Abstract
Background
Programmatic treatment outcome data for people living with human immunodeficiency virus type 2 (HIV-2) in West Africa, where the virus is most prevalent, are scarce.
Methods
Adults with HIV-2 initiating or receiving antiretroviral therapy (ART) through the Senegalese national AIDS program were invited to participate in this prospective, longitudinal observational cohort study. We analyzed HIV-2 viral loads, CD4 cell counts, antiretroviral drug resistance, loss to follow-up, and mortality. We also examined changes in treatment guidelines over time and assessed progress toward the Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90 targets for HIV-2.
Results
We enrolled 291 participants at 2 sites for 926.0 person-years of follow-up over 13 years. Median follow-up time was 2.2 years per participant. There were 21 deaths reported (7.2%), and 117 individuals (40.2%) were lost to follow-up, including 43 (14.7%) who had an initial visit but never returned for follow-up. CD4 counts and HIV-2 viral suppression (< 50 copies/mL) at enrollment increased over calendar time. Over the study period, 76.7% of plasma viral loads for participants receiving ART were suppressed, and median CD4 gain was 84 cells/μL in participants’ first 2 years on study. Since the UNAIDS 90-90-90 strategy was published, 88.1% of viral loads were suppressed. Fifteen percent of patients experienced virologic failure with no known resistance mutations, while 56% had evidence of multiclass drug resistance.
Conclusions
Participants in the Senegalese national AIDS program are initiating ART earlier in the course of disease, and more modern therapeutic regimens have improved outcomes among those receiving therapy. Despite these achievements, HIV-2 treatment remains suboptimal, and significant challenges to improving care remain.
Keywords: HIV-2, antiretroviral therapy, viral suppression, 90-90-90, HIV treatment
This study summarizes 13 years’ experience following people with Human Immunodeficiency Virus Type 2 (HIV-2) receiving antiretroviral therapy (ART) in Senegal. Earlier ART initiation and more modern therapeutic regimens have improved outcomes. However, HIV-2 treatment remains suboptimal, and viral suppression rates remain below 90%.
(See the Viewpoints by Berzow et al on pages 503–9 and the Editorial Commentary by Jenny-Avital on pages 510–2.)
Human immunodeficiency virus (HIV) type 2 (HIV-2) is a neglected public health problem in West Africa [1]. Compared to HIV type 1 (HIV-1), HIV-2 infection is characterized by lower plasma viral loads, slower CD4 cell count decline, lower rates of mother-to-child and sexual transmission, longer asymptomatic stage, and slower disease progression [2–6]. However, the majority of untreated people with HIV-2 (PWHIV2) will progress to AIDS and death [7, 8], and any strategy for combatting AIDS must include HIV-2.
The Joint United Nations Programme on HIV/AIDS’ (UNAIDS) ambitious 90-90-90 goals for ending the AIDS epidemic implicitly target HIV-1; however, HIV-2 has been largely overlooked [1, 9]. PWHIV2 can benefit significantly from antiretroviral therapy (ART) [10], yet no antiretrovirals (ARVs) have been specifically developed or approved for the treatment of HIV-2. Guidelines for HIV-2 treatment [10] are based primarily on in vitro data, observational cohort studies, 2 single-arm trials [11, 12], and extrapolation from HIV-1. HIV-2 is intrinsically resistant to nonnucleoside reverse transcriptase inhibitors (NNRTIs) [13] and the fusion inhibitor enfuvirtide [14], displays reduced sensitivity to many protease inhibitors [15], and exhibits a low genetic barrier to nucleoside reverse transcriptase inhibitor (NRTI) resistance [16], limiting treatment options [17]. Integrase inhibitors are potent against HIV-2 [11, 12], and rollout of fixed-dose combination tenofovir-lamivudine-dolutegravir is planned in West Africa in 2020. Currently however, most HIV-2 treatment programs in West Africa rely on regimens containing ritonavir-boosted lopinavir (LPV/r) plus 2 NRTIs. Patient care decisions in most low- and middle- income countries (LMICs) are further complicated by a lack of HIV-2 viral load and drug resistance testing capacity outside of research settings [10, 18–20].
Despite up to 20 years of programmatic ART availability for HIV-2 infection in West Africa and 5 years since the UNAIDS 90-90-90 targets were announced, therapeutic outcomes for HIV-2 remain poorly characterized. To this end, we present programmatic, immunologic, and virologic data from 13 years of follow-up of ART for HIV-2 in the Senegalese national AIDS program.
METHODS
Study Design and Participant Population
Study participants were adults (≥ 18 years old) infected with HIV-2, and were either already receiving ART or eligible for treatment under the Senegalese Antiretroviral Drug Access Initiative (ISAARV), which began in 1998. PWHIV2 who were ineligible for ART under ISAARV guidelines at the time of screening were excluded; rescreening and enrollment as individuals became eligible or guidelines changed were allowed. Pregnant or breastfeeding women, as well as individuals who were HIV-1 seropositive or HIV-1/HIV-2 dually seropositive at screening, were excluded.
The Service des Maladies Infectieuses et Tropicales, Centre Hospitalier National Universitaire de Fann, Dakar, started enrolling participants in November 2005. The Centre de Sante de Ziguinchor, Casamance, started enrolling in January 2010. The study ended in September 2018.
Study Procedures
HIV testing in Senegal evolved over the study period. Participants were screened for HIV infection by serology using combination antibody testing (GenScreen [Bio-Rad] or Determine [Alere]), with confirmatory testing using HIV-1/HIV-2 immunodifferentiation assays (MultiSpot [Sanofi Pasteur], Immunocomb II HIV-1&2 BiSpot [Orgenics], or SD Bioline HIV-1/2 3.0 [Alere]), and/or HIV-2–specific Western blot or enzyme immunoassay (Bio-Rad or Genetic Systems). Individuals with positive HIV-2 tests who met ISAARV criteria for ART were invited to participate. At screening, enrollment, and follow-up visits 1 month postenrollment and every 4 months thereafter, participants underwent standardized interviews including demographic characteristics and routine medical histories, including prior ART where applicable. Where exact dates, such as ART initiation, were unknown, the midpoint of the month or year provided was used. At each visit, physical examinations were performed and blood was collected by venipuncture for safety and monitoring laboratory tests: blood counts including T-cell subsets, and chemistries, using standard methods. Repeat HIV serologic testing and lipid panels were performed annually. Sexually transmitted infection (with syndromic management per Senegalese guidelines) and urine pregnancy testing were performed as needed. Retrospective HIV-2 plasma viral load testing was performed in Seattle, Washington, using a research assay developed at Roche Molecular Systems (Pleasanton, California) (limit of detection [LOD] = 40 copies/mL) (2005–2011) [6] or using the University of Washington HIV-2 Abbott m2000 assay (LOD = 10 copies/mL) (2011–2018) [21]. The m2000 assay was transferred to Dakar in 2014; subsequent viral load testing was performed in Dakar or Seattle. Genotypic resistance testing of the reverse transcriptase (RT) and protease (PR) genes was performed in Seattle as described previously [15, 22]. For participants without virologic failure resulting in detectable plasma RNA, resistance testing was performed using total nucleic acid from peripheral blood mononuclear cells or dried blood spots. Participants were considered to have multiclass resistance if they had any major mutation in RT and any major mutation in PR, even if they were in genotypes from different visits.
The study was conducted according to procedures approved by the institutional review boards at the University of Washington and Universite de Cheikh Anta Diop de Dakar, and the Senegalese National Ethics Committee for Health Research. All participants provided written informed consent for study participation.
Definitions and Statistical Analyses
Study social workers attempted to contact participants who missed appointments. Participants were considered lost to follow-up (LTFU) if there was no contact for > 1 year, and were censored at their last visit. Participants who were reported to have died while on study were censored at the date of death if known, or date of last visit if unknown. All remaining participants were censored on 30 September 2018. For Kaplan-Meier analyses, participants who enrolled but did not attend a follow-up visit were considered to contribute 1 day of follow-up and were thereafter considered LTFU unless reported dead. HIV-2 “viral load suppression” was defined as viral load < 50 copies/mL to conform to HIV-1 US Food and Drug Administration Snapshot definitions. Because follow-up visits did not occur exactly every 4 months, visits were binned within 2 months before or after the target date to allow for graphing cohort CD4 cell count trajectories and rates of viral suppression. Changes in CD4 counts were calculated using baseline (either initiating ART or enrollment) CD4 count and CD4 count at 24 and 60 months (± 2 months). Categorical variables were compared using Pearson χ 2 test and continuous variables were compared by nonparametric Mann-Whitney U test or Kruskal-Wallis test. The level of statistical significance used for all analyses was P < .05. All analyses were conducted in StataSE version 14.2 software (StataCorp, College Station, Texas).
RESULTS
Guidelines for HIV-2 treatment in Senegal have evolved over time (Table 1) [23]. Generally, these recommendations have shifted from treating those only with AIDS in the early years, to a universal test-and-treat strategy implemented in 2016.
Table 1.
Summary of Human Immunodeficiency Virus Type 2 Treatment Guidelines From the Senegalese Antiretroviral Drug Access Initiative, 1998–2018
| Guideline and Years | Recommendation |
|---|---|
| ART initiation timing | |
| 1998–2010 | Clinical AIDS (WHO stage 4) irrespective of CD4 count |
| Clinical symptoms (WHO stage 3) and CD4 count ≤ 350 cells/μL | |
| CD4 count ≤ 200 cells/μL irrespective of clinical criteria | |
| 2011–2013 | Clinical AIDS or symptoms (WHO stage 3 or 4) irrespective of CD4 count |
| CD4 count ≤ 350 cells/μL irrespective of clinical criteria | |
| 2013–2015 | Clinical AIDS or symptoms (WHO stage 3 or 4) irrespective of CD4 count |
| CD4 count ≤ 500 cells/μL irrespective of clinical criteria | |
| 2016–2018 | Universal “test and treat” for all persons living with HIV, irrespective of clinical symptoms or CD4 counta |
| First-line therapy recommendations | |
| 1998–2010 | ZDV, 3TC, IDV |
| 2011–2013 | ZDV, 3TC, LPV/r |
| 2014–2018 | TDF, 3TC, LPV/r |
| Second-line therapy recommendations | |
| 2009–2013 | TDF, 3TC, ATV/r or ddI, ABC, ATV/r |
| 2014–2018 | TDF, 3TC, DRV/r |
| Alternate therapeutic recommendations | |
| 1998–2010 | Use d4T or ddI instead of ZDV in participants with severe anemia (Hb < 8 g/dL) |
| 2010–2018 | Use TDF instead of ZDV in participants with severe anemia |
| 1998–2018 | Use ABC instead of ZDV or TDF in participants with renal insufficiency (CrCl < 50 mg/dL) |
| 1998–2018 | Use ABC instead of PI in participants receiving concurrent TB treatment |
| 2010–2018 | Use TDF instead of ZDV in participants with hepatitis B virus coinfection |
Abbreviations: 3TC, lamivudine; ABC, abacavir; ART, antiretroviral therapy; ATV/r, ritonavir-boosted atazanavir; AZT, zidovudine; CrCl, creatinine clearance; d4T, stavudine; ddI, didanosine; DRV/r, ritonavir-boosted darunavir; Hb, hemoglobin; HIV, human immunodeficiency virus; IDV, indinavir; LPV/r, ritonavir-boosted lopinavir; PI, protease inhibitor; TB, tuberculosis; TDF, tenofovir disoproxil fumarate; WHO, World Health Organization; ZDV, zidovudine.
aTATARSEN (Test All, Treat All, and Retain–Senegal).
The study enrolled 291 participants contributing 926.0 person-years of follow-up, with Dakar contributing approximately twice as much follow-up as Ziguinchor (601.4 vs 324.6 person-years). Baseline demographic, clinical, and immunovirologic characteristics of the participants who enrolled are shown in Table 2. Overall, 74.0% of participants were female, and the median age was 49 years. Consistent with the changing national treatment guidelines to start ART earlier, participants enrolling in Ziguinchor (starting in 2010) tended to have earlier-stage disease and higher body mass index (BMI) and were more frequently enrolled already receiving ART, compared to those in Dakar (starting in 2005). Even among those who were ART naive, participants in Ziguinchor had higher CD4 counts than those in Dakar (Table 2). In total, 38.9% of ART-naive participants, and 76.3% of those receiving ART, had viral loads at enrollment that were < 50 copies/mL (considered as “virologically suppressed”). The majority of participants from both sites were receiving an ART regimen (whether initiated on study or previously) containing either indinavir (IDV) or LPV/r plus 2 NRTIs, consistent with national program guidelines. Fourteen participants received 3 NRTIs due to concurrent tuberculosis infection and treatment. Two participants were receiving an NNRTI-based regimen at study enrollment, and 10 more had previously received an NNRTI due to HIV type misclassification.
Table 2.
Baseline Demographic, Immunovirologic, and Treatment Data of Participants Enrolling in a Study of Antiretroviral Therapy for Human Immunodeficiency Virus Type 2 Infection in Senegal, 2005–2018
| Characteristic | Dakar (n = 142) | Ziguinchor (n = 149) |
|---|---|---|
| Female sexa | 101 (71.6) | 110 (76.4) |
| Age, y, median (IQR) | 49 (43–54) | 50 (42–56) |
| Year of HIV diagnosis, median (range) | 2007 (1992–2018) | 2012 (1999–2018) |
| WHO stage | ||
| 1 | 11 (8.3) | 37 (26.6) |
| 2 | 43 (32.3) | 26 (18.7) |
| 3 | 58 (43.6) | 71 (51.1) |
| 4 | 21 (15.8) | 5 (3.6) |
| BMI category (kg/m2) | ||
| Underweight/malnourished (< 18.5) | 36 (30.8) | 24 (18.3) |
| Normal weight (18.5–24.9) | 58 (49.6) | 59 (45.0) |
| Overweight (25–29.9) | 12 (10.3) | 32 (24.4) |
| Obese (≥ 30.0) | 11 (9.4) | 16 (12.2) |
| ART-naive subjects | 54 (38.0) | 22 (14.8) |
| CD4 count, cells/μL, median (IQR) | 194 (97–363) | 378 (156–496) |
| Plasma viral load < 50 copies/mLb | 15 (34.9) | 6 (54.6) |
| Plasma viral load, log10 copies/mL, median (IQR)c | 3.1 (2.5–3.6) | 2.6 (2.1–3.7) |
| ART-experienced subjects | 88 (62.0) | 127 (85.2) |
| CD4 count, cells/μL, median (IQR) | 289 (136–532) | 398 (213–618) |
| Plasma viral load < 50 copies/mLb | 50 (70.4) | 37 (86.1) |
| Plasma viral load, log10 copies/mL, median (IQR)c | 3.1 (1.9–3.3) | 1.8 (1.8–2.4) |
| Baseline ARV regimen | ||
| 2 NRTIsd | 2 (1.4) | 0 (0) |
| 3 NRTIs | 7 (4.9) | 5 (3.4) |
| Indinavir + 2 NRTIs | 45 (31.7) | 0 (0) |
| Ritonavir-boosted lopinavir + 2 NRTIs | 76 (53.5) | 144 (96.6) |
| Raltegravir + 2 NRTIs | 12 (8.5) | 0 (0) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral; BMI, body mass index; HIV, human immunodeficiency virus; IQR, interquartile range; NRTI, nucleoside reverse transcriptase inhibitor; WHO, World Health Organization.
aSex was not recorded for 1 participant from Dakar and 5 participants from Ziguinchor.
bPercentage of participants with viral load data available. Missing data: n = 11 naive, n = 17 experienced (Dakar); n = 11 naive, n = 84 experienced (Ziguinchor).
cAmong those with nonsuppressed viral loads.
dHIV type misdiagnoses treated with 2 NRTIs + nonnucleoside reverse transcriptase inhibitor.
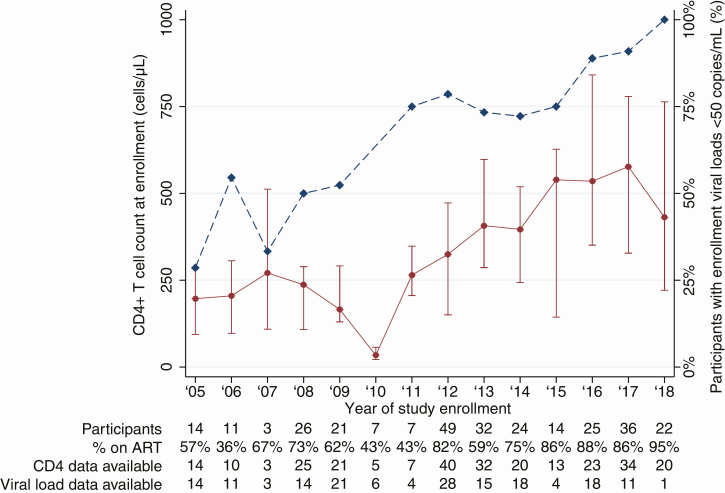
A calendar time bias in enrollment CD4 count and viral load suppression, consistent with increasing ART uptake, is shown in Figure 1. The median CD4 count increased from 198 cells/μL in participants who enrolled in 2005, to 431 cells/μL in participants who enrolled in 2018. Similarly, 28.6% of participants enrolling in 2005 had suppressed viral loads, compared to 90.9% of those enrolling in 2017. There were insufficient viral load data available for participants enrolling in 2018 for comparison.
Figure 1.
Enrollment CD4+ T-cell count and viral load suppression for participants receiving antiretroviral therapy (ART) through the Senegalese national program, by study enrollment year. Median (interquartile range) CD4 count (cells/μL) of study participants (left y-axis, solid line) and proportion of study participants with viral load suppression (< 50 copies/mL, right y-axis, dashed line) as a function of enrollment year. Number of participants enrolled per year, percentage of subjects enrolling already receiving ART, and number of participants for whom CD4 count and viral load data are available are listed below the plot.
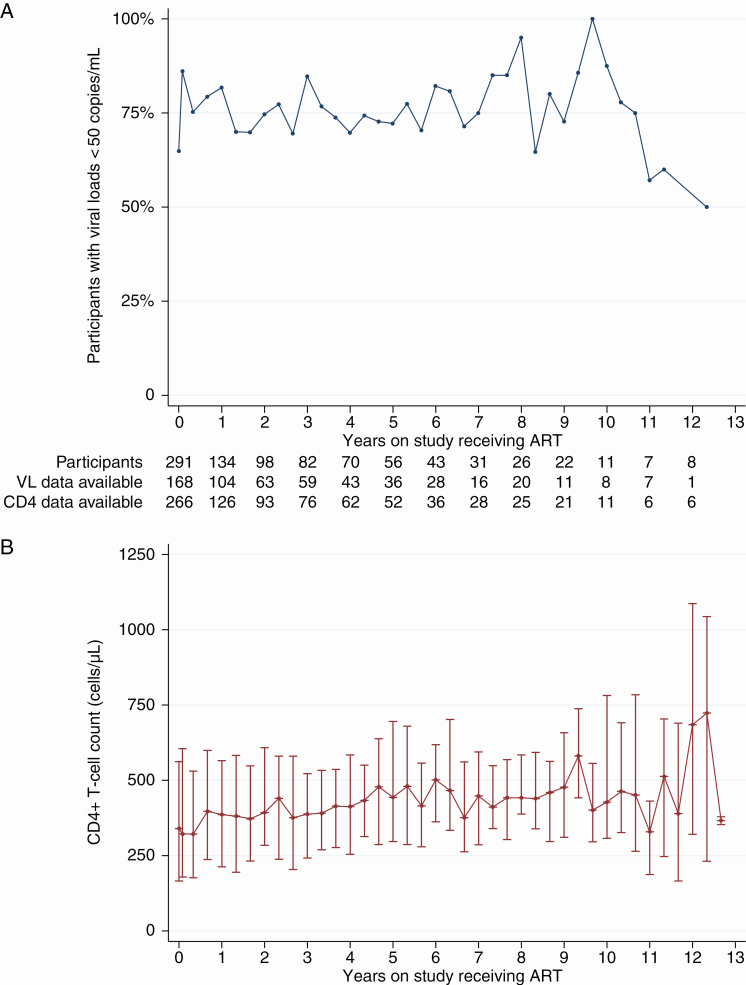
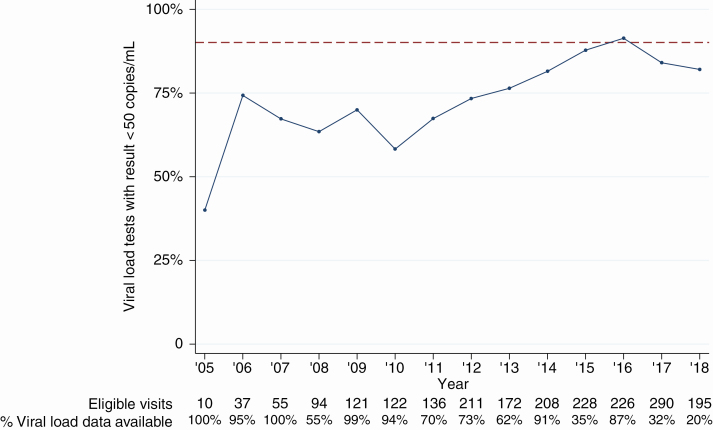
Rates of viral suppression as a function of participant time on study receiving ART (as opposed to calendar time) are shown in Figure 2A. Data were binned and plotted by trimester to match planned study visit timing of approximately every 4 months. Over the entire study period, 76.7% of all viral load tests (n = 1466) from participants receiving ART, regardless of duration, were < 50 copies/mL. To assess improvement on the third “90” benchmark (90% of participants receiving ART having a suppressed viral load), we also plotted proportion of viral load tests with a result < 50 copies/mL against calendar year for any participant who had been receiving ART for > 6 months (Figure 3). Since the 90-90-90 goals were set by UNAIDS in late 2014, 83.1% of 77 participants in Dakar and 93.9% of 33 participants in Ziguinchor with viral load testing available were suppressed at the last study visit for which a result was obtained (2015–2018, data not shown). However, 4.9% and 68.6% of participants in Dakar and Ziguinchor, respectively, had no viral load testing performed in that period.
Figure 2.
Viral load (VL) suppression and CD4+ T-cell counts among participants receiving antiretroviral therapy (ART) through the Senegalese national program. Rates of VL suppression (< 50 copies/mL) (A) and median (interquartile range) CD4 counts (cells/μL) (B) are shown as a function of time on study. Data shown are from enrollment, month 1 postenrollment, and trimesterly visits thereafter. Due to variability in interval between visits, data are binned within 2 months before and after target visit date. Number of participants with a visit occurring within 2 months of the annual anniversaries of their enrollment, and number of data available for each variable at those visits, are shown between panels.
Figure 3.
Viral load suppression for participants receiving antiretroviral therapy (ART) through the Senegalese national program, by calendar year. Proportion of viral load results < 50 copies/mL from ART-experienced (> 6 months) study participants, shown as a function of calendar year of the visit. Dashed line indicates 90%. Number of eligible visits and percentage of visits with viral load data available are listed below the plot.
The per-participant median increases in CD4 count over 2 and 5 years on study receiving ART were 84 (interquartile range [IQR], −9 to 181) cells/μL and 159 (IQR, −3 to 343) cells/μL, respectively, with 29.4% and 25.5% of participants experiencing a decline in CD4 count (data not shown). The cohort median CD4 count as a function of time on study is shown in Figure 2B, and the median CD4 count of participants who were ART naive at enrollment, for the duration of the first regimen, is shown in Supplementary Figure 1B.
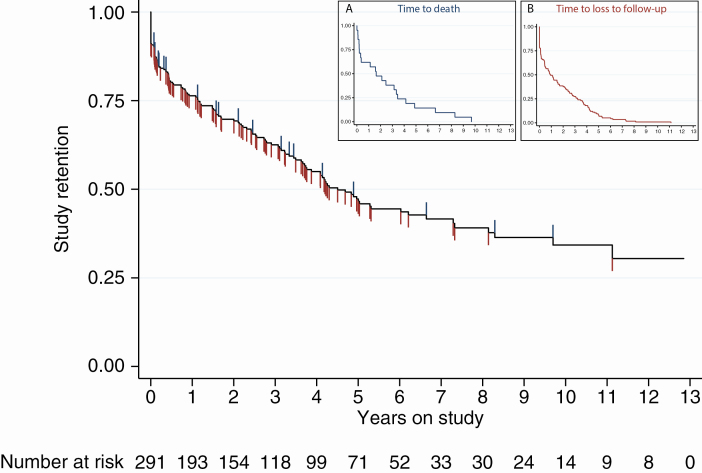
Participant outcomes at study conclusion are shown in Table 3. Overall, the median follow-up time was 2.2 years (IQR, 0.5–5.0 years). Seventeen participants in Dakar and 4 in Ziguinchor were reported to have died during the follow-up period. Participants in Dakar had a median of 2.9 years of on-study follow-up (IQR, 1.1–6.6 years; maximum, 12.8) whereas those in Ziguinchor had a median of 1.4 years of follow-up on study (IQR, 0.2–4.1 years; maximum, 6.7). Rates of LTFU were high at both sites (35.9% in Dakar, 44.3% in Ziguinchor), and a total of 43 participants (14.8%) enrolled in the study but never attended a follow-up visit. At both sites, the maximum time any participant had been receiving ART was approximately 18 years. A Kaplan-Meier plot for study retention is shown in Figure 4, indicating that fewer than half of participants were retained on study by 5 years postenrollment. Insets show actual time to death or LTFU among participants with the corresponding outcome (eg, time to death of participants who died) and demonstrate very similar curves with median time to death or time to follow-up of < 2 years.
Table 3.
Outcomes of Senegalese Participants With Human Immunodeficiency Virus Type 2 Receiving Antiretroviral Therapy Through the Senegalese National Program, 2005–2018
| Outcome | Dakar (n = 142) | Ziguinchor (n = 149) |
|---|---|---|
| Years receiving ART, median (IQR) | 4.1 (2.4–7.5) | 3.6 (1.3–6.3) |
| Years of on-study follow-up, median (IQR) | 2.9 (1.1–6.6) | 1.4 (0.2–4.1) |
| All-cause mortality, No. (%) | 17 (12.0) | 4 (2.7) |
| Years to death, median (IQR) | 2.5 (0.4–4.1) | 0.1 (0.0a–0.6) |
| LTFU, No. (%) | 51 (35.9) | 66 (44.3) |
| Years to LTFU, median (IQR) | 1.7 (0.4–4.1) | 0.5 (0.0b–2.8) |
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range; LTFU, lost to follow-up.
aFifteen days.
bOne day (enrolled and did not return).
Figure 4.
Kaplan-Meier estimation of study retention. The probability of participant retention on study, where both loss to follow-up (blue hash marks) and death (red hash marks) are considered as failures, is plotted. Participants were considered lost to follow-up if they had not come to a follow-up visit for > 1 year, and were censored at last visit date. Participants who were reported dead were censored at date of death if known, or date of last clinic visit if not. All remaining participants were censored at study closure. Number of participants at risk is shown below. Insets show actual time to death among 21 participants who died (A) and time to loss to follow-up among 117 participants (B).
Those participants who were LTFU tended to have CD4 counts, viral loads, BMI, and World Health Organization (WHO) stage somewhere between those retained on study and those who died (Table 4). Lower CD4 counts both at baseline and last visit, higher viral loads (particularly at last visit), lower BMI at last visit, and more advanced disease at entry and at last visit were significantly associated with death or LTFU. Enrollment ART status (naive or on-ART) was not associated with negative outcomes. Women were more likely to be retained on study than men. Women were also more likely to have suppressed viral loads than men, but no other sex-based differences were identified in programmatic or immunovirologic outcomes (Supplementary Table 1).
Table 4.
Factors Associated With Death or Loss to Follow-up in Senegalese Participants With Human Immunodeficiency Virus Type 2 Receiving Antiretroviral Therapy Through the Senegalese National Program
| Factor | On Study (n = 153) | LTFU (n = 117) | LTFU vs On Study, P Value | Dead (n = 21) | Dead vs On Study, P Value | Dead vs LTFU, P Value | Global P Value |
|---|---|---|---|---|---|---|---|
| Sex, female, No. (%) | 118 (79.7) | 81 (69.8) | .064 | 12 (57.1) | .022 | .252 | .035 |
| Enrollment age, y, median (IQR) | 49 (42–55) | 50 (43–54) | .797 | 50 (47–53) | .496 | .457 | .739 |
| Baseline | |||||||
| CD4 count, cells/μL, median (IQR) | 397 (205–633) | 285 (156–476) | .002 | 135 (103–287) | < .001 | .012 | < .001 |
| Plasma VL < 50 copies/mL, No. (%)a | 59 (72.0) | 42 (60.9) | .149 | 7 (41.2) | .014 | .142 | .041 |
| Plasma VL, copies/mL, median (IQR)b | 819 (185–4113) | 893 (74–2190) | .414 | 898 (96–2955) | .557 | .973 | .686 |
| BMI, kg/m2, median (IQR) | 22.4 (19.2–25.6) | 21.1 (18.4–25.6) | .398 | 20.6 (15.6–22.7) | .067 | .166 | .166 |
| WHO stage 3 or 4, No. (%) | 68 (48.2) | 71 (64.0) | .013 | 16 (80.0) | .008 | .162 | .004 |
| On ART, No. (%) | 111 (72.6) | 89 (76.1) | .513 | 15 (71.4) | .914 | .650 | .780 |
| Last visit | |||||||
| CD4 count, cells/μL, median (IQR) | 559 (378–770) | 334 (154–507) | < .001 | 135 (26–409) | < .001 | .003 | < .001 |
| Plasma VL < 50 copies/mL, No. (%)a | 35 (85.4) | 40 (58.0) | .003 | 8 (57.1) | .027 | .954 | .009 |
| Plasma VL, copies/mL, median (IQR)b | 651 (193–1847) | 246 (96–1969) | .484 | 3639 (1810–6625) | .025 | .020 | .042 |
| BMI, kg/m2, median (IQR) | 23.1 (20.6–26.4) | 21.6 (18.1–25.8) | .012 | 17.2 (14.9–21.1) | < .001 | .001 | < .001 |
| WHO stage 3 or 4, No. (%) | 82 (54.0) | 85 (72.7) | .002 | 19 (90.5) | .001 | .081 | < .001 |
Values in bold indicate statistical significance (P <.05).
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; IQR, interquartile range; LTFU, lost to follow-up; VL, viral load; WHO, World Health Organization.
aPercentage of participants with VL data available.
bAmong those with nonsuppressed VL.
Documented HIV-associated or AIDS-defining opportunistic infections and HIV- or ART- associated clinical and laboratory adverse events are shown in (Supplementary Appendix A). Exploratory analyses examining predictors of death or LTFU are shown in (Supplementary Appendix B).
We assessed the occurrence of major HIV-2 drug resistance–associated mutations, including PR V47A, I50V, I54M, L90M, and RT K65R, Q151M, and M184I/V. We genotyped PR/RT from 21 ART-naive participants and observed no pretreatment drug resistance among these participants. We also obtained 232 genotypes from 53 ART-treated participants, of which 107 genotypes were from 34 participants experiencing virologic failure (viral load ≥ 50 copies/mL after > 6 months on their current ART regimen). Considering all 53 ART-experienced participants (whether or not experiencing virologic failure), we observed PR changes V47A in sequences from 8 participants (15.1%), I50V in 2 (3.8%), I54M in 12 (22.6%), and L90M in 6 (11.3%). In RT, we observed K65R in sequences from 8 participants (15.1%), Q151M in 10 (18.9%), and M184I/V in 32 (60.4%). Of the 34 participants experiencing virologic failure, genotypes from 5 participants (14.7%) contained no known resistance-associated mutations, 10 (29.4%) contained mutations conferring resistance to NRTIs only, and 19 (55.9%) contained evidence of multiclass resistance, with changes known to confer resistance to both NRTIs and protease inhibitors.
DISCUSSION
Few studies have attempted to quantify progress toward the UNAIDS 90-90-90 goals for HIV-2 or describe HIV-2 ART outcomes for West African HIV/AIDS treatment programs. We examined virologic and immunologic outcomes among 2 cohorts of adults with HIV-2 in Senegal, West Africa, being treated with ART under the Senegalese national AIDS program over a 13-year period.
Encouragingly, changing national guidelines for ART over time have resulted in participants initiating ART with higher CD4 counts, lower plasma viral loads, and earlier WHO stage. Since the 90-90-90 strategy was announced in 2014, 86.3% of our study participants with measured viral loads had achieved viral suppression at the last visit for which testing was available. However, 25%–40% of ART-naive PWHIV2 naturally have suppressed viral loads [6, 11, 24], so achieving the UNAIDS third “90” target for HIV-2 should, in theory, be less challenging than for HIV-1. Despite naturally occurring “viral suppression” in a significant proportion of PWHIV-2, declines in CD4 counts still occur naturally or on ART (albeit slowly) [6, 25, 26]. Consequently, there is no consensus regarding the HIV-2 plasma viral load that should be considered “virologic failure,” indicative of a need to switch ART, for HIV-2, and it has been proposed that a combined viral load and CD4 count measure should be used to define treatment success [12]. Moreover, HIV-2 viral load monitoring is not routinely available in Senegal or elsewhere in West Africa. Existing HIV-2 viral load testing platforms require expensive equipment and considerable technical expertise [21], and are not ideal for community health clinics in LMICs. Instead, simple, easy-to-use point-of-care testing is urgently needed for HIV-2 treatment monitoring [27].
Our study confirms previous studies reporting poor immune reconstitution in ART-treated PWHIV2 [28, 29]. Although the majority of participants who remained on study had viral loads < 50 copies/mL, the median CD4 gains per year were modest, including > 25% of participants who experienced CD4 declines, and opportunistic infections, laboratory abnormalities, and clinical adverse events were reported for a number of participants. In addition, rates of viral suppression and CD4 increases may be artificially inflated by the LTFU. The high rates of LTFU (40.2%) and short median follow-up times in our study mirror those reported in other West African studies [30, 31], and likely reflect a combination of patient mobility, deaths, and withdrawing to seek care elsewhere, including other national program clinics or traditional healers [32]. Transfers out were not routinely assessed in our study, so it is possible that some portion of the lost participants are alive, on ART, and virally suppressed. However, participants who were LTFU tended to have more profound immunosuppression and worse health status than those retained on study, suggesting that a substantial proportion of them may instead have died.
Virologic failure both without known resistance mutations, as well as with multiclass drug resistance, was observed and has important ramifications for reaching and maintaining 90% suppression. We found a substantial proportion (14.7%) of participants with virologic failure with apparently wild-type virus, agreeing with previous studies [15, 22, 33]. Although it is probable that those failing with wild-type virus are nonadherent to their ART, it is also possible that “wild type” viruses may simply harbor as-yet unknown resistance mutations. Few studies have attempted to link particular ARVs with the development of key resistance-associated mutations in HIV-2; importantly, HIV-2 drug resistance patterns, particularly in group B virus, remain incompletely characterized [15, 17]. Another factor warranting consideration is that clinicians may temporarily switch drugs or stop therapy altogether during drug stockouts (unpublished observations); a recent survey reported ARV stockouts at 21.8% of Senegalese health facilities visited [34]. The large proportion of participants failing with multiclass resistance is problematic for second-line treatment switches, and access to second-line ARVs is limited.
Changes in the national guidelines for HIV-2 treatment complicate comparisons between study participants who enrolled early and those who enrolled later. Notably, phenotypic data and other studies have demonstrated that IDV is inferior to LPV/r for the treatment of HIV-2 [15, 35–37]. ISAARV made a programmatic switch from IDV-based regimens to LPV/r-based regimens in 2008–2009; although all participants receiving an IDV-based regimen switched to LPV/r-based regimens, some switched due to immunologic or clinical failure, while others switched due to programmatic change. Later on, atazanavir, darunavir, and raltegravir were occasionally available and recommended to treat HIV-2 infection in Senegal, although darunavir and raltegravir are only sporadically available and HIV-2 is minimally susceptible to atazanavir [15, 38]. In many cases, the reasons for switching individual participants’ regimens are unknown. A study of algorithmic switching to second-line ART based on HIV-2 resistance testing is currently under way in Senegal (RESIST-2, ClinicalTrials.gov: NCT03394196).
Compared to HIV-1, observational studies of HIV-2 treatment outcomes in LMICs are few in number, frequently small, and either cross-sectional in nature or with limited follow-up duration, and provide an incomplete picture of programmatic outcomes [24, 28–30, 35–37, 39, 40]. Only 1 study that we know of has looked at rates of HIV-2 viral suppression in West Africa in the last decade, making it difficult to assess West African progress toward the 90-90-90 benchmarks. A cross-sectional analysis of participants from Burkina Faso, Cote d’Ivoire, and Mali in 2002 reported that 84.3% of 220 participants receiving ART had a viral load of < 100 copies/mL [24].
Improving care for PWHIV2 is critical if this population is to reach the ambitious 90-90-90 goals set by UNAIDS [1]. Particularly for PWHIV2 in LMICs, treatment challenges are numerous. Increasing the quality of care for PWHIV2 will require a multifaceted approach incorporating point-of-care technologies, new regimens such as fixed-dose combination tenofovir-lamivudine-dolutegravir, and improvement to existing capacity.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: Conference on Retroviruses and Opportunistic Infections, Boston, Massachusetts, 3–6 March 2014. Abstract 573.
Author contributions. G. S. G., P. S. S., N. B. K., and S. E. H. conceived and designed the study. D. N. R. performed the analysis. D. N. R., G. S. G., R. A. S., and S. E. H. interpreted the data and wrote the manuscript. F. S., M. T., and E. I. S. recruited participants and managed study sites. S. B., O. C., K. D., I. T. T., C. N., N. M. D. B., L. F., M. B. D., D. F., M. D. S., I. N., A. N., J. M., P. S. S., and M. S. oversaw patient care. M. P. S., B. D., J. S., and R. B. assisted in patient care. H. A. D., K. F., J. P. D., M. C., and R. W. C. performed laboratory testing. All authors approved the final submitted version.
Acknowledgments. The authors thank the study participants, without whom these studies would not be possible. The University of Washington (UW)–Senegal HIV-2 Study Group also includes Fatou Traore, Samba Cisse, Ousseynou Ndiaye, Babacar Faye, Fatou Simal, Ndeye Astou Diop, Amadou Bale Diop, Marianne Fadam Diome (Clinique des Maladies Infectieuses Ibrahima Diop Mar, Centre Hospitalier Universitaire de Fann, Universite’ Cheikh Anta Diop de Dakar, Dakar, Senegal); Juliette Gomis, Therese Dieye (Région Médicale de Ziguinchor, Ziguinchor, Casamance, Senegal), Noelle Benzekri, John Lin, Donna Kenney, Alison Starling, Cathy Critchlow, Steve Cherne, Jennifer Song, Robbie Nixon, Pallas Burhen, Chris Zavala, Vincent Wu, Sara Masoum, Sally Leong, Alex Montano, Mariah Oakes, Julia Olson, Lindsey Blankenship, Charlotte Pan, Kara Parker, Kate Parker, Alex Hernandez, Brad Church, Moon Kim, Paul Lu, Stefanie Sorensen, Kim Wong, James Mullins (UW, Seattle, Washington). HIV-2 sequences generated for genotype resistance testing have been deposited under the following accession numbers: FJ812523–FJ812621, FJ812624–FJ812692, and KC768350–KC768705.
Financial support. This study was supported by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) (grant numbers 2R01-AI060466 and R01-AI120765 to G. S. G.); the AIDS Clinical Trials Group (grant numbers UM1-AI-068636 and UM1-AI-106701); and the UW Center for AIDS Research Retrovirology Core, an NIH-funded program which is supported by NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, and NIDDK (grant number P30-AI-027757).
Potential conflicts of interest. G. S. G. has received research grants and research support from the NIH, UW, the Bill & Melinda Gates Foundation, Gilead Sciences, Alere Technologies, Merck & Co, Janssen Pharmaceutica, Cerus Corporation, ViiV Healthcare, Bristol-Myers Squibb, Roche Molecular Systems, Abbott Molecular Diagnostics, and TheraTechnologies/TaiMed Biologics, Inc. M. S. has received grant funds and clinical support from the France Recherche Nord & sud Sida-hiv Hepatites, GlaxoSmith Kline, and Gilead Sciences. P. S. S. is a current employee of Gilead Sciences. S. E. H. has received research grants and research support from the NIH, UW, and the Bill & Melinda Gates Foundation. M. D. reports salary support through UW from the NIAID/NIH. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
for the University of Washington–Senegal HIV-2 Study Group:
Fatou Traore, Samba Cisse, Ousseynou Ndiaye, Babacar Faye, Fatou Simal, Ndeye Astou Diop, Amadou Bale Diop, Marianne Fadam Diome, Juliette Gomis, Therese Dieye, Noelle Benzekri, John Lin, Donna Kenney, Alison Starling, Cathy Critchlow, Steve Cherne, Jennifer Song, Robbie Nixon, Pallas Burhen, Chris Zavala, Vincent Wu, Sara Masoum, Sally Leong, Alex Montano, Mariah Oakes, Julia Olson, Lindsey Blankenship, Charlotte Pan, Kara Parker, Kate Parker, Alex Hernandez, Brad Church, Moon Kim, Paul Lu, Stefanie Sorensen, Kim Wong, and James Mullins
References
- 1. Gottlieb GS, Raugi DN, Smith RA. 90-90-90 for HIV-2? Ending the HIV-2 epidemic by enhancing care and clinical management of patients infected with HIV-2. Lancet HIV 2018; 5:e390–9. [DOI] [PubMed] [Google Scholar]
- 2. Simon F, Matheron S, Tamalet C, et al. Cellular and plasma viral load in patients infected with HIV-2. AIDS 1993; 7:1411–7. [DOI] [PubMed] [Google Scholar]
- 3. Adjorlolo-Johnson G, De Cock KM, Ekpini E, et al. Prospective comparison of mother-to-child transmission of HIV-1 and HIV-2 in Abidjan, Ivory Coast. JAMA 1994; 272:462–6. [PubMed] [Google Scholar]
- 4. Kanki PJ, Travers KU, MBoup S, et al. Slower heterosexual spread of HIV-2 than HIV-1. Lancet 1994; 343:943–6. [DOI] [PubMed] [Google Scholar]
- 5. Marlink R, Kanki P, Thior I, et al. Reduced rate of disease development after HIV-2 infection as compared to HIV-1. Science 1994; 265:1587–90. [DOI] [PubMed] [Google Scholar]
- 6. Gottlieb GS, Sow PS, Hawes SE, et al. Equal plasma viral loads predict a similar rate of CD4+ T cell decline in human immunodeficiency virus (HIV) type 1- and HIV-2-infected individuals from Senegal, West Africa. J Infect Dis 2002; 185:905–14. [DOI] [PubMed] [Google Scholar]
- 7. Schim van der Loeff MF, Jaffar S, Aveika AA, et al. Mortality of HIV-1, HIV-2 and HIV-1/HIV-2 dually infected patients in a clinic-based cohort in The Gambia. AIDS 2002; 16:1775–83. [DOI] [PubMed] [Google Scholar]
- 8. Esbjornsson J, Mansson F, Kvist A, et al. Long-term follow-up of HIV-2-related AIDS and mortality in Guinea-Bissau: a prospective open cohort study. Lancet HIV 2018. doi: 10.1016/S2352-3018(18)30254-6. [DOI] [PubMed] [Google Scholar]
- 9. Joint United Nations Programme on HIV/AIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: UNAIDS, 2014. [Google Scholar]
- 10. World Health Organization. Guidelines: HIV. Geneva, Switzerland: WHO, 2017. [Google Scholar]
- 11. Ba S, Raugi DN, Smith RA, et al. University of Washington–Dakar HIV-2 Study Group A trial of a single-tablet regimen of elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate for the initial treatment of human immunodeficiency virus type 2 infection in a resource-limited setting: 48-week results from Senegal, West Africa. Clin Infect Dis 2018; 67:1588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matheron S, Descamps D, Gallien S, et al. France Recherche Nord & Sud Sida-Hiv Hépatites (ANRS) 159 HIV-2 Trial Study Group First-line raltegravir/emtricitabine/tenofovir combination in human immunodeficiency virus type 2 (HIV-2) infection: a phase 2, noncomparative trial (ANRS 159 HIV-2). Clin Infect Dis 2018; 67:1161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Witvrouw M, Pannecouque C, Switzer WM, Folks TM, De Clercq E, Heneine W. Susceptibility of HIV-2, SIV and SHIV to various anti-HIV-1 compounds: implications for treatment and postexposure prophylaxis. Antivir Ther 2004; 9:57–65. [PubMed] [Google Scholar]
- 14. Poveda E, Rodes B, Toro C, Soriano V. Are fusion inhibitors active against all HIV variants? AIDS Res Hum Retroviruses 2004; 20:347–8. [DOI] [PubMed] [Google Scholar]
- 15. Raugi DN, Smith RA, Ba S, et al. University of Washington-Dakar HIV-2 Study Group Complex patterns of protease inhibitor resistance among antiretroviral treatment-experienced HIV-2 patients from Senegal: implications for second-line therapy. Antimicrob Agents Chemother 2013; 57:2751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith RA, Anderson DJ, Pyrak CL, Preston BD, Gottlieb GS. Antiretroviral drug resistance in HIV-2: three amino acid changes are sufficient for classwide nucleoside analogue resistance. J Infect Dis 2009; 199:1323–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Menéndez-Arias L, Alvarez M. Antiretroviral therapy and drug resistance in human immunodeficiency virus type 2 infection. Antiviral Res 2014; 102:70–86. [DOI] [PubMed] [Google Scholar]
- 18. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 11 October 2019. [Google Scholar]
- 19. Gilleece Y, Chadwick DR, Breuer J, et al. BHIVA Guidelines Subcommittee British HIV Association guidelines for antiretroviral treatment of HIV-2-positive individuals 2010. HIV Med 2010; 11:611–9. [DOI] [PubMed] [Google Scholar]
- 20. France Recherche Nord & sud Sida-hiv Hepatites. Prise en charge medicale des personnes vivant avec le VIH. Infection VIH-2; Diversite des VIH-1. Conseil national du sida et des hepatites virales. Available at: https://cns.sante.fr/wp-content/uploads/2017/01/experts-vih_diversite.pdf. Accessed 11 October 2019. [Google Scholar]
- 21. Chang M, Gottlieb GS, Dragavon JA, et al. Validation for clinical use of a novel HIV-2 plasma RNA viral load assay using the Abbott m2000 platform. J Clin Virol 2012; 55:128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gottlieb GS, Badiane NM, Hawes SE, et al. University of Washington-Dakar HIV-2 Study Group Emergence of multiclass drug-resistance in HIV-2 in antiretroviral-treated individuals in Senegal: implications for HIV-2 treatment in resource-limited West Africa. Clin Infect Dis 2009; 48:476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Conseil National de Lutte Contre le SIDA du Senegal. Home page Available at: www.cnls-senegal.org. Accessed 24 March 2019.
- 24. Ekouévi DK, Avettand-Fènoël V, Tchounga BK, et al. IeDEA West Africa Collaboration Plasma HIV-2 RNA according to CD4 count strata among HIV-2-infected adults in the IeDEA West Africa collaboration. PLoS One 2015; 10:e0129886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thiébaut R, Matheron S, Taieb A, Brun-Vezinet F, Chêne G, Autran B; Immunology Group of the ANRS CO5 HIV-2 Cohort Long-term nonprogressors and elite controllers in the ANRS CO5 HIV-2 cohort. AIDS 2011; 25:865–7. [DOI] [PubMed] [Google Scholar]
- 26. Sousa AE, Carneiro J, Meier-Schellersheim M, Grossman Z, Victorino RM. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J Immunol 2002; 169:3400–6. [DOI] [PubMed] [Google Scholar]
- 27. Chang M, Steinmetzer K, Raugi DN, et al. Detection and differentiation of HIV-2 using the point-of-care Alere q HIV-1/2 Detect nucleic acid test. J Clin Virol 2017; 97:22–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Drylewicz J, Eholie S, Maiga M, et al. International Epidemiologic Databases to Evaluate AIDS (IeDEA) West Africa Collaboration First-year lymphocyte T CD4+ response to antiretroviral therapy according to the HIV type in the IeDEA West Africa collaboration. AIDS 2010; 24:1043–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ekouevi DK, Tchounga BK, Coffie PA, et al. Antiretroviral therapy response among HIV-2 infected patients: a systematic review. BMC Infect Dis 2014; 14:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tchounga BK, Hønge BL, Eholie SP, et al. IeDEA West Africa Collaboration Effect of sex and age on outcomes among HIV-2-infected patients starting antiretroviral therapy in West Africa. AIDS 2016; 30:2707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Auld AF, Ekra KA, Shiraishi RW, et al. Temporal trends in treatment outcomes for HIV-1 and HIV-2-infected adults enrolled in Côte d’Ivoire’s national antiretroviral therapy program. PLoS One 2014; 9:e98183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Benzekri NA, Sambou JF, Ndong S, et al. UW-Senegal Research Collaboration Traditional healers, HIV outcomes, and mortality among people living with HIV in Senegal, West Africa. AIDS 2019; 33:1521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Charpentier C, Eholié S, Anglaret X, et al. IeDEA West Africa Collaboration Genotypic resistance profiles of HIV-2-treated patients in West Africa. AIDS 2014; 28:1161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mosime WO, Oberth G, Baptiste S, Manouan A, et al. Understanding gaps in the HIV treatment cascade in 11 West African countries: findings from the regional community treatment observatory. In: 10th IAS Conference on HIV Science. Mexico City, Mexico, 2019. [Google Scholar]
- 35. Bénard A, Damond F, Campa P, et al. ANRS CO5 HIV-2 Cohort Study Group Good response to lopinavir/ritonavir-containing antiretroviral regimens in antiretroviral-naive HIV-2-infected patients. AIDS 2009; 23:1171–3. [DOI] [PubMed] [Google Scholar]
- 36. Benard A, van Sighem A, Taieb A, et al. ACHIEV2E Collaboration Study Group Immunovirological response to triple nucleotide reverse-transcriptase inhibitors and ritonavir-boosted protease inhibitors in treatment-naive HIV-2-infected patients: The ACHIEV2E Collaboration Study Group. Clin Infect Dis 2011; 52:1257–66. [DOI] [PubMed] [Google Scholar]
- 37. Balestre E, Ekouevi DK, Tchounga B, et al. International Epidemiological Database to Evaluate AIDS (IeDEA) West Africa Collaboration Immunologic response in treatment-naïve HIV-2-infected patients: the IeDEA West Africa cohort. J Int AIDS Soc 2016; 19:20044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cavaco-Silva J, Aleixo MJ, Van Laethem K, et al. Portuguese HIV-2 Resistance Study Group Mutations selected in HIV-2-infected patients failing a regimen including atazanavir. J Antimicrob Chemother 2013; 68:190–2. [DOI] [PubMed] [Google Scholar]
- 39. Jallow S, Alabi A, Sarge-Njie R, et al. Virological response to highly active antiretroviral therapy in patients infected with human immunodeficiency virus type 2 (HIV-2) and in patients dually infected with HIV-1 and HIV-2 in the Gambia and emergence of drug-resistant variants. J Clin Microbiol 2009; 47:2200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wittkop L, Arsandaux J, Trevino A, et al. COHERE in EuroCoord and ACHIeV2e Study Group CD4 cell count response to first-line combination ART in HIV-2+ patients compared with HIV-1+ patients: a multinational, multicohort European study. J Antimicrob Chemother 2017; 72:2869–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.