Abstract
Background
Although miRNA-183-5p plays a critical role in many cancer types, including gastric cancer, hepatocellular carcinoma, prostate cancer, renal cell cancer and breast cancer, its role in osteosarcoma remains unclear.
Methods
Expression levels of miR-183-5p were detected in osteosarcoma tissues and cell lines using qRT-PCR. The effect of miR-183-5p on the survival and recurrence of osteosarcoma patients was analyzed in a cohort of 80 patients using Kaplan–Meier curves and Cox regression analysis. Effects of miR-183-5p on cell proliferation, migration and invasion abilities were evaluated using CCK-8, crystal violet and transwell assays.
Results
The expression of miR-183-5p was found to be upregulated in human osteosarcoma tissues and cell lines. Moreover, miR-183-5p expression was observed to be closely associated with tumor size, TNM stage and lung metastasis. Notably, high expression of miR-183-5p was shown to be able to predict unfavorable clinical prognosis in osteosarcoma patients. Additionally, whilst overexpression of miR-183-5p was observed to significantly promote the proliferation, migration and invasion of osteosarcoma cells; an inhibitory effect was observed with knockdown of miR-183-5p.
Conclusion
This study demonstrated that miR-183-5p acts as an oncogene and plays a critical role in the regulation of osteosarcoma tumor progression, and our results suggest a novel potential prognostic and therapeutic value of miR-183-5p in osteosarcoma.
Keywords: miR-183-5p, osteosarcoma, progression, prognosis, biomarker
Introduction
Accounting for nearly 60% of malignant bone cancers, osteosarcoma is one of the most frequently diagnosed malignant bone tumors that primarily affects children and young adults.1,2 Approximately 80% of newly diagnosed osteosarcoma patients are found to have developed metastasis, especially lung metastasis, which is a challenge in tumor prognosis and treatment.3 Although current treatment intervention using surgery with combined chemotherapy and radiotherapy has shown greater clinical outcome in osteosarcoma patients, long-term prognosis remains poor with an approximate 5-year overall survival of only 65%.4 With an increased knowledge on osteosarcoma initiation and progression, the underlying molecular mechanisms, such as oncogene activation and suppressor gene silencing that play a vital role in osteosarcoma tumor progression, has gained considerable research attention.5 Therefore, it is necessary to explore reliable biological markers for the diagnosis, treatment and prognosis of osteosarcoma.
MicroRNAs (miRNAs) are endogenous small non-coding RNAs with a length of 19 to 25 nucleotides. A number of recent studies have extensively elucidated the expression of miRNAs in tumor cells and the roles of miRNAs in tumor progression, and the results have revealed that abnormal expression of miRNAs can regulate cell proliferation, differentiation, development and apoptosis in multiple cancers.6–8 Moreover, there has been an increased understanding on the diagnostic, prognostic, and biological functions of miRNAs in osteosarcoma patients.9,10 As one of the vital members of the miR-183 family, miR-183-5p located on human chromosome 7 has been widely reported as an oncogene in many cancer types, such as gastric cancer,11 hepatocellular carcinoma, prostate cancer,12 renal cell cancer13 and breast cancer.14 In addition, miR‐183-5p has been proposed to play a tumor suppressor role in other cancers, including lung cancer,15 cervical cancer,16 endometrial cancer17 and acute myeloid leukemia.18 Previously, miR-183 has also been determined in the initiation and progression of osteosarcoma;19–22 nevertheless, the prognostic and biological roles of miR‐183-5p in osteosarcoma remain unclear.
In this study, we explored the prognostic and biological functions of miR-183-5p in the progression of osteosarcoma. We revealed that miR-183-5p was upregulated in human primary osteosarcoma tissues and cell lines, and that high expression of miR-183-5p was associated with unfavorable prognosis in osteosarcoma patients. In addition, miR-183-5p was found to play a role in promoting osteosarcoma cell proliferation, migration and invasion in vitro.
Materials and Methods
Clinical Samples
A total of 80 osteosarcoma patients from the Renmin Hospital of Wuhan University (Hubei, China) between January 2012 and December 2015 were enrolled in this study. All patients were pathologically confirmed and underwent routine surgery without receiving pre-surgery anticancer treatments, like chemotherapy or radiotherapy. Osteosarcoma tissues and their adjacent normal muscle tissues collected from patients were immediately frozen in liquid nitrogen before further analyses. Clinicopathological information and survival information were recorded during follow-ups. Patients were staged according to tumor-node-metastasis (TNM) classification. Regular physical examinations were conducted for all patients to determine tumor recurrence. This study was censored in December 2019. All included patients have sighed their informed consents. This study was approved by the ethics committee of the Renmin Hospital of Wuhan University and complied with the Declaration of Helsinki.
Cell Culture
Human osteosarcoma cell lines HOS, MG-63, U2OS and one osteoblastic cell line (hFOB1.19) were obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). Osteosarcoma cells were cultured at 37 ◦C in 5% CO2 in DMEM medium (Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA), 100 U/mL penicillin and 100 g/mL streptomycin (Sangong Biotech).
RNA Extraction and Reverse Transcription‑Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA from clinical tissue and cell line samples was conducted using RNeasy Maxi Kit (Qiagen GmbH, Inc.). Then, the RNA concentration was detected under BioDrop (BioDrop). Reverse transcription was carried out using miScript II RT Kit (Qiagen GmbH). MiR-183-5p expression level was detected using RT-qPCR on the ABI PRISM 7500 Sequence Detection System (Applied Biosystems) with the following thermal cycling conditions: 95°C for 2 min, followed by 40 cycles of 95°C for 15 sec, 60°C for 30 sec, and 72°C for 30 sec. During this assay, miScript SYBR-Green PCR Kit from Qiagen GmbH was used. The relative expression of miR-183-5p level was calculated using the 2−∆∆Ct method normalized to U6. The sequences of oligonucleotides (RiboBio, Guangzhou, China) used in this study are as follows: 5′-ACACTCCAGCTGGGTATGGCACTGGTAGAATT-3′ (miR-183-5p forward), 5′- CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAGTGAATT-3′ (miR-183-5p reverse), 5′-CTCGCTTCGGCAGCACATATACT-3′ (U6 forward) and 5′- ACGCTTCACGAATTTGCGTGTC-3′ (U6 reverse).
Cell Transfection
MiR-183-5p mimics, miR-183-5p inhibitor and their corresponding negative controls (miR-NCs) were obtained from GenePharma (Shanghai, China). Osteosarcoma cells were seeded in a 6-well plate (3×105 per well) and cultured to 70‑80% density. Next, the cell transfection process was performed using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). Lipofectamine 2000 (Thermo Fisher Scientific, Inc.) was prepared in a sterile Eppendorf tube, and 5 µL of Lipofectamine 2000 and 100 µL of serum‑free medium were incubated at room temperature for 5 min. siRNA (50 nmol) and 100 µL of serum‑free medium were incubated at room temperature for 20 min. The cells in the culture flask were washed. Serum-free medium (without antibiotics) was added to the complex, which was then mixed, and the mixture was added into the 50 mL culture flask for transfection. The flask was placed in an incubator containing 5% CO2 at 37°C for 6–8 h, and the reagent was then replaced with complete culture medium. Further experiments were conducted following 48 h after transfection.
Cell Counting Kit-8 (CCK-8) Assays
CCK-8 assays were conducted to measure cell viability of experimental cells. Three replicates of transfected osteosarcoma cells (5×103 cells in 100 μL culture medium per well) were seeded in 96-well plates. Then, 10 μL of CCK-8 solution (Dojindo, Japan) was added in each well at 24, 48, 72, and 96 h, and incubated for 2 h at 37 °C. Subsequently, the optical density (OD) values were measured at 450 nm using a microplate reader (ELx800, Bio-Tek, USA, USA).
Crystal Violet Assays
Transfected osteosarcoma cells were seeded in 6-well plates (1000 cells per well) with medium containing 10% FBS. The culture medium was changed every 3 days for 2 weeks, and then the culture medium was removed and cells were stained with 0.1% crystal violet at room temperature for 10 min. Fixed cells were then washed with phosphate-buffered saline (PBS) and photographed. Optical density (OD) values were measured at 570 nm using a microplate reader (ELx800, Bio-Tek, USA, USA).
Transwell Assays
The polyethylene membranes (24-well inserts; 8.0 μm; Corning, Inc.) were utilized to detect the migration and invasion abilities of osteosarcoma cells. For cell invasion assay, the upper surface of the membrane was coated with 50 µL Matrigel, incubated at 37°C for 5 h, while the membrane was uncoated during cell migration assay. The mixed cell suspension solution (1 × 105 cells in a volume of 150 μL serum-free medium) was added to the upper chamber, and then a total of 500 μL culture medium containing 10% FBS was added to the lower chamber. Cells were cultured at 5% CO2 and 37°C for 48 hours. The cells on the upper layer were wiped off with a cotton swab. Next, the chamber was fixed with methanol at room temperature for 10 min, stained with 0.1% crystal violet solution for 10 min. Five visual fields were randomly selected for image capture, and the cells were counted under a light microscope (Olympus Corporation) at ×400 magnification.
Statistical Analyses
All measurement data were expressed as mean ± standard deviation. Statistical analysis was performed with SPSS 20.0 (IBM SPSS Inc., Chicago, IL, USA). An unpaired Student’s t-test was used to analyze differences between the two groups. The chi-square test was adopted to investigate the association between the miR-183-5p expression and the clinicopathological features of osteosarcoma patients. The overall survival (OS) and recurrence-free survival (RFS) of patients with osteosarcoma were evaluated by Kaplan-Meier curves and were compared by the Log Rank test. Univariate analysis and multivariate Cox regression analysis with stepwise selection was performed to detect independent predictors of OS and DFS. The significant prognostic factors on univariate analysis (P< 0.05) were subjected to multivariate analysis using the Cox proportional hazards regression model. A statistically significant difference was considered when P< 0.05.
Results
MiR-183-5p Was Upregulated in Osteosarcoma Tissues and Cell Lines
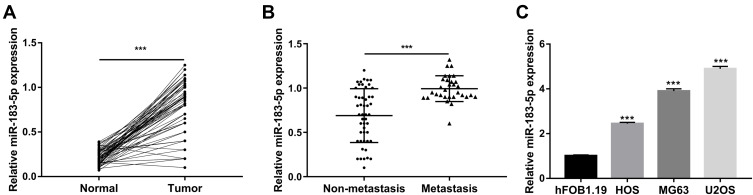
First, the expression of miR-183-5p in 80 pairs of osteosarcoma tissues and paired adjacent normal tissues was determined using qRT-PCR. As shown in Figure 1A, the expression of miR-183-5p was significantly increased in tumor tissues compared to that in matched normal tissues (P< 0.001). Moreover, the expression of miR-183-5p was observed to be significantly higher in the tumor tissues of patients with lung metastasis than those without lung metastasis (P< 0.001, Figure 1B). In addition, the miR-183-5p was also found to be expressed in several osteosarcoma cell lines (HOS, MG-63, U2OS) and one osteoblastic cell line (hFOB1.19). As shown in Figure 1C, the expression of miR-183-5p was significantly higher in osteosarcoma cell lines than that in hFOB1.19 (all P< 0.001).
Figure 1.
Upregulation of miR-183-5p expression in osteosarcoma tissues and cell lines. (A) Relative expression of miR-183-5p in 80 pairs of tumor tissues and adjacent normal tissues determined by qRT-PCR. (B) Relative expression of miR-183-5p in tumor tissues with or without lung metastasis determined by qRT-PCR. (C) Relative expression of miR-183-5p in osteosarcoma cells determined by qRT-PCR. ***P< 0.001; qRT-PCR, reverse transcription‑quantitative polymerase chain reaction. Data are presented as mean ± SD of three independent experiments.
The association between miR-183-5p expression and clinicopathologic features in patients with osteosarcoma was analyzed. Using the median value of miR-183-5p expression as the cut-off value, osteosarcoma patients were divided into two groups: miR-183-5p low (below the median, 40 patients) and miR-183-5p high (above the median, 40 patients). As shown in Table 1, the number of patients with a tumor size of ≥5 cm was higher in patients with high miR-183-5p expression compared to that in patients with low miR-183-5p expression (P= 0.012). The number of patients in TNM stage III-IV was higher in patients with high miR-183-5p expression (P= 0.003). In addition, patients with lung metastasis were more commonly found in the high miR-183-5p expression group than those in the low miR-183-5p expression group (P= 0.003). However, there was no significant difference on age, gender and tumor location between the two groups.
Table 1.
The Relationship Between miR-183-5p Expression and Clinical Features in Patients with Osteosarcoma
| Variables | Cases (n=80) | miR-183-5p, n (%) | P-value | |
|---|---|---|---|---|
| Low Expression (n=40) | High Expression (n=40) | |||
| Age, years | 0.651 | |||
| <18 | 46 (57.5) | 24 (60.0) | 22 (55.0) | |
| ≥18 | 34 (42.5) | 16 (40.0) | 18 (45.0) | |
| Gender | 0.822 | |||
| Male | 45 (56.3) | 22 (55.0) | 23 (57.5) | |
| Female | 35 (43.8) | 18 (45.0) | 17 (42.5) | |
| Location | 0.606 | |||
| Femur/Tibia | 60 (75.0) | 29 (72.5) | 31 (77.5) | |
| Others | 20 (25.0) | 11 (27.5) | 9 (22.5) | |
| Tumor size, cm | 0.012 | |||
| <5 | 33 (41.3) | 22 (55.0) | 11 (27.5) | |
| ≥5 | 47 (58.8) | 18 (45.0) | 29 (72.5) | |
| TNM stage | 0.003 | |||
| I–II | 33 (41.3) | 23 (57.5) | 10 (25.0) | |
| III–IV | 47 (58.8) | 17 (42.5) | 30 (75.0) | |
| Lung metastasis | 0.003 | |||
| Yes | 31 (38.8) | 9 (22.5) | 22 (55.0) | |
| No | 49 (61.3) | 31 (77.5) | 18 (45.0) | |
Note: The bold values denote P-value less than 0.05 with statistical significance.
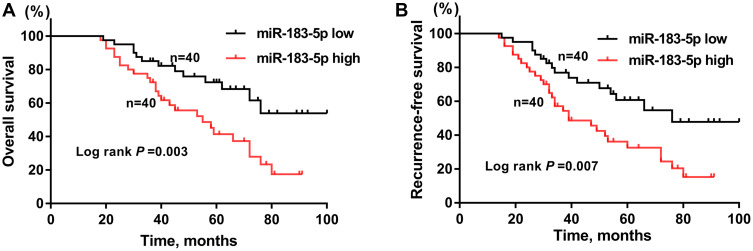
Next, the prognostic value of miR-183-5p in osteosarcoma patients was investigated. Kaplan-Meier curves indicated that osteosarcoma patients with high miR-183-5p expression were associated with poor OS (Figure 2A; P= 0.003) and RFS (Figure 2B; P= 0.007) compared to those with low miR-183-5p expression. The prognostic factors for OS and RFS were subsequently analyzed using multivariate Cox regression analysis. The results obtained for OS (Table 2) were as follows: TNM stage (hazard ratio (HR): 2.21, 95% confidence interval (CI): 1.23–3.94, P= 0.001); lung metastasis (HR: 1.61, 95% CI: 1.06–2.34, P= 0.004); miR-183-5p expression (HR: 1.72, 95% CI: 1.16–2.69, P= 0.007). For RFS (Table 3), the following results were obtained: TNM stage (HR: 2.01, 95% CI: 1.54–2.63, P< 0.001); lung metastasis (HR: 1.28, 95% CI: 1.04–1.65, P= 0.037); miR-183-5p expression (HR: 1.65, 95% CI: 1.17–2.15, P = 0.012). Taken together, the above findings showed that high expression of miR-183-5p was associated with unfavorable prognosis in osteosarcoma patients.
Figure 2.
Osteosarcoma patients with high miR-183-5p expression (miR-183-5p high, n= 40) had worse overall survival (A) and recurrence-free survival (B) than those with low miR-183-5p expression (miR-183-5p low, n= 40).
Table 2.
Univariate and Multivariate Analyses of Prognostic Factors Associated with Overall Survival
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (<18 vs ≥18 years) | 1.01 (0.73–1.65) | 0.621 | ||
| Gender (male vs female) | 0.97 (0.42–1.79) | 0.678 | ||
| Location (femur/tibia vs others) | 1.06 (0.55–2.04) | 0.763 | ||
| Tumor size (≥5 vs <5 cm) | 1.34 (0.86–2.05) | 0.065 | ||
| TNM stage (III–IV vs I–II) | 2.67 (1.64–4.34) | <0.001 | 2.21 (1.23–3.94) | 0.001 |
| Lung metastasis (yes vs no) | 1.65 (1.10–2.47) | 0.001 | 1.61 (1.06–2.34) | 0.004 |
| miR-183-5p expression (high vs low) | 1.82 (1.21–2.73) | 0.003 | 1.72 (1.16–2.69) | 0.007 |
Note: The bold values denote P-value less than 0.05 with statistical significance.
Abbreviations: HR, hazard ratio; CI, confidence interval.
Table 3.
Univariate and Multivariate Analyses of Prognostic Factors Associated with Recurrence-Free Survival
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (<18 vs ≥18 years) | 1.23 (0.97–1.57) | 0.098 | ||
| Gender (male vs female) | 1.12 (0.83–1.51) | 0.444 | ||
| Location (femur/tibia vs others) | 1.21 (0.92–1.60) | 0.168 | ||
| Tumor size (≥5 vs <5 cm) | 1.21 (0.76–2.15) | 0.135 | ||
| TNM stage (III–IV vs I–II) | 1.97 (1.53–2.54) | <0.001 | 2.01 (1.54, 2.63) | <0.001 |
| Lung metastasis (yes vs no) | 1.35 (1.12–1.69) | 0.010 | 1.28 (1.04–1.65) | 0.037 |
| miR-183-5p expression (high vs low) | 1.71 (1.25–2.32) | 0.001 | 1.65 (1.17–2.15) | 0.012 |
Note: The bold values denote P-value less than 0.05 with statistical significance.
Abbreviations: HR, hazard ratio; CI, confidence interval.
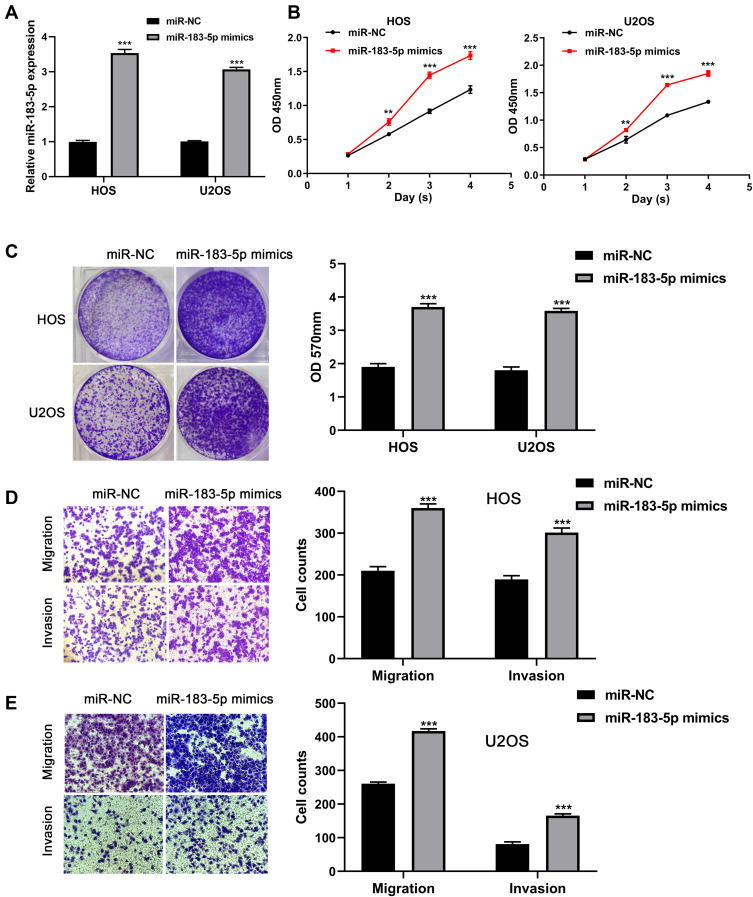
Based on the clinical findings, further experiments were conducted to explore the biological functions of miR-183-5p in the tumor progression of osteosarcoma. Firstly, miR-183-5p was overexpressed in HOS and U2OS cell lines using miR-183-5p mimics. As shown in Figure 3A, as indicated by RT-qPCR, the expression of miR-183-5p was significantly increased in HOS and U2OS cells with miR-183-5p mimics (P< 0.001). CCK-8 assays showed that the absorbance values of HOS and U2OS cells at 2, 3 and 4 days in the miR-183-5p mimics group were significantly higher than the negative control group (all P< 0.01, Figure 3B). Moreover, crystal violet assays demonstrated that miR-183-5p overexpression significantly promoted the proliferation ability of HOS and U2OS cells (P < 0.001, Figure 3C). Next, the migratory and invasive capacity of HOS cells were evaluated using transwell assays. As shown in Figure 3D and E, miR-183-5p overexpression notably promoted the migration and invasion of HOS and U2OS cells (all P< 0.001).
Figure 3.
MiR-183-5p overexpression promotes the proliferation, migration, and invasion capacity of osteosarcoma cells. (A) Expression of miR-183-5p detected in HOS and U2OS cells after transfection with miR-183-5p mimics and negative controls. (B) Effect of miR-183-5p overexpression on the proliferation of HOS and U2OS cells assessed by CCK-8 assays. (C) Effect of miR-183-5p overexpression on the proliferation of HOS and U2OS cells assessed by crystal violet assays; the OD values of crystal violet assays was shown in right panel. (D) Effects of miR-183-5p overexpression on the migration of HOS and U2OS cells assessed by transwell assays (magnification: 400×); the calculation of cells that migrated through the filter was shown in right panel. (E) Effects of miR-183-5p overexpression on the invasion of HOS and U2OS cells assessed by transwell assays (magnification: 400×); the calculation of cells that invaded through the filter was shown in right panel. **P< 0.01; ***P< 0.001. Data are presented as mean ± SD of three independent experiments.
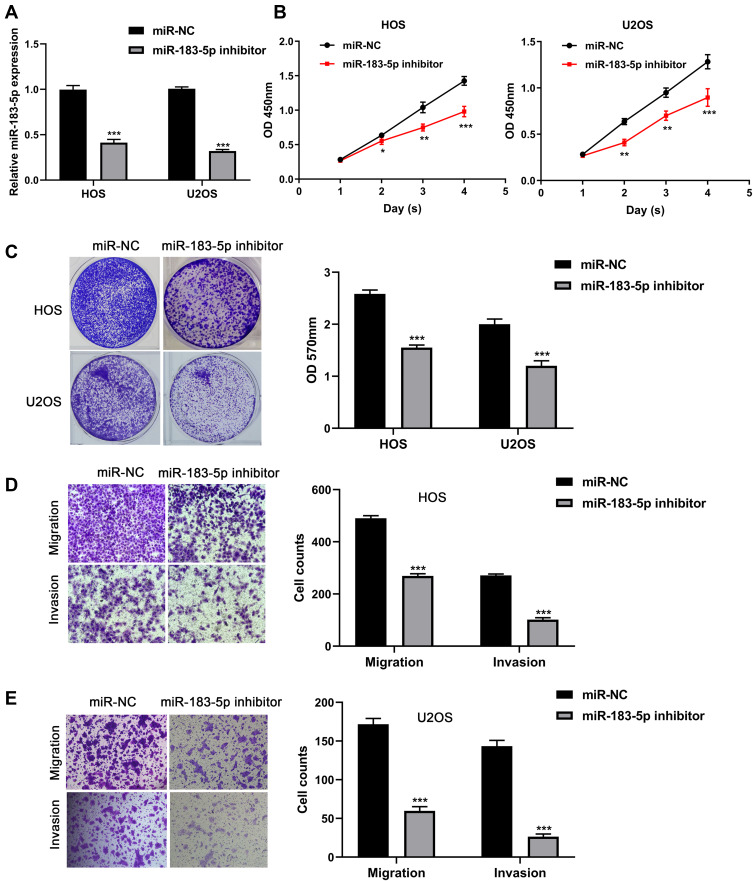
In order to downregulate the expression of miR-183-5p in HOS and U2OS cell lines, miR-183-5p inhibitor was used. The efficiency of miR-183-5p downregulation was confirmed by qRT-PCR (P < 0.001, Figure 4A). As shown in Figure 4B and C, CCK-8 assays and crystal violet assays demonstrated that downregulation of miR-183-5p significantly inhibited the proliferation ability of HOS and U2OS cells (all P< 0.05). In addition, transwell assays indicated that the cell migratory and invasive capacity of HOS and U2OS cells transfected with miR-183-5p inhibitor were significantly decreased compared with those transfected with negative control (all P< 0.001, Figure 4D and E).
Figure 4.
Knockdown of miR-183-5p inhibits proliferation, migration and invasion capacity of osteosarcoma cells. (A) Expression of miR-183-5p detected in HOS and U2OS cells after transfection with miR-183-5p inhibitor and negative controls. (B) Effect of miR-183-5p knockdown on the proliferation of HOS and U2OS cells assessed by CCK-8 assays. (C) Effect of miR-183-5p knockdown on the proliferation of HOS and U2OS cells assessed by crystal violet assays; the OD values of crystal violet assays was shown in right panel. (D) Effects of miR-183-5p knockdown on the migration of HOS and U2OS cells assessed by transwell assays (magnification: 400×); the calculation of cells that migrated through the filter was shown in right panel. (E) Effects of miR-183-5p knockdown on the invasion of HOS and U2OS cells assessed by transwell assays (magnification: 400×); the calculation of cells that invaded through the filter was shown in right panel. *P< 0.05; **P< 0.01; ***P< 0.001. Data are presented as mean ± SD of three independent experiments.
Discussion
A variety of miRNAs that exert fundamental roles in the progression of osteosarcoma have recently been identified to be diagnostic or prognostic biomarkers.23 For instance, a recent study has shown that there are 15 differentially expressed miRNAs in osteosarcoma patients compared with those in healthy controls, and that miR-215-5p and miR-642a-5p, both overexpressed in the serum of osteosarcoma patients, can be used as potential biomarkers for the diagnosis of osteosarcoma.24 Yang and colleagues have reported that miR‑328‑3p overexpression can enhance the radio-sensitivity of osteosarcoma cells via direct targeting of H2AX.25
In this study, we documented that miR-183-5p was upregulated in human osteosarcoma tissues and cell lines compared with that in adjacent normal tissues and osteoblastic cell line, respectively. Moreover, we demonstrated that miR-183-5p expression was closely associated with tumor size, TNM stage and lung metastasis. Notably, high miR-183-5p expression was found to be capable of predicting unfavorable clinical prognosis in patients with osteosarcoma. These findings demonstrate the prognostic value and oncogenic role of miR-183-5p in osteosarcoma patients. Subsequently, we overexpressed/knocked-down the expression of miR-183-5p in two osteosarcoma cell lines using miR-183-5p mimics/inhibitors, respectively, and analyzed the effects of miR-183-5p on cell proliferation, migration and invasion. We observed that whilst miR-183-5p overexpression promoted the proliferation, migration and invasion of osteosarcoma cells, miR-183-5p downregulation exerted an opposite inhibitory effect.
The oncogenic role of miR-183-5p in some other cancers has been confirmed previously. For example, miR-183-5p has been identified as a potential prognostic biomarker that is capable of predicting worse survival in patients with gastric cancer11 and renal cell cancer.13 Miao and colleagues have reported that miR-183-5p overexpression can promote the proliferation, invasion and metastasis of pancreatic adenocarcinoma cells.26 Meanwhile, Li and colleagues have demonstrated that miR-183-5p upregulation inhibits cell apoptosis in human hepatocellular carcinoma by repressing the expression of programmed cell death 4 (PDCD4).27 Moreover, miR-183-5p has been reported to promote the growth of non-small cell lung cancer cells through the inhibition of FoxO1 expression.28 However, one previous study has demonstrated that miR-183-5p was downregulated in osteosarcoma and acts as a tumor suppressor,29 which is opposite to our findings. In this study, for the first time, we report the prognostic and oncogenic role of miR-183-5p in the progression of osteosarcoma.
In conclusion, this study demonstrates miR-183-5p as an oncogene that plays a critical role in regulating the progression of osteosarcoma cells. Thus, our results suggest a novel potential prognostic and therapeutic value of miR-183-5p in osteosarcoma.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gianferante DM, Mirabello L, Savage SA. Germline and somatic genetics of osteosarcoma - connecting aetiology, biology and therapy. Nat Rev Endocrinol. 2017;13(8):480–491. doi: 10.1038/nrendo.2017.16 [DOI] [PubMed] [Google Scholar]
- 2.Morrow JJ, Khanna C. Osteosarcoma genetics and epigenetics: emerging biology and candidate therapies. Crit Rev Oncog. 2015;20(3–4):173–197. doi: 10.1615/CritRevOncog.2015013713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaste SC, Pratt CB, Cain AM, Jones-Wallace DJ, Rao BN. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer. 1999;86(8):1602–1608. doi: [DOI] [PubMed] [Google Scholar]
- 4.Anderson ME. Update on survival in osteosarcoma. Orthop Clin North Am. 2016;47(1):283–292. doi: 10.1016/j.ocl.2015.08.022 [DOI] [PubMed] [Google Scholar]
- 5.Letson GD, Muro-Cacho CA. Genetic and molecular abnormalities in tumors of the bone and soft tissues. Cancer Control. 2001;8(3):239–251. doi: 10.1177/107327480100800304 [DOI] [PubMed] [Google Scholar]
- 6.Tutar Y. miRNA and cancer; computational and experimental approaches. Curr Pharm Biotechnol. 2014;15(5):429. doi: 10.2174/138920101505140828161335 [DOI] [PubMed] [Google Scholar]
- 7.Qadir MI, Faheem A. miRNA: a diagnostic and therapeutic tool for pancreatic cancer. Crit Rev Eukaryot Gene Expr. 2017;27(3):197–204. doi: 10.1615/CritRevEukaryotGeneExpr.2017019494 [DOI] [PubMed] [Google Scholar]
- 8.Sun Z, Shi K, Yang S, et al. Effect of exosomal miRNA on cancer biology and clinical applications. Mol Cancer. 2018;17(1):147. doi: 10.1186/s12943-018-0897-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Liu S, Shi J, et al. The role of miRNA in the diagnosis, prognosis, and treatment of osteosarcoma. Cancer Biother Radiopharm. 2019;34(10):605–613. doi: 10.1089/cbr.2019.2939 [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Yan YG, Wang C, Zhang SJ, Yu XH, Wang WJ. MicroRNAs in osteosarcoma. Clinica Chimica Acta. 2015;444:9–17. doi: 10.1016/j.cca.2015.01.025 [DOI] [PubMed] [Google Scholar]
- 11.Li W, Cui X, Qi A, Yan L, Wang T, Li B. miR-183-5p acts as a potential prognostic biomarker in gastric cancer and regulates cell functions by modulating EEF2. Pathol Res Pract. 2019;215(11):152636. doi: 10.1016/j.prp.2019.152636 [DOI] [PubMed] [Google Scholar]
- 12.Waseem M, Ahmad MK, Serajuddin M, Bhaskar V, Sankhwar SN, Mahdi AA. MicroRNA-183-5p: a new potential marker for prostate cancer. Indian J Clin Biochem. 2019;34(2):207–212. doi: 10.1007/s12291-017-0731-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Pan X, Gui Y, et al. Upregulation of miR-183-5p predicts worse survival in patients with renal cell cancer after surgery. Cancer Biomarkers. 2019;24(2):153–158. doi: 10.3233/CBM-182047 [DOI] [PubMed] [Google Scholar]
- 14.Cheng Y, Xiang G, Meng Y, Dong R. MiRNA-183-5p promotes cell proliferation and inhibits apoptosis in human breast cancer by targeting the PDCD4. Reprod Biol. 2016;16(3):225–233. doi: 10.1016/j.repbio.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 15.Meng F, Zhang L. miR-183-5p functions as a tumor suppressor in lung cancer through PIK3CA inhibition. Exp Cell Res. 2019;374(2):315–322. doi: 10.1016/j.yexcr.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Zhang M, Liu L, Jin D, Wang P, Hu J. MicroRNA-183-5p inhibits aggressiveness of cervical cancer cells by targeting integrin subunit beta 1 (ITGB1). Med Sci Monit. 2018;24:7137–7145. doi: 10.12659/MSM.910295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan H, Sun BM, Zhang YY, et al. Upregulation of miR-183-5p is responsible for the promotion of apoptosis and inhibition of the epithelial-mesenchymal transition, proliferation, invasion and migration of human endometrial cancer cells by downregulating Ezrin. Int J Mol Med. 2018;42(5):2469–2480. doi: 10.3892/ijmm.2018.3853 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Zheng Z, Zheng X, Zhu Y, et al. miR-183-5p inhibits occurrence and progression of acute myeloid leukemia via targeting erbin. Mol Ther. 2019;27(3):542–558. doi: 10.1016/j.ymthe.2019.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu J, Feng Y, Ke Z, et al. Down-regulation of miR-183 promotes migration and invasion of osteosarcoma by targeting Ezrin. Am J Pathol. 2012;180(6):2440–2451. doi: 10.1016/j.ajpath.2012.02.023 [DOI] [PubMed] [Google Scholar]
- 20.Yang X, Wang L, Wang Q, Li L, Fu Y, Sun J. MiR-183 inhibits osteosarcoma cell growth and invasion by regulating LRP6-Wnt/β-catenin signaling pathway. Biochem Biophys Res Commun. 2018;496(4):1197–1203. doi: 10.1016/j.bbrc.2018.01.170 [DOI] [PubMed] [Google Scholar]
- 21.Golbakhsh MR, Boddouhi B, Hatami N, et al. Down-regulation of microRNA-182 and microRNA-183 predicts progression of osteosarcoma. Arch Med Sci. 2017;6(6):1352–1356. doi: 10.5114/aoms.2016.60091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mu Y, Zhang H, Che L, Li K. Clinical significance of microRNA-183/Ezrin axis in judging the prognosis of patients with osteosarcoma. Med Oncol (Northwood, London, England). 2014;31(2):821. doi: 10.1007/s12032-013-0821-3 [DOI] [PubMed] [Google Scholar]
- 23.Sasaki R, Osaki M, Okada F. MicroRNA-based diagnosis and treatment of metastatic human osteosarcoma. Cancers. 2019;11(4):553. doi: 10.3390/cancers11040553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monterde-Cruz L, Ramirez-Salazar EG, Rico-Martinez G, et al. Circulating miR-215-5p and miR-642a-5p as potential biomarker for diagnosis of osteosarcoma in Mexican population. Hum Cell. 2018;31(4):292–299. doi: 10.1007/s13577-018-0214-1 [DOI] [PubMed] [Google Scholar]
- 25.Yang Z, Wa QD, Lu C, Pan W, Lu Z, Ao J. miR‑328‑3p enhances the radiosensitivity of osteosarcoma and regulates apoptosis and cell viability via H2AX. Oncol Rep. 2018;39(2):545–553. doi: 10.3892/or.2017.6112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miao F, Zhu J, Chen Y, Tang N, Wang X, Li X. MicroRNA-183-5p promotes the proliferation, invasion and metastasis of human pancreatic adenocarcinoma cells. Oncol Lett. 2016;11(1):134–140. doi: 10.3892/ol.2015.3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Fu H, Xu C, et al. miR-183 inhibits TGF-beta1-induced apoptosis by downregulation of PDCD4 expression in human hepatocellular carcinoma cells. BMC Cancer. 2010;10:354. doi: 10.1186/1471-2407-10-354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Quan H, Wang S, Li X, Che X. MiR-183 promotes growth of non-small cell lung cancer cells through FoxO1 inhibition. Tumour Biol. 2015;36(10):8121–8126. doi: 10.1007/s13277-015-3550-8 [DOI] [PubMed] [Google Scholar]
- 29.Shao JH, Yuan S, Qian QR, Zhu J. MicroRNA-183-5p suppresses the malignant progression of osteosarcoma via binding to AKT. Eur Rev Med Pharmacol Sci. 2019;23(19):8203–8210. doi: 10.26355/eurrev_201910_19127 [DOI] [PubMed] [Google Scholar]