Abstract
COVID-19 has become a global pandemic and there is an urgent call for developing drugs against the virus (SARS-CoV-2). The 3C-like protease (3CLpro) of SARS-CoV-2 is a preferred target for broad spectrum anti-coronavirus drug discovery. We studied the anti-SARS-CoV-2 activity of S. baicalensis and its ingredients. We found that the ethanol extract of S. baicalensis and its major component, baicalein, inhibit SARS-CoV-2 3CLpro activity in vitro with IC50’s of 8.52 µg/ml and 0.39 µM, respectively. Both of them inhibit the replication of SARS-CoV-2 in Vero cells with EC50’s of 0.74 µg/ml and 2.9 µM, respectively. While baicalein is mainly active at the viral post-entry stage, the ethanol extract also inhibits viral entry. We further identified four baicalein analogues from other herbs that inhibit SARS-CoV-2 3CLpro activity at µM concentration. All the active compounds and the S. baicalensis extract also inhibit the SARS-CoV 3CLpro, demonstrating their potential as broad-spectrum anti-coronavirus drugs.
Keywords: COVID-19, SARS-CoV-2, 3C-like protease, Scutellaria baicalensis, baicalein
1. Introduction
Coronaviruses (CoVs) are single stranded positive-sense RNA viruses that cause severe infections in respiratory, hepatic, and various organs in humans and many other animals1,2. Within the 20 years of the twenty-first century, there are already three outbreaks of CoV-causing global epidemics, including SARS, MERS, and COVID-19. The newly emerged CoV infectious disease (COVID-19) has become a worldwide pandemic that needs to be controlled. There is an urgent call for drug and vaccine research and development against COVID-19.
COVID-19 was confirmed to be caused by a new coronavirus (SARS-CoV-2), whose genome was sequenced in early January 20203,4. The genomic sequence of SARS-CoV-2 is highly similar to that of SARS-CoV with about 79.6% sequence identity5 and remains stable up to now6. However, the sequence identities vary significantly for different viral proteins5. For instance, the spike proteins (S-protein) in CoVs are diverse in sequences and even in the host receptors that bind due to the rapid mutations and recombination7. Although it has been confirmed that both SARS-CoV and SARS-CoV-2 use ACE2 as receptor and occupy the same binding site, their binding affinities to ACE2 vary due to subtle interface sequence variations8. On the contrary, the 3C-like proteases (3CLpro) in CoVs are highly conserved. The 3CLpro in SARS-CoV and SARS-CoV-2 share a sequence identity of 96.1%, making it an ideal target for broad spectrum anti-CoV therapy. Although many inhibitors have been reported for SARS-CoV and MERS-CoV 3CLpro 9–12, unfortunately none of them has entered clinical trial. Inspired by the previous studies, several covalent inhibitors were rationally designed and experimentally shown to inhibit the 3CLpro activity and viral replication of SARS-CoV-2, with some of the complex crystal structures solved13–15. In addition, a number of clinically used HIV and HCV protease inhibitors have been proposed as possible cure for COVID-1916 and some of them are now processed to clinically trials17. Most of the reported SARS-CoV-2 3CLpro inhibitors covalently target the active site cysteine. Highly potent SARS-CoV-2 3CLpro inhibitors with diverse chemical structures and mode of action need to explored.
Traditional Chinese medicine (TCM) herbs and formulae have long been used in treating viral diseases. Some of them have been clinically tested to treat COVID-1918. Scutellariae radix (Huangqin in Chinese), the root of Scutellaria baicalensis Georgi, has been reported for widely used in TCM for heat clearing, fire purging, detoxification, and haemostasis. Huangqin is officially recorded in Chinese Pharmacopoeia (2015 Edition)19 and European Pharmacopoeia (10th Edition)20. Its anti-tumour, antiviral, anti-microbial, and anti-inflammatory activities have been reported21. Remarkably, the extracts of S. baicalensis have exhibited broad spectrum anti-viral activities, including ZIKA22, H1N123, HIV24, and DENV25. In addition, a multicentre, retrospective analysis demonstrated that S. baicalensis exhibits more potent antiviral effects and higher clinical efficacy than ribavirin for the treatment of hand, foot, and mouth disease26. Several S. baicalensis derived mixtures or pure compounds have been approved as antiviral drugs, such as Baicalein capsule (to treat hepatitis) and Huangqin tablet (to treat upper respiratory infection) in China. Most of the S. baicalensis ingredients are flavonoids27. Flavonoids from other plants were also reported to mildly inhibit SARS and MERS-CoV 3CLpro 28,29. Here, we studied the anti-SARS-CoV-2 activity of S. baicalensis and its ingredients. We found that the ethanol extract of S. baicalensis inhibits SARS-CoV-2 3CLpro activity and the most active ingredient baicalein exhibits an IC50 of 0.39 µM. Both the ethanol extract of S. baicalensis and baicalein effectively inhibit the replication of SARS-CoV-2 in cell assay. The anti-SARS-CoV-2 3CLpro activity and antiviral activity were also reported by a recent publication30. We further studied the structure and activity relationship of baicalein analogues and identified four new active compounds from other herbs that inhibit SARS-CoV-2 3CLpro activity at µM concentration.
2. Materials and methods
S. baicalensis were purchased from Tong Ren Tang Technologies Co. Ltd. (Beijing, China). Baicalein and compounds not listed below were from J&K Scientific (Beijing, China). 5,6-Dihydroxyflavone was purchased from Alfa Aesar (Haverhill, MA). 6,7-Dihydroxyflavone was synthesised by Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). Myricetin, quercetagetin, and herbacetin were purchased from MCE (Shanghai, China). Dihydromyricetin and myricetin were purchased from Targetmol (Boston, MA).
2.1. Construction of plasmid SARS-CoV-2 pET 3CL-21x, protein expression, and purification
The DNA of SARS-CoV-2 3CLpro (referred to GenBank, accession number MN908947) was synthesised (Hienzyme Biotech, Changsha, China) and amplified by PCR using primers n3CLP-Nhe (5′-CATGGCTAGCGGTTTTAGAAAAATGGCATTCCC-3′) and n3CLP-Xho (5′-CACTCTCGAGTTGGAAAGTAACACCTGAGC-3′). The PCR product was digested with Nhe I/Xho I and cloned into the pET 21a DNA as reported previously31. The resulting SARS-CoV-2 pET 3CL-21x plasmid encodes a 35,064 Da SARS-CoV-2 3CLpro with a C-terminal 6xHis-tag. The SARS-CoV-2 pET 3CL-21x plasmid was further transformed to E. coli BL21 < DE3> for protein expression as reported31. The recombinant protein was purified through a nickel-nitrilotriacetic acid column (GE Healthcare, Chicago, IL) and subsequently loaded on a gel filtration column Sephacryl S-200 HR (GE Healthcare, Chicago, IL) for further purification as previously described32.
2.2. Preparation of the ethanol extract of S. baicalensis
After minced, 5 g S. baicalensis was extracted with 100 ml of 70% ethanol in a 300 ml flask after sonication for 1 h at 25 °C. The solvent was filtrated and completely removed under reduced pressure at room temperature. The residue was freeze-dried overnight. The dried herbal residue was then dissolved completely to obtain a 100 mg/ml stock solution in DMSO which was stored at −20 °C before use.
2.3. Enzyme inhibition assay
A colorimetric substrate Thr-Ser-Ala-Val-Leu-Gln-pNA (GL Biochemistry Ltd., Shanghai, China) and assay buffer (40 mM PBS, 100 mM NaCl, 1 mM EDTA, 0.1% Triton 100, pH 7.3) was used for the inhibition assay. Stock solutions of the inhibitors were prepared with 100% DMSO. The 100 µl reaction systems in assay buffer contain 0.5 µM protease and 5% DMSO or inhibitor to the final concentration. First, SARS-CoV-2 3CLpro was diluted with assay buffer to the desired concentration. Five microlitres DMSO or inhibitor at various concentrations was pre-incubated with 85 µl diluted SARS-CoV-2 3CLpro for 30 min at room temperature. Then 10 µl 2 mM substrate Thr-Ser-Ala-Val-Leu-Gln-pNA (dissolved in water) was added into the above system to the final concentration of 200 µM to initiate the reaction. Increase in absorbance at 390 nm was recorded for 20 min at interval of 30 s with a kinetics mode program using a plate reader (Synergy, Biotek, Winooski, VT). The percent of inhibition was calculated by Vi/V0, where V0 and Vi represent the mean reaction rate of the enzyme incubated with DMSO or compounds. IC50 was fitted with Hill1 function.
2.4. Molecular docking
The structure of SARS-CoV-2 3CLpro (PDB ID 6LU7)13 and S. baicalensis components were prepared using Protein Preparation Wizard and LigPrep module, respectively. Then, the binding site was defined as a 20 × 20 × 20 Å3 cubic box centred to the centroid of C145. After that, molecular docking was performed using Glide. Extra precision (XP) and flexible ligand sampling were adopted. Post-docking minimisation was performed to further refine the docking results. All the above-mentioned modules were implemented in Schrödinger version 2015-4 (Schrödinger Software Suite, L.L.C., New York, NY, 2015).
2.5. Cell culture and virus
Vero cell line (ATCC, CCL-81) was cultured at 37 °C in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Grand Island, NY) supplemented with 10% foetal bovine serum (FBS, Gibco, Grand Island, NY) in the atmosphere with 5% CO2. Cells were digested with 0.25% trypsin and uniformly seeded in 96-well plates with a density of 2 × 104 cells/well prior infection or drug feeding. The virus (C-Tan-nCoV Wuhan strain 01) used is a SARS-CoV-2 clinically isolated virus strain. These viruses were propagated in Vero cells.
2.6. Antiviral activity assay
The cytotoxicity of S. baicalensis extract and baicalein on Vero cells were determined by CCK8 assays (DOJINDO, Kumamoto, Japan). We then evaluated the antiviral efficiency of S. baicalensis extract and baicalein against SARS-CoV-2 (C-Tan-nCoV Wuhan strain 01) virus in vitro. Cells were seeded into 96-well plates at a density of 2 × 104 cells/well and then grown for 24 h. For the full-time inhibition test, cells were pre-treated with indicated concentrations of S. baicalensis extract or baicalein for 1 h, and the virus (MOI of 0.01, 200 PFU/well) was subsequently added to allow infection for 2 h at 37 °C. Virus input was washed with DMEM and then the cells were treated with medium containing the drugs at various concentrations for 48 h. For the entry inhibition test, after viral infection for 2 h, both the virus input and the drugs were washed out with DMEM and the cells were treated with fresh medium without drugs for 48 h. For the post-entry inhibition test, the drugs were added only after 2 h of viral infection and maintained until the end of the experiment. The supernatant was collected and the RNA was extracted and analysed by relative quantification using RT-PCR as in the previous study33.
2.7. RNA extraction and RT-qPCR
Viral RNA was extracted from 100 μl supernatant of infected cells using the automated nucleic acid extraction system (TIANLONG, Hangzhou, China), following the manufacturer’s recommendations. SARS-CoV-2 virus detection was performed using the One Step PrimeScript RT-PCR kit (TaKaRa, Shiga, Japan) on the LightCycler 480 Real-Time PCR system (Roche, Rotkreuz, Switzerland). ORF 1ab was amplified from cDNA and cloned into MS2-nCoV-ORF1ab and used as the plasmid standard after its identity was confirmed by sequencing. A standard curve was generated by determination of copy numbers from serially dilutions (103–109 copies) of plasmid. The following primers used for quantitative PCR were 1ab-F: 5′-AGAAGATTGGTTAGATGATGATAGT-3′; 1ab-R: 5′-TTCCATCTCTAATTGAGGTTGAACC-3′; and probe 5′-FAM-TCCTCACTGCCGTCTTGTTG ACCA-BHQ1-3′. The individual EC50 values were calculated by the Origin 2018 software.
3. Results and discussion
3.1. The ethanol extract of S. baicalensis strongly inhibits SARS-CoV-2 3CLpro
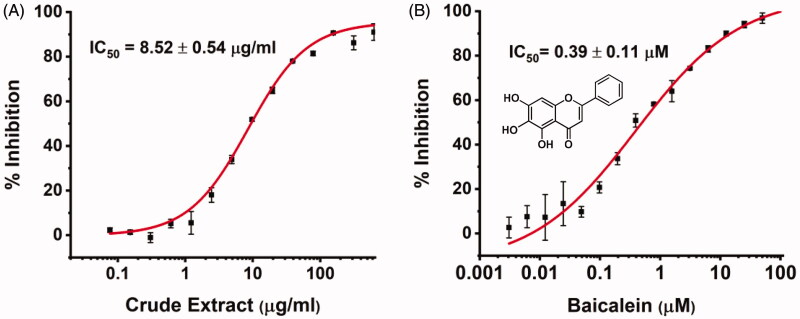
We first prepared the 70% ethanol extract of S. baicalensis and tested its inhibitory activity against SARS-CoV-2 3CLpro. We expressed SARS-CoV-2 3CLpro and performed activity assay using a peptide substrate (Thr-Ser-Ala-Val-Leu-Gln-pNA) according to the published procedure of SARS-CoV 3CLpro assay10,34. As shown in Figure 1(A), the crude extract exhibits significant inhibitory effect with an IC50 of 8.5 µg/ml, suggesting that S. baicalensis contains candidate inhibitory ingredients against SARS-CoV-2 3CLpro.
Figure 1.
The in vitro anti-SARS-CoV-2 3CLpro activity of S. baicalensis ethanol extract (A) and baicalein (B).
3.2. Baicalein is the major active ingredient in S. baicalensis that inhibits SARS-CoV-2 3CLpro
We then tested the inhibitory activity of four major ingredients from S. baicalensis: baicalein, baicalin, wogonin, and wogonoside in vitro. Baicalein showed the most potent anti-SARS-CoV-2 3CLpro activity with an IC50 of 0.39 µM (Figure 1(B) and Table 1). Baicalin inhibited SARS-CoV-2 3CLpro activity for about 41% at 50 µM with an IC50 of 83.4 µM (Figure S1), while wogonin and wogonoside were not active at this concentration. The ratio of baicalein in the crude extract determined by HPLC was 2.07% (Table S2) which is in consistent with previous reports35. To eliminate possible non-specific effect, the inhibitory activity of ethanol extract and all the compounds were measured in the buffer with 0.1% Triton X-100.
Table 1.
The SARS-CoV-2 3CLpro inhibition activity of four major flavones derived from S. baicalensis.
| Compound | Chemical structure | IC50 (μM) | % Inhibition at 50 μM |
|---|---|---|---|
| Baicalein |
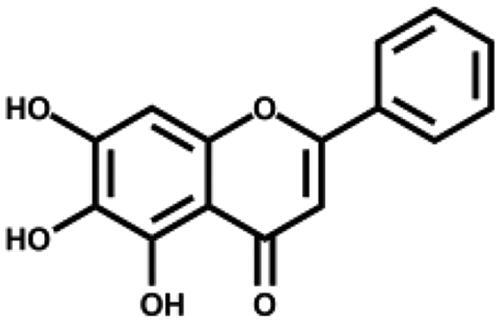
|
0.39 ± 0.12 | – |
| Baicalin |
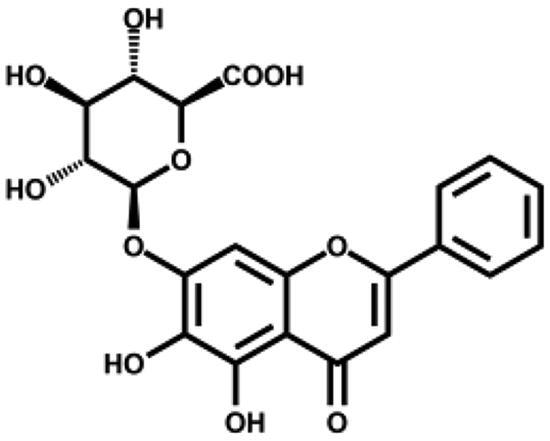
|
83.4 ± 0.9 | 41.5 ± 0.6 |
| Wogonin |
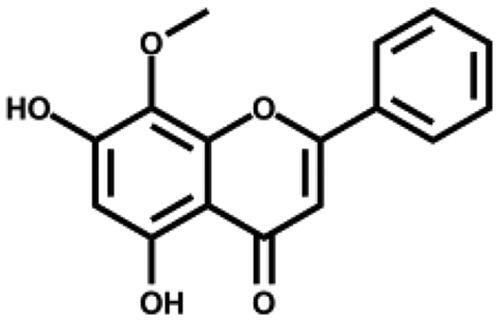
|
– | 6.1 ± 0.8 |
| Wogonoside |
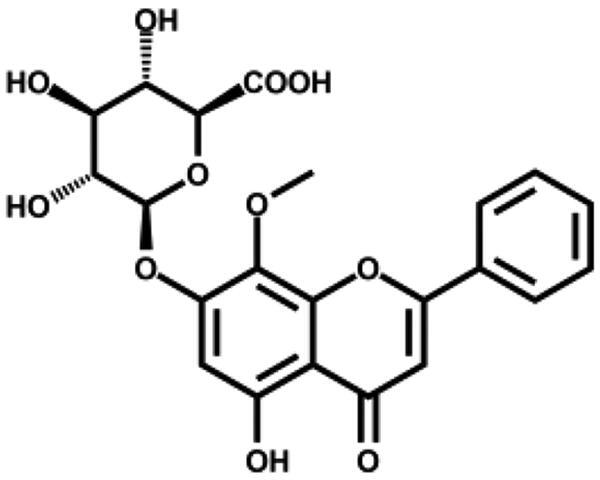
|
– | 8.5 ± 3.3 |
3.3. S. baicalensis extract and baicalein inhibit the replication of SARS-CoV-2 in Vero cells
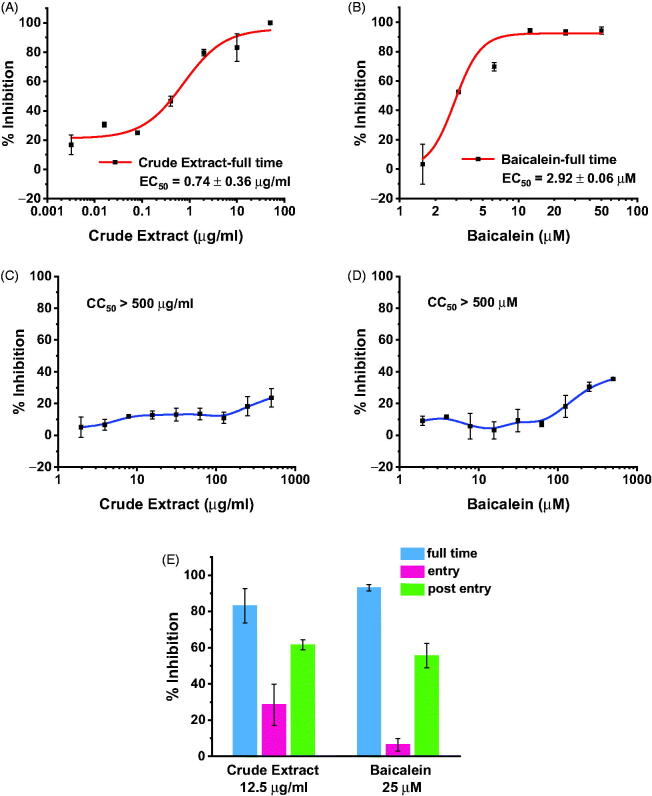
We further tested the antiviral activity of S. baicalensis ethanol extract and baicalein against SARS-CoV-2 using RT-qPCR. Vero cells were pre-treated with the extract or baicalein for 1 h, followed by virus infection for 2 h. Virus input was then washed out and the cells were treated with medium containing the extract or baicalein. Viral RNA was extracted from the supernatant of the infected cells and quantified by RT-PCR. The S. baicalensis ethanol extract significantly reduced the growth of the virus with an EC50 of about 0.74 µg/ml with low cytotoxicity (SI > 675, Figure 2(A,C)). Baicalein inhibits the replication of SARS-CoV2 with an EC50 of 2.9 µM and SI > 172 (Figure 2(B,D)).
Figure 2.
The antiviral activity and cytotoxicity of S. baicalensis extract (A, C) and baicalein (B, D) against SARS-CoV-2 in Vero cells. The effects of S. baicalensis extract and baicalein in different viral infection periods were also detected (E).
As the synthesis and function of SARS-CoV-2 3CLpro take place inside the host cell, its inhibitors should be effective mainly at the post-entry stage. Since the above data were obtained by full-time incubation, we further tested the inhibition activity of baicalein and S. baicalensis extract at the entry or post-entry stage (see Materials and Methods section in Supporting Information for details). Both baicalein and the extract are active at post-entry stage, while the extract also shows noticeable inhibition activity at the viral entry stage. The high activity and multiple action stages of S. baicalensis crude extract in the antiviral assay implies that it may also interact with other viral or host targets in addition to SARS-CoV-2 3CLpro inhibition, which can be further explored in the future.
3.4. Baicalein binds SARS-CoV-2 3CLpro to inhibit its activity
To confirm that baicalein and the crude extract inhibit viral replication by directly targeting SARS-CoV-2 3CLpro in cells, we tested their anti-SARS-CoV-2 3CLpro activity under complex cell environment. As the expression of SARS-CoV-2 3CLpro is toxic to mammalian cells36, we used the mixture of HEK293T cell lysate and purified SARS-CoV-2 3CLpro for the test. Both baicalein and the crude extract showed inhibition activity under this condition (Figure S2).
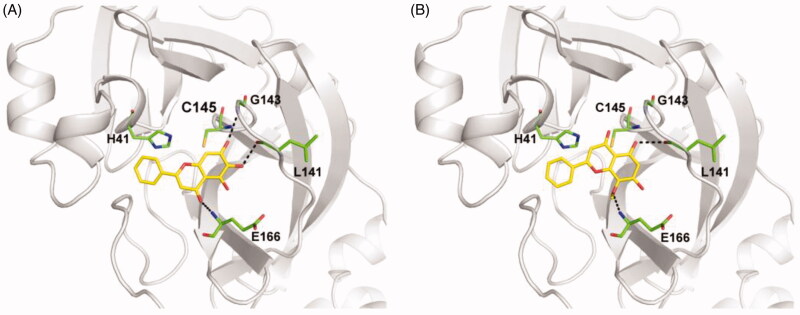
We performed molecular docking to understand the inhibitory activity of S. baicalensis ingredients. The docking scores are listed in Table S1. In the docking model, baicalein binds well in the substrate binding site of SARS-CoV-2 3CLpro with its 6-OH and 7-OH forming hydrogen bond interactions with the carbonyl group of L141 and the backbone amide group of G143, respectively (Figure 3(A)). In addition, the carbonyl group of baicalein is hydrogen bonded with the backbone amide group of E166. The catalytic residues H41 and C145 are well covered by baicalein, accounting for its inhibitory effect. This computational model is in consistent with the recently reported crystal structure of SARS-CoV-2 3CLpro complexed with bacailein30. As the 7-OH in baicalin is in close contact with the protein, there may not be enough space for glycosyl modification, explaining the low activity of baicalin. As for wogonin, the absence of 6-OH together with its additional 8-methoxyl group alters the binding orientation and weakens the binding strength (Figure 3(B)). Hydrogen bond is observed between its 5-OH and the backbone carbonyl group of L141, while the interaction with E166 by its 8-methoxy group is weaker than that formed by the carbonyl group in baicalein.
Figure 3.
The interactions between SARS-CoV-2 3CLpro and S. baicalensis ingredients baicalein (A) and wogonin (B) in the docking models. The overall structure and key residues of SARS-CoV-2 3CLpro are shown as grey cartoon and green sticks, respectively. S. baicalensis ingredients are displayed as yellow sticks.
3.5. Searching for baicalein analogues that inhibit SARS-CoV-2 3CLpro
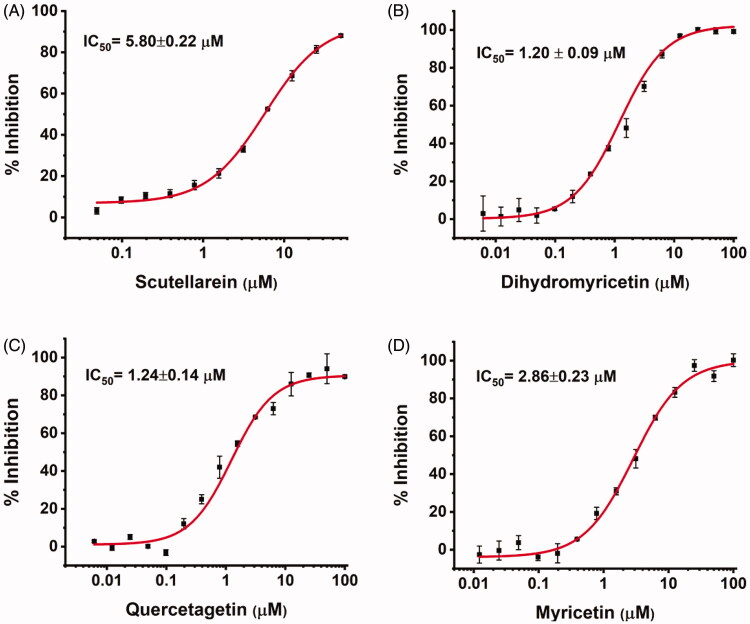
Based on the docking analysis, we searched for baicalein analogues from available flavonoid suppliers and selected eight flavonoids and two glycosides for experimental testing (Table 2). The docking scores are given in Table S1. Four flavonoid compounds were found to be potent SARS-CoV-2 3CLpro inhibitors. Among them, scutellarein is mainly distributed in genus Scutellaria and Erigerontis herba (Dengzhanxixin or Dengzhanhua in Chinese) in its glucuronide form, scutellarin. Scutellarin has long been used in cardiovascular disease treatment for its ability to improve cerebral blood supply37. As shown in Figure 4, scutellarein inhibits SARS-CoV-2 3CLpro with an IC50 value of 5.8 µM, while scutellarin showed mild inhibitory activity at 50 µM concentration. The other three flavonoid compounds, dihydromyricetin, quercetagetin, and myricetin derived from Ampelopsis japonica (Bailian in Chinese), Eriocaulon buergerianum (Gujingcao in Chinese), and Polygoni avicularis (Bianxu in Chinese), respectively, inhibit SARS-CoV-2 3CLpro with IC50 values of 1.20, 1.24, and 2.86 µM. Interestingly, scutellarein and myricetin were reported to inhibit the SARS-CoV, indicating their potential as multi-target anti-SARS-CoV-2 agents38.
Table 2.
The anti-SARS-CoV-2 3CLpro activity of baicalein analogue flavonoids.
| Compound | Chemical structure | IC50 (μM) | % Inhibition at 50 μM |
|---|---|---|---|
| Scutellarein |
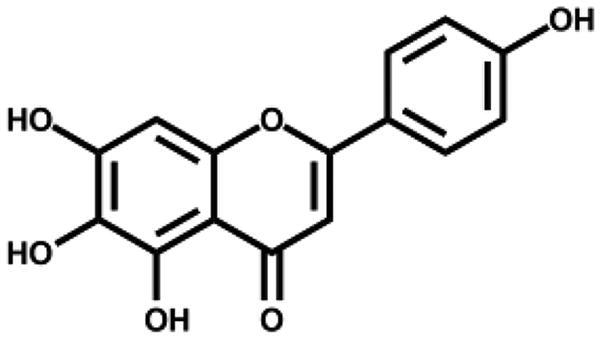
|
5.80 ± 0.22 | – |
| Dihydromyricetin |
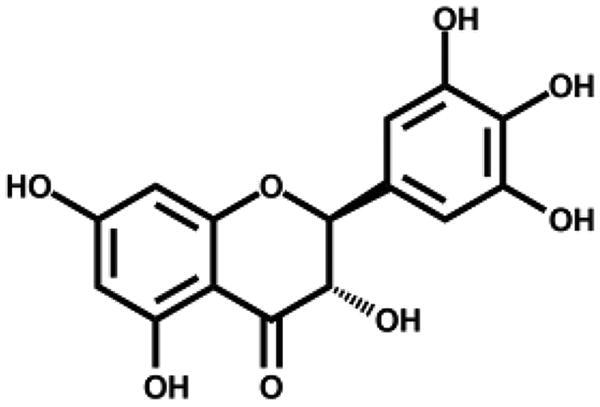
|
1.20 ± 0.09 | – |
| Quercetagetin |
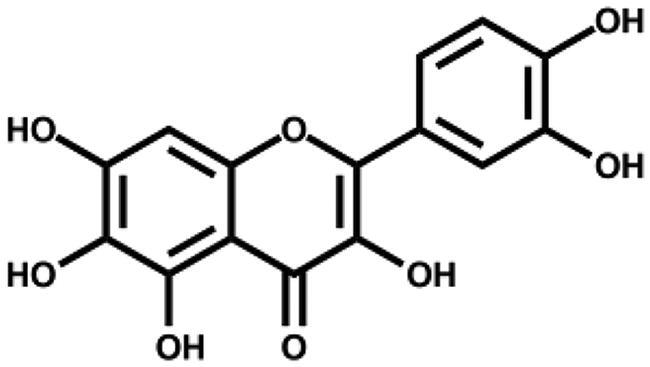
|
1.24 ± 0.14 | – |
| Myricetin |
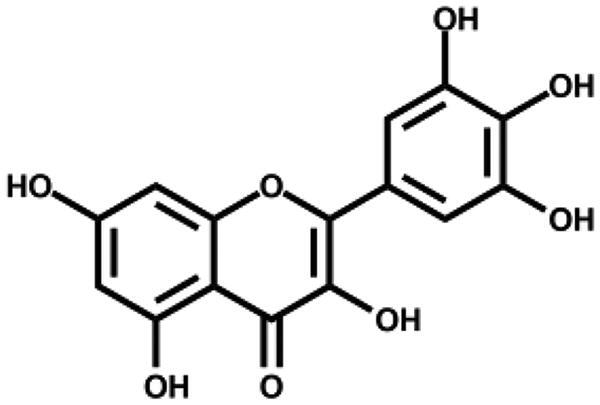
|
2.86 ± 0.23 | – |
| Scutellarin |
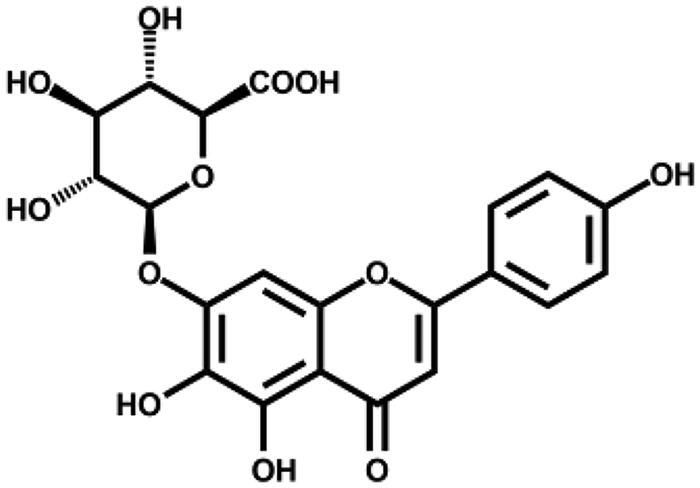
|
– | 28.9 ± 1.6 |
| 5,6-Dihydroxyflavone |
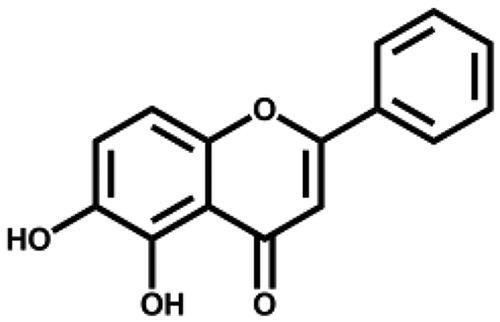
|
– | 26.6 ± 0.4 |
| 6,7-Dihydroxyflavone |
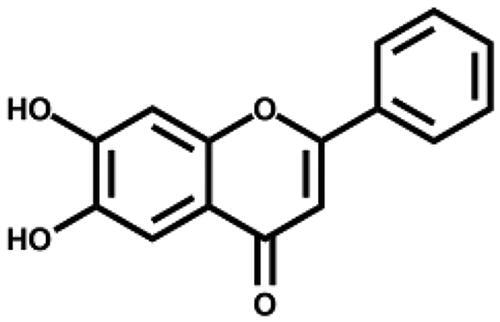
|
– | 56.7 ± 2.0 |
| Chrysin |
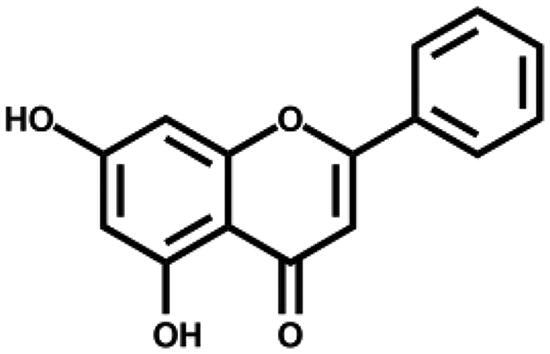
|
– | 2.6 ± 1.1 |
| Myricetin |
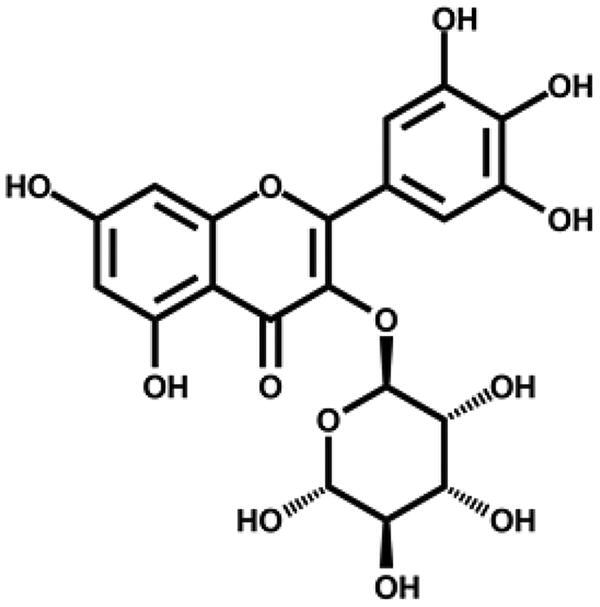
|
– | 30.8 ± 4.6 |
| Herbacetin |
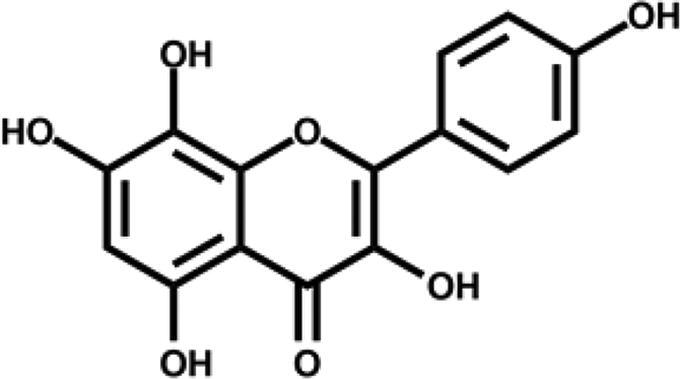
|
– | 59.1 ± 1.9 |
Figure 4.
The SARS-CoV-2 3CLpro inhibition activity of (A) scutellarein, (B) dihydromyricetin, (C) quercetagetin, and (D) myricetin.
We further showed that S. baicalensis extract, baicalein, and the four active baicalein analogue compounds also inhibit the activity of SARS-CoV 3CLpro in vitro (Figure S3), demonstrating their potential as broad spectrum anti-CoV agents. For future development and in vivo studies, the bioavailability of these compounds needs to be considered. Although the bioavailability of baicalein is low in oral administration, its concentration can be increased at lung tissue through intratracheal administration30. We suggest that these compounds can be further optimised or used to search for other TCM herbs containing these compounds or substructures to develop effective treatments for COVID-19 and other coronavirus infectious diseases.
Supplementary Material
Funding Statement
This work was supported in part by the Ministry of Science and Technology of China [2016YFA0502303, 2016YFD0500301], the National Natural Science Foundation of China [21633001], and the Fundamental Research Funds for the Central Universities of China.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Zumla A, Chan JF, Azhar EI, et al. Coronaviruses – drug discovery and therapeutic options. Nat Rev Drug Discov 2016;15:327–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adedeji AO, Sarafianos SG.. Antiviral drugs specific for coronaviruses in preclinical development. Curr Opin Virol 2014;8:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020;579:265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang X, Wu C, Li X, et al. On the origin and continuing evolution of SARS-CoV-2. Natl Sci Rev 2020;7:1012–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol 2016;3:237–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lan J, Ge J, Yu J, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020;581:215–20. [DOI] [PubMed] [Google Scholar]
- 9.Pillaiyar T, Manickam M, Namasivayam V, et al. An overview of severe acute respiratory syndrome-coronavirus (SARS-CoV) 3CL protease inhibitors: peptidomimetics and small molecule chemotherapy. J Med Chem 2016;59:6595–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou L, Liu Y, Zhang W, et al. Isatin compounds as noncovalent SARS coronavirus 3C-like protease inhibitors. J Med Chem 2006;49:3440–3. [DOI] [PubMed] [Google Scholar]
- 11.Yang H, Xie W, Xue X, et al. Design of wide-spectrum inhibitors targeting coronavirus main proteases. PLoS Biol 2005;3:e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu CY, Jan JT, Ma SH, et al. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc Natl Acad Sci U S A 2004;101:10012–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin Z, Du X, Xu Y, et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors . Nature 2020;582:289–93. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Lin D, Sun X, et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 2020;368:409–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai W, Zhang B, Jiang XM, et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science 2020;368:1331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li G, De Clercq E.. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov 2020;19:149–50. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Wang Y, Qi C, et al. Clinical trial analysis of 2019-nCoV therapy registered in China. J Med Virol 2020;92:540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo H, Tang QL, Shang YX, et al. Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin J Integr Med 2020;26:243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chinese Pharmacopoeia Commission . Pharmacopoeia of the People's Republic of China. 2015 ed. Beijing (China): China Medical Science and Technology Press; 2015. [Google Scholar]
- 20.European Directorate for the Quality of Medicines of European Council. European Pharmacopoeia. 10th ed. Strasbourg (France): European Directorate for the Quality of Medicines of European Council; 2019. [Google Scholar]
- 21.Wang ZL, Wang S, Kuang Y, et al. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm Biol 2018;56:465–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oo A, Teoh BT, Sam SS, et al. Baicalein and baicalin as Zika virus inhibitors. Arch Virol 2019;164:585–93. [DOI] [PubMed] [Google Scholar]
- 23.Ji S, Li R, Wang Q, et al. Anti-H1N1 virus, cytotoxic and Nrf2 activation activities of chemical constituents from Scutellaria baicalensis. J Ethnopharmacol 2015;176:475–84. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Tang X, Chen H.. Inhibition of HIV replication by baicalin and S. baicalensis extracts in H9 cell culture. Chin Med Sci J 1991;6:230–2. [PubMed] [Google Scholar]
- 25.Zandi K, Lim TH, Rahim NA, et al. Extract of Scutellaria baicalensis inhibits dengue virus replication. BMC Complement Altern Med 2013;13:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin H, Zhou J, Lin K, et al. Efficacy of Scutellaria baicalensis for the treatment of hand, foot, and mouth disease associated with encephalitis in patients infected with EV71: a multicenter, retrospective analysis. Biomed Res Int 2016;2016:5697571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiao X, Li R, Song W, et al. A targeted strategy to analyze untargeted mass spectral data: rapid chemical profiling of Scutellaria baicalensis using ultra-high performance liquid chromatography coupled with hybrid quadrupole orbitrap mass spectrometry and key ion filtering. J Chromatogr A 2016;1441:83–95. [DOI] [PubMed] [Google Scholar]
- 28.Jo S, Kim S, Shin DH, et al. Inhibition of SARS-CoV 3CL protease by flavonoids. J Enzyme Inhib Med Chem 2020;35:145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jo S, Kim H, Kim S, et al. Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors. Chem Biol Drug Des 2019;94:2023–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su HX, Yao S, Zhao WF, et al. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacol Sin 2020;41:1167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan K, Wei P, Feng Q, et al. Biosynthesis, purification, and substrate specificity of severe acute respiratory syndrome coronavirus 3C-like proteinase. J Biol Chem 2004;279:1637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C, Qi Y, Teng X, et al. Maturation mechanism of severe acute respiratory syndrome (SARS) coronavirus 3C-like proteinase. J Biol Chem 2010;285:28134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang C, Wei P, Fan K, et al. 3C-like proteinase from SARS coronavirus catalyzes substrate hydrolysis by a general base mechanism. Biochemistry 2004;43:4568–74. [DOI] [PubMed] [Google Scholar]
- 35.Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev 2009;35:57–68. [DOI] [PubMed] [Google Scholar]
- 36.Resnick SJ, Iketani S, Hong SJ, et al. A simplified cell-based assay to identify coronavirus 3CL protease inhibitors. bioRxiv. 2020. [Google Scholar]
- 37.Gao J, Chen G, He H, et al. Therapeutic effects of breviscapine in cardiovascular diseases: a review. Front Pharmacol 2017;8:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu MS, Lee J, Lee JM, et al. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorg Med Chem Lett 2012;22:4049–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.