Abstract
Objective
To determine the extent to which disease duration, alone or in combination with other baseline clinical and non-clinical factors, explains variations in outcome of tocilizumab initiated in biologic-naïve patients with established RA.
Methods
In this pooled analysis of phase 3 and 4 clinical trials conducted by the sponsor, predictors of response, including demographics, disease characteristics at baseline (start of tocilizumab dosing) and study characteristics (e.g. patient inclusion criteria, tocilizumab dosing regimen) were evaluated. Response was measured as change from baseline to week 24 in Clinical Disease Activity Index (CDAI) and HAQ–Disability Index (HAQ-DI) scores and as the proportions of patients who experienced ≥50% improvement based on ACR criteria (ACR50) and CDAI remission (≤2.8) rates at week 24.
Results
Improvements in all outcomes investigated were observed in patients receiving tocilizumab. Although disease duration was statistically significant in the models, it accounted for <2% of variation in CDAI and HAQ-DI score changes from baseline to week 24; baseline CDAI and HAQ-DI values accounted for 32% and 15% of variations, respectively. Doubling of disease duration reduced the odds of achieving an ACR50 response by only 9%, and each additional 5-year period of disease duration decreased the odds of achieving CDAI remission by only 15%.
Conclusion
RA duration, alone or in combination with other baseline characteristics, had a statistically significant but clinically small effect on the outcomes of tocilizumab initiated in biologic-naïve patients with established RA.
Keywords: biological therapies, clinical trials and methods, cytokines and inflammatory mediators, DMARDs, rheumatoid arthritis
Rheumatology key messages
Disease duration had statistically significant but clinically small effects on efficacy outcomes with tocilizumab treatment.
Quantified effects and P-values are important to evaluate practical implications of predictors of response.
Introduction
The addition of a biologic to conventional synthetic DMARD (csDMARD) treatment regimens of patients with RA allows those who have inadequate responses the potential to achieve low disease activity and remission. Although the efficacy and safety of biologics are well established in csDMARD-inadequate responder (IR) patients with RA, predictors of response are less well recognized.
Results from observational studies, registry data and retrospective analyses indicate that duration of RA is a predictor of response to treatment; patients are more likely to achieve remission or sustained remission if csDMARD and/or biologic treatment is initiated earlier in their disease course [1–12]. In real-world clinical practice, patients whose disease duration is <5 years and who are receiving csDMARDs, alone or in combination with biologics, have the greatest likelihood of achieving remission according to DAS assessing 28 joints (DAS28) and Clinical Disease Activity Index (CDAI), although the odds of achieving remission decrease with longer duration of disease [1]. Other characteristics reportedly associated with achievement of remission include baseline levels of physical function and quality-of-life outcomes [HAQ–Disability Index (HAQ-DI) and the 36-Item Short Form Health Survey, respectively] and of disease activity measures [2, 4, 6, 8, 11, 13].
Tocilizumab, a humanized anti-IL-6 receptor-α mAb, has been approved for the treatment of patients with RA, GCA, polyarticular and systemic JIA and cytokine release syndrome; its long-term efficacy, safety and tolerability are well established in RA [14–16].
The aim of this study was to investigate the extent to which disease duration alone and in combination with other variables, such as inflammation, disease burden and other baseline factors, might explain variations in clinical outcomes in biologic-naïve patients with RA who are treated with tocilizumab.
Methods
Patients
Data were pooled from all phase 3 and 4 clinical studies conducted by the sponsor that met the selection criteria; in all there were 12 studies. Patients with established RA were included who initiated i.v. or s.c. tocilizumab treatment either as monotherapy or in combination with MTX/csDMARDs (Table 1) [17–28]. To ensure that pooled patients were all at a similar stage of therapy (i.e. that tocilizumab was their first biologic) and because the intention was to look into the effect of disease duration within a specific treatment line, patients with previous exposure to biologics were excluded from this analysis. All patient data from the trials were imported into one data set, as similarity of study protocols, schedules of treatment and assessment and patient selection criteria allowed for pooling on the patient level. All included studies had been conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation/Good Clinical Practice, or both, and had been approved by the ethics committees/institutional review boards of the investigational centres as published. All patients provided written informed consent to participate in the individual studies.
Table 1.
Studies included in the analysis
| Study | Patient population | Patients who received tocilizumab, n |
|---|---|---|
| ACT-RAY [17] | MTX-IR | 552 |
| ACT-STAR [18] | csDMARD-IR | 145 |
| ACT-SURE [19] | csDMARD-IR | 949 |
| ADACTA [20] | MTX-IR or MTX-intolerant | 161 |
| AMBITION [21] | MTX-naïve or MTX-free for ≥6 monthsa | 143 |
| BREVACTA [22] | csDMARD-IR | 358 |
| COMP-ACT [23] | csDMARD-IR | 624 |
| LITHE [24] | MTX-IR | 353 |
| OPTION [25] | MTX-IR | 194 |
| ROSE [26] | csDMARD-IR | 253 |
| SUMMACTA [27] | csDMARD-IR | 1070 |
| TOWARD [28] | csDMARD-IR | 660 |
| Total | – | 5462 |
Included patients with previous exposure to MTX who had discontinued MTX for reasons other than toxicity or lack of efficacy. csDMARD-IR: conventional synthetic DMARD inadequate responders; MTX-IR: MTX inadequate responders.
Assessments
Characteristics considered potential predictors of response are shown in supplementary Table S1, available at Rheumatology online, and include demographic and patient characteristics (age, sex, region, race, weight, height, BMI and tobacco use), baseline disease and therapy characteristics [disease duration, number of previous csDMARDs (confounded with study), previous MTX use (confounded with study), glucocorticoid use and dose, DAS28 joint count, HAQ-DI, Routine Assessment of Patient Index Data 3 (RAPID3), CDAI, CRP, RF status, patient pain visual analogue scale, patient global health visual analogue scale, physician global health visual analogue scale, tender joint count, swollen joint count and ESR] and study medication characteristics [tocilizumab administered i.v. every 4 weeks or s.c. weekly or every 2 weeks (per study) and tocilizumab monotherapy or combination therapy with MTX or other csDMARDs]. RF status was not assessed in three studies (ROSE, ACT-STAR and ACT-SURE) and was available only in a minority of patients in ACT-RAY, HAQ-DI data were not assessed in two studies (ROSE and ACT-STAR) and BMI was not assessed in one study (ACT-SURE).
Outcomes of these pooled analyses were change from baseline to week 24 in CDAI and HAQ-DI and proportion of patients with ≥50% improvement in ACR criteria (ACR50 response) and CDAI remission (CDAI ≤2.8) rates at week 24.
Statistical analysis
Using a combination of clinically informed and mathematically driven variable-selection techniques, models were built to optimally fit and explain outcome variance. Mixed-model analysis of covariance (ANCOVA) with predictors as fixed effects and ‘study’ as a random effect to account for intracorrelation of observations within each study was used to model continuously distributed outcomes (week 24 score or change from baseline in CDAI and HAQ-DI) and estimate the least squares mean at week 24. A logistic regression approach was used to model binary outcomes (achievement or not of ACR50 and of CDAI remission at week 24), also using ‘study’ as a random effect in addition to the predictors. The effect of disease duration on outcomes was examined by estimating outcomes without any predictor, with RA duration at baseline as the only predictor and with RA duration plus additional baseline predictors. The effect of the baseline values of each continuously distributed outcome (i.e. HAQ-DI, CDAI) on the week 24 outcomes was explored by comparing the fit of models with only the baseline value of the outcome (i.e. unadjusted models) with those of models that also included other covariates (i.e. adjusted models).
Model fit was optimized by applying Bayes information criterion for ANCOVA and quasi-Akaike information criterion for logistic regression; models with the lowest Bayes information criterion and quasi-Akaike information criterion values were preferred. Explained outcome variance was operationalized by the residual variance method (ANCOVA) and Harrell’s C (logistic regression), respectively. To further determine the unique contribution of RA duration, models including only the baseline value of the outcome (if statistically significant; P < 0.05) were compared with those including only RA duration and those excluding all RA duration parameters. SAS version 9.2 (SAS Institute Inc, Cary, NC, USA) was used to perform all analyses.
Results
Baseline demographics and disease characteristics
In total, 5462 tocilizumab-treated patients from 12 studies (Table 1) were included in the statistical analyses: MTX-IR (n = 1099), csDMARD-IR (n = 4059), MTX-naïve or MTX-free for ≥6 months (n = 143), and MTX-intolerant (n = 161).
Baseline (i.e. before initiation of tocilizumab) demographics and patient and disease characteristics are presented according to RA duration categories in Table 2. Patients with longer disease duration were older and had been exposed to more csDMARDs than patients with shorter disease duration. Use of glucocorticoids was similar across the disease duration categories, with approximately half the patients using glucocorticoids at baseline. Patients with disease duration >5 years had a higher prevalence of RF positivity and slightly higher mean HAQ-DI scores than those with shorter disease duration. Three-quarters of the patients were from Europe and North America.
Table 2.
Baseline characteristics of tocilizumab-treated patients (N = 5462)a, classified according to RA duration
| Baseline characteristics | RA duration |
|||||
|---|---|---|---|---|---|---|
| <6 months, n = 44 | 6 months– 2 years, n = 1279 | >2–5 years, n = 1274 | >5–10 years, n = 1173 | >10 years, n = 1692 | All patients, n = 5462 | |
| Female, n (%) | 35 (79.5) | 982 (76.8) | 1027 (80.6) | 962 (82.0) | 1414 (83.6) | 4420 (80.9) |
| White, n (%) | 36 (81.8) | 1037 (81.1) | 1012 (79.4) | 901 (76.8) | 1381 (81.6) | 4367 (80.0) |
| Age, mean (s.d.), years | 51.1 (15.1) | 51.7 (13.2) | 51.5 (12.9) | 52.3 (12.4) | 56.7 (10.3) | 53.3 (12.3) |
| Region, n (%) | ||||||
| Europe | 21 (47.7) | 450 (35.2) | 508 (39.9) | 507 (43.2) | 670 (39.6) | 2156 (39.5) |
| North America | 21 (47.7) | 590 (46.1) | 451 (35.4) | 335 (28.6) | 558 (33.0) | 1955 (35.8) |
| South America | 2 (4.5) | 139 (10.9) | 196 (15.4) | 204 (17.4) | 229 (17.7) | 840 (15.4) |
| Rest of world | 0 | 100 (7.8) | 119 (9.3) | 127 (10.8) | 165 (9.8) | 511 (9.4) |
| BMI, mean (s.d.), kg/m2 | 28.7 (7.7) | 29.1 (7.1) | 28.3 (6.6) | 27.9 (6.4) | 27.4 (5.7) | 28.1 (6.4) |
| No. of previous csDMARDs, mean (s.d.) | 1.2 (0.5) | 1.4 (0.7) | 1.6 (0.8) | 1.7 (0.9) | 1.9 (1.1) | 1.7 (0.9) |
| Use of oral glucocorticoids, n (%) | 24 (54.5) | 656 (51.3) | 639 (50.2) | 628 (53.5) | 892 (52.7) | 2839 (52.0) |
| Oral glucocorticoid dose, mean (s.d.), mg/dayb | 7.3 (2.5) | 7.0 (3.1) | 6.7 (3.0) | 6.9 (4.6) | 6.7 (5.3) | 6.8 (4.2) |
| RF positive, n (%) | 19 (59.4) | 554 (64.4) | 638 (75.3) | 633 (80.5) | 955 (81.6) | 2779 (75.8) |
| CRP, mean (s.d.), mg/dl | 2.3 (2.6) | 2.0 (2.6) | 1.9 (2.5) | 2.1 (2.7) | 1.9 (2.3) | 2.0 (2.5) |
| HAQ-DI, mean (s.d.) | 1.4 (0.7) | 1.5 (0.6) | 1.5 (0.6) | 1.5 (0.6) | 1.6 (0.6) | 1.5 (0.6) |
| DAS28, mean (s.d.) | 6.3 (1.1) | 6.4 (1.1) | 6.4 (1.1) | 6.5 (1.0) | 6.4 (1.1) | 6.4 (1.1) |
| CDAI, mean (s.d.) | 37.5 (13.4) | 39.8 (13.7) | 39.0 (13.9) | 39.0 (13.3) | 39.4 (13.6) | 39.3 (13.6) |
RF positivity was not assessed in three studies (ROSE, ACT-STAR and ACT-SURE) and was only available in a minority of patients in ACT-RAY; HAQ-DI data were not assessed in two studies (ROSE and ACT-STAR); BMI was not assessed in one study (ACT-SURE). bIn prednisone equivalents. CDAI: Clinical Disease Activity Index; csDMARD: conventional synthetic DMARD; DAS28: DAS based on 28 joints; HAQ-DI: HAQ–Disability Index.
Effect of baseline characteristics on outcomes after tocilizumab treatment
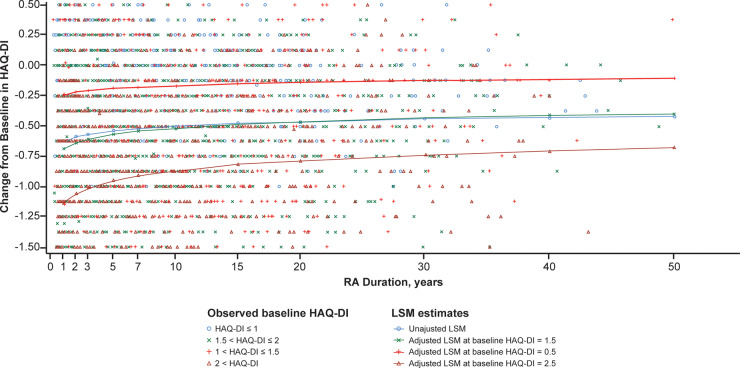
In all outcomes investigated, improvements were observed in patients receiving tocilizumab treatment (Figs. 1 and 2). Statistical modeling showed that from baseline to week 24, disease duration accounted for <2% of the variation in changes of HAQ-DI (Table 3, Fig. 1) and CDAI (Table 3, Fig. 2). The overall mean (s.d.) change from baseline to week 24 in HAQ-DI was –0.54 (2.07). The mean change decreased with increasing disease duration, indicating that patients with RA of longer duration experienced smaller improvements (Fig. 1). In the unadjusted model that only included baseline HAQ-DI as predictor, 15% of the variation in HAQ-DI change from baseline was explained. When comparing the adjusted models with and without RA duration, RA duration explained an estimated 1.7% of the variation in HAQ-DI change from baseline to week 24. Among the variables tested, variation in change from baseline to week 24 in HAQ-DI in the adjusted model was influenced, alone or in interaction terms, by baseline HAQ-DI, RA duration, CRP level, body weight, region, age and sex (Table 3).
Fig. 1.
ANCOVA for estimated mean change in HAQ-DI from baseline to week 24
ANCOVA: analysis of covariance; HAQ-DI: HAQ–Disability Index; LSM: least squares mean. HAQ-DI data were not assessed in two studies (ROSE and ACT-STAR).
Table 3.
ANCOVA results for changes from baseline to week 24 in HAQ-DI (N = 5064)a and CDAI (N = 5462)
| HAQ-DI |
CDAI |
||
|---|---|---|---|
| Estimate (95% CI), p | Estimate (95% CI), p | ||
| Base Model | |||
| BIC | 8912.0 | 44 424.3 | |
| Explained varianceb | 0.95% | 0.001% | |
| RA durationc | 0.05 (0.04, 0.07), P < 0.0001 | 0.17 (–0.18 to 0.53), P = 0.3421 | |
| Model with only baseline HAQ-DI and CDAI, respectively | |||
| BIC | 8177.9 | 42379.0 | |
| Explained varianceb | 14.97% | 31.72% | |
| Final model with RA duration and other predictors | |||
| BIC | 7810.0 | 41 268.9 | |
| Explained varianceb | 21.38% | 35.38% | |
| Main effects |
|
||
| Two-way interaction |
|
||
| Three-way interaction | RA duration c and age and sex |
|
|
| Final model with RA duration as variable removede | |||
| BIC | 7901.3 | 41 288.5 | |
| Explained varianceb | 19.7% | 35.2% | |
Patients treated with any biologic before enrolment were excluded. Missing values for HAQ-DI and CDAI were imputed using the last available postbaseline value. Variables shown in italics are statistically significant (P < 0.05). aHAQ-DI data were not assessed in two studies (ROSE and ACT-STAR). bAssessed using residual variance method. cTransformed by natural logarithm. dRegion is the location category of patients (North America, South America, Europe and rest of world). eModel with RA duration and all interaction terms with RA duration removed. ANCOVA: analysis of covariance; BIC: Bayes Information Criterion; CDAI: Clinical Disease Activity Index; csDMARD: conventional synthetic DMARD; HAQ-DI: HAQ–Disability Index; RAPID3: Routine Assessment of Patient Index Data 3.
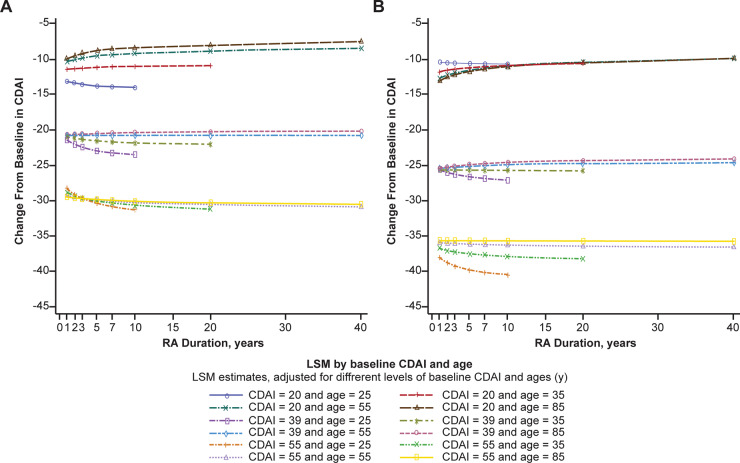
Fig. 2.
ANCOVA for change in CDAI from baseline to week 24 according to age and region
(A) North America and (B) Europe, South America and rest of the world. ANCOVA: analysis of covariance; CDAI: Clinical Disease Activity Index; LSM: least squares mean.
The overall mean (s.d.) change from baseline to week 24 in CDAI was –23.5 (33.3). The unadjusted model that included only baseline CDAI explained 32% of this change. In the adjusted model that included RA duration plus additional baseline predictors, the explained variance was 35.4%. In the model with RA duration and interactions involving RA duration removed, the explained variance was 35.2%, which means that disease duration accounted for only 0.2% additional variation in CDAI change from baseline to week 24. Among the variables tested, variation in change from baseline to week 24 in CDAI in the adjusted model was influenced significantly, alone or in interaction terms, by baseline CDAI, CRP level, RAPID3, RA duration, glucocorticoid use, number of previous csDMARDs, region, age, sex and body weight (Table 3, Fig. 2). This more comprehensive model explained only an additional 3.7% of variance in change of CDAI, compared with the model with only baseline CDAI.
In total, 11.5% (629/5462) of patients achieved CDAI remission by week 24. The odds of achieving CDAI remission of the adjusted model decreased by 15% for each additional 5 years of RA duration (odds ratio 0.85; 95% CI: 0.79, 0.91) (Table 4). Each additional 10 units of baseline CDAI score decreased the odds of achieving CDAI remission at week 24 by 22% (odds ratio 0.78; 95% CI: 0.71, 0.86) (Table 4). Among all variables tested, achievement of CDAI remission at week 24 in the adjusted model was influenced significantly, alone or in interaction terms, by baseline RA duration, CDAI, RAPID3, CRP levels, oral glucocorticoid use, number of previous csDMARDs, region, age, sex and body weight (Table 4).
Table 4.
Logistic regression analysis of CDAI remission and ACR50 response at week 24 (N = 5462)
| Adjusted model for CDAI remission | Odds ratio (95% CI)a |
|---|---|
| RA duration (per 5 years) | 0.85 (0.79, 0.91) |
| Baseline CDAI (per 10 score units) | 0.78 (0.71, 0.86) |
| Baseline CRP (per mg/dl) | 1.07 (1.04, 1.09) |
| Baseline RAPID3 (per unit score) | 0.90 (0.85, 0.95) |
| Region | |
| North America vs Europe | 0.65 (0.46, 0.91) |
| South America vs Europe | 0.64 (0.47, 0.89) |
| Rest of world vs Europe | 1.01 (0.77, 1.35) |
| Age (per decade) | 1.04 (0.98, 1.10) |
| Sex (male vs female) | 1.17 (1.05, 1.30) |
| Weight (per 10 kg) | 0.92 (0.89, 0.96) |
| No. of previous csDMARDs | 0.99 (0.87, 1.12) |
| Baseline oral glucocorticoids (yes vs no) | 0.94 (0.75, 1.17) |
| Adjusted model for ACR50 response | Odds ratio (95% CI)b |
|---|---|
| RA duration (doubling)c | 0.91 (0.87, 0.95) |
| Baseline DAS28 (per unit score) | 0.99 (0.92, 1.05) |
| Baseline CRP (per mg/dl) | 1.06 (1.04, 1.08) |
| Age (per decade) | 0.96 (0.92, 1.01) |
| Sex (male vs female) | 1.21 (1.06, 1.38) |
| Region | |
| North America vs Europe | 0.60 (0.53, 0.68) |
| Rest of worlddvs Europe | 1.07 (0.80, 1.42) |
| Weight (per 10 kg) | 0.93 (0.89, 0.97) |
| Previous csDMARDs, n | 0.94 (0.85, 1.05) |
| Baseline oral glucocorticoids (yes vs no) | 0.95 (0.86, 1.07) |
Patients treated with any biologic before enrolment were excluded. Missing week 24 values for CDAI remission and ACR50 were imputed as no CDAI remission and no ACR50 response, respectively. The adjusted logistic regression model for CDAI remission included RA duration as a fixed predictor and additional fixed baseline covariates of CDAI, sex, region, age, weight, number of previous csDMARDs, oral glucocorticoid use, CRP, RAPID3 and two-way interactions of RA duration with sex, age and number of previous csDMARDs, age with sex, sex with glucocorticoid use and glucocorticoid use with number of previous csDMARDs. Study was included as a random effect. The adjusted logistic regression model for ACR50 response included RA duration as a fixed predictor and additional fixed baseline covariates of DAS28, sex, region, age, weight, number of previous csDMARDs, oral glucocorticoid use, CRP and two-way interactions of RA duration with sex, weight and number of previous csDMARDs, age with sex, glucocorticoid use with number of previous csDMARDs and region with weight. Study was included as a random effect. aQuasi-AIC, 3628; Harrell’s C, 0.68. bQuasi-AIC, 7238; Harrell’s C, 0.62. cTransformed by natural logarithm. dRest of world includes South America. ACR50: ≥50% improvement in ACR criteria; AIC: Akaike information criterion; CDAI: Clinical Disease Activity Index; csDMARD: conventional synthetic DMARD; DAS28: DAS at 28 joints; RAPID3: Routine Assessment of Patient Index Data 3.
Overall, 43% (2370/5462) of patients achieved ACR50 response by week 24. Doubling the duration of RA decreased the odds of achieving ACR50 response at week 24 by 9.2% (odds ratio 0.91; 95% CI: 0.87, 0.95). Among all variables tested, achievement of ACR50 response at week 24 in the adjusted model was influenced significantly, alone or in interaction terms, by baseline RA duration, CRP levels, region (North America vs Europe), sex and weight (Table 4).
Discussion
This pooled analysis of data from 5462 biologic-naïve patients with established RA who initiated tocilizumab i.v. or s.c., either as monotherapy or in combination with csDMARDs, in 12 phase 3 or 4 clinical trials, is the first of its kind. It determined the effects of disease duration and of other baseline factors on clinical efficacy and HAQ-DI outcomes in patients with RA initiating tocilizumab. The effect of disease duration and early treatment initiation on outcomes has been investigated in observational, registry and retrospective studies [1–3, 7, 10–12, 29–32], as well as in meta-analyses [6, 33], generally showing a statistically significantly negative effect of disease duration on outcomes with RA therapies. However, our pooled analysis in a large population of clinical trials is more robust. It provided a unique opportunity to investigate to what extent disease duration and other baseline factors of inflammation and disease burden influence clinical and patient-reported outcomes of tocilizumab treatment. Statistical tests among large samples might yield statistically significant P-values for very small, clinically not relevant effects; therefore, P-values alone should not be used as a basis for scientific or clinical conclusions [34, 35]. By quantifying effects in our analysis, we were able to put weakly predictive factors that were ‘significant’ according to P-values into relevant clinical context and perspective. This provided the clinically important insight that, although longer disease duration had a statistically significantly negative effect, in line with the literature, on efficacy outcomes following tocilizumab treatment, outcomes were not heavily influenced by disease duration among patients with established RA. In our study, the odds ratios of achieving efficacy outcomes decreased slightly with RA of longer duration. In 4992 patients from a Norwegian registry treated with csDMARDs, relatively longer disease duration (>6 vs ≤6 months) was shown to decrease the odds of achieving CDAI remission by ∼20% [5]. In 3179 patients from the Consortium of Rheumatology Researchers of North America registry initiating TNF inhibitors, the odds of achieving CDAI remission decreased by 12% for every 5-year increase in disease duration [1]. A relatively short disease duration (<5 years) was shown to increase the odds of achieving Boolean remission by more than 2-fold in 123 patients with RA treated with tocilizumab, compared with a longer disease duration [2]. These modest decreases in remission rates of 12 and 20% reported with longer disease duration are similar to our result of 15%. The 2-fold (100%) increase for Boolean remission reported with shorter disease duration was a result from a small study in Japan [2].
It might be speculated that in multivariable analyses such as those performed in the current study, the effect of longer disease duration in patients with established RA would negate the effect of the number of csDMARDs used because the two are correlated. However, as has been shown in other studies [32], the number of csDMARDs used had an independent effect on treatment outcomes, likely reflecting the fact that the number of csDMARDs is a surrogate marker of difficult-to-treat RA [36].
Outcomes at week 24 were more heavily influenced by baseline values of HAQ-DI and CDAI in our analysis, showing that greater disability and higher disease activity at baseline were associated with larger changes in week 24 outcomes. Our findings contrast with those of other studies which showed that higher levels of certain disease activity measures at baseline were negatively associated with treatment outcomes in patients with RA [31]. A possible explanation could be that all studies, except one, included in our analysis enrolled biologic-naïve patients with inadequate response to MTX or other csDMARDs and that therefore, in our study population, high baseline values are indicative of patients with greater potential to achieve an improvement in disease activity on their first biologic.
Limitations of study
Several study limitations should be considered when drawing conclusions from these results. For most but not all of the included studies, RA disease duration of ≥6 months was an eligibility criterion; therefore, the findings of these analyses may not precisely characterize shorter disease durations. Although heterogeneity in our study was minimized by limiting the analyses primarily to patients who had received similar treatment (MTX/csDMARDs-IR with tocilizumab as first-line biologic), the pooled patient population might not have been completely homogeneous with respect to disease state. This might have contributed to unexplained variation in outcomes. Because csDMARD-naïve patients were excluded to minimize heterogeneity, the findings and conclusions of this study are most relevant primarily to csDMARDs-IR patients with established RA. In addition, given that our analyses included only patients with established RA from tocilizumab groups of clinical trials and not comparator groups, the findings may not be generalizable to other therapies. Although several potential predictors were considered in the models, it is possible that some variability might be predicted by other factors that were not assessed in most of the trials (e.g. other proteins, peptides, inflammatory markers, subjective factors). Not all variables had been assessed in the 12 trials. RF status was not included in the models because it was not available in three studies and only available in a minority of patients in another study. However, in the eight studies that consistently assessed RF positivity, it showed a modest interaction with RA duration on CDAI and HAQ change models, reducing the effect of disease duration even more. Finally, the models used were those that best fitted the data and were not necessarily the models that would generalize precisely across other data sets in terms of variance explained or of clinical practice.
In conclusion, tocilizumab treatment outcomes from clinical trials were not heavily influenced by disease duration, either alone or in combination with other baseline characteristics, in patients with established RA. Rather, baseline values of outcome variables were stronger predictors of outcome variables. Additional studies may be warranted to further elucidate the potential of other patient and disease factors to predict responses to tocilizumab and other medications.
Supplementary Material
Acknowledgements
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. A.R.-R. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. A.R.-R., D.A., J.D., P.N.S., Y.L., M.D.E. and J.W.G.J. were responsible for study conception and design. D.A., J.D., P.N.S. and M.D.E. were responsible for acquisition of data. A.R.-R., D.A., J.D., P.N.S., Y.L. and M.D.E. were responsible for analysis and interpretation of data. Medical writing assistance was provided by Maxwell Chang, MSc, Jacqueline Kolston, PhD, and Sara Duggan, PhD, of ApotheCom, on behalf of F. Hoffmann-La Roche Ltd.
Funding: This work was sponsored by F. Hoffmann-La Roche Ltd. The sponsor was involved in the study design; the collection, analysis and interpretation of data; writing the manuscript; and the decision to submit the manuscript for publication.
Disclosure statement: A.R.-R. reports personal fees and honoraria from Roche for lectures and consultations. D.A. reports personal fees from AbbVie, Merck, Union Chimique Belge (UCB), Janssen, Bristol Myers Squibb (BMS), Pfizer, Medac, Roche and Merck Sharp & Dohme (MSD), and grants from MSD, BMS and AbbVie outside the submitted work. J.D. reports employment with Roche. P.N.S. reports employment with and stock ownership in Genentech, a member of the Roche group. Y.L. reports employment with F. Hoffmann-La Roche. M.D.E. reports employment with Everest, which has received consulting fees from Genentech. J.W.G.J. reports reimbursements from Roche Nederland BV for patients included in the UActEarly study.
Data availability statement
Qualified researchers may request access to data through the clinical study data request platform (www.clinicalstudydatarequest.com). Further details on Roche’s criteria for eligible studies are available here: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx. For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Furst DE, Pangan AL, Harrold LR. et al. Greater likelihood of remission in rheumatoid arthritis patients treated earlier in the disease course: results from the Consortium of Rheumatology Researchers of North America registry. Arthritis Care Res (Hoboken) 2011;63:856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kojima T, Kaneko A, Hirano Y. et al. Early aggressive intervention with tocilizumab for rheumatoid arthritis increases remission rate defined using a Boolean approach in clinical practice. Mod Rheumatol 2012;22:370–5. [DOI] [PubMed] [Google Scholar]
- 3. Gremese E, Salaffi F, Bosello SL. et al. Very early rheumatoid arthritis as a predictor of remission: a multicentre real life prospective study. Ann Rheum Dis 2013;72:858–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoshi D, Nakajima A, Shidara K. et al. Disability is the major negative predictor for achievement of Boolean-based remission in patients with rheumatoid arthritis treated with tocilizumab. Mod Rheumatol 2013;23:1205–10. [DOI] [PubMed] [Google Scholar]
- 5. Uhlig T, Lie E, Norvang V. et al. Achievement of remission and low disease activity definitions in patients with rheumatoid arthritis in clinical practice: results from the NOR-DMARD study. J Rheumatol 2016;43:716–23. [DOI] [PubMed] [Google Scholar]
- 6. Hamann P, Holland R, Hyrich K. et al. Factors associated with sustained remission in rheumatoid arthritis in patients treated with anti-tumor necrosis factor. Arthritis Care Res (Hoboken) 2017;69:783–93. [DOI] [PubMed] [Google Scholar]
- 7. Harrold LR, Litman HJ, Connolly SE. et al. A window of opportunity for abatacept in RA: is disease duration an independent predictor of low disease activity/remission in clinical practice? Clin Rheumatol 2017;36:1215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miwa Y, Takahashi R, Ikari Y. et al. Clinical characteristics of rheumatoid arthritis patients achieving functional remission with six months of biological DMARDs treatment. Intern Med 2017;56:903–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hattori Y, Kida D, Kaneko A.. Comparison of physical function in early rheumatoid arthritis patients treated with biologics for 1 year who achieved clinical remission or low disease activity. Clin Rheumatol 2017;36:2607–12. [DOI] [PubMed] [Google Scholar]
- 10. Murakami K, Sekiguchi M, Hirata S. et al. Predictive factors for structural remission using abatacept: results from the ABROAD study. Mod Rheumatol 2019;29:406–12. [DOI] [PubMed] [Google Scholar]
- 11. Einarsson JT, Willim M, Ernestam S. et al. Prevalence of sustained remission in rheumatoid arthritis: impact of criteria sets and disease duration, a Nationwide Study in Sweden. Rheumatology (Oxford) 2019;58:227–36. [DOI] [PubMed] [Google Scholar]
- 12. Anderson JJ, Wells G, Verhoeven AC, Felson DT.. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum 2000;43:22–9. [DOI] [PubMed] [Google Scholar]
- 13. Lee KE, Choi SE, Xu H. et al. HAQ score is an independent predictor of sustained remission in patients with rheumatoid arthritis. Rheumatol Int 2017;37:2027–34. [DOI] [PubMed] [Google Scholar]
- 14. Genovese MC, Rubbert-Roth A, Smolen JS. et al. Longterm safety and efficacy of tocilizumab in patients with rheumatoid arthritis: a cumulative analysis of up to 4.6 years of exposure. J Rheumatol 2013;40:768–80. [DOI] [PubMed] [Google Scholar]
- 15. Kivitz A, Wallace T, Olech E. et al. Long-term safety and efficacy of subcutaneously administered tocilizumab for adult rheumatoid arthritis: a multicenter phase 3b long-term extension study. Rheumatol Ther 2016;3:291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bykerk VP, stör AJK, Alvaro-Gracia J. et al. Long-term safety and effectiveness of tocilizumab in patients with rheumatoid arthritis and inadequate responses to csDMARDs and/or TNF inhibitors: an open-label study close to clinical practice. Clin Rheumatol 2019;38:2411–21. [DOI] [PubMed] [Google Scholar]
- 17. Dougados M, Kissel K, Conaghan PG. et al. Clinical, radiographic, and immunogenic effects after 1 year of tocilizumab based treatment strategy with and without methotrexate in rheumatoid arthritis: the ACT RAY study. Ann Rheum Dis 2013;71:185.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weinblatt ME, Kremer J, Cush J. et al. Tocilizumab as monotherapy or in combination with nonbiologic disease-modifying antirheumatic drugs: twenty-four-week results of an open-label, clinical practice study. Arthritis Care Res (Hoboken) 2013;65:362–71. [DOI] [PubMed] [Google Scholar]
- 19. Bykerk VP, stör AJ, Alvaro-Gracia J. et al. Tocilizumab in patients with active rheumatoid arthritis and inadequate responses to DMARDs and/or TNF inhibitors: a large, open-label study close to clinical practice. Ann Rheum Dis 2012;71:1950–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gabay C, Emery P, van Vollenhoven R et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. The Lancet 2013;381:1541–50. 10.1016/S0140-6736(13)60250-0 [DOI] [PubMed] [Google Scholar]
- 21. Jones G, Gu JR, Lowenstein M. et al. Tocilizumab monotherapy is superior to methotrexate monotherapy in reducing disease activity in patients with rheumatoid arthritis: the AMBITION study. Ann Rheum Dis 2010;69:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kivitz A, Olech E, Borofsky M. et al. Subcutaneous tocilizumab versus placebo in combination with disease modifying antirheumatic drugs in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2014;66:1653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kremer JM, Rigby W, Singer NG. et al. Sustained response following discontinuation of methotrexate in patients with rheumatoid arthritis treated with subcutaneous tocilizumab: results from a randomized, controlled trial. Arthritis Rheumatol 2018;70:1200–8. [DOI] [PubMed] [Google Scholar]
- 24. Kremer JM, Blanco R, Brzosko S. et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum 2011;63:609–21. [DOI] [PubMed] [Google Scholar]
- 25. Smolen JS, Beaulieu A, Rubbert-Roth A. et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 2008;371:987–97. [DOI] [PubMed] [Google Scholar]
- 26. Yazici Y, Curtis JR, Ince A. et al. Efficacy of tocilizumab in patients with moderate to severe active rheumatoid arthritis and a previous inadequate response to disease-modifying antirheumatic drugs: the ROSE study. Ann Rheum Dis 2012;71:198–205. [DOI] [PubMed] [Google Scholar]
- 27. Burmester GR, Rubbert-Roth A, Cantagrel A. et al. A randomised, double-blind, parallel-group study of the safety and efficacy of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional disease-modifying antirheumatic drugs in patients with moderate to severe rheumatoid arthritis (SUMMACTA study). Ann Rheum Dis 2014;73:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Genovese MC, McKay JD, Nasonov EL. et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum 2008;58:2968–80. [DOI] [PubMed] [Google Scholar]
- 29. Lard LR, Visser H, Speyer I. et al. Early versus delayed treatment in patients with recent-onset rheumatoid arthritis: comparison of two cohorts who received different treatment strategies. Am J Med 2001;111:446–51. [DOI] [PubMed] [Google Scholar]
- 30. Molina E, Del Rincon I, Restrepo JF, Battafarano DF, Escalante A.. Association of socioeconomic status with treatment delays, disease activity, joint damage, and disability in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2015;67:940–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Narváez J, Magallares B, Diaz Torne C. et al. Predictive factors for induction of remission in patients with active rheumatoid arthritis treated with tocilizumab in clinical practice. Semin Arthritis Rheum 2016;45:386–90. [DOI] [PubMed] [Google Scholar]
- 32. Aletaha D, Maa JF, Chen S. et al. Effect of disease duration and prior disease-modifying antirheumatic drug use on treatment outcomes in patients with rheumatoid arthritis. Ann Rheum Dis 2019;78:1609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Nies JA, Krabben A, Schoones JW. et al. What is the evidence for the presence of a therapeutic window of opportunity in rheumatoid arthritis? A systematic literature review. Ann Rheum Dis 2014;73:861–70. [DOI] [PubMed] [Google Scholar]
- 34. Wasserstein RL, Lazar NA.. The ASA statement on p-values: context, process, and purpose. Am Stat 2016;70:129–33. [Google Scholar]
- 35. Kuffner TA, Walker SG.. Why are p-values controversial? Am Stat 2019;73:1–3. [Google Scholar]
- 36. de Hair MJH, Jacobs JWG, Schoneveld JLM, van Laar JM.. Difficult-to-treat rheumatoid arthritis: an area of unmet clinical need. Rheumatology (Oxford) 2018;57:1135–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.