize the carriers since folate receptor (FR) have been reported highly expressed in many cancer cells but rarely in normal cells (Narmani et al., 2019). Therefore, FA conjugated DDSs such as carbon nanotubes (Wen et al., 2013), liposomes (Liu, Gao, et al., 2014), mesoporous silica nanoparticles (Datz et al., 2016; Yan et al., 2020) has been developed in recent years. For instance, Liu, Gao, et al. (2014) demonstrated FA conjugated malachite green carbinol-based liposome with highly efficient cellular and pH-sensitive doxorubicin release. Datz et al. (2016) synthesized pH-responsive mesoporous silica nanoparticles combined with FA for KB cell targeting and delivery of actinomycin D. Other kinds of pH-responsive DDSs such as anti-EGFR nanoparticles and HN-1 peptide grafted nanoparticles for HNC tumor cells accumulation has also show great potential of specific drug delivery (Wang, Wan, et al., 2017; Huang, Huang, et al., 2019). Other targeting agents like antibodies, nucleic acids, receptor ligands, etc. are also worth investigating in the future development of pH-responsive DDSs (Salahpour Anarjan, 2019).
The multidrug resistance (MDR) of HNC tumor cells has become one of the major problems of chemotherapy (Zhou et al., 2018). Beside of more specific drug delivery, several other strategies have also been applied in the pH-responsive DDSs research. Small molecules inhibitors like autophagy inhibitors, MTH1 inhibitors, P-glycoprotein inhibitors, etc. have been reported co-loading with chemotherapeutic agents in the DDSs (Saiyin et al., 2014; Zhao et al., 2015; Li et al., 2017). Another co-loading therapy was reported by Tan et al. (2020). The metal–organic framework-based hydrogel was loaded with doxorubicin and a nonsteroidal anti-inflammatory drug, celecoxib, which demonstrated synergistic effect and outstanding tumor inhibition efficiency. Other adjuvant therapies such as oxygen delivery systems has also been developed. Song et al. (2019) designed oxygen nanobubbles with pH-responsive release of oxygen in the TME, which could help overcome the hypoxia-induced resistance.
In recent years multifunctional DDSs have drawn intense attention as well. With integrated targeted imaging and treatment agents, the DDSs could provide simultaneous diagnoses and treatment for HNC. Huang, Huang, et al. (2019) designed 5-fluorouracil and gadolinium chelates loaded selenium nanoparticles covered with EGFR-targeted drugs Cet. The DDS showed increased intracellular accumulation, acid activated drug release and excellent magnetic resonance imaging capability. Tawfik et al. (2018) reported pH-activated upconversion nanoparticles loaded with doxorubicin, which demonstrated enhanced luminescence intensity for NIR imaging and significantly inhibit the viability KB cancer cells. These researches demonstrated new strategies with the combination of diagnoses and treatment of HNC, which provides great potential for further research and clinical application.
Matrix metalloproteinases (MMPs)-responsive DDSs
Matrix metalloproteinases (MMPs), a family of proteolytic enzymes which could degrade extracellular matrix protein. They are in low quantity and activity in normal situation but could be upregulated in many kinds of cancers including HNC, which have been considered closely related to cancer initiation, growth, and metastasis (Coussens et al., 2002; Gabriel et al., 2011). Especially, MMP-2, MMP-9, and Membrane type 1-MMP are most reported in the HNC research (Rosenthal & Matrisian, 2006; Chien et al., 2013; Kaomongkolgit, 2013; Virós et al., 2013). Thus, MMPs have been considered as biomarkers for cancer diagnostics and therapeutic targets. MMPs have also served as a type trigger for stimuli-responsive DDSs in recent years (Xiong & Gao, 2017; Yao et al., 2018).
In the research of HNC therapy, MMP-responsive DDSs for the local chemotherapy were reported recently. The DDSs carriers were usually incorporated with short linear peptide that could be degraded by MMPs (Figure 1(B)). For instance, Li, Tao, et al. (2019) designed doxorubicin micelles loading MMP-responsive hyaluronic acid (HA) hydrogels crosslinked by an MMP-2 degradable peptide for the treatment of OSCC. The hydrogel DDSs demonstrated MMP-2-responsive drug release and significant antitumor effect in vitro and in vivo. Damiani et al. (2017) fabricated a novel nano-ferritin complex, loaded with doxorubicin as well. The carrier was linked with a polypeptide shield and a short motif sequence for enhanced tumor accumulation and MMPs triggered drug delivery. The DDS showed enhances antitumor effects on several HNSCC cell lines. Many kinds of MMP-responsive DDSs based on carriers like liposomes, dendrimers, polymetric nanoparticles, etc. were reported for cancer therapy in recent years. Ligands incorporated DDSs and cell penetrating peptides linked DDSs for more specific intracellular tumor targeting were also investigated. MMP-responsive DDSs provide another strategy for intelligent drug delivery (Xiong & Gao, 2017; Yao et al., 2018). Therefore, more research on various kinds of MMP-responsive DDSs for HNC is of great potential for further study.
Reactive oxygen species (ROS)-responsive DDSs
ROS, including hydrogen peroxide (H2O2), singlet oxygen (1O2), superoxide (O2−), and hydroxyl radicals (HO•), are oxygen-carrying active molecules produced by mammalian mitochondria, endoplasmic reticulum, and NADPH oxidase. In normal situation, ROS in tissue play an important role in modulating the of functions of proteins, regulating cell signaling, killing pathogens, etc. (Burgoyne et al., 2013; Shim & Xia, 2013; Li, Wen, Wen et al., 2016). However, unbalanced ROS levels could be related to many diseases. It has been reported that the ROS production is upregulated during the progression of several types of cancers including HNC (Qian et al., 2018). The mitochondrial dysfunction, abnormal metabolic process metabolism and genetic mutations could cause the accumulation of oxidized protein, DNA, and lipids (Trachootham et al., 2009). Therefore, the enhanced ROS production could serve as a potential trigger for intelligent drug delivery. ROS-responsive DDSs have gained increasing attention in the treatment for HNC. ROS-cleavable groups such as organoborane-based groups and sulfur-containing groups were incorporated to carriers for ROS-triggered drug delivery (Saravanakumar et al., 2017) (Figure 1(C)). Thioketal groups were mostly reported in the ROS-responsive DDSs for HNC. They are a kind of sulfur-containing linkers that could be degraded through oxidative means, thus leading to ROS-activated drug release. Li, Wen, You, et al. (2016) constructed doxorubicin and alpha-TOS loading ROS-triggered drug delivery copolymer nanoplatform linked with TK groups for effective oral cancer therapy. Integrin αvβ3 targeting groups were also incorporated for active drug delivery. The nanoparticles showed improved cellular uptake and ROS-triggered drug release. The release of alpha-TOS could help stimulate cellular ROS. The DDS demonstrated significant inhibition of oral tongue Cal27 cancer cell line and antitumor. Similarly, Wang, Wang, et al. (2019) developed TK linked nanoparticles modified with another commonly used target, FA, for selective entry into cancer cells and ROS-responsive drug delivery. With specific release of doxorubicin, the DDS induced enhanced apoptosis of OSCC cells, which demonstrated robust therapeutic performance. ROS-responsive DDSs has shown great potential in the treatment for HNC. Yet the research is still limited and further research on in vivo application and more controllable in situ ROS level is desirable. Some researchers have tried to combined ROS-responsive DDSs with light-responsive for enhance ROS level in TME which will be described below.
Redox-responsive DDSs
As mentioned above, the increased generation of ROS induced high levels of oxidative stress in cancer cells, which also results in the compensatory upregulation of antioxidant. The reducing environment of tumors has been considered as an important target and biomarker of cancer, including HNC. The glutathione (GSH)/glutathione disulfide (GSSG) couple has been reported the most abundant redox couple in cancer cells. The concentration of GSH in tumor tissues has been found much higher than that in normal tissue (Bansal & Simon, 2018; Guo et al., 2018). The research on redox-responsive DDSs showed another novel strategy for the treatment of HNC. The redox-responsive linkers are incorporated in the carriers such as disulfide bonds and diselenide bonds, which could be cleavage by GSH, leading to controlled drug release (Huo et al., 2014) (Figure 1(D)). Sun et al. (2018) designed redox-responsive nanoscale micelles carrying doxorubicin for the treatment of laryngopharyngeal carcinoma. The micelles based on the heparosan (HEP) and deoxycholic acid conjugates (HSDs) could be internalized by human pharynx squamous carcinoma cell lines (FaDu cell) through clathrin-mediated endocytosis. With the link of disulfide bonds, the DDS demonstrated GSH-triggered drug release and significantly inhibited tumor cell growth. Fan, Wang, et al. (2020) reported a GSH-sensitive conjugated with disulfide bonds as well. The folate-targeted nanoparticles loaded with paclitaxel for the OSCC showed enhanced antitumor effect in vitro and in vivo. The research on redox-responsive DDSs is still limited right now. More research on multifunctional redox-responsive DDSs could be further conducted in the future.
External-stimuli responsive DDSs
Light-responsive DDSs
Light responsive DDSs has been widely studied in recent researches. Specific wavelength of light (such as ultraviolet, visible and infrared/near-infrared (NIR) fluorescence, etc.) represents as promising exogenous stimulus in the HNC tumor therapy, for its noninvasive feature and possibility of spatio-temporal control (Son et al., 2019; Wang et al., 2020). Especially near infrared (NIR) laser with better penetration and minor damage to tissues has been applied in quite many reports. The light responsive DDSs were mainly used in photodynamic therapy and photothermal therapy (Figure 2(A)). Some other light-trigged drug release systems were also developed (Gao et al., 2016; Civantos et al., 2018; Li et al., 2020).
Photodynamic therapy (PDT) has been recognized as an important therapy for HNC, with low side effect and little influence on the tissue structure and function of HNC patients (Meulemans et al., 2019). In the PDT process, photosensitizers (PSs) are activated by the light of specific wavelength and convert oxygen into singlet oxygen or other reactive oxygen species (ROS), leading to apoptosis, necrosis or autophagy of tumor cells. There are mainly two generations of PS, which is represented by photofrin and 5-aminolevulinic acid (Fan, Zhu, et al., 2020). However, the light-associated toxicity and hypoxia -induced drug resistance limits application of PDT (Agostinis et al., 2011). In recent years, increasing number of researchers have designed PS loading DDSs and proved these DDSs could help reduce light-associated toxicity and enhance the drug accumulation. Various kinds of nanocarriers have been developed for the more specific drug delivery and better biocompatibility, such as lipid-based nanoparticles, calcium phosphate nanoparticles, magnetic nanoparticles and other copolymer nano-carriers (Wang, Fei, et al., 2014; Haedicke et al., 2015; He et al., 2015; Hong et al., 2016).
Photothermal therapy (PTT) refers to induced hyperthermia in the targeted tissues under light irradiation for anti-neoplasm effect, which is considered as a promising strategy for HNC treatment (Curry et al., 2014; Fan, Zhu, et al., 2020). Optical absorbing materials have been used to convert light energy into heat under external illumination, among which gold nanoparticles (AuNPs) are most commonly applied. AuNPs have been reported to exhibit strong surface plasmon resonance (SPR) and thus convert light to heat efficiently (Link & El-Sayed, 2000). Gold-coated magnetic nanoparticles have also been applied in PTT researches. Moreover, some other gold loading DDSs have been developed as well. For example, Rao et al. Rao et al. (2018) reported a platelet-facilitated PTT (PLT–PTT). Gold nanorods (AuNRs) were loaded into PLTs for tumor sites targeting and enhanced PTT effect. Table 2 listed current reports of light-triggered DDSs for HNC therapy.
Table 2.
Multifunctional light-responsive DDSs for HNC therapy.
| DDS carriers | Anticancer agentsa | Functions | Refs. |
|---|---|---|---|
| Fibronectin-mimetic peptide conjugated ION | Photosensitizer (Pc 4) | Tumor targeting; MRI imaging and PDT | Wang, Fei, et al. (2014) |
| Self-assembled core–shell nanoparticles | CDDP; pyrolipid | Chemotherapy and PDT | He et al. (2015) |
| Polyglutamate nanoparticles | ICG | PDT and PTT | Tarassoli et al. (2017) |
| Human serum albumin nanoparticles | ICG; CDDP | Tumor targeting; PDT, PTT and chemotherapy | Wang, Xie, et al. (2019) |
| RGDfK peptide conjugated calcium phosphate nanoparticles | Temoporfin; fluorescent dye molecule (DY682-NHS) | Tumor targeting; NIR fluorescence imaging and PDT | Haedicke et al. (2015) |
| Gold nanorods | siRNA oligos | Gene therapy and PTT | Wang, Yu, et al. (2016) |
| Evans blue derivative-functionalized gold nanorods | Hydroxycamptothecin | tumor targeting; PTT and chemotherapy | Wang et al. (2018) |
| PDPN antibody-conjugated gold nanoparticles | DOX | tumor targeting; PTT and chemotherapy | Liu et al. (2020) |
| Gold nanorods | Rose Bengal molecules | PDT and PTT | Wang, Wang, et al. (2014) |
| Au nanoring | Sulfonated aluminum phthalocyanines | PDT and PTT | Chu et al. (2016) |
aThe anticancer agents are shown in the following abbreviations: CDDP: cisplatin; ICG: indocyanine green; DOX: doxorubicin.
Light-stimuli released systems have also been demonstrated in recent DDSs researches. Copolymers with light sensitive and degradable groups could serve as carriers, loaded with various antitumor agents especially chemotherapy drugs. Xing et al. (2015) reported doxorubicin loading spiropyran-containing upconversion nanoparticles. The spiropyran amphiphilic group could be shift to a hydrophilic one with NIR exposure and detach from the copolymer carriers, leading to drug release. The DDS demonstrated active targeting to KB cells and NIR-triggered drug release.
Furthermore, multifunctional DDSs have shown great potential in the HNC treatment in recent years. In the research of light-responsive DDSs, the combination of PDT and PTT were mostly reported, of which the synergistic therapeutic effect were verified. Other therapy modalities like chemotherapy and gene therapy were also combined to the phototherapy (Agostinis et al., 2011). For instance, Song et al. (2020) designed chlorin e6 (Ce6) linked DDS co-loaded with cisplatin (CDDP) and metformin for Head and Neck Squamous Cell Carcinoma. The PS Ce6 showed laser-triggered PTT and PDT effect while CDDP and metformin served as the chemotherapeutic core. Nam et al. (2018) loaded polydopamine (PDA)-coated spiky gold nanoparticles (SGNPs) with a subtherapeutic dose of doxorubicin (DOX) for the chemo-photothermal therapy (chemo-PTT) or HNSCCs. The nanoparticles elicit robust antitumor T-cell immunity and exert strong therapeutic efficacy against tumors.
Indocyanine green (ICG), a kind of clinically used NIR-absorbing dye was reported able to convert light energy into heat due to internal conversion. Meanwhile, ICG was also reported cytotoxic effect on tumor cells with the generation of ROS (Wang, Xie, et al., 2017; Wang, Xiao, et al., 2019). Therefore, several studies on the multifunctional light-responsive DDSs loaded with ICG were reported. Tarassoli et al. (2017) developed copolymer nanoparticles loaded with ICG for NIR-trigged head and neck cancer therapy with improved drug accumulation and antitumor effect. Wang, Xie, et al. (2019) fabricated ICG-cisplatin nanoparticles for the treatment of OSCC, the coordination bond of which could be cleaved by a NIR-induced photothermal effect of ICG. The results showed that the nanoparticles showed synergistic effects of PTT/PDT and chemotherapy.
As mentioned before, gene therapy is considered as a promising HNC. Multifunctional light-responsive DDSs combined with gene therapy has been fabricated by several researches, among which RNA interference by the small-interfering RNA (siRNA) were mostly used strategy. Wang et al. (2016) developed gold nanorods linked with siRNA targeting Bcl-2 associated athanogene domain 3(BAG3) BAG3 could be induced by the heat shock response in PTT, leading to thermoresistance. The multifunctional nano-complex showed silence of the BAG3 expression in the Cal27 and improved PTT effect. Ma et al. (2017) reported Ce6 linked nanoparticle carrying Wnt-1 siRNA for oral cancer. Aberrant activated Wnt-1/β-catenin pathway was considered closely related to epithelial–mesenchymal transition (EMT) and progression of tumors. The DDS were proved to reduce the expression of Wnt-1, β-catenin and vimentin. The PDT effect was also enhanced. Ma et al. (2014) fabricated a star-shaped copolymer loaded with chemotherapeutic drugs docetaxel and MMP-9 shRNA plasmid for nasopharyngeal cancer therapy. The DDS showed light-enhanced gene transfection efficiency as the carrier was functionalized with porphyrin–arginine. The MMP-9 protein expression in HNE-1 cells was significantly reduced and the DDS showed synergetic antitumor effect compared to docetaxel or MMP-9 plasmid used alone.
Positive targeting was also reported in the light-responsive DDSs research. Ligands conjugated carriers were been fabricated for more specific drug delivery. For instance, Akbarzadeh et al. (2018) reported a FA-conjugated nanoparticle for the delivery of PS 5-aminolevulinic acid which demonstrated selective endocytosis into KB cells and increased effect of PDT. The SGNPs constructed by Nam et al. (2018) for chemo-photothermal therapy were also modified with low-density lipoprotein receptor (LDLR) for active tumor targeting.
Multifunctional light-responsive DDSs has been applied in the design of integrated materials of diagnosis and treatment for HNC as well. Near-infrared fluorescence dyes could be co-loaded in the DDSs for tumor imaging and tumor ablation. Haedicke et al. (2015) fabricated calcium phosphate nanoparticles conjugated with the PS temoporfin and fluorescent dye molecule DY682-NHS, which combined NIRF optical imaging and PDT therapy. Wang et al. (2018) developed Evans Blue derivative gold nanoparticles for imaging-guided cancer therapy. DDSs based on magnetic nanoparticles were also utilized as multifunctional tools for phototherapy and magnetic resonance imaging (MRI). For instance, Wang, Fei, et al. (2014) developed iron oxide nanoparticles loaded with PDT drug, Pc 4, showing improved MRI contrast and enhanced PDT efficacy. Further researches on multifunctional light-responsive DDSs are worth conducting.
Magnetic field-responsive DDSs
The magnetic nanoparticle (MNPs)-based DDSs that could respond to external magnetic field have been extensively studied in diagnosis and treatment of the cancer (Figure 2(B)). They could serve as carriers for the delivery of antitumor agents controlled by external magnetic field, which could also be modified with various kinds of functional groups for stable drug loading, gene delivery, active tumor targeting and so on (Cardoso et al., 2018). In the treatment of HNC, MNPs have drawn increasing attention. Zhang et al. (2020) fabricated bleomycin loading hollow mesoporous magnetic nanoparticles for the treatment of HNC. The DDS were targeted to the focal area under the external magnetic field and demonstrated sustained drug release, which could induce the apoptosis of several kinds of head and neck cancer cell lines. Miao et al. (2014) have developed new PEI-modified Fe3O4 nanoparticles as a gene transfer vector to mediate transfection of OSCC by the targeting plasmids transport with an external magnetic field. The improved transfection efficiency and antitumor effect was verified in vitro and in vivo.
Furthermore, another important of MNPs is the heat generation induced by alternating magnetic field (LeBrun & Zhu, 2018). Therefore, MNPs could also serve as hyperthermia agents in the magnetic field-responsive DDSs, leading to antitumor effect. Su et al. (2019) designed the anti-CD44 antibody-modified superparamagnetic iron oxide nanoparticles targeting cancer stem cells of HNC. The nanoparticles could penetrate into the cells through the cell membrane, and inhibit grafted tumor growth in mice by hyperthermia induced in a magnetic field.
Other multifunctional magnetic field-responsive DDSs has also been reported with the development of the modification of MNPs, such as DDS with combination of diagnostics and treatment, ligand linked DDS and MNP-based multistimuli-responsive DDS which will be describe below.
Dual and multi stimuli-responsive DDSs
As is showed above, great progress has been made in the stimuli-responsive DDSs for the treatment of HNC in recent years. Researches have also made great efforts to modify stimuli-responsive DDSs with multiple functions for more controllable drug delivery, better biocompatibility and less drug resistance. The carriers were modified with functional segments for active tumor targeting, better stability of DDS, more drug loading, etc. Various kinds of diagnostic and/or therapeutic agents were co-loaded to improve the treatment efficiency, overcome drug resistance, simplify the diagnosis and treatment procedures, and so on.
Moreover, another important strategy to further finetune drug release and enhance therapeutic efficacy of stimuli-responsive DDSs has gained great attention in recent years; that is, the development of novel dual or multi stimuli activated DDSs, which could respond to more than one internal and/or external trigger simultaneously or in sequence (Bhatnagar & Venuganti, 2015; Qiao et al., 2019). Table 3 lists recent research of emerging dual or multistimuli responsive DDSs for HNC therapy. DDSs with response to multiple TME triggers like pH, ROS, redox were reported with improved TME sensitivity. Zhang et al. (2016) prepared A pH and redox responsive mesoporous silica nanoparticles loaded with doxorubicin (DOX). The disulfide bond and H-bond could be degraded by GSH and acidic condition leading to increased drug release and inhibition of human tongue squamous cell carcinoma TCA8113 cell lines. Liu et al. (2019) constructed another kind of pH and redox responsive DDS for advanced nasopharyngeal carcinoma. The multifunctional DDS also showed folate-targeted codelivery of docetaxel (DOC) and tissue factor pathway inhibitor 2 TFPI2, which made cancer cells more sensitive to DOC and enhanced the antitumor effect compared with monochemotherapy.
Table 3.
Dual/multiresponsive DDSs for HNC therapy.
| Stimuli | Druga | Target cellb/model | Mechanism | Remark | Ref |
|---|---|---|---|---|---|
| pH, redox | DOX | TCA8113 | Sulfur–sulfur bonding of the molecule in SMIP decomposed by an acidic pH and GSH | Inhibited tumor cells growth, low cytotoxicity and great biocompatibility | Zhang et al. (2016) |
| pH, redox | DOC and TFPI-2 | HNE-1 | The hydrazone bond split by acidity and the disulfide linkages degraded in the cytosol increase the drug release | Induced cell apoptosis, reduced cell invasion and decreased the tumor size | Liu et al. (2019) |
| pH, light | Photosensitizer (C60) | KB | DMA blocks cleavage-activated drug release and cytotoxic singlet oxygen generation | Enhanced photodynamic tumor inhibition | Kim et al. (2014) |
| pH, light | Photosensitizer (ce6) | KB | DEAP protonation-mediated drug release and high-yield generation of cytotoxic singlet oxygen | Enhanced photodynamic tumor inhibition | Lee, Oh, et al. (2014) |
| Light, MMPs | DOX | SCC-15 | MMP2-responsive accelerated degradation of the hydrogel | Increased antitumor efficacy and fluorescence imaging | Wang, Fu, et al. (2019) |
| Light, ROS | DOX | KB | ROS-responsive self-destruction and following H2O2-responsive drug release | Increased antitumor efficacy and decreased systemic toxicity | Wang, Zhai, et al. (2019) |
| Light, ROS | Photosensitizer (Cu2−xS) | HeLa tumor cell line-derived xenograft and HNSCC patient-derived xenograft | MnS-mediated O2 production overcomes hypoxia and NIR-triggered Cu2−xS leads to enhanced PD effect | Increased antitumor efficacy and magnetic resonance imaging | Huang, Deng, et al. (2019) |
| Light, ROS | DOX and photosensitizer (HP) | CAL-27 | ROS-responsive thioketal bond cleavage leading to release of drug and photosensitizer for PDT | Increased antitumor efficacy | Shi et al. (2018) |
| Light, magnet | SiNC | KB | A magnetic field guide the release of photosensitizer at tumor for synergistic PDT/PTT | Increased antitumor efficacy and magnetic resonance imaging | Bhana et al. (2015) |
| Thermo, magnet | DOX | SQ20B | magnetic hyperthermia-mediated drug release | Increased antitumor efficacy and magnetic resonance imaging | Kim et al. (2013) |
| pH, magnet | BTZ | SCC-7 | pH-responsive drug release and magnet-triggered hyperthermia | Increased antitumor efficacy | Sasikala et al. (2015) |
| pH, ROS, light | DOX | WSU-HN6 | pH-triggered Fe2+ coordination bonds cleavage for drug release and photothermal therapy; H2O2-induced catalytic generation of hydroxyl radicals | Combined photothermal therapy, chemotherapy, and Fenton reaction-based tumor therapy | Jin et al. (2018) |
| pH, redox, light | Taxol | KB | pH-responsive boronate ester and DTT-responsive disulfide bonds for triggered drug release | Increased antitumor efficacy and confocal laser scanning microscopy imaging | Lee et al. (2014) |
aThe drugs are shown in the following abbreviations: DOX: doxorubicin; DOC: docetaxel; TFPI: tissue factor pathway inhibitor; ce6: chlorin e6; Cu2−xS: copper sulfide with numerous copper-deficient stoichiometries; HP: hematoporphyrin; SiNC: silicon 2,3-naphthalocyannie dihydroxide; BTZ: bortezomib.
bCell lines: KB cell, HNE-1, Human nasopharyngeal carcinoma cell lines; SCC-7, SCC-15, SQ20B, TCA8113, WSU-HN6, CAL-27, human oral squamous cell carcinoma cell lines.
More researches were conducted on the intelligent DDSs with combined response to internal and external triggers, since physical exogenous stimuli could be more controllable and flexible. Such dual stimuli-responsive DDSs were widely applied in the phototherapy for HNC. PDT or PTT agents were loaded in pH or MMP sensitive drug carriers for TME controlled drug release and light triggered antitumor treatment (Tarassoli et al., 2017). Meanwhile, chemotherapy agents were also reported co-delivered for the improved chemophoto therapy (Wang, Hu, et al., 2019). Fluorescence dyes could also be co-loaded for simultaneous tumor detection. Furthermore, in view of the ROS generation triggered by light during the PDT for HNC, light and ROS dual responsive DDSs were considered promising in recent researches. Light-responsive agents were loaded in ROS-sensitive carriers, which lead to enhanced ROS generation for PDT and synergistical promotion of drug release combined with endogenous ROS. For instance, Shi et al. (2018) developed ROS-responsive nanoparticles co-loaded with doxorubicin (DOX) and photosensitizer hematoporphyrin for the treatment of oral tongue squamous cell carcinoma. The ROS-induced rupture of thioketal linkage caused the TME-responsive drug release. The DDSs showed significant antitumor effect after laser irradiation. Wang, Zhai, et al. (2019) synthesized ROS-sensitive DDS for OSCC, composed of the self-degrading polymeric carrier and photosensitizer chlorin e6 (Ce6). The significant PDT effect and a cascade reaction for the release of loaded DOX was verified in the research. Another form of light and ROS dual responsive DDS was reported by Huang, Deng, et al. (2019) The nanoplatform was integrated by plasmonic Cu2 − xS and magnetic manganese compounds. The Cu2 − xS core demonstrated PTT and PDT effect under NIR irradiation while the MnS shell showed ROS-responsive O2 production through Fenton-like pathways.
External magnetic field is another important physical stimulus applied in the research of multistimuli responsive DDSs. Magnetic nanoparticles have been widely studied as drug carriers in the diagnosis and treatment for HNC. In recent research, they were also reported combined with phototherapy, yielding magnetic field and light stimuli-responsive DDSs. For example, Bhana et al. (2015) presented iron oxide–gold nanostructures with magnetic field-guided drug delivery of NIR absorbing photosensitizer for targeting PDT and PTT, which significantly inhibited the KB cell viability. The researchers also mentioned the nanoparticle could be used for MRI and serve as a platform for tumor imaging and therapy. Moreover, considering the heat-generating properties of magnetic nanoparticles, iron oxide-based dual stimuli-responsive DDSs has also be reported for the hyperthermia therapy. Kim et al. (2013) reported multifunctional thermo and magnet responsive micelles encapsulated with superparamagnetic iron oxide nanoparticles. The DDS exhibited magnetic hyperthermia-mediated doxorubicin release and enhancement abilities of MRI contrast. With the integrin β4 antibody linked on the carriers, the DDS also demonstrated active targeting to squamous head and neck carcinoma cells. Sasikala et al. (2015) developed a smart magnetic nanoplatform for the pH-responsive delivery of anticancer drug Bortezomib (BTZ), which simultaneously showed hyperthermia effect triggered by the alternating magnetic field.
Beside of dual-stimuli responsive DDSs, other triple or multistimuli responsive DDSs have also been reported in recent years. Jin et al. (2018) conducted researches about core–satellite materials in response to pH, ROS and light stimuli for the treatment of OSCC. The nanoparticles showed NIR-induced PTT effect and pH-triggered doxorubicin (DOX) release. The ferrous iron linked on the nanoparticles could also catalyze the decomposition of ROS in tumor cells by a Fenton-like reaction. He et al. synthesized multistimuli responsive expansile nanogel DDS targeting head and neck tumor. The DDS exhibited intracellular release of photosensitizer PC4 triggered by acidic pH, high temperature, and elevated GSH and significant PDT effect under red light irradiation. Lee, Lee, In, et al. (2014) developed pH, redox and light responsive polymeric micelles for the delivery of anticancer drug Taxol and tumor cell imaging. The dual and multistimuli responsive DDSs exhibited great potential in the treatment of HNC, which deserve more research for improved material stability and synergistic antitumor effect.
Conclusion and future perspectives
In this article, current progress of stimuli-responsive DDSs for the treatment of HNC were reviewed, which could respond to various kinds of triggers including the internal TME of HNC, external stimuli or multi stimuli. Their advantages and limitations were also summarized. It revealed that stimuli-responsive DDSs could be a promising nanomedicine platform for the anticancer drug delivery with improved therapeutic effect and systemic side effects over traditional drugs administration. Yet there are still many challenges which desire future studies are desirable. As mentioned before, although TME is quite different from normal, the condition is sometimes unstable and uncontrollable, which might limit the application of the internal stimuli-responsive DDSs. More strategies are desired to improve the sensitivity and controllability of stimuli-responsive DDSs. The combination of external stimuli responsive ability could be a useful option. Yet more research is still required for proper tissue penetration, more specific action range and less adverse effect on normal tissue around. Meanwhile, although the active targeting stimuli responsive DDSs has drawn growing attention in the HNC therapy, the research is still on the way to further exploration. Particularly, more preclinical tests are needed to investigated the antitumor effect and biocompatibility in the complex in vivo condition. Additionally, multifunctional and multi stimuli-responsive DDSs have showed great potential in theranostic applications, combined antitumor therapy, inhibition of MDR and other kinds of treatments for NHC as mention before. The fabrication of stimuli-responsive DDSs with optimized structure, simplified preparation process and improved in vivo therapeutic effect might become one of the development directions.
To sum up, stimuli-responsive DDSs have great prospect of clinical translation and more exploration, which provide a great alternative for NHC therapy. It is believed that with the development of fabrication techniques and further research, more stimuli-responsive DDSs will be applied for the treatment of HNC in clinic in the near future.
Funding Statement
This work was supported by National Natural Science Foundation of China [82071106].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Agostinis P, Berg K, Cengel KA, et al. (2011). Photodynamic therapy of cancer: an update. CA Cancer J Clin 61:250–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarzadeh F, Khoshgard K, Arkan E, et al. (2018). Evaluating the photodynamic therapy efficacy using 5-aminolevulinic acid and folic acid-conjugated bismuth oxide nanoparticles on human nasopharyngeal carcinoma cell line. Artif Cells Nanomed Biotechnol 46:S514–S23. [DOI] [PubMed] [Google Scholar]
- Bali A, Bali D, Sharma A. (2013). An overview of gene therapy in head and neck cancer. Indian J Hum Genet 19:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A, Simon MC. (2018). Glutathione metabolism in cancer progression and treatment resistance. J Cell Biol 217:2291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhana S, Lin G, Wang L, et al. (2015). Near-infrared-absorbing gold nanopopcorns with iron oxide cluster core for magnetically amplified photothermal and photodynamic cancer therapy. ACS Appl Mater Interfaces 7:11637–47. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Venuganti VVK. (2015). Cancer targeting: responsive polymers for stimuli-sensitive drug delivery. J Nanosci Nanotechnol 15:1925–45. [DOI] [PubMed] [Google Scholar]
- Burgoyne JR, Oka S, Ale-Agha N, et al. (2013). Hydrogen peroxide sensing and signaling by protein kinases in the cardiovascular system. Antioxid Redox Signal 18:1042–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso VF, Francesko A, Ribeiro C, et al. (2018). Advances in magnetic nanoparticles for biomedical applications. Adv Healthcare Mater 7:1700845. [DOI] [PubMed] [Google Scholar]
- Chen M, He X, Wang K, et al. (2014). A pH-responsive polymer/mesoporous silica nano-container linked through an acid cleavable linker for intracellular controlled release and tumor therapy in vivo. J Mater Chem B 2:428–36. [DOI] [PubMed] [Google Scholar]
- Chi AC, Day TA, Neville BW. (2015). Oral cavity and oropharyngeal squamous cell carcinoma – an update. CA Cancer J Clin 65:401–21. [DOI] [PubMed] [Google Scholar]
- Chien M-H, Lin C-W, Cheng C-W, et al. (2013). Matrix metalloproteinase-2 as a target for head and neck cancer therapy. Expert Opin Ther Targets 17:203–16. [DOI] [PubMed] [Google Scholar]
- Chu C-K, Tu Y-C, Hsiao J-H, et al. (2016). Combination of photothermal and photodynamic inactivation of cancer cells through surface plasmon resonance of a gold nanoring. Nanotechnology 27:115102. [DOI] [PubMed] [Google Scholar]
- Civantos FJ, Karakullukcu B, Biel M, et al. (2018). A review of photodynamic therapy for neoplasms of the head and neck. Adv Ther 35:324–40. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Fingleton B, Matrisian LM. (2002). Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 295:2387–92. [DOI] [PubMed] [Google Scholar]
- Curry T, Kopelman R, Shilo M, et al. (2014). Multifunctional theranostic gold nanoparticles for targeted CT imaging and photothermal therapy: theranostic au nanoparticles for targeted CT and PTT. Contrast Media Mol Imaging 9:53–61. [DOI] [PubMed] [Google Scholar]
- Damiani V, Falvo E, Fracasso G, et al. (2017). Therapeutic efficacy of the novel stimuli-sensitive nano-ferritins containing doxorubicin in a head and neck cancer model. Int J Mol Sci 18:1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datz S, Argyo C, Gattner M, et al. (2016). Genetically designed biomolecular capping system for mesoporous silica nanoparticles enables receptor-mediated cell uptake and controlled drug release. Nanoscale 8:8101–10. [DOI] [PubMed] [Google Scholar]
- Davoodi P, Lee LY, Xu Q, et al. (2018). Drug delivery systems for programmed and on-demand release. Adv Drug Deliv Rev 132:104–38. [DOI] [PubMed] [Google Scholar]
- Epstein JB, Thariat J, Bensadoun R-J, et al. (2012). Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA Cancer J Clin 62:400–22. [DOI] [PubMed] [Google Scholar]
- Fan H, Zhu Z, Zhang W, et al. (2020). Light stimulus responsive nanomedicine in the treatment of oral squamous cell carcinoma. Eur J Med Chem 199:112394. [DOI] [PubMed] [Google Scholar]
- Fan L, Wang J, Xia C, et al. (2020). Glutathione-sensitive and folate-targeted nanoparticles loaded with paclitaxel to enhance oral squamous cell carcinoma therapy. J Mater Chem B 8:3113–22. [DOI] [PubMed] [Google Scholar]
- Feller L, Lemmer J. (2012). Oral squamous cell carcinoma: epidemiology, clinical presentation and treatment. J Cancer Ther 03:263–8. [Google Scholar]
- Fitzmaurice C. (2018). Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 2006 to 2016: a systematic analysis for the Global Burden of Disease study. J Clin Oncol 36:1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel D, Zuluaga MF, Lange N. (2011). On the cutting edge: protease-sensitive prodrugs for the delivery of photoactive compounds. Photochem Photobiol Sci 10:689–703. [DOI] [PubMed] [Google Scholar]
- Gao S, Zheng M, Ren X, et al. (2016). Local hyperthermia in head and neck cancer: mechanism, application and advance. Oncotarget 7:57367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies RJ, Raghunand N, Garcia-Martin ML, et al. (2004). pH imaging. A review of pH measurement methods and applications in cancers. IEEE Eng Med Biol Mag 23:57–64. [DOI] [PubMed] [Google Scholar]
- Godin B, Tasciotti E, Liu X, et al. (2011). Multistage nanovectors: from concept to novel imaging contrast agents and therapeutics. Acc Chem Res 44:979–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Chen M, Zheng Y, et al. (2012). Polymeric micelles drug delivery system in oncology. J Control Release 159:312–23. [DOI] [PubMed] [Google Scholar]
- Greish K. (2010). Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. In: Grobmyer SR, Moudgil BM, eds. Cancer nanotechnology. Totowa, NJ: Humana Press, 25–37. [DOI] [PubMed] [Google Scholar]
- Guo X, Cheng Y, Zhao X, et al. (2018). Advances in redox-responsive drug delivery systems of tumor microenvironment. J Nanobiotechnol 16:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haedicke K, Kozlova D, Gräfe S, et al. (2015). Multifunctional calcium phosphate nanoparticles for combining near-infrared fluorescence imaging and photodynamic therapy. Acta Biomater 14:197–207. [DOI] [PubMed] [Google Scholar]
- Hajebi S, Rabiee N, Bagherzadeh M, et al. (2019). Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater 92:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Liu D, Lin W. (2015). Self-assembled core–shell nanoparticles for combined chemotherapy and photodynamic therapy of resistant head and neck cancers. ACS Nano 9:991–1003. [DOI] [PubMed] [Google Scholar]
- Hong Z, Liu H, Zhao M, et al. (2016). Photodynamic therapy of tumors with pyropheophorbide-a-loaded polyethylene glycol-poly(lactic-co-glycolic acid) nanoparticles. Int J Nanomedicine 11:4905–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossen S, Hossain MK, Basher MK, et al. (2019). Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: a review. J Adv Res 15:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Huang W, Zhang Z, et al. (2019). Highly uniform synthesis of selenium nanoparticles with EGFR targeting and tumor microenvironment-responsive ability for simultaneous diagnosis and therapy of nasopharyngeal carcinoma. ACS Appl Mater Interfaces 11:11177–93. [DOI] [PubMed] [Google Scholar]
- Huang X, Deng G, Han Y, et al. (2019). Right Cu2-x S@MnS core–shell nanoparticles as a photo/H2O2-responsive platform for effective cancer theranostics. Adv Sci 6:1901461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo M, Yuan J, Tao L, et al. (2014). Redox-responsive polymers for drug delivery: from molecular design to applications. Polym Chem 5:1519–28. [Google Scholar]
- Jin R, Liu Z, Bai Y, et al. (2018). Core–satellite mesoporous silica–gold nanotheranostics for biological stimuli triggered multimodal cancer therapy. Adv Funct Mater 28:1801961. [Google Scholar]
- Kalaydina R-V, Bajwa K, Qorri B, et al. (2018). Recent advances in “smart” delivery systems for extended drug release in cancer therapy. Int J Nanomed 13:4727–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamala M, Wilson WR, Yang M, et al. (2016). Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: a review. Biomaterials 85:152–67. [DOI] [PubMed] [Google Scholar]
- Kaomongkolgit R. (2013). Alpha-mangostin suppresses MMP-2 and MMP-9 expression in head and neck squamous carcinoma cells. Odontology 101:227–32. [DOI] [PubMed] [Google Scholar]
- Karimi M, Ghasemi A, Sahandi Zangabad P, et al. (2016). Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem Soc Rev 45:1457–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketabat F, Pundir M, Mohabatpour F, et al. (2019). Controlled drug delivery systems for oral cancer treatment—current status and future perspectives. Pharmaceutics 11:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D-H, Vitol EA, Liu J, et al. (2013). Stimuli-responsive magnetic nanomicelles as multifunctional heat and cargo delivery vehicles. Langmuir 29:7425–32. [DOI] [PubMed] [Google Scholar]
- Kim JO, Tran TH, Ramasamy T, et al. (2015). Tumor-targeting, pH-sensitive nanoparticles for docetaxel delivery to drug-resistant cancer cells. Int J Nanomed 10: 5249–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Lee DJ, Kwag DS, et al. (2014). Acid pH-activated glycol chitosan/fullerene nanogels for efficient tumor therapy. Carbohydr Polym 101:692–8. [DOI] [PubMed] [Google Scholar]
- Kim SK, Youn YS, Oh KT, et al. (2017). Development of pH-responsive starch–glycol chitosan nanogels for proapoptotic (KLAKLAK)2 peptide delivery. J Bioactive Compat Polym 32:345–54. [Google Scholar]
- Koo OM, Rubinstein I, Onyuksel H. (2005). Role of nanotechnology in targeted drug delivery and imaging: a concise review. Nanomedicine 1:193–212. [DOI] [PubMed] [Google Scholar]
- Koontongkaew S. (2013). The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J Cancer 4:66–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBrun A, Zhu L. (2018). Magnetic nanoparticle hyperthermia in cancer treatment: history, mechanism, imaging-assisted protocol design, and challenges. In: Shrivastava D, ed. Theory and applications of heat transfer in humans. Chichester, UK: John Wiley & Sons Ltd, 631–67. [Google Scholar]
- Lee JO, Lee MJ, Kim D, et al. (2014). A molecular zipping/unzipping nano-vehicles sensitive to tumor extracellular pH. J Bioactive Compat Polym 29:368–81. [Google Scholar]
- Lee JO, Oh KT, Kim D, et al. (2014). pH-sensitive short worm-like micelles targeting tumors based on the extracellular pH. J Mater Chem B 2:6363–70. [DOI] [PubMed] [Google Scholar]
- Lee SY, Lee H, In I, et al. (2014). pH/redox/photo responsive polymeric micelle via boronate ester and disulfide bonds with spiropyran-based photochromic polymer for cell imaging and anticancer drug delivery. Eur Polym J 57:1–10. [Google Scholar]
- Li L, Yang W-W, Xu D-G. (2019). Stimuli-responsive nanoscale drug delivery systems for cancer therapy. J Drug Target 27:423–33. [DOI] [PubMed] [Google Scholar]
- Li Q, Wen Y, Wen J, et al. (2016). A new biosafe reactive oxygen species (ROS)-responsive nanoplatform for drug delivery. RSC Adv 6:38984–9. [Google Scholar]
- Li Q, Wen Y, You X, et al. (2016). Development of a reactive oxygen species (ROS)-responsive nanoplatform for targeted oral cancer therapy. J Mater Chem B 4:4675–82. [DOI] [PubMed] [Google Scholar]
- Li W, Tao C, Wang J, et al. (2019). MMP-responsive in situ forming hydrogel loaded with doxorubicin-encapsulated biodegradable micelles for local chemotherapy of oral squamous cell carcinoma. RSC Adv 9:31264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li L, Huang Y, et al. (2017). Synergistic therapy of chemotherapeutic drugs and MTH1 inhibitors using a pH-sensitive polymeric delivery system for oral squamous cell carcinoma. Biomater Sci 5:2068–78. [DOI] [PubMed] [Google Scholar]
- Li X, Lovell JF, Yoon J, et al. (2020). Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol 17:657–18. [DOI] [PubMed] [Google Scholar]
- Lin Q, Yang Y, Hu Q, et al. (2017). Injectable supramolecular hydrogel formed from α-cyclodextrin and PEGylated arginine-functionalized poly(l-lysine) dendron for sustained MMP-9 shRNA plasmid delivery. Acta Biomater 49:456–71. [DOI] [PubMed] [Google Scholar]
- Link S, El-Sayed MA. (2000). Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals. Int Rev Phys Chem 19:409–53. [Google Scholar]
- Liu T, Chen S, Wu X, et al. (2019). Folate-targeted pH and redox dual stimulation-responsive nanocarrier for codelivering of docetaxel and TFPI-2 for nasopharyngeal carcinoma therapy. ACS Appl Bio Mater 2:1830–41. [DOI] [PubMed] [Google Scholar]
- Liu T, Xue W, Ke B, et al. (2014). Star-shaped cyclodextrin-poly(l-lysine) derivative co-delivering docetaxel and MMP-9 siRNA plasmid in cancer therapy. Biomaterials 35:3865–72. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gao F-P, Zhang D, et al. (2014). Molecular structural transformation regulated dynamic disordering of supramolecular vesicles as pH-responsive drug release systems. J Control Release 173:140–7. [DOI] [PubMed] [Google Scholar]
- Liu Z, Shi J, Zhu B, et al. (2020). Development of a multifunctional gold nanoplatform for combined chemo-photothermal therapy against oral cancer. Nanomedicine 15:661–76. [DOI] [PubMed] [Google Scholar]
- Ma C, Shi L, Huang Y, et al. (2017). Nanoparticle delivery of Wnt-1 siRNA enhances photodynamic therapy by inhibiting epithelial–mesenchymal transition for oral cancer. Biomater Sci 5:494–501. [DOI] [PubMed] [Google Scholar]
- Ma D, Lin Q-M, Zhang L-M, et al. (2014). A star-shaped porphyrin–arginine functionalized poly(l-lysine) copolymer for photo-enhanced drug and gene co-delivery. Biomaterials 35:4357–67. [DOI] [PubMed] [Google Scholar]
- Marur S, Forastiere AA. (2016). Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc 91:386–96. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Maeda H. (1986). A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent Smancs. Cancer Res 46:6387–92. [PubMed] [Google Scholar]
- Mehanna H, Paleri V, West CML, et al. (2011). Head and neck cancer – part 1: epidemiology, presentation, and preservation. Clin Otolaryngol 36:65–8. [DOI] [PubMed] [Google Scholar]
- Meulemans J, Delaere P, Vander Poorten V. (2019). Photodynamic therapy in head and neck cancer: indications, outcomes, and future prospects. Curr Opin Otolaryngol Head Neck Surg 27:136–41. [DOI] [PubMed] [Google Scholar]
- Miao L, Liu C, Ge J, et al. (2014). Antitumor effect of TRAIL on oral squamous cell carcinoma using magnetic nanoparticle-mediated gene expression. Cell Biochem Biophys 69:663–72. [DOI] [PubMed] [Google Scholar]
- Nam J, Son S, Ochyl LJ, et al. (2018). Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat Commun 9:1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narmani A, Rezvani M, Farhood B, et al. (2019). Folic acid functionalized nanoparticles as pharmaceutical carriers in drug delivery systems. Drug Dev Res 80:404–24. [DOI] [PubMed] [Google Scholar]
- Olusanya T, Haj Ahmad R, Ibegbu D, et al. (2018). Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules 23:907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Sayáns M, Somoza-Martín JM, Barros-Angueira F, et al. (2010). Multidrug resistance in oral squamous cell carcinoma: the role of vacuolar ATPases. Cancer Lett 295:135–43. [DOI] [PubMed] [Google Scholar]
- Qian X, Nie X, Yao W, et al. (2018). Reactive oxygen species in cancer stem cells of head and neck squamous cancer. Semin Cancer Biol 53:248–57. [DOI] [PubMed] [Google Scholar]
- Qiao Y, Wan J, Zhou L, et al. (2019). Stimuli-responsive nanotherapeutics for precision drug delivery and cancer therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol 11:e1527. [DOI] [PubMed] [Google Scholar]
- Rao L, Bu L-L, Ma L, et al. (2018). Platelet-facilitated photothermal therapy of head and neck squamous cell carcinoma. Angew Chem 130:998–1003. [DOI] [PubMed] [Google Scholar]
- Rofstad EK, Mathiesen B, Kindem K, et al. (2006). Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res 66:6699–707. [DOI] [PubMed] [Google Scholar]
- Rosenthal EL, Matrisian LM. (2006). Matrix metalloproteases in head and neck cancer. Head Neck 28:639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiyin W, Wang D, Li L, et al. (2014). Sequential release of autophagy inhibitor and chemotherapeutic drug with polymeric delivery system for oral squamous cell carcinoma therapy. Mol Pharm 11:1662–75. [DOI] [PubMed] [Google Scholar]
- Salahpour Anarjan F. (2019). Active targeting drug delivery nanocarriers: ligands. Nano-Struct Nano-Objects 19:100370. [Google Scholar]
- Saravanakumar G, Kim J, Kim WJ. (2017). Reactive-oxygen-species-responsive drug delivery systems: promises and challenges. Adv Sci 4:1600124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasikala ARK, GhavamiNejad A, Unnithan AR, et al. (2015). A smart magnetic nanoplatform for synergistic anticancer therapy: manoeuvring mussel-inspired functional magnetic nanoparticles for pH responsive anticancer drug delivery and hyperthermia. Nanoscale 7:18119–28. [DOI] [PubMed] [Google Scholar]
- Shi S, Zhang L, Zhu M, et al. (2018). Reactive oxygen species-responsive nanoparticles based on PEGlated prodrug for targeted treatment of oral tongue squamous cell carcinoma by combining photodynamic therapy and chemotherapy. ACS Appl Mater Interfaces 10:29260–72. [DOI] [PubMed] [Google Scholar]
- Shim MS, Xia Y. (2013). A reactive oxygen species (ROS)-responsive polymer for safe, efficient, and targeted gene delivery in cancer cells. Angew Chem 125:7064–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son J, Yi G, Yoo J, et al. (2019). Light-responsive nanomedicine for biophotonic imaging and targeted therapy. Adv Drug Deliv Rev 138:133–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Tang C, Xu W, et al. (2020). Hypoxia-targeting multifunctional nanoparticles for sensitized chemotherapy and phototherapy in head and neck squamous cell carcinoma. Int J Nanomedicine 15:347–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Peng S, Lin Q, et al. (2019). pH-responsive oxygen nanobubbles for spontaneous oxygen delivery in hypoxic tumors. Langmuir 35:10166–72. [DOI] [PubMed] [Google Scholar]
- Su Z, Liu D, Chen L, et al. (2019). CD44-targeted magnetic nanoparticles kill head and neck squamous cell carcinoma stem cells in an alternating magnetic field. Int J Nanomed 14:7549–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Li X, Du X, et al. (2018). Redox-responsive micelles for triggered drug delivery and effective laryngopharyngeal cancer therapy. Int J Biol Macromol 112:65–73. [DOI] [PubMed] [Google Scholar]
- Taghizadeh B, Taranejoo S, Monemian SA, et al. (2015). Classification of stimuli-responsive polymers as anticancer drug delivery systems. Drug Deliv 22:145–55. [DOI] [PubMed] [Google Scholar]
- Tan G, Zhong Y, Yang L, et al. (2020). A multifunctional MOF-based nanohybrid as injectable implant platform for drug synergistic oral cancer therapy. Chem Eng J 390:124446. [Google Scholar]
- Tarassoli SP, de Pinillos Bayona AM, Pye H, et al. (2017). Cathepsin B-degradable, NIR-responsive nanoparticulate platform for target-specific cancer therapy. Nanotechnology 28:055101. [DOI] [PubMed] [Google Scholar]
- Tawfik SM, Sharipov M, Huy BT, et al. (2018). Naturally modified nonionic alginate functionalized upconversion nanoparticles for the highly efficient targeted pH-responsive drug delivery and enhancement of NIR-imaging. J Ind Eng Chem 57:424–35. [Google Scholar]
- Trachootham D, Alexandre J, Huang P. (2009). Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8:579–91. [DOI] [PubMed] [Google Scholar]
- Virós D, Camacho M, Zarraonandia I, et al. (2013). Prognostic role of MMP-9 expression in head and neck carcinoma patients treated with radiotherapy or chemoradiotherapy. Oral Oncol 49:322–5. [DOI] [PubMed] [Google Scholar]
- Wang B, Wang J-H, Liu Q, et al. (2014). Rose-Bengal-conjugated gold nanorods for in vivo photodynamic and photothermal oral cancer therapies. Biomaterials 35:1954–66. [DOI] [PubMed] [Google Scholar]
- Wang B-K, Yu X-F, Wang J-H, et al. (2016). Gold-nanorods-siRNA nanoplex for improved photothermal therapy by gene silencing. Biomaterials 78:27–39. [DOI] [PubMed] [Google Scholar]
- Wang D, Fei B, Halig LV, et al. (2014). Targeted iron-oxide nanoparticle for photodynamic therapy and imaging of head and neck cancer. ACS Nano 8:6620–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Wang M, Zhao L, et al. (2019). A new biosafe reactive oxygen species responsive nanoplatform for targeted oral squamous cell carcinoma therapy. Mat Expr 9:1076–81. [Google Scholar]
- Wang H, Fu Z, Li W, et al. (2019). The synthesis and application of nano doxorubicin–indocyanine green matrix metalloproteinase-responsive hydrogel in chemophototherapy for head and neck squamous cell carcinoma. Int J Nanomedicine 14:623–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Xiao Y, Li Y, et al. (2019). Reactive oxygen species and near-infrared light dual-responsive indocyanine green-loaded nanohybrids for overcoming tumour multidrug resistance. Eur J Pharm Sci 134:185–93. [DOI] [PubMed] [Google Scholar]
- Wang M, Zhai Y, Ye H, et al. (2019). High co-loading capacity and stimuli-responsive release based on cascade reaction of self-destructive polymer for improved chemo-photodynamic therapy. ACS Nano 13:7010–23. [DOI] [PubMed] [Google Scholar]
- Wang X, Gao S, Qin Z, et al. (2018). Evans Blue derivative-functionalized gold nanorods for photothermal therapy-enhanced tumor chemotherapy. ACS Appl Mater Interfaces 10:15140–9. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wan G, Li Z, et al. (2017). PEGylated doxorubicin nanoparticles mediated by HN-1 peptide for targeted treatment of oral squamous cell carcinoma. Int J Pharm 525:21–31. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xie D, Pan J, et al. (2019). A near infrared light-triggered human serum albumin drug delivery system with coordination bonding of indocyanine green and cisplatin for targeting photochemistry therapy against oral squamous cell cancer. Biomater Sci 7:5270–82. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xie Y, Li J, et al. (2017). Tumor-penetrating nanoparticles for enhanced anticancer activity of combined photodynamic and hypoxia-activated therapy. ACS Nano 11:2227–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Meng Q, Li S. (2020). The role of NIR fluorescence in MDR cancer treatment: from targeted imaging to phototherapy. CMC 27:5510–29. [DOI] [PubMed] [Google Scholar]
- Wei Z, Yin X, Cai Y, et al. (2018). Antitumor effect of a Pt-loaded nanocomposite based on graphene quantum dots combats hypoxia-induced chemoresistance of oral squamous cell carcinoma. Int J Nanomed 13:1505–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg F, Ramnath N, Nagrath D. (2019). Reactive oxygen species in the tumor microenvironment: an overview. Cancers 11:1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen S, Liu H, Cai H, et al. (2013). Targeted and pH-responsive delivery of doxorubicin to cancer cells using multifunctional dendrimer-modified multi-walled carbon nanotubes. Adv Healthc Mater 2:1267–76. [DOI] [PubMed] [Google Scholar]
- Wu T-T, Zhou S-H. (2015). Nanoparticle-based targeted therapeutics in head-and-neck cancer. Int J Med Sci 12:187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Q, Li N, Jiao Y, et al. (2015). Near-infrared light-controlled drug release and cancer therapy with polymer-caged upconversion nanoparticles. RSC Adv 5:5269–76. [Google Scholar]
- Xiong J, Gao H. (2017). Matrix metalloproteases-responsive nanomaterials for tumor targeting diagnosis and treatment. J Microencapsul 34:440–53. [DOI] [PubMed] [Google Scholar]
- Yan T, He J, Liu R, et al. (2020). Chitosan capped pH-responsive hollow mesoporous silica nanoparticles for targeted chemo-photo combination therapy. Carbohydr Polym 231:115706. [DOI] [PubMed] [Google Scholar]
- Yao Q, Kou L, Tu Y, et al. (2018). MMP-responsive ‘smart’ drug delivery and tumor targeting. Trends Pharmacol Sci 39:766–81. [DOI] [PubMed] [Google Scholar]
- Zhang K, Guan X, Qiu Y, et al. (2016). A pH/glutathione double responsive drug delivery system using molecular imprint technique for drug loading. Appl Surf Sci 389:1208–13. [Google Scholar]
- Zhang W. (2015). Supramolecular hydrogels co-loaded with camptothecin and doxorubicin for sustainedly synergistic tumor therapy. J Mater Chem B 3:2127–36. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhuang L, Lin Y, et al. (2020). Novel drug delivery system based on hollow mesoporous magnetic nanoparticles for head and neck cancers – targeted therapy in vitro and in vivo. Am J Cancer Res 10:350–64. [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Zhou Y, Wang D, et al. (2015). pH-responsive polymeric micelles based on poly(2-ethyl-2-oxazoline)-poly(d,l-lactide) for tumor-targeting and controlled delivery of doxorubicin and P-glycoprotein inhibitor. Acta Biomater 17:182–92. [DOI] [PubMed] [Google Scholar]
- Zhou L, Wang H, Li Y. (2018). Stimuli-responsive nanomedicines for overcoming cancer multidrug resistance. Theranostics 8:1059–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Zhang L, Yang T, et al. (2018). Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int J Nanomed 13:2921–42. [DOI] [PMC free article] [PubMed] [Google Scholar]