Abstract
Objectives
To assess whether the presence of arthritis autoantibodies alongside IgG ACPA predicts clinically suspect arthralgia in ACPA-positive subjects without RA.
Methods
In the population-based Lifelines cohort (n = 40 136), 308 IgG ACPA-positive individuals without RA were present. Serum levels of IgA ACPA, IgA and IgM RF, and IgG anti-carbamylated antibodies were measured at baseline. Individuals were divided based on the Connective tissue disease Screening Questionnaire after 2 years follow-up. Antibodies to Porphyromonas gingivalis were determined at baseline and related to presence of periodontitis and joint complaints at 2 years follow-up.
Results
Of 308 subjects 53.6% were also seropositive for IgA ACPA, 42.2% for IgM RF, 23.7% for IgA RF and 13.6% for anti-carbamylated antibodies. We defined 75 persons with clinically suspect arthralgia at risk for RA based on CTD Screening Questionnaire at follow-up. Significantly more seropositivity for IgM RF and higher levels of IgG ACPA, IgA ACPA and IgM RF were found in clinically suspect arthralgia compared with no-clinically suspect arthralgia. In multivariate logistic regression correcting for age, gender and never smoking, positivity for three or more extra autoantibodies was significantly associated with clinically suspect arthralgia. Although levels of anti-P. gingivalis were not different between groups, they were significantly correlated to levels of both RFs, and both ACPAs in clinically suspect arthralgia.
Conclusions
ACPA-positive individuals without RA who develop clinically suspect arthralgia have more and higher levels of other arthritis autoantibodies at baseline. Levels of anti-P. gingivalis are not related to self-reported periodontitis or clinically suspect arthralgia, but are correlated to arthritis autoantibodies in clinically suspect arthralgia.
Keywords: arthritis autoantibodies, arthralgia, P. gingivalis, periodontitis
Rheumatology key messages
ACPA-positive persons with clinically suspect arthralgia have more and higher levels of arthritis autoantibodies.
Seropositivity for multiple arthritis autoantibodies is significantly associated with development of clinically suspect arthralgia.
Antibodies to P. gingivalis are correlated to arthritis autoantibodies in persons with clinically suspect arthralgia.
Introduction
RA is a chronic autoimmune disease, characterized by inflammation in the joints, causing pain, cartilage and bone damage, and disability [1], and presence of the autoantibodies RF and ACPA are part of the classification criteria for RA [2].
The development of RA is considered a multistep process. EULAR has identified several phases: (i) presence of genetic and environmental risk factors, (i) systemic autoimmunity associated with RA, (iii) symptoms without clinical arthritis, (iv) unclassified arthritis and finally (v) RA [3]. Genetic and environmental risk factors include shared epitope (HLA-SE) and other genetic loci, smoking, periodontitis and gut dysbiosis, which are discussed by Karlson et al. [4]. Regarding the relation between periodontitis and arthritis, much attention has been given to Porphyromonas gingivalis, an oral bacterium that possesses a peptidyl arginine deiminase enzyme that is able to citrullinate bacterial and human proteins [5]. Antibodies to P. gingivalis have been regarded as a measure for periodontitis, and may be of predictive value for development of RA.
RF and ACPA are present years before clinical diagnosis of RA [6], although <50% of ACPA- and RF-positive arthralgia patients develop RA in a median follow-up time of 28 months [7]. Anti-carbamylated protein antibodies (anti-CarP) are also present in RA [8]. Carbamylation is a posttranslational modification, converting lysines into homocitrullines [9]. Anti-CarP antibodies can also be detected before diagnosis [10] and may also be present in patients seronegative for RF and ACPA [8]. These antibodies may be of additional value in the diagnosis of RA. In a recent literature study [11], it was reported that triple positivity for ACPAs, RFs and anti-CarP antibodies resulted in a higher specificity for RA (98–100%), but with lower sensitivity (11–39%). Anti-CarP antibodies have been detected in arthralgia patients [12]. Another recent publication, however, demonstrated no additional value for RA development of measuring anti-CarP in arthralgia patients in clinical practice, so the data of Verheul et al. [11] cannot be extrapolated to the setting of populations with symptoms [13].
In the above-mentioned phases defined by the EULAR, the symptomatic phase preceding clinical arthritis is the first opportunity to recognize patients at risk for progression to RA. This phase is less studied compared with the other phases. Recently, a EULAR taskforce defined the clinical characteristics of patients with arthralgia who are considered at risk for RA, described as clinically suspect arthralgia [14]. These clinical characteristics, of which five are from history-taking and two from physical examination, can be used to establish risk of progression to RA. The history-taking questions involved joint symptoms of recent onset (<1 year), symptoms located in MCP joints, duration of morning stiffness ≥60 min, most severe symptoms present in the early morning and presence of a first-degree relative (FDR) with RA. Physical examination included difficulty of making a fist and positive squeeze test of MCP joints [14].
In a previous study [15], presence of ACPA in Lifelines, a population-based cohort from the northeast of the Netherlands, was investigated. In a large cohort of >40.000 participants, 311 individuals were identified that were ACPA positive (Phadia ACPA-CCP2 levels ≥6.2 U/ml), but did not have RA. The aim of the present study was to assess whether presence of arthritis associated autoantibodies next to IgG ACPA can predict clinically suspect arthralgia in ACPA positive subjects without RA. In addition, it was assessed whether levels of anti-P. gingivalis are related to self-reported periodontitis and joint complaints or arthritis-associated autoantibodies.
Methods
Study design and study population
Lifelines is a multi-disciplinary prospective population-based cohort study examining in a unique three-generation design the health and health-related behaviour of 167 729 persons living in the north of the Netherlands. It employs a broad range of investigative procedures in assessing the biomedical, socio-demographic, behavioural, physical and psychological factors that contribute to the health and disease of the general population, with a special focus on multi-morbidity and complex genetics [16]. Information on application and data access procedure is summarized on http://www.Lifelines.nl. The Lifelines Cohort Study was conducted according to the principles of the Declaration of Helsinki, approved by the local ethics committee of the University Medical Center Groningen. All participants provided written informed consent. In a previous study [15] cross-sectional data from 40 136 participants were included at baseline visits (2012–13). ACPA detection was performed by measuring IgG anti-CCP2 on the Phadia-250 analyser (ThermoFisher Scientific, Freiburg, Germany) with levels ≥6.2 U/ml considered positive. The baseline questionnaire was taken on demographic and clinical information, including smoking, periodontal health and early symptoms of musculoskeletal disorders. RA was defined by a combination of self-reported RA, medication use for the indication of rheumatism and visiting a medical specialist within the last year. Of 40 136 unselected individuals, 401 (1.0%) had an ACPA level ≥6.2 U/ml, of which 80 persons (22.4%) had RA (15.2% had defined RA according to the criteria and 7.2% self-reported RA only). In participants without RA (non-RA), 311 persons (0.8% of the total cohort) were ACPA-positive.
For the present study, serum samples were tested from non-RA participants who were IgG ACPA-positive. No serum samples were available from three participants, so 308 persons were included. In the obtained sera, levels of IgA ACPA, IgM and IgA RF, and IgG anti-CarP were measured, as well as levels of antibodies to P. gingivalis.
CTD Screening Questionnaire
Lifelines participants were invited to complete the CTD Screening Questionnaire (CSQ) [17] during the follow-up assessment, which had taken place ∼2 years after the baseline assessment. For the 308 non-RA participants this was done in 23 months (median; interquartile range 23–25). The CSQ comprises 30 questions designed at identifying potential CTDs. The RA-related questions were the following: (1) ‘Do you have joint stiffness in the morning’, (2) ‘Do you have joint complaints for >3 months’, (3) ‘Do you have joint stiffness in the morning lasting at least 1 h for >6 weeks’, (4) ‘Do you have complaints in the same joints on both sides of your body’, (5) ‘Do you have joint pain’, (6) ’Do you have pain in the wrist or hand’, (7) ‘Do you have swollen hands or wrists’ and (8) ‘Is it difficult for you to make a fist’.
Persons that answered ‘yes’ to one or more of these questions were considered to have joint complaints. Of these, questions 3, 6, 7 and 8 overlap with the EULAR taskforce questions, therefore persons who answered ‘yes’ to these questions and/or had an FDR with RA reported at baseline were considered clinically suspect for arthralgia at risk for RA (clinically suspect arthralgia) and analysed separately.
Laboratory measurements
IgA ACPA measurements were performed using a modification of the anti-CCP2 kit (Euro Diagnostica, Arnhem, The Netherlands) as described previously [18]. A pool of four high-level IgA ACPA sera served as standard curve expressed in arbitrary units (U/ml). Seropositivity was defined as greater than mean + 2 s.d. of healthy controls, and set to 1 U/ml [18].
Levels of IgG anti-CarP antibodies against carbamylated fetal calf serum were assessed using the protocol as described by Shi et al. [8]. Seropositivity was defined as greater than mean + 2 s.d. of a distinctive healthy control cohort from the same study.
IgM and IgA RF levels were assessed using a validated in-house ELISA. Levels were expressed in IU/ml, and seropositivity was defined as ≥10 IU/ml for IgM RF and ≥25 IU/ml for IgA RF for diagnostic purposes [19]. In a recent publication, IgM RF levels ≥3.5 IU/ml were also considered positive [13].
Antibodies to P. gingivalis were assessed as described previously [20]. In short, antibodies to P. gingivalis were measured by an in-house ELISA, in which a pooled lysate of four randomly selected clinical isolates of P. gingivalis from patients with periodontitis was used as antigen. Standard curves were made from protein standards (N protein-standard SL; Siemens Healthcare Diagnostics, den Haag, the Netherland). Detection of anti-P. gingivalis antibodies was carried out with mouse anti-human IgG (Fc fragment specific, clone JDC-10; Southern Biotech, Uithoorn, the Netherlands). Arbitrary cut-off values for anti-P. gingivalis positivity was 5 mg/l, defined as mean values + 2 s.d. of healthy subjects without periodontitis and without cultivable subgingival P. gingivalis [healthy control, n = 36, mean (s.d.) age 34 (15) years, 53% female, 14% current smoker] as described previously [21].
Statistics
Statistics were performed with The Statistical Product and Service Solutions (SPSS, version 25; IBM Corp., Armonk, NY, USA). Results were expressed as numbers of subjects (percentages), mean (s.d.) or median (interquartile range) for categorical, normally and non-normally distributed data, respectively. χ2 test, independent samples t test, and Mann–Whitney U test were used as appropriate to compare characteristics between participants with and without RA. Multivariable logistic regression with clinically suspect arthralgia based on questions related to the EULAR definition (yes/no) as dependent variable was performed to correct the association with the presence of joint complaints and autoantibodies for age, gender and never smoking. P-values <0.05 were considered as statically significant.
Results
Distribution of RA-related autoantibodies
Arthritis-associated autoantibodies were measured in serum samples of 308 IgG ACPA-positive individuals without RA. Of these persons, 166 (53.6%) were also positive for IgA ACPA, 73 (23.7%) for IgA RF, 78 (25.3%) for IgM RF using a cut-off of 10 IU/ml and 130 (42.2%) using a cut-off of 3.5 IU/ml, and 42 (13.6%) for anti-CarP. There was a strong correlation (Spearman ρ between 0.42 and 0.70, all P < 0.0001) between all autoantibodies.
From the 308 ACPA-positive individuals, 178 (57.8%) had responded to the CSQ. Baseline characteristics of this group and the group with missing CSQ group are summarized in Table 1. It can be seen from the table that the persons for whom CSQ data were available were older, had lower BMI, were less often smokers and more often had an FDR with rheumatism. However, there were no significant differences between autoantibody positivity or autoantibody levels between these groups.
Table 1.
Baseline information of all ACPA-positive participants without RA, divided by availability of CSQ data at follow up (median 23 months)
| Total group (N = 308) | CSQ available (N = 178) | Missing CSQ (N = 130) | P-value, CSQ yes/no | |
|---|---|---|---|---|
| Age, years, median (IQR) | 46 (35–53) | 47 (37–53) | 44 (33–50) | 0.05 |
| Gender (female), n (%) | 191 (62.0) | 112 (62.9) | 79 (60.8) | 0.70 |
| BMI, kg/m2, median (IQR) | 25.4 (23.2–27.8) | 24.8 (22.9–27.3) | 26.0 (23.8–28.7) | 0.002 |
| Smoking | ||||
| Never smoker, n (%) | 124 (940.3) | 76 (42.6) | 48 (36.9) | 0.31 |
| Former smoker, n (%) | 107 (34.7) | 67 (37.6) | 40 (30.8) | 0.21 |
| Current smoker, n (%) | 77 (25.0) | 35 (19.7) | 42 (32.3) | 0.01 |
| Alcohol (g/day), median (IQR) | 3.4 (0–8.6) | 3.6 (0–7.4) | 2.9 (0–10.8) | 0.74 |
| Periodontitis (self-reported), n (%) | 44 (14.3) | 27 (15.2) | 17 (13.1) | 0.61 |
| Joint complaints: pain hands and/or feet, n (%) | 65 (21.1) | 39 (21.9) | 26 (20) | 0.82 |
| Joint complaints: stiffness in hands and/or feet, n (%) | 63 (20.5) | 37(20.7) | 26 (20) | 0.97 |
| FDR with rheumatism, n (%) | 47 (15.3) | 34 (19.1) | 13 (10.0) | 0.03 |
| ACPA IgG pos, n (%) | 308 (100) | 178 (100) | 130 (100) | 1.00 |
| ACPA IgG level, median (IQR) | 16 (9.0–61) | 17.5 (9.0–61.5) | 14 (9.0–60.5) | 0.75 |
| ACPA IgA pos, n (%) | 166 (53.9) | 97 (54.5) | 69 (53.1) | 0.85 |
| ACPA IgA level, median (IQR) | 1.1 (0.6–2.3) | 1.1 (0.6–2.1) | 1.1 (0.6–2.4) | 0.67 |
| RF IgA pos, n (%) | 73 (23.7) | 40 (22.5) | 33 (25.4) | 0.55 |
| RF IgA level median (IQR) | 4.5 (1.5–19.2) | 4.0 (1.5–18.7) | 5.3 (1.4–21.5) | 0.59 |
| RF IgM pos (>3.5), n (%) | 130 (42.2) | 70 (39.3) | 60 (46.2) | 0.23 |
| RF IgM pos (>10.0), n (%) | 78 (25.3) | 48 (27.0) | 30 (23.1) | 0.44 |
| RF IgM level, median (IQR) | 2.7 (0.8–10.7) | 2.5 (0.8–11.2) | 3.0 (0.8–8.4) | 0.89 |
| Anti-CarP pos, n (%) | 42 (13.6) | 25 (14.0) | 17 (13.1) | 0.81 |
| Anti-CarP level, median (IQR) | 101 (44–208) | 95 (43–206) | 104 (51–212) | 0.54 |
Significant P values are shown in bold. CSQ: CTD Screening Questionnaire; IQR: interquartile range; anti-CarP: anti-carbamylated protein antibody; FDR: first-degree relative; pos: positive.
Evaluation of arthritis autoantibodies and joint complaints
Scores on questions regarding self-reported joint complaints are listed in Table 2. In total, 83 out of 178 persons (46.6%) scored positive for one or more joint complaints. Most participants reported joint pain and joint complaints for >3 months, and around 25% of the persons suffered from morning stiffness and from joint complaints on both sides of their body.
Table 2.
Joint complaints in ACPA-positive participants with CSQ data during follow-up (median 23 months)
|
N = 178 | |
|---|---|
| Joint complaints, n (%) | |
| 1: Joint stiffness in the morning | 48 (26.9) |
| 2: Joint complaints for >3 months | 62 (34.8) |
| 3: Joint stiffness in the morning lasting at least 1 h for >6 weeks | 18 (10.1) |
| 4: Complaints in the same joints on both sides of your body | 45 (25.2) |
| 5: Joint pain | 59 (33.1) |
| 6: Pain in the wrist or hand | 47 (26.4) |
| 7: Swollen hands or wrist | 11 (6.2) |
| 8: Difficult to make a fist | 14 (7.9) |
CSQ: CTD Screening Questionnaire.
Next, differences in autoantibody profile were evaluated between persons with and without joint complaints. Participants with joint complaints were significantly older and were more often female (Table 3). Furthermore, IgA RF levels (P = 0.03) and both IgM RF levels (P = 0.03) and IgM RF positivity (≥3.5 IU/ml; P = 0.005) were significantly higher in the joint complaint group. IgG ACPA and anti-CarP levels and positivity were equal in the groups. Of the persons with joint complaints at the 2-year follow-up, 39.8 and 34.9% had reported pain or stiffness at baseline, respectively. There was no difference between the groups regarding positivity for more than one autoantibody.
Table 3.
Baseline information of ACPA-positive participants divided by CSQ data concerning joint complaints (N = 178)
| Baseline | Joint complaints (N = 83) | No joint complaints (N = 95) | P-value |
|---|---|---|---|
| Age, years, median (IQR) | 49 (43–56) | 44 (34–51) | 0.005 |
| Gender (female), n (%) | 60 (72.2) | 52 (54.7) | 0.01 |
| BMI, kg/m2, median (IQR) | 25.1 (23.2–27.70 | 24.5 (22.6–26.9) | 0.21 |
| Smoking | |||
| Never smoker, n (%) | 30 (36.1) | 46 (48.4) | 0.10 |
| Former smoker, n (%) | 37 (44.6) | 30 (31.6) | 0.08 |
| Current smoker, n (%) | 16 (19.3) | 19 (20) | 0.90 |
| Alcohol (g/day), median (IQR) | 3.57 (0–7.14) | 3.57 (0–8.57) | 0.89 |
| Periodontitis (self-reported), n (%) | 14 (16.8) | 13 (13.7) | 0.56 |
| Joint complaints: pain hands and/or feet, n (%) | 33 (39.8) | 6 (6.3) | <0.001 |
| Joint complaints: stiffness in hands and/or feet, n (%) | 29 (34.9) | 8 (8.4) | <0.001 |
| FDR with rheumatism, n (%) | 21 (25.3) | 13 (13.6) | 0.05 |
| ACPA IgG pos, n (%) | 83 (100) | 95 (100) | NA |
| ACPA IgG level, median (IQR) | 19 (9.0–64.0) | 17.0(8.0–61.0) | 0.39 |
| ACPA IgA pos n (%) | 47 (56.6) | 50 (52.6) | 0.61 |
| ACPA IgA level, median (IQR) | 1.1 (0.6–2.4) | 1.1 (0.6–1.9) | 0.61 |
| RF IgA pos, n (%) | 21 (25.3) | 19 (20.0) | 0.42 |
| RF IgA level, median (IQR) | 6.7 (2.0–25.5) | 3.0 (1.2–17.3) | 0.03 |
| RF IgM pos (>3.5), n (%) | 42 (50.6) | 28(29.5) | 0.005 |
| RF IgM pos (>10.0), n (%) | 28 (33.7) | 20 (21.1) | 0.06 |
| RF IgM level, median (IQR) | 4.0 (1.1–17.5) | 2.3 (0.6–8.5) | 0.03 |
| Anti-CarP pos, n (%) | 13 (15.6) | 12 (12.6) | 0.44 |
| Anti-CarP level, median (IQR) | 100 (46–237) | 89 (43–287) | 0.46 |
Significant P values are shown in bold. CSQ: CTD Screening Questionnaire; IQR: interquartile range; anti-CarP: anti-carbamylated protein antibody; FDR: first-degree relative; pos: positive; NA: not applicable.
Development of RA
Ten participants (5.6%) reported having developed RA between baseline and the 2-year follow-up. They all reported positively on the CSQ regarding joint stiffness, and joint, hand and wrist pain; nine reported complaints for >3 months and on both sides of the body, five reported difficulty of making a fist, four had hand and wrist swollen, and three had morning stiffness for >6 weeks. Of these 10 persons, 7 were also seropositive for IgA ACPA and IgM RF, 5 for IgA RF and 2 for anti-CarP at baseline.
EULAR definition: clinically suspect arthralgia
It was found that 75 persons were defined as clinically suspect arthralgia positive (of which 62 were also in the joint complaints group), 102 as no-clinically suspect arthralgia and 1 had missing data. In Table 4, the characteristics between these groups are compared and, next to the already detected differences in the joint complaints group, the number of never smokers was higher in the no-clinically suspect arthralgia group, and levels of IgG ACPA, IgA ACPA and IgM RF were significantly higher in clinically suspect arthralgia group. In Fig. 1, the distribution of arthritis autoantibodies in clinically suspect arthralgia and no-clinically suspect arthralgia groups is shown.
Table 4.
Baseline information of ACPA-positive participants divided by clinically suspect arthralgia characteristics (N = 177)
| Baseline | Clinically suspect arthralgia (N = 75) | No-clinically suspect arthralgia (N = 102) | P-value |
|---|---|---|---|
| Age, years, median (IQR) | 49 (42–55) | 45 (34–51) | 0.005 |
| Gender (female), n (%) | 53 (70.7) | 58 (56.9) | 0.06 |
| BMI, kg/m2, median (IQR) | 25.1 (23.3–27.4) | 24.6 (22.4–27.3) | 0.31 |
| Smoking | |||
| Never smoker, n (%) | 22 (29.3) | 53 (52.0) | 0.003 |
| Former smoker, n (%) | 36 (48.0) | 31 (29.4) | 0.02 |
| Current smoker, n (%) | 17 (22.7) | 18 (17.6) | 0.41 |
| Alcohol (g/day), median (IQR) | 4.1 (0.0–9.3) | 3.1 (0.0–7.1) | 0.50 |
| Periodontitis (self-reported), n (%) | 13 (17.3) | 14 (13.7) | 0.51 |
| Joint complaints: pain hands and/or feet, n (%) | 27 (36.0) | 12 (11.8) | <0.001 |
| Joint complaints: stiffness in hands and/or feet, n (%) | 26 (34.7) | 11 (10.8) | <0.001 |
| ACPA IgG pos, n (%) | 75 (100) | 102 (100) | NA |
| ACPA IgG level, median (IQR) | 22 (10–122) | 16.5 (8–44) | 0.04 |
| ACPA IgA pos, n (%) | 44 (58.7)) | 50 (49.0) | 0.20 |
| ACPA IgA level, median (IQR) | 1.39 (0.61–3.93) | 1.00 (0.52–1.71) | 0.02 |
| RF IgA pos, n (%) | 22 (29.3) | 18 (17.6) | 0.07 |
| RF IgA level, median (IQR) | 5.1 (1.6–46.3) | 3.4 (1.4–15.1) | 0.13 |
| RF IgM pos (>3.5), n (%) | 37 (49.3) | 32 (31.4) | 0.02 |
| RF IgM pos (>10.0), n (%) | 26 (38.7) | 22 (21.5) | 0.05 |
| RF IgM level, median (IQR) | 3.1 (1.2–17.5) | 2.1 (0.6–7.2) | 0.02 |
| Anti-CarP pos, n (%) | 13 (17.3) | 12 (11.7) | 0.06 |
| Anti-CarP level, median (IQR) | 110 (53–258) | 88 (37–166) | 0.30 |
| Positivity for number of arthritis autoantibodies, n (%) | |||
| Only IgG ACPA pos | 26 (34.7) | 39 (38.2) | 0.63 |
| 1 extra autoAb pos | 15 (20.0) | 34 (33.3) | 0.05 |
| 2 extra autoAb pos | 10 (13.3) | 13 (12.7) | 0.91 |
| 3 or 4 extra autoAb pos | 24 (34.6) | 16 (15.7) | 0.01 |
| IgG ACPA + IgM RF + anti-CarP | 12 (16) | 6 (5.9) | 0.03 |
Significant P values are shown in bold. QR: interquartile range; anti-CarP: anti-carbamylated protein antibody; pos: positive; autoAb: autoantibody; NA: not applicable.
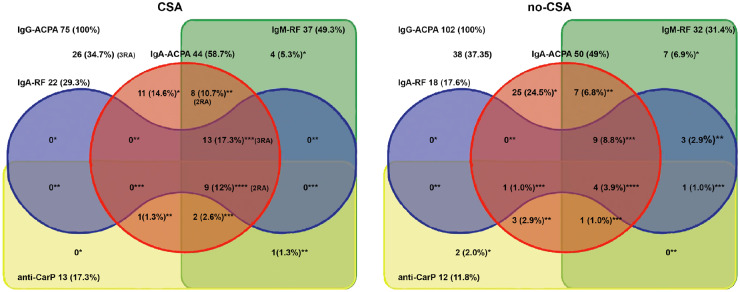
Fig. 1.
Distribution of numbers of seropositivity (and percentages) for autoantibodies in clinically suspect arthralgia and no-clinically suspect arthralgia persons
Distribution of numbers of seropositivity (and percentages) for IgA ACPA, IgM RF, IgA RF and anti-CarP in IgG ACPA-positive individuals without RA. Persons are divided into clinically suspect arthralgia and no-clinically suspect arthralgia, based on data from follow-up questionnaires. Persons who developed RA during follow-up and their autoantibody profile are placed at the appropriate place. anti-CarP: anti-carbamylated protein antibody; * seropositive for one extra (besides IgG ACPA) autoantibody; ** seropositive for two extra autoantibodies; *** seropositive for three extra autoantibodies; **** seropositive for four extra autoantibodies.
In the clinically suspect arthralgia group, significantly more persons were seropositive for the combination of IgG ACPA, IgM RF and anti-CarP, and more persons were seropositive for three or more autoantibodies. Autoantibody positivity and levels were investigated in the clinically suspect arthralgia group in persons with an FDR only (n = 21) and in those with FDR and/or joint complaints (n = 53). Of note, FDR only persons may have responded positively to the joint complaint questions that were not used in our clinically suspect arthralgia definition. FDR only persons tended to have lower positivity for and lower levels of IgM RF and IgA RF; however, these were not statistically significant. No further differences were found between these groups (data not shown).
In multivariate logistic regression correcting for age, gender and never smoking, joint complaints [pain hands and/or feet (odds ratio: 3.88, 95% CI: 1.74–8.65); and stiffness in hands and/or feet (odds ratio: 3.72, 95% CI: 1.63–8.50)] and three or more autoantibodies positive (odds ratio: 2.63, 95% CI: 1.04–6.65) remained significantly associated with clinically suspect arthralgia. Furthermore, there was a trend for ACPA IgA (P = 0.05). ACPA IgG level, RF IgM positivity and RF IgM level did not remain significantly different between clinically suspect arthralgia and no-clinically suspect arthralgia.
Evaluation of anti-P. gingivalis antibodies, joint complaints and periodontitis
Self-reported periodontitis and levels of anti-P. gingivalis at baseline were not different between persons that completed the CSQ or not, nor between the persons with and without joint complaints, or between clinically suspect arthralgia nor no-clinically suspect arthralgia groups.
Of note, anti-P. gingivalis levels were significantly correlated to IgM RF (ρ = 0.30, P = 0.006) and IgA RF levels (ρ = 0.30, P = 0.007) in the joint complaints group, and there was a trend (ρ = 0.21, P = 0.06) for a positive correlation with IgG ACPA. In the clinically suspect arthralgia group, anti-P. gingivalis levels were significantly correlated to IgG ACPA (ρ = 0.28, P = 0.014), IgA ACPA (ρ = 0.23, P = 0.05) and IgM RF (ρ = 0.36, P = 0.001), and highly significantly to IgA RF levels (ρ = 0.36, P = 0.0006). There were no significant correlations with anti-P. gingivalis levels in the no joint complaints and no-clinically suspect arthralgia groups.
When looking at the association between anti-P. gingivalis and specific periodontitis-related questions, significantly more individuals had visible teeth roots in the joint complaints group (30.1 vs 14.7%) and in the clinically suspect arthralgia group (29.3 vs 14.7%). The number of persons with loose teeth and bleeding gums were not significantly different between clinically suspect arthralgia and no-clinically suspect arthralgia groups (Table 5).
Table 5.
Periodontal complaints and anti-P. gingivalis levels in ACPA-positive participants divided by clinically suspect arthralgia characteristics
| N = 177 | Clinically suspect arthralgia (N = 75) | No-clinically suspect arthralgia (N = 102) | P-value | |
|---|---|---|---|---|
| Signs of periodontitis | ||||
| Roots of teeth visible, n (%) | 39 (22.0) | 22 (29.3) | 17 (16.7) | 0.05 |
| Loose teeth, n (%) | 7 (3.9) | 2 (2.6) | 5 (4.7) | 0.45 |
| Easily bleeding gum, n (%) | 23 (13.0) | 9 (12.0) | 14 (13.7) | 0.74 |
| Level of anti-P. gingivalis (U/ml), median (IQR) | 1.97 (1.05–3.77) | 2.12 (1.05–3.83) | 1.92 (1.04–3.77) | 0.99 |
Significant P values are shown in bold. QR: interquartile range.
Discussion
ACPA-positive individuals without defined or self-reported RA but with clinically suspect arthralgia have higher levels of multiple arthritis autoantibodies than ACPA-positive individuals without arthralgia. In the clinically suspect arthralgia group levels of anti-P. gingivalis were also correlated to other arthritis autoantibodies, but not in the no-clinically suspect arthralgia group.
It its acknowledged that early recognition of RA is important to prevent joint destruction and various comorbidities [22]. Development to RA Is a multistep process with different known phases, as mentioned previously [3]. At-risk patients often present with arthralgia, and in 2017 a EULAR study group defined the most important characteristics describing clinically suspect arthralgia at risk for RA [14]. Since the current study is part of a large longitudinal cohort study that started in 2007–13, with first inclusion and in 2012–16 with the follow up CSQ [16], the exact questions as formulated by the EULAR taskforce are not included in the questionnaires used in Lifelines. We chose the questions from the CSQ that, in our opinion, overlapped with the EULAR taskforce characteristics, and used these in the present study.
To investigate systemic autoimmunity in RA, an expansion of the autoantibody repertoire was chosen, including IgA ACPA, IgM and IgA RF, and anti-CarP. Presence of multiple autoantibodies have been previously described in pre-symptomatic individuals. Brink et al. [23] showed seropositivity for IgG ACPA (30%), IgM RF (26%) and IgA RF (25%) in a median pre-dating time of 6.2 years before onset of symptoms. In a previous study of the same group, it was shown that IgA RF occurred at a higher frequency and that it was a good predictor in the early pre-symptomatic period [24]. Kelmenson et al. [25] recently showed that in pre-RA individuals, occurrence of autoantibodies before diagnosis was as follows: IgG ACPA 17.9 years, IgA RF 14.2 years, IgM RF 7.2 years, IgA ACPA 6.2 years, and IgM ACPA and IgA RF both at 5.0 years. Prevalence of IgA autoantibodies is not surprising, since a dominance of IgA-positive plasmablasts was found in a cohort of autoantibody-positive at-risk persons, suggesting that a subset of RA-related autoantibodies may arise from mucosal immune responses [26]. Recently, Holers et al. [27] proposed the so-called mucosal-origin hypothesis, suggesting that local production of IgA autoantibodies at mucosal sites may systemically spread due to inflammatory processes, and are then serologically detectable. Interestingly, we recently showed that in healthy persons with periodontitis, local IgA ACPA production was found in gingival crevicular fluid, indicating that periodontitis may play a role in local ACPA production and onset of arthritis [28].
Antibodies recognizing carbamylated (homocitrulline-containing) antigens have been described for the first time in sera of >45% of RA patients, and in 16% of sera of ACPA-negative patients [8]. Moreover, it was suggested that the presence of anti-CarP antibodies was predictive for a more severe clinical course in ACPA-negative RA patients. Recently, in a large cohort of undifferentiated arthritis the additive value of measuring anti-CarP for predicting RA development was determined [29]. It was found that anti-CarP antibodies had no additional value when RA was defined according to the 2010 criteria, but anti-CarP may be useful for prediction in ACPA-negative and RF-negative patients. In our study, anti-CarP was not different between persons with and without joint complaints, but there was a significant difference between clinically suspect arthralgia and no-clinically suspect arthralgia groups. As described in the study by ten Brinck et al. [13], positivity for IgM RF was of greater influence than measuring additional anti-CarP levels.
Porphyromonas gingivalis is hypothesized to play a role in RA risk by inducing the production of ACPA, either by molecular mimicry or epitope spreading [30]. A recent meta-analysis determining the relation between anti-P. gingivalis and ACPA showed not only that anti-P. gingivalis levels were higher in RA compared with healthy controls, but also that anti-P. gingivalis was positively correlated to ACPA [31]. In the clinically suspect arthralgia patients we also found a positive correlation with both ACPA isotypes as well as with both RF isotypes, while this was not the case for the no-clinically suspect arthralgia group. Our current study shows that anti-P. gingivalis levels did not correlate with self-reported periodontitis or with specific periodontitis-related questions. Previous studies showed higher levels of anti-P. gingivalis in periodontitis patients compared with no periodontitis patients [32–34], but anti-P. gingivalis levels cannot be regarded as a measure for periodontal disease [35]. Whether anti-P. gingivalis levels can be regarded as predictive for development of RA is questionable as well. In a cohort of arthralgia patients who were seropositive for IgM RF and/or ACPA, anti-P. gingivalis levels at baseline were not different in patients who did or did not develop RA during 12 months follow-up [21]. However, a year may be too short to study RA development in seropositive arthralgia patients. The follow-up time in our present study may also not be long enough. Development to RA may require more time, since Nielen et al. [6] showed that autoantibodies may be present up to 14 years before diagnosis.
In our analysis, we found an important contribution of smoking to clinically suspect arthralgia, meaning that persons who were previous or current smokers had a significantly higher risk. In the past years many studies, reviewed by Sparks and Karlson [36], investigating cigarette smoking and RA risk, as well as transitions between phases of preclinical RA, were helpful in establishing the lung as a potential initiating site in the pathogenesis of seropositive RA. In our study, we saw a 2.6 higher risk in clinically suspect arthralgia in persons that were previous or current smokers.
So, in conclusion, measuring multiple arthritis autoantibodies in combination with clinical characteristics identifying clinically suspect arthralgia may help in the early identification of RA development. Levels of anti-P. gingivalis are not related to self-reported periodontitis or clinically suspect arthralgia, but are correlated to arthritis autoantibodies in clinically suspect arthralgia.
Acknowledgements
The Lifelines initiative has been made possible by subsidy form the Dutch Ministry of Health, Welfare and Sport, the Dutch Ministry of Economic Affairs, the University Medical Center Groningen (UMCG), Groningen University and the Provinces in the North of the Netherlands (Drenthe, Friesland, Groningen).
Funding: This work was supported by the Dutch Arthritis Society, number 16-2-201.
Disclosure statement: The authors declare no conflicts of interest.
References
- 1. Smolen JS, Aletaha D, McInnes IB.. Rheumatoid arthritis. Lancet 2016;388:2023–38. [DOI] [PubMed] [Google Scholar]
- 2. Aletaha D, Neogi T, Silman AJ. et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 3. Gerlag DM, Raza K, van Baarsen LG. et al. EULAR recommendations for terminology and research in individuals at risk of rheumatoid arthritis: report from the Study Group for Risk Factors for Rheumatoid Arthritis. Ann Rheum Dis 2012;71:638–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karlson EW, van Schaardenburg D, van der Helm-van Mil AH.. Strategies to predict rheumatoid arthritis development in at-risk populations. Rheumatology (Oxford) 2016;55:6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koziel J, Mydel P, Potempa J.. The link between periodontal disease and rheumatoid arthritis: an updated review. Curr Rheumatol Rep 2014;16:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nielen MM, van SD, Reesink HW. et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004;50:380–6. [DOI] [PubMed] [Google Scholar]
- 7. Bos WH, Wolbink GJ, Boers M. et al. Arthritis development in patients with arthralgia is strongly associated with anti-citrullinated protein antibody status: a prospective cohort study. Ann Rheum Dis 2010;69:490–4. [DOI] [PubMed] [Google Scholar]
- 8. Shi J, Knevel R, Suwannalai P. et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci USA 2011;108:17372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shi J, van Veelen PA, Mahler M. et al. Carbamylation and antibodies against carbamylated proteins in autoimmunity and other pathologies. Autoimmun Rev 2014;13:225–30. [DOI] [PubMed] [Google Scholar]
- 10. Gan RW, Trouw LA, Shi J. et al. Anti-carbamylated protein antibodies are present prior to rheumatoid arthritis and are associated with its future diagnosis. J Rheumatol 2015;42:572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verheul MK, Böhringer S, van Delft MAM. et al. Triple positivity for anti-citrullinated protein autoantibodies, rheumatoid factor, and anti-carbamylated protein antibodies conferring high specificity for rheumatoid arthritis: implications for very early identification of at-risk individuals. Arthritis Rheumatol 2018;70:1721–31. [DOI] [PubMed] [Google Scholar]
- 12. Shi J, van de Stadt LA, Levarht EW. et al. Anti-carbamylated protein antibodies are present in arthralgia patients and predict the development of rheumatoid arthritis. Arthritis Rheum 2013;65:911–5. [DOI] [PubMed] [Google Scholar]
- 13. ten Brinck RM, Trouw LA, van der Helm-van Mil A.. Screening for two or three autoantibodies in persons at risk for RA: implications of current data for clinical practice. Rheumatology (Oxford) 2019;58:914–5. [DOI] [PubMed] [Google Scholar]
- 14. van Steenbergen HW, Aletaha D, Beaart-van de Voorde LJ. et al. EULAR definition of arthralgia suspicious for progression to rheumatoid arthritis. Ann Rheum Dis 2017;76:491–6. [DOI] [PubMed] [Google Scholar]
- 15. van Zanten A, Arends S, Roozendaal C. et al. Presence of anticitrullinated protein antibodies in a large population-based cohort from the Netherlands. Ann Rheum Dis 2017;76:1184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scholtens S, Smidt N, Swertz MA. et al. Cohort profile: LifeLines, a three-generation cohort study and biobank. Int J Epidemiol 2015;44:1172–80. [DOI] [PubMed] [Google Scholar]
- 17. Karlson EW, Sanchez-Guerrero J, Wright EA. et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol 1995;5:297–302. [DOI] [PubMed] [Google Scholar]
- 18. Janssen KM, de Smit MJ, Brouwer E. et al. Rheumatoid arthritis-associated autoantibodies in non-rheumatoid arthritis patients with mucosal inflammation: a case-control study. Arthritis Res Ther 2015;17:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Leeuwen MA, Westra J, van Riel PL, Limburg PC, van Rijswijk MH.. IgM, IgA, and IgG rheumatoid factors in early rheumatoid arthritis predictive of radiological progression? Scand J Rheumatol 1995;24:146–53. [DOI] [PubMed] [Google Scholar]
- 20. Smit MD, Westra J, Vissink A. et al. Periodontitis in established rheumatoid arthritis patients: a cross-sectional clinical, microbiological and serological study. Arthritis Res Ther 2012;14:R222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Smit M, van de Stadt LA, Janssen KM, Doornbos-van der Meer B. et al. Antibodies against Porphyromonas gingivalis in seropositive arthralgia patients do not predict development of rheumatoid arthritis. Ann Rheum Dis 2014;73:1277–9. [DOI] [PubMed] [Google Scholar]
- 22. van Steenbergen HW, da Silva JAP, Huizinga TWJ, van der Helm-van Mil A.. Preventing progression from arthralgia to arthritis: targeting the right patients. Nat Rev Rheumatol 2018;14:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brink M, Hansson M, Mathsson-Alm L. et al. Rheumatoid factor isotypes in relation to antibodies against citrullinated peptides and carbamylated proteins before the onset of rheumatoid arthritis. Arthritis Res Ther 2016;18:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rantapää-Dahlqvist S, de Jong BA, Berglin E. et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003;48:2741–9. [DOI] [PubMed] [Google Scholar]
- 25. Kelmenson LB, Wagner BD, McNair BK. et al. Timing of elevations of autoantibody isotypes in rheumatoid arthritis prior to disease diagnosis. Arthritis Rheumatol 2019;72:251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kinslow JD, Blum LK, Deane KD, Demoruelle MK. et al. Elevated IgA plasmablast levels in subjects at risk of developing rheumatoid arthritis. Arthritis Rheumatol 2016;68:2372–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holers VM, Demoruelle MK, Kuhn KA. et al. Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction. Nat Rev Rheumatol 2018;14:542–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rahajoe PS, Smit M, Schuurmans G. et al. Increased IgA ACPA in the periodontal inflammatory exudate of healthy individuals compared to rheumatoid arthritis patients. J Clin Periodontol 2020;47:552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boeters DM, Trouw LA, van der Helm-van Mil AHM, van Steenbergen HW.. Does information on novel identified autoantibodies contribute to predicting the progression from undifferentiated arthritis to rheumatoid arthritis: a study on anti-CarP antibodies as an example. Arthritis Res Ther 2018;20:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Smit MJ, Westra J, Brouwer E. et al. Periodontitis and rheumatoid arthritis: what do we know? J Periodontol 2015;86:1013–9. [DOI] [PubMed] [Google Scholar]
- 31. Bae SC, Lee YH.. Association between anti-Porphyromonas gingivalis antibody, anti-citrullinated protein antibodies, and rheumatoid arthritis: a meta-analysis. Z Rheumatol 2018;77:522–32. [DOI] [PubMed] [Google Scholar]
- 32. Lange L, Thiele GM, McCracken C. et al. Symptoms of periodontitis and antibody responses to Porphyromonas gingivalis in juvenile idiopathic arthritis. Pediatr Rheumatol Online J 2016;14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mikuls TR, Payne JB, Reinhardt RA. et al. Antibody responses to Porphyromonas gingivalis (P. gingivalis) in subjects with rheumatoid arthritis and periodontitis. Int Immunopharmacol 2009;9:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Damgaard C, Reinholdt J, Enevold C. et al. Immunoglobulin G antibodies against Porphyromonas gingivalis or Aggregatibacter actinomycetemcomitans in cardiovascular disease and periodontitis. J Oral Microbiol 2017;9:1374154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kinane DF, Mooney J, Ebersole JL.. Humoral immune response to Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in periodontal disease. Periodontol 2000 1999;20:289–340. [DOI] [PubMed] [Google Scholar]
- 36. Sparks JA, Karlson EW.. The roles of cigarette smoking and the lung in the transitions between phases of preclinical rheumatoid arthritis. Curr Rheumatol Rep 2016;18:15. [DOI] [PMC free article] [PubMed] [Google Scholar]