ABSTRACT
Remdesivir is an antiviral drug currently being studied as a potential treatment of pneumonia caused by infection with SARS-CoV-2. The Adaptive Covid-19 Treatment Trial (ACTT-1) by NIH and the SIMPLE study by Gilead Sciences are two major trials that showed promising results of Remdesivir in the non-pregnant population. We are presenting the case of a pregnant patient who was diagnosed with COVID-19 pneumonia and successfully treated with Remdesivir.
KEYWORDS: COVID-19 pneumonia, pregnancy, Remdesivir, hydroxychloroquine
1. Introduction
Remdesivir inhibits viral replication by inhibiting RNA-dependent RNA-polymerase. Recent studies have shown a shortened time to recovery in patients with COVID-19 pneumonia who were treated with Remdesivir. Unfortunately, pregnant patients were excluded from the Adaptive Covid-19 Treatment Trial (ACTT-1) and the SIMPLE trial, which showed promising results of Remdesivir treatment in SARS-CoV-2 infection. Currently, there are no evidence-based studies on the effect of Remdesivir in pregnant patients diagnosed with COVID-19 pneumonia. Here, we present a 39-year-old pregnant patient, on hydroxychloroquine (HCQ) for Sjogren’s syndrome, diagnosed with COVID-19 pneumonia and successfully discharged home after treatment with Remdesivir at our institution.Table 1 and Table 2
Table 1.
Inflammatory markers, labs and oxygen requirement during the Remdesivir therapy
| Days since admission | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| IV Remdesivir (mg) | 200 | 100 | 100 | 100 | 100 | |
| Oxygen | 2 L | 2 L | 2 L | 1 L | 1 L | Room air |
| WBC (4.00–11.00 103/ul) | 14.7 | 15.8 | 11.2 | 10.3 | 12.4 | 9.33 |
| Creatinine (0.40–1.10 mg/dl) | 0.45 | 0.44 | 0.36 | 0.38 | 0.36 | 0.40 |
| AST (4–31 IU/L) | 18 | 21 | 21 | 21 | 25 | 34 |
| ALT (4–31 IU/L) | 12 | 12 | 10 | 11 | 14 | 22 |
| ALP (39–117 IU/L) | 115.7 | 123.3 | 117.4 | 133.7 | 124.7 | 124.5 |
| Ferritin (15–150 ng/ml) | 95.08 | 140 | 156 | 128 | 106 | 79.16 |
| D-dimer (≤0.50 ug/ml IFEU) | 1.40 | 1.55 | 0.90 | 0.84 | 1.02 | 0.98 |
| LDH (81–216 IU/L) | 311 | –- | –- | 305 | –- | –- |
| CRP (≤0.50 mg/dl) | 19 | –- | –- | 6.30 | 3.00 | 2.36 |
Table 2.
Summary of Remdesivir initiation and discontinuation criteria at our institute
| Initiation criteria | Hospital stay ≤7 days |
| Weight ≥40 kg with confirmed COVID-19 requiring oxygen supplementation* | |
| Estimated glomerular filtration rate >30 mL/min | |
| AST, ALT, bilirubin and alkaline phosphatase ≤3 times the upper limit of normal at baseline | |
| Not on >1 vasopressor or inotropic support | |
| Discontinuation criteria | ALT increase or decrease 5x upper limit of normal |
| ALT elevation along with signs or symptoms of liver inflammation or increasing conjugated bilirubin, alkaline phosphatase or INR | |
| Renal function declines to eGFR <30 mL/min or patient requires dialysis | |
| Serious adverse event |
Note: Only the important criteria have been mentioned.
*Excluding use of high-flow oxygen devices, noninvasive ventilation nor invasive ventilation.
2. Case description
A 39-year-old woman, Gravida 3, Para 2 at 29 weeks gestation presented to the emergency department with complaints of fever, chills and dry cough since 3 days. Her past medical history was significant for Rheumatoid arthritis treated with Methotrexate for 5 years, Non-Hodgkin’s lymphoma treated with chemotherapy 10 years ago and is in remission, Sjogren’s syndrome (diagnosed 1 year ago) and Rheumatoid arthritis (relapsed 2 years ago), currently on Hydroxychloroquine. On admission, she was febrile with a temperature of 102.2 degrees Fahrenheit, tachycardic at 126 beats per minute, tachypneic at 24 breaths per minute and mildly hypoxic at 94% on room air. She was mildly hypertensive with a blood pressure of 141/76. Her oxygen saturation improved to 100% after oxygen supplementation with 2 liters via nasal cannula.
Initial labs were significant for a leukocytosis of 14.75 x 103/µl with neutrophilia, a normal lymphocyte count, elevated C-reactive protein (CRP), lactate dehydrogenase and D-dimer. Procalcitonin was normal. Blood cultures were collected. A nasopharyngeal swab sample was taken and tested positive for COVID-19 by polymerase chain reaction. CT angiogram of the chest revealed extensive severe bilateral focal ground-glass infiltrates (Figures 1 and 2) without evidence of pulmonary embolism. Given the patient’s history of autoimmune conditions, current pregnancy, severe imaging findings and overall septic picture, she was admitted to the Intermediate Care Unit for close monitoring despite minimal oxygen requirements. Since the patient meets the criteria for the severe disease defined as requiring supplemental oxygen, as per our institution's policy, Infectious disease (ID) consultation was requested to evaluate if the patient meets the criteria for the use of Remdesivir set forth by the Emergency use authorization (EUA) released by the FDA [1]. Patient met the criteria and ID physician suggested to initiate Remdesivir after discussing the risks and benefits and obtaining the patient's consent since the teratogenic side effects of Remdesivir are not known. Patient has been provided information regarding the possible side effects including unknown teratogenicity and provided the copy of the patient information released by the Gilead sciences. Patient consented for the use of the Remdesivir. After following the standard of work flow for the use of Remdesivir at our institute which includes approval by hospital pharmacy as per the EUA guidelines for the Remdesivir issued by the FDA, she received 200 mg of IV Remdesivir on Day 1 followed by 100 mg daily for the next 4 days. Since the patient is symptomatic for less than 7 days at the time of admission, she did not meet the criteria to receive the steroid therapy as per the RECOVERY trial.
Figure 1.
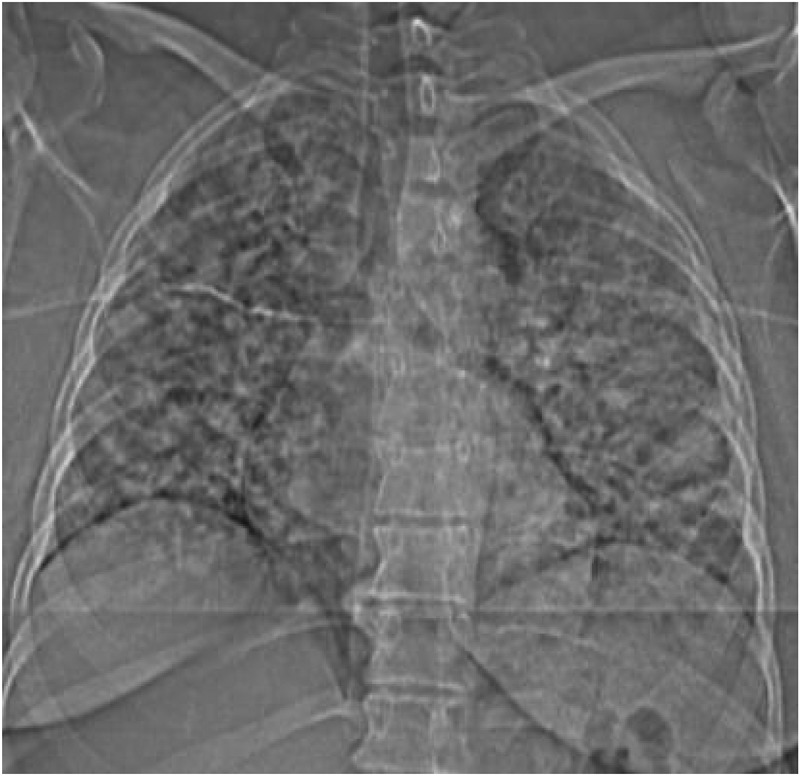
Chest computerized tomography coronal view showing bilateral infiltrates in the lungs
Figure 2.
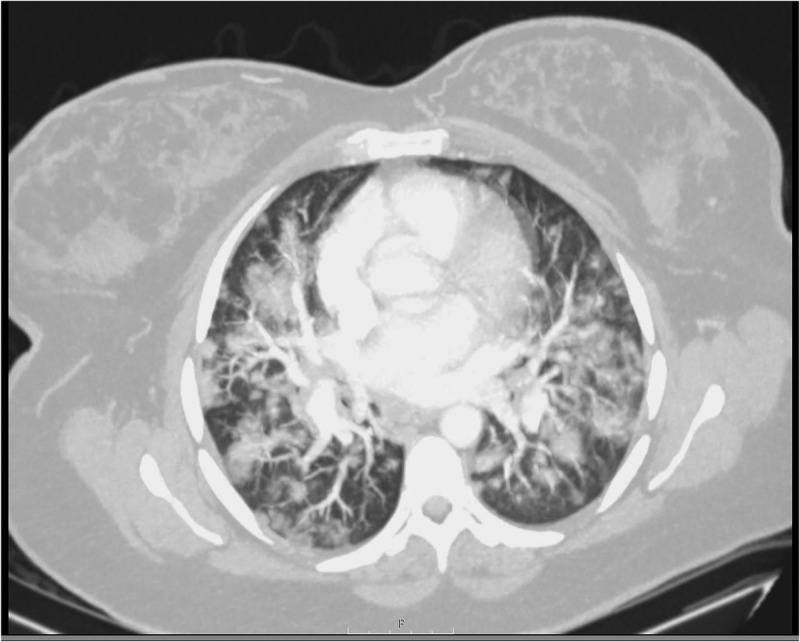
Chest computerized tomography showing extensive severe bilateral focal ground-glass infiltrates typical characteristics of COVID-19 (axial view)
She successfully completed the course of Remdesivir without any side effects and showed steady improvement throughout the hospital stay. Fetal activity was monitored every 8 hours using the external fetal monitor, with no abnormalities detected. As per the obstetrician’s recommendations, supplemental oxygen was provided to maintain saturations >95%. Oxygen saturations were around 98−100% while on 2 L via NC and weaned down to room air with saturations >95% by the time of discharge on day six.
3. Discussion
Remdesivir is an antiviral medication that achieves its effect by inhibiting the RNA-dependent RNA polymerase and viral nucleic acid synthesis [2]. It has shown superiority over placebo in shortening the time to recovery in patients with COVID-19 infection. Two major trials, the Adaptive Covid-19 Treatment Trial (ACTT-1) by NIH [3] and the SIMPLE study by Gilead sciences [4], have shown promising results with the use of Remdesivir. An Emergency Use Authorization (EUA) has been issued by the US Food and Drug Administration (FDA) for using Remdesivir to treat COVID-19 patients.
Despite there being a considerable number of pregnant women diagnosed with COVID-19 pneumonia, the ACTT-1 trial excluded pregnant patients. Historically, pregnant patients are less likely to be enrolled in clinical trials due to the potential for deleterious effects on the developing fetus, which is likely the case in studies of Remdesivir in COVID-19 thus far [5]. Gilead Sciences has not yet done a proper study on the pregnant patient but has recently released some of its data analysis from the compassionate use program of Remdesivir in pregnant patients and is planning to collaborate on a study of pregnant patients. Zaigham et al. (2020) and Pierce et al. (2020) in their systematic review and cohort study respectively, on pregnant patients with COVID-19 infection showed that these patients are at risk for severe disease, that 50% delivered successfully during active infection [6,7] and that vertical transmission has been reported [8]. Pregnancy is a unique condition with many changes in the immune system, which may lead to increased susceptibility to severe infections involving the activation of the immune system. We believe this is highly likely to be the case in COVID-19 pneumonia as the immune system and cytokine storm play a major role in the pathophysiology [9]. According to recent update from the CDC MMWR, among the women of reproductive age between 15 and 44 years with symptomatic COVID-19 infection, pregnant patients are significantly more likely to have severe disease including ICU admission, invasive ventilation, receive ECMO and die from the infection [10]. All these factors stress the importance of including pregnant patients in clinical trials involving COVID-19 therapies [11].
Our patient was considered high-risk given her pregnancy, autoimmune comorbidities and diffuse bilateral ground glass lung opacities requiring supplemental oxygen. With limited available data for the treatment of COVID-19 pneumonia, the decision was made to proceed with Remdesivir. After discussing the risks and benefits, a 5-day course of IV Remdesivir therapy was given. As per FDA guidelines, HCQ was held as co-administration has been found to decrease the effectiveness of Remdesivir therapy [1]. As shown, her inflammatory markers continued to improve with therapy, particularly the CRP levels. The patient tolerated the therapy without any side effects. Daily levels of AST, ALT and serum creatinine were obtained to monitor the side effects of Remdesivir therapy and stayed within an acceptable range. Her oxygen requirement was weaned from 2 L on Day 1 to 1 L on Day 4 and she was successfully weaned off oxygen by Day 6. After the initial observation in the Intermediate Care Unit, she was transferred to the general medical floor and was successfully discharged home with precautionary instructions. Patient did not experience side effects since the discharge and had complete symptomatic recovery from the COVID-19 infection, followed regularly with the Obstetrician and delivered a healthy infant at 39 weeks of gestation via C-section. Infant did not show any illness, signs of the respiratory infection after delivery and therefore not tested for the COVID-19 infection. Post pregnancy till date patient is in her usual state of health prior to the COVID-19 infection and no follow-up imaging has been done yet to evaluate the progression of the pulmonary lesions.
4. Conclusion
In summary, we present a pregnant patient with COVID-19 infection and significant bilateral ground glass opacities requiring supplemental oxygen who demonstrated both clinical and laboratory improvement after a 5-day course of intravenous Remdesivir. Given the fact that pregnant patients are at similar risk of contracting COVID-19 to the general population, higher risk to have severe infection including death and with the potential risk of vertical transmission to the fetus, there is an urgent need to evaluate the efficacy of COVID-19 pneumonia treatment in this group of patients. Even though there are few published case reports about compassionate use of the Remdesivir in pregnant with clinical improvement and uncomplicated deliveries [12–14] like ours, a randomized controlled trial to provide evidence-based treatment guidelines for this unique patient group is desperately needed especially with a recent CDC report indicating pregnant patient are at higher risk for the severe disease including death.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Fact sheet for health care providers: emergency use authorization (EUA) of VEKLURY (Remdesivir).
- [2].Gordon CJ, Tchesnokov EP, Woolner E, et al. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem. 2020;295(20):6785–6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 – preliminary report [published online ahead of print, 2020 May 22]. N Engl J Med. 2020. DOI: 10.1056/NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- [4].Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe covid-19 [published online ahead of print, 2020 May 27]. N Engl J Med. 2020;NEJMoa2015301. DOI: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Smith DD, Pippen JL, Adesomo AA, et al. Exclusion of pregnant women from clinical trials during the coronavirus disease 2019 pandemic: a review of international registries. Am J Perinatol. 2020;37(8):792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zaigham M, Andersson O.. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020;99(7):823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].RAM P-W, Burd J, Felder L, et al. Clinical course of severe and critical COVID-19 in hospitalized pregnancies: a US cohort study [published online ahead of print, 2020 May 8]. Am J Obstet Gynecol MFM. 2020;100134. DOI: 10.1016/j.ajogmf.2020.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Alzamora MC, Paredes T, Caceres D, et al. During pregnancy and possible vertical transmission. Am J Perinatol. 2020;37(8):861–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu H, Wang LL, Zhao SJ, et al. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J Reprod Immunol. 2020;139:103122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status — USA, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].LaCourse S, John-Stewart G, Adams Waldorf KM. Importance of Inclusion of pregnant and breastfeeding women in COVID-19 therapeutic trials. Clin Infect Dis. 2020;71(15):879–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Naqvi MMD, Zakowski PMD, Glucksman LMD, et al. MPH tocilizumab and Remdesivir in a pregnant patient with coronavirus disease 2019 (COVID-19). Obstetrics Gynecol. 2020. November;136(5):1025–1029. [DOI] [PubMed] [Google Scholar]
- [13].Maldarelli GA, Savage M, Mazur S, et al. Remdesivir treatment for severe COVID-19 in third-trimester pregnancy: case report and management discussion. Open Forum Infect Dis. 2020. September;7(9):ofaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Igbinosa I, Miller S, Bianco K, et al. Use of Remdesivir for pregnant patients with severe novel coronavirus disease 2019. Am J Obstet Gynecol. 2020;223(5):768–770. [DOI] [PMC free article] [PubMed] [Google Scholar]


