Abstract
Background
Head and neck squamous cell carcinoma (HNSCC) affects health-related quality of life (HRQoL); few treatments have demonstrated clinically meaningful HRQoL benefit. KEYNOTE-040 evaluated pembrolizumab vs standard of care (SOC) in patients with recurrent and/or metastatic HNSCC whose disease recurred or progressed after platinum-containing regimen.
Methods
Patients received pembrolizumab 200 mg or SOC (methotrexate, docetaxel, or cetuximab). Exploratory HRQoL analyses used European Organisation for Research and Treatment of Cancer (EORTC) 30 quality-of-life, EORTC 35-question quality-of-life head and neck cancer-specific module, and EuroQoL 5-dimensions questionnaires.
Results
The HRQoL population comprised 469 patients (pembrolizumab = 241, SOC = 228). HRQoL compliance for patients in the study at week 15 was 75.3% (116 of 154) for pembrolizumab and 74.6% (85 of 114) for SOC. The median time to deterioration in global health status (GHS) and QoL scores were 4.8 months with pembrolizumab and 2.8 months with SOC (hazard ratio = 0.79, 95% confidence interval [CI] = 0.59 to 1.05). At week 15, GHS / QoL scores were stable for pembrolizumab (least squares mean [LSM] = 0.39, 95% CI = –3.00 to 3.78) but worsened for SOC (LSM = −5.86, 95% CI = −9.68 to −2.04); the LSM between-group difference was 6.25 points (95% CI = 1.32 to 11.18; nominal 2-sided P = .01). A greater difference in the LSM for GHS / QoL score occurred with pembrolizumab vs docetaxel (10.23, 95% CI = 3.15 to 17.30) compared with pembrolizumab vs methotrexate (6.21, 95% CI = −4.57 to 16.99) or pembrolizumab vs cetuximab (−1.44, 95% CI = −11.43 to 8.56). Pembrolizumab-treated patients had stable functioning and symptoms at week 15, with no notable differences from SOC.
Conclusions
GHS / QoL scores were stable with pembrolizumab but declined with SOC in patients at week 15, supporting the clinically meaningful benefit of pembrolizumab in recurrent and/or metastatic HNSCC.
Health-related quality of life (HRQoL) is a critical component of measuring patients’ overall health status (1). Head and neck squamous cell carcinoma (HNSCC), occurring in structurally complex and functionally important areas, can profoundly affect patients’ social interactions and psychological well-being, compounding their cancer symptoms of pain and fatigue and resulting in diminished HRQoL (2,3). Additionally, prognosis is poor for patients with recurrent and/or metastatic (RM) HNSCC. Until recently, systemic treatment options for platinum-refractory RM HNSCC were limited to single-agent chemotherapy or cetuximab, with median overall survival (OS) of 6 months or less (4–9). Although HRQoL is an independent prognostic factor of OS in RM HNSCC, it has been assessed in few clinical trials, and few treatments have demonstrated clinically meaningful HRQoL benefit (9–14). Thus, therapies that prolong OS while preserving HRQoL are needed (12,15).
Targeting the programmed death 1 (PD-1) receptor, using pembrolizumab or nivolumab, has demonstrated clinical benefit in patients with RM HNSCC (16–20). Nivolumab prolonged OS over investigator’s choice of standard of care (SOC) therapy while maintaining HRQoL from baseline to weeks 9 and 15 in platinum-refractory RM HNSCC; however, low compliance at later time points limited HRQoL analyses beyond week 15 (16,17).
In KEYNOTE-040, pembrolizumab prolonged OS versus investigator’s choice of SOC (hazard ratio = 0.80, 95% confidence interval [CI] = 0.65 to 0.98; nominal 1-sided P = .016), while resulting in fewer treatment-related adverse events in patients with platinum-refractory RM HNSCC (20). Results of prespecified exploratory HRQoL analyses of KEYNOTE-040 are presented here.
Methods
Study Design and Treatment
KEYNOTE-040 (ClinicalTrials.gov, NCT02252042) is a randomized phase III trial evaluating pembrolizumab versus SOC in patients with RM HNSCC that progressed during or after platinum-containing treatment (20). In brief, patients were randomly allocated (1:1) to pembrolizumab or SOC (methotrexate, docetaxel, or cetuximab). Investigators chose 1 of these 3 drugs based on product characteristics and in accordance with local guidelines before patients were randomly assigned to treatment. Study protocol and amendments were approved by appropriate ethics review committees, and the study was conducted in accordance with the Declaration of Helsinki.
Patients
Detailed eligibility criteria for the KEYNOTE-040 trial are published (20). Patients with RM HNSCC of the oral cavity, oropharynx, hypopharynx, or larynx who had platinum-refractory disease were eligible. All patients provided written informed consent.
HRQoL Assessments
HRQoL data were collected at baseline; weeks 3, 6, and 9; every 6 weeks thereafter up to 1 year (51 weeks) or end of treatment (whichever came first); and at the 30-day safety follow-up. At each visit, 3 validated HRQoL instruments were administered before all other study procedures: 3-level version of the EuroQoL 5-dimensions questionnaire(EQ-5D), European Organisation for Research and Treatment of Cancer core 30 quality-of-life questionnaire (EORTC QLQ-C30), and EORTC 35-question head and neck cancer-specific module (EORTC QLQ-H&N35) (21–23). Additional details on HRQoL instruments and scoring are provided in the supplement.
Key HRQoL analyses assessed time to deterioration (TTD) and mean change from baseline in individual scores of EORTC QLQ-C30, EORTC QLQ-H&N35, and EQ-5D. Deterioration in all scales of both EORTC questionnaires was defined as at least a 10-point decline from baseline in HRQoL scores. Changes from baseline in EORTC QLQ-C30 scores were interpreted according to recent subscale-specific guidelines, which indicate that clinically meaningful differences vary by scale; a mean difference of 5 to 10 points was defined as a small but clinically meaningful change in global health status (GHS) and QoL scores (24–26). For the EQ-5D, deterioration was defined as a decline from baseline of at least 0.08 in the utility index and a decline from baseline of at least 7 on the EQ-5D visual analog scale (27).
Statistical Analysis
No formal power calculations were performed for these exploratory outcomes. The overall HRQoL analysis population included all patients who received at least 1 dose of study therapy and completed at least 1 HRQoL assessment. Compliance was defined as the proportion that completed at least 1 HRQoL assessment among those expected to complete the instruments at each visit (excluding patients who discontinued treatment). Completion was defined as the proportion that completed at least 1 HRQoL assessment among the overall HRQoL analysis population.
The median TTD of individual EORTC QLQ-C30, EORTC QLQ-H&N35, and EQ-5D scores was estimated using Kaplan–Meier analysis. HRs were estimated using a stratified (by Eastern Cooperative Oncology Group performance status [ECOG PS], human papillomavirus infection status, and programmed death ligand 1 [PD-L1] expression status) Cox proportional hazards model. Consistent with current recommendations (17,28), deterioration was applied at the individual patient level; confirmation was not required at a subsequent visit, deaths were not included as events, and patients ongoing or discontinued from the study without deterioration were censored at the last assessment.
Treatment effect of change from baseline in the EORTC QLQ-C30, QLQ-H&N35, and EQ-5D scores was evaluated primarily at week 15, selected because a low completion rate based on disease progression was expected after week 15 for the SOC group. Change in least squares mean (LSM) score from baseline to week 15 was assessed using a constrained longitudinal data analysis (cLDA) model, with HRQoL score as the response variable and treatment-by-time interaction and trial stratification factors as covariates (29,30). The cLDA model implicitly treats missing data as missing at random, although they could be missing not at random given that increased patient attrition occurs because of disease progression or death. Therefore, to compare the estimated treatment differences for the EORTC QLQ-C30 GHS and QoL scores with the cLDA model results, 5 sensitivity analyses using more conservative assumptions on missing data were performed according to the control-based mean imputation method (31) and 2 rank-based nonparametric methods (Wilcoxon rank-sum test and aligned rank test) with 2 imputation strategies for missing data (32,33). Details on the cLDA methodology and each of the sensitivity analysis methods are provided in the Supplementary Methods (available online). For all statistical analyses, nominal 2-sided P values were reported with statistical significancetesting at the .05 level.
To further analyze trends observed at week 15, descriptive analyses of mean change from baseline (and 95% CIs) in GHS and QoL scores were summarized for patients who remained in the studyand completed questionnaires at each time point through week 51. Additionally, LSM change from baseline in GHS and QoL scores according to progressive disease status was evaluated for patients in the study at week 15 and compared between groups to assess the association of response to therapy on GHS and QoL scores. Subgroup analyses of LSM change from baseline according to investigator’s choice of SOC therapy and PD-L1 biomarker expression (combined positive score [CPS] ≥1 vs CPS <1 and tumor proportion score [TPS] ≥50% vs TPS <50%) in EORTC QLQ-C30, QLQ-H&N35, and EQ-5D scores were performed to further assess potential factors associated with HRQoL outcomes.
Descriptive analyses of postbaseline EORTC QLQ-C30 and EORTC QLQ-H&N35 scores at week 15 were classified as improved, stable, or deteriorated based on at least a 10-point change relative to baseline and were summarized using numbers and proportions. Proportions were calculated based on multiply imputed datasets assuming missing at random and then synthesized based on Rubin’s rule.
The data cutoff date was May 15, 2017 (final analysis).
Results
HRQoL Instrument Completion and Compliance
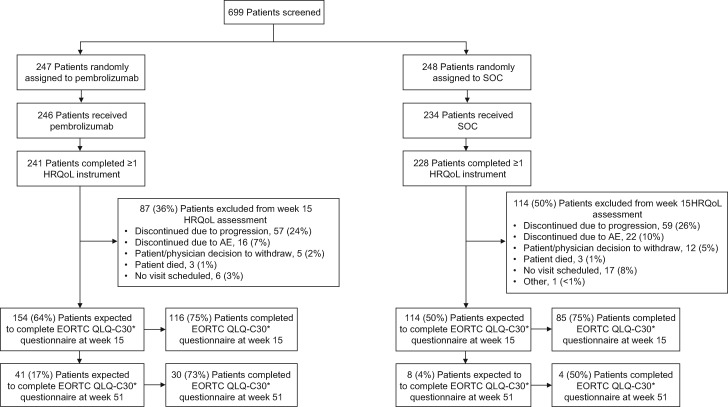
Of 495 patients enrolled, the overall HRQoL population included 469 patients (94.7%: pembrolizumab, N = 241; SOC, N = 228) (Figure 1). Median duration of follow-up was 7.5 months (interquartile range, 3.4 to 13.3) (20). EORTC QLQ-C30 compliance rates were 95.9% (231 of 241) for pembrolizumab and 94.3% (215 of 228) for SOC at baseline and 75.3% (116 of 154) for pembrolizumab and 74.6% (85 of 114) for SOC for patients in the study at week 15 (Supplementary Table 1, available online). Completion rates decreased at week 15 based on treatment discontinuation because of disease progression, intolerable toxicity, physician or patient decision to withdraw, or death (Figure 1). At week 15, the EORTC QLQ-C30 completion rate was 48.1% with pembrolizumab and 37.3% with SOC (Supplementary Table 1, available online). EORTC QLQ-H&N35 and EQ-5D compliance and completion rates were similar to those observed for EORTC QLQ-C30.
Figure 1.
CONSORT diagram. *European Organisation for Research and Treatment of Cancer 35-question quality of life head and neck cancer-specific module and EuroQoL 5-dimensions compliance rates were nearly identical to those observed for European Organisation for Research and Treatment of Cancer scale 30 quality-of-life questionnaire (EORTC QLQ-C30). AE = adverse event; HRQoL = health-related quality of life; SOC = standard of care.
Baseline Characteristics of the HRQoL Population
Baseline characteristics were generally balanced between treatment groups (20); baseline characteristics of the HRQoL population followed the same trend overall and at week 15 with certain exceptions (Table 1). At week 15, imbalances across treatment groups in the distribution of the investigator’s choice of methotrexate and cetuximab were seen because of the proportionately higher dropout for patients on methotrexate. In contrast, the proportion of patients assigned to SOC of docetaxel remained balanced at week 15. Relative to the overall HRQoL population, a slightly higher proportion of patients at week 15 had a PD-L1 TPS of at least 50% and a CPS greater than or equal to 1 status in the pembrolizumab group and an ECOG PS of 0 in both groups; between-group comparisons of the HRQoL scores incorporated stratification for PD-L1 and ECOG PS to address these imbalances.
Table 1.
Baseline characteristics of the overall HRQoL populationa and the HRQoL population at week 15b
| Characteristic | Overall HRQoL populationa |
HRQoL population at week 15b |
||
|---|---|---|---|---|
| Pembrolizumab | SOC | Pembrolizumab | SOC | |
| N = 241 | N = 228 | N = 116 | N = 85 | |
| Age, median (range), y | 60.0 (19-85) | 60.0 (34-78) | 60.5 (31-85) | 60.0 (36-78) |
| Sex, No. (%) | ||||
| Male | 204 (84.6) | 188 (82.5) | 96 (82.8) | 65 (76.5) |
| Female | 37 (15.4) | 40(17.5) | 20 (17.2) | 20 (23.5) |
| Race, No. (%) | ||||
| American Indian or Alaska Native | 2 (0.8) | 0 (0) | 2 (1.7) | 0 (0) |
| Black or African American | 3 (1.2) | 7 (3.1) | 1 (0.9) | 5 (5.9) |
| White | 201 (83.4) | 189 (82.9) | 98 (84.5) | 68 (80.0) |
| Asian | 15 (6.2) | 15 (6.6) | 6 (5.2) | 6 (7.1) |
| Multiracial | 4 (1.7) | 3 (1.3) | 1 (0.9) | 0 (0) |
| Unknown | 16 (6.6) | 14 (6.1) | 8 (6.9) | 6 (7.1) |
| Ethnicity, No. (%) | ||||
| Hispanic or Latino | 20 (8.3) | 12 (5.3) | 11 (9.5) | 3 (3.5) |
| Not Hispanic or Latino | 179 (74.3) | 178 (78.1) | 81 (69.8) | 68 (80.0) |
| Not reported or unknown | 42 (17.4) | 38 (16.7) | 11 (9.5) | 5 (5.9) |
| Region, No. (%) | ||||
| Europe | 142 (58.9) | 144 (63.2) | 71 (61.2) | 54 (63.5) |
| North America | 72 (29.9) | 54 (23.7) | 31 (26.7) | 17 (20.0) |
| Rest of world | 27 (11.2) | 30 (13.2) | 14 (12.1) | 14 (16.5) |
| ECOG PS, No. (%) | ||||
| 0 | 71 (29.5) | 63 (27.6) | 48 (41.4) | 32 (37.6) |
| 1 | 170 (70.5) | 165 (72.4) | 68 (58.6) | 53 (62.4) |
| Smoking status, No. (%) | ||||
| Never smoked | 67 (27.8) | 58 (25.4) | 26 (22.4) | 21 (24.7) |
| Former smoker | 143 (59.3) | 136 (59.6) | 72 (62.1) | 50 (58.8) |
| Current smoker | 31 (12.9) | 34 (14.9) | 18 (15.5) | 14 (16.5) |
| HPV status, No. (%) | ||||
| Positive | 58 (24.1) | 51 (22.4) | 24 (20.7) | 16 (18.8) |
| Negative | 183 (75.9) | 177 (77.6) | 92 (79.3) | 69 (81.2) |
| PD-L1 TPS status, No. (%) | ||||
| 0% | 100 (41.5) | 85 (37.3) | 45 (38.8) | 36 (42.4) |
| 1% ≤ TPS < 50% | 76 (31.5) | 83 (36.4) | 35 (30.2) | 29 (34.1) |
| ≥50% | 64 (26.6) | 57 (25.0) | 35 (30.2) | 19 (22.4) |
| Missing | 1 (0.4) | 3 (1.3) | 1 (0.9) | 1 (1.2) |
| PD-L1 CPS status, No. (%) | ||||
| <1 | 48 (19.9) | 48 (21.1) | 16 (13.8) | 19 (22.4) |
| ≥1 | 192 (79.7) | 177 (77.6) | 99 (85.3) | 65 (76.5) |
| Missing | 1 (0.4) | 3 (1.3) | 1 (0.9) | 1 (1.2) |
| Current disease overall stage, No. (%) | ||||
| II | 5 (2.1) | 7 (3.1) | 3 (2.6) | 4 (4.7) |
| III | 9 (3.7) | 16 (7.0) | 8 (6.9) | 9 (10.6) |
| IV | 82 (34.0) | 69 (30.3) | 32 (27.6) | 28 (32.9) |
| IV A | 22 (9.1) | 28 (12.3) | 11 (9.5) | 8 (9.4) |
| IV B | 11 (4.6) | 12 (5.3) | 8 (6.9) | 3 (3.5) |
| IV C | 112 (46.5) | 96 (42.1) | 54 (46.6) | 33 (38.8) |
| Investigator’s choice of SOC before randomization, c No. (%) | ||||
| Methotrexate | 70 (29.0) | 63 (27.6) | 35 (30.2) | 15 (17.6) |
| Docetaxel | 118 (49.0) | 95 (41.7) | 51 (44.0) | 39 (45.9) |
| Cetuximab | 53 (22.0) | 70 (30.7) | 30 (25.9) | 31 (36.5) |
| Setting of previous systemic therapy, No. (%) | ||||
| Adjuvant, neoadjuvant, or definitive | 33 (13.7) | 38 (16.7) | 22 (19.0) | 20 (23.5) |
| First line | 138 (57.3) | 130 (57.0) | 64 (55.2) | 43 (50.6) |
| Second line | 67 (27.8) | 58 (25.4) | 28 (24.1) | 21 (24.7) |
| Third line | 3 (1.2) | 2 (0.9) | 2 (1.7) | 1 (1.2) |
| Most recent oncologic radiation, No. (%) | ||||
| Neoadjuvant | 22 (9.1) | 29 (12.7) | 12 (10.3) | 12 (14.1) |
| Adjuvant | 120 (49.8) | 118 (51.8) | 53 (45.7) | 42 (49.4) |
| In combination with first-line treatment | 29 (12.0) | 15 (6.6) | 14 (12.1) | 10 (11.8) |
| In combination with second-line treatment | 3 (1.2) | 3 (1.3) | 3 (2.6) | 1 (1.2) |
| Control of metastatic or recurrent disease or refractory | 13 (5.4) | 10 (4.4) | 7 (6.0) | 1 (1.2) |
| Palliative treatment or symptom control | 25 (10.4) | 19 (8.3) | 12 (10.3) | 5 (5.9) |
| No radiation | 29 (12.0) | 34 (14.9) | 15 (12.9) | 14 (16.5) |
| HRQoL score | ||||
| EORTC QLQ-C30 GHS and QoL, mean (SD) | 56.02d (21.24) | 55.81d (21.63) | 62.03 (20.66) | 59.18 (19.59) |
The overall health-related quality of life (HRQoL) analysis population included all patients who received at least 1 dose of study treatment and completed at least 1 HRQoL assessment. CPS = combined positive score; ECOG PS = Eastern Cooperative Oncology Group performance status; EORTC QLQ-C30 = European Organisation for Research and Treatment of Cancer core 30 quality-of-life questionnaire; GHS and QoL = global health status and quality of life; HPV = human papillomavirus; PD-L1 = programmed death ligand 1; SOC = standard of care; TPS = tumor proportion score.
The HRQoL population at week 15 included all patients who received at least 1 dose of study treatment and completed the HRQoL assessment at week 15.
Investigators chose between methotrexate, docetaxel, and cetuximab based on product characteristics and according to local guidelines before patients were randomly assigned to receive pembrolizumab or SOC. For patients randomly assigned to SOC, the investigator’s choice of SOC also reflected the regimen assigned and delivered.
Mean scores are reported in the HRQoL population, who completed the EORTC QLQ-C30 questionnaire at baseline; n = 231 for pembrolizumab and n = 215 for SOC.
Baseline (mean [SD]) GHS and QoL scores of EORTC QLQ-C30 were similar for pembrolizumab (56.0 [21.2]) and SOC (55.8 [21.6]) in the overall HRQoL population. For the HRQoL population at week 15, baseline mean GHS and QoL scores appeared slightly higher for pembrolizumab (62.0 [20.66]) than for SOC (59.18 [19.59]) and the overall HRQoL population. Thus, sensitivity analyses in the between-group comparisons of the GHS and QoL scores were applied to vary assumptions on the missing GHS and QoL scores at week 15 (Supplementary Table 2, available online).
TTD in HRQoL Scores
Median TTD in the GHS and QoL scores were 4.8 months with pembrolizumab and 2.8 months with SOC (Figure 2), resulting in a trend toward prolonged TTD with pembrolizumab versus SOC (hazard ratio = 0.79, 95% CI = 0.59 to 1.05; nominal 2-sided P = .10). Although few clinically meaningful differences occurred in TTD across individual EORTC QLQ-C30, QLQ-H&N35, and EQ-5D scales, with few exceptions trends in longer TTD tended to favor pembrolizumab (Supplementary Figure 1, available online).
Figure 2.
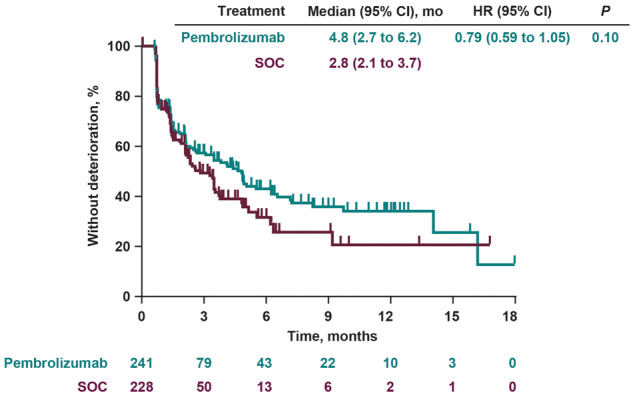
Time to deterioration in the European Organisation for Research and Treatment of Cancer scale 30 quality-of-life questionnaire global health status and quality of life scores. The nominal 2-sided P value was calculated using a log-rank test. CI = confidence interval; HR = hazard ratio; SOC = standard of care.
Change From Baseline in HRQoL Scores
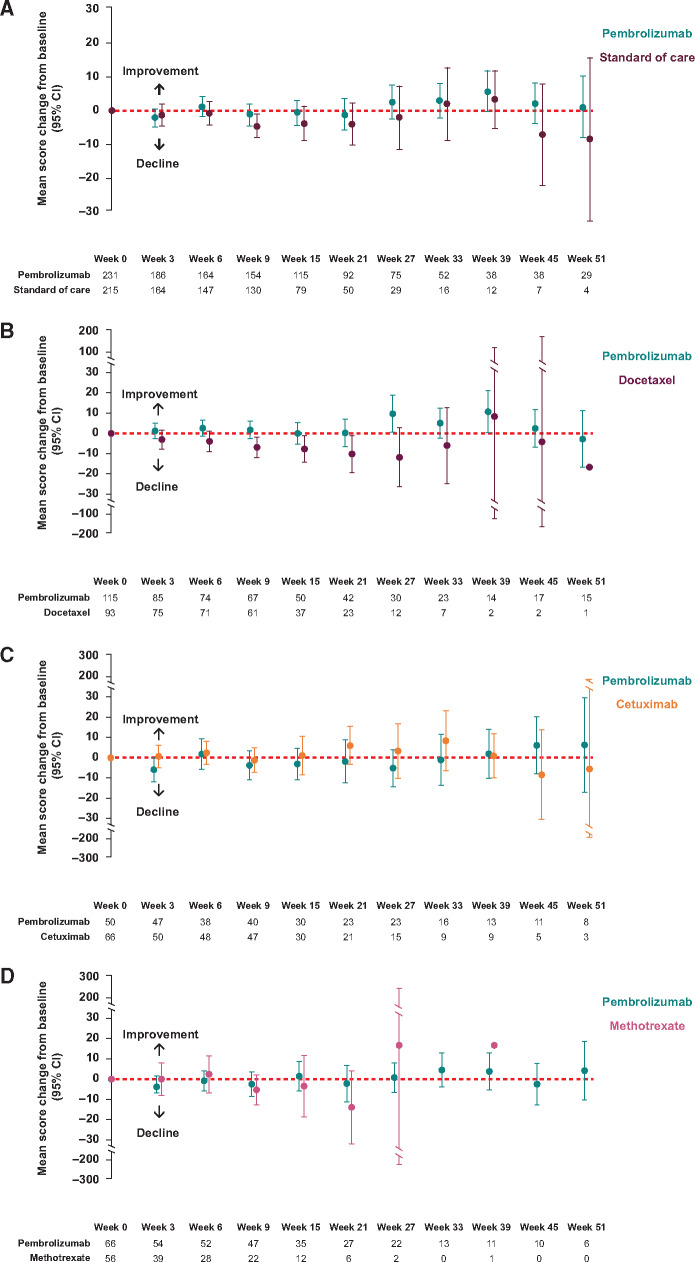
EORTC QLQ-C30 GHS and QoL scores remained stable relative to baseline for patients treated with pembrolizumab who remained in the study at week 15, with an LSM change of 0.4 points (95% CI = −3.00 to 3.78) (Table 2). By contrast, the GHS and QoL scores worsened in patients treated with SOC, with an LSM change of –5.9 points (95% CI = −9.68 to −2.04). The difference in LSM between groups was 6.3 points (95% CI = 1.32 to 11.18; nominal 2-sided P = .01), indicating modest improvement with pembrolizumab versus SOC. Sensitivity analyses using the control-based mean imputation method further identified a modest improvement in mean GHS and QoL scores between pembrolizumab and SOC (mean difference, 5.2 points; 95% CI = 1.51 to 8.88; nominal 2-sided P = .008), and the 2 rank-based methods produced similar findings (Supplementary Table 2, available online). When stratified by time of dropout, trends in mean GHS and QoL scores with pembrolizumab remained stable for patients who sequentially completed questionnaires through weeks 6-15, whereas a trend in decline was observed with SOC over time (Supplementary Figure 2, available online). As expected, patients able to complete HRQoL assessments until week 15 exhibited higher GHS and QoL scores at baseline and over time in both treatment groups. Descriptive analysis of mean change from baseline further revealed that the GHS and QoL scores were stable relative to baseline at each time point through week 51 in both treatment groups for those who were in the study and able to complete questionnaires at later time points (Figure 3A).
Table 2.
Difference in LSM change from baseline in the EORTC QLQ-C30 GHS and QoL scores by investigator’s choice of SOC in patients who remained in the study at week 15
| Treatment | Baseline score, mean (SD)a | Week 15 score, mean (SD)a | Change from baseline to week 15, LSM (95% CI) b,c | Difference in LSMs (95% CI)c |
|---|---|---|---|---|
| Pembrolizumab vs SOC | ||||
| Pembrolizumab | 6.25 (1.32 to 11.18)f | |||
| No. | 231d | 116d | 241e | |
| Mean (SD) | 56.02 (21.24) | 61.71 (19.72) | 0.39 (−3.00 to 3.78) | |
| SOC | ||||
| No. | 215d | 85d | 228e | |
| Mean (SD) | 55.81 (21.63) | 55.69 (22.02) | −5.86 (−9.68 to −2.04) | |
| Pembrolizumab vs docetaxelg | ||||
| Pembrolizumab | 10.23 (3.15 to 17.30) | |||
| No. | 115d | 51d | 118e | |
| Mean (SD) | 53.33 (21.08) | 60.62 (18.34) | 0.51 (−4.24 to 5.36) | |
| Docetaxel | ||||
| No. | 93d | 39d | 95e | |
| Mean (SD) | 59.50 (21.14) | 53.63 (21.10) | −9.71 (−15.12 to −4.31) | |
| Pembrolizumab vs cetuximabg | ||||
| Pembrolizumab | –1.44 (–11.43 to 8.56) | |||
| No. | 50d | 30d | 53e | |
| Mean (SD) | 58.50 (21.20) | 58.06 (19.01) | −3.23 (−10.55 to 4.10) | |
| Cetuximab | ||||
| No. | 66d | 31d | 70e | |
| Mean (SD) | 55.43 (20.49) | 58.06 (24.95) | −1.79 (−8.90 to 5.33) | |
| Pembrolizumab vs methotrexateg | ||||
| Pembrolizumab | 6.21 (–4.57 to 16.99) | |||
| No. | 66d | 35d | 70e | |
| Mean (SD) | 58.84 (21.28) | 66.43 (21.81) | 2.76 (−3.75 to 9.27) | |
| Methotrexate | ||||
| No. | 56d | 15d | 63e | |
| Mean (SD) | 50.15 (21.17) | 56.11 (18.49) | −3.45 (−12.45 to 5.64) | |
Mean scores were calculated among patients with available scores at each time point. CI = confidence interval; cLDA = constrained longitudinal data analysis; ECOG PS = Eastern Cooperative Oncology Group performance status; EORTC QLQ-C30 = European Organisation for Research and Treatment of Cancer core 30 quality-of-life questionnaire; GHS = global health status; HPV = human papillomavirus; HRQoL = health-related quality of life; LSM = least squares mean; PD-L1 = programmed death ligand 1; QoL = quality of life; SOC = standard of care; TPS = tumor proportion score.
Based on cLDA model with the HRQoL scores as the response variable and treatment-by-study-visit interaction and stratification factors (ECOG PS [0 or 1], HPV status [positive vs negative], and PD-L1 status [TPS ≥50% vs TPS <50%]) as covariates.
Positive GHS and QoL scores indicate improvement, whereas negative scores indicate decline. A mean difference of 5-10 points was defined as a small but clinically meaningful change in GHS and QoL scores (24, 25).
Number of patients in each group who completed the EORTC QLQ-C30 questionnaire at that time point.
Number of patients in the total HRQoL analysis population.
P = .01.
Analyses were conducted in the subgroup of patients for whom investigators chose SOC of methotrexate, docetaxel, or cetuximab before patients were randomly assigned to receive pembrolizumab or SOC. The division of pembrolizumab-treated patients was based on the corresponding SOC treatment chosen by the investigator before randomization.
Figure 3.
Change from baseline in the European Organisation for Research and Treatment of Cancer scale 30 quality-of-life questionnaire global health status and quality of life scores over time by investigator’s choice of standard of care (SOC) in patients who were in the study at each time point. A) Pembrolizumab vs SOC. Week 51: pembrolizumab, 95% confidence interval (CI) = –7.85 to 10.14; SOC, 95% CI = –32.54 to 15.88. B) Pembrolizumab vs docetaxel. Week 39: pembrolizumab 95% CI = –6.86 to 11.76; docetaxel 95% CI = –97.55 to 114.22. Week 45: pembrolizumab 95% CI = –0.30 to 21.12; docetaxel 95% CI = –162.99 to 154.66. C) Pembrolizumab vs cetuximab. Week 45: pembrolizumab 95% CI = –7.90 to 20.02; cetuximab 95% CI = –30.28 to 13.62. Week 51: pembrolizumab 95% CI = –16.93 to 29.43; cetuximab 95% CI = –48.65 to 37.54. D) Pembrolizumab vs methotrexate. Week 21: pembrolizumab 95% CI = –11.19 to 6.87; methotrexate 95% CI = –31.95 to 4.18. Week 27: pembrolizumab 95% CI = –6.54 to 8.05; methotrexate 95% CI = –195.10 to 228.44. One patient receiving methotrexate did not complete a questionnaire at week 33 but did so at week 39. For B, C, and D, analyses were conducted in the subgroup of patients for whom investigators chose SOC of methotrexate, docetaxel, or cetuximab before patients were randomly assigned to receive pembrolizumab or SOC. The division of pembrolizumab-treated patients in panels B, C, and D was based on the corresponding SOC treatment chosen by the investigator before randomization.
Descriptive trends in change from baseline in GHS and QoL scores at each time point through week 51 appeared to differ according to the investigator’s choice of SOC (Figure 3, B-D). EORTC QLQ-C30 GHS and QoL scores worsened relative to baseline for patients treated with docetaxel but were generally stable for cetuximab and methotrexate (descriptive analysis only), although numbers of patients receiving SOC therapies were very low beyond the 15-week time point. From baseline to week 15, the LSM change in EORTC QLQ-C30 GHS and QoL scores were stable for patients treated with cetuximab (–1.79 points, 95% CI = −8.90 to 5.33 points) and methotrexate (−3.45 points, 95% CI = −12.54 to 5.64 points), whereas a notable decline of −9.71 points was observed among patients treated with docetaxel (95% CI = −15.12 to −4.31 points) (Table 2). Consequently, a greater clinically meaningful difference between groups in LSM change in GHS and QoL scores was observed with pembrolizumab versus docetaxel (difference in LSM = 10.2 points, 95% CI = 3.15 to 17.30 points) than with pembrolizumab versus methotrexate (6.21 points, 95% CI = −4.57 to 16.99 points) or cetuximab (−1.44 points, 95% CI = −11.43 to 8.56 points) (Table 2). Notably, imbalances across treatment groups in the distribution of investigator’s choice of methotrexate and cetuximab were seen at week 15 (Table 1), which could have affected the validity of these comparisons; in contrast, the proportion of patients assigned to SOC of docetaxel remained balanced at week 15. Additional sensitivity analyses of the GHS and QoL scores by investigator’s choice of SOC therapy are provided in Supplementary Table 2 (available online).
Descriptive analyses conducted at week 15 indicated greater improvement in GHS and QoL scores with pembrolizumab versus SOC in patients without disease progression (difference in LSM = 9.40 points, 95% CI = 3.83 to 14.97 points), whereas no clinically meaningful difference in GHS and QoL scores between treatment groups was observed for patients with disease progression (difference in LSM = 6.27 points, 95% CI = –4.87 to 17.41 points) (Supplementary Table 3, available online). GHS and QoL remained stable at week 15 for patients treated with pembrolizumab whose disease did not progress (LSM change = 4.30 points, 95% CI = 0.48 to 8.12 points) and for patients whose disease progressed (LSM change = −3.56 points, 95% CI = −7.39 to 0.26 points). In contrast, in patients treated with SOC, those whose disease did not progress experienced moderate decline in GHS and QoL scores from baseline to week 15 (LSM change = −5.09 points, 95% CI = −9.35 to −0.83 points), and those whose disease progressed experienced greater decline from baseline to week 15 (LSM change = −9.8 points, 95% CI = −14.03 to −5.63 points) (Supplementary Table 3, available online).
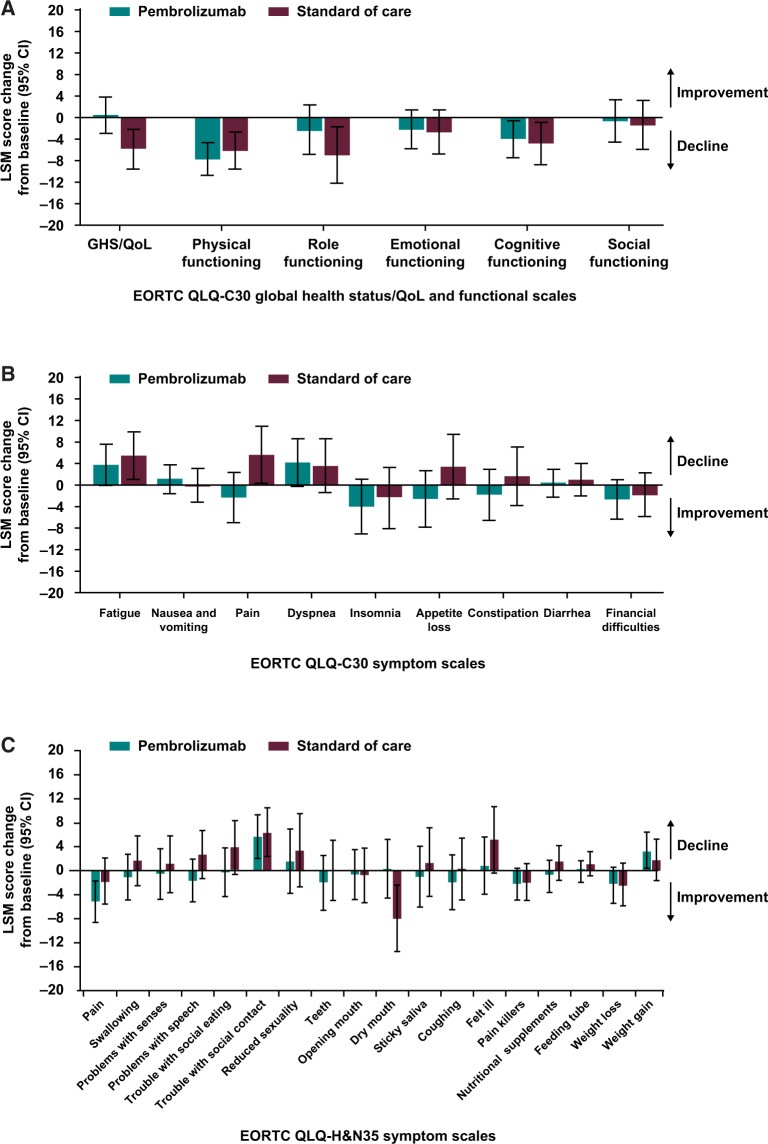
For those in the study at week 15, pembrolizumab-treated patients exhibited stable functioning and stable symptom scores for EORTC QLQ-C30 and QLQ-H&N35, with few exceptions (decline in physical and cognitive functioning, decline in social contact scores; Figure 4, A-C). SOC-treated patients exhibited declines from baseline in the physical, role, cognitive functioning, fatigue, pain, and social contact symptoms of EORTC QLQ-C30 and QLQ-H&N35 but otherwise stable functioning and stable symptom scores at week 15. No notable between-group differences were observed (Figure 4, A-C). EQ-5D utility index and visual analog scale scores were also stable at week 15 for pembrolizumab-treated patients, with no notable differences versus SOC (Supplementary Figure 3, A and B, available online). Subgroup analyses according to SOC choice of therapy for the functioning, symptom, and health status scales of the EORTC QLQ-C30, EORTC QLQ-H&N35, and EQ-5D instruments were generally consistent with the main results; no notable between-group differences were observed (Supplementary Figure 4, available online).
Figure 4.
Difference in least squares mean (LSM) from baseline in European Organisation for Research and Treatment of Cancer core 30 quality-of-life questionnaire (EORTC QLQ-C30) and European Organisation for Research and Treatment of Cancer 35-question quality of life head and neck cancer-specific module (EORTC QLQ-H&N35) global health status (GHS) and quality of life (QoL) functional and symptom scales for patients who remained in the study at week 15. A) EORTC QLQ-C30 GHS and QoL and functional scales. A positive GHS and QoL or functioning score indicates improvement in health-related quality of life or function, whereas a negative score indicates decline. B) EORTC QLQ-C30 symptom scales. A positive symptom score indicates decline or more severe symptoms, whereas a negative score indicates symptom improvement. C) EORTC QLQ-H&N35 multi-item and single-item symptom scales. A positive symptom score indicates decline or more severe symptoms, whereas a negative score indicates symptom improvement. CI = confidence interval.
When assessed by PD-L1 biomarker status at week 15, LSM differences between treatment groups were similar to overall treatment effects for each of the EORTC QLQ-C30, EORTC QLQ-H&N35, and EQ-5D scales, suggesting no evidence of a differential HRQoL benefit according to PD-L1 status (Supplementary Figures 5 and 6, available online).
Proportion With Deteriorated, Stable, and Improved HRQoL Scores
Smaller proportions of patients treated with pembrolizumab than SOC experienced deterioration based on a change from baseline of at least 10 points in the GHS and QoL scores for those in the study at week 15 (24.9% vs 42.5%) (Supplementary Figure 7A, available online). For the functioning and symptom scales of EORTC QLQ-C30 and QLQ-H&N35, the proportion of patients who experienced deterioration in the pembrolizumab group was generally smaller than or similar to that in the SOC group at week 15, with few exceptions (dry mouth at week 15) (Supplementary Figure 7, B-D, available online).
Discussion
Therapies that prolong survival without reducing HRQoL are needed for RM HNSCC. In KEYNOTE-040, pembrolizumab demonstrated clinically meaningful improvements in OS and a better safety profile over SOC in patients with RM HNSCC (20). In this analysis, patients treated with pembrolizumab who remained in the study at week 15 demonstrated stable GHS and QoL, whereas those treated with SOC experienced a small but clinically meaningful decline. Pembrolizumab-treated patients also had stable functioning and symptoms over 15 weeks, further underscoring the clinical benefits of pembrolizumab in RM HNSCC.
Previous HRQoL analyses in pembrolizumab trials across several tumor types have consistently demonstrated HRQoL benefit. In the KEYNOTE-024 study of non–small cell lung cancer (NSCLC), pembrolizumab-treated patients who remained in the study at week 15 demonstrated greater improvement or maintenance in HRQoL than those receiving chemotherapy (34). In the KEYNOTE-045 study of urothelial cancer, prolonged TTD in GHS and QoL score and greater stability or improvement in HRQoL and symptom scores were observed with pembrolizumab versus chemotherapy among those in the study at week 15 (35). In KEYNOTE-002, fewer patients with advanced or treatment-refractory melanoma exhibited deterioration in HRQoL scores with pembrolizumab versus chemotherapy among those in the study at week 12 (36).
Recently, HRQoL has been investigated in patients with RM HNSCC treated with nivolumab. In an exploratory analysis of CheckMate 141 in patients with platinum-refractory RM HNSCC, nivolumab stabilized GHS and QoL, symptoms, and functioning for patients in the study at weeks 9 and 15, whereas SOC chemotherapy led to clinically meaningful deterioration (17). In this analysis, pembrolizumab-treated patients also exhibited stable GHS and QoL at week 15, whereas a modest decline in GHS and QoL was observed with SOC. To our knowledge, this analysis of KEYNOTE-040 is novel and shows that the descriptive trend in stable GHS and QoL extends for as long as 51 weeks in patients with RM HNSCC treated with pembrolizumab.
HRQoL has also been assessed in phase III trials of the targeted therapies afatinib and gefitinib in platinum-refractory RM HNSCC (9,13). Both agents demonstrated stable HRQoL relative to baseline and modest improvements in HRQoL versus methotrexate, but not in OS (9,13), as observed in trials investigating pembrolizumab and nivolumab (16,20).
Interestingly, in this study, a greater between-group difference in GHS and QoL was observed in the comparison of pembrolizumab with docetaxel (difference in LSM = 10.2, 95% CI = 3.15 to 17.30) than with either methotrexate (difference in LSM = 6.2, 95% CI = −4.57 to 16.99) or cetuximab (difference in LSM = −1.4, 95% CI = 11.43 to 8.56) for patients who remained in the study at week 15. This is important because docetaxel was also determined to be the most efficacious of the SOC therapies in both KEYNOTE-040 and CheckMate 141 (16). In addition, in situations in which anti–PD-1 therapy is not available to patients, docetaxel is the most likely therapy to be administered in real-world practice (37–41). These data evaluating PD-1 inhibitors versus docetaxel are consistent with results from NSCLC in KEYNOTE-010; patients treated with pembrolizumab versus docetaxel showed improved survival and HRQoL (42,43). Similarly, in the CheckMate 017 study in NSCLC, nivolumab improved both survival and HRQoL versus docetaxel in the second-line setting (44,45). Indeed, toxicity of systemic agents in patients with RM HNSCC varies, as does their burden on HRQoL .
In this study, differences between groups in HRQoL appeared to be correlated with response to therapy. For patients in the study at week 15, greater improvement in GHS and QoL was observed with pembrolizumab than SOC in patients without disease progression; however, no such clinically meaningful difference in GHS and QoL was found in patients with progression. GHS and QoL remained stable in pembrolizumab-treated patients with and without disease progression, whereas GHS and QoL declined with SOC regardless of progression status at week 15. The overall treatment effect on HRQoL was similar among PD-L1 subgroups. These findings are consistent with those of CheckMate 141, which noted no meaningful influence of PD-L1 status on HRQoL (17).
Limitations of these analyses include the open-label trial design, which might have influenced patient responses and may explain why HRQoL was not worse in the experimental arm in this and other open-label trials in this indication (9,13,17). Further, as is common with HRQoL assessments (17), analyses were limited to week 15 to ensure sufficient completion rates. Although formal statistical analyses on change from baseline in GHS and QoL scores were limited to week 15, sensitivity analyses testing different assumptions about missing data consistently confirmed a modest improvement in GHS and QoL with pembrolizumab versus SOC. In addition, trends observed at week 15 remained consistent through week 51, indicating stable GHS and QoL with pembrolizumab for those in the study at later time points. Last, the exploratory nature of the HRQoL analyses should be interpreted in light of the multiple comparisons performed, which might have contributed to the possibility of false findings.
Pembrolizumab-treated patients had stable GHS and QoL, whereas SOC-treated patients who remained in the study at week 15 experienced modest declines in GHS and QoL. Additionally, pembrolizumab-treated patients exhibited stable functioning and symptoms at week 15. Along with previously presented efficacy and safety results, these data support the clinically meaningful benefit of pembrolizumab in patients with RM HNSCC.
Funding
This study was funded by Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ, USA.
Notes
Role of the funder: The sponsor collaborated with the senior academic authors in the study design, and collection, analysis, and interpretation of the results. The corresponding author had full access to all study data, and all authors had final responsibility for the decision to submit this manuscript for publication. The sponsor funded medical writing and/or editorial assistance for this manuscript.
Disclosures: KJ Harrington: Honoraria and consultant/advisory role: AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Merck Serono, Merck Sharp & Dohme, and Pfizer. Speakers bureau: Bristol-Myers Squibb, Merck Serono, and Merck Sharp & Dohme. Research funding: AstraZeneca, and Merck Sharp & Dohme. D Soulières: Honoraria, consulting/advisory role, and research funding: Merck. C Le Tourneau: Grant support to the institution from Merck Sharp & Dohme for clinical research related to the submitted work. Personal fees for serving as a consultant or adviser or for lectures from Amgen, Bristol-Myers Squibb, Merck Serono, MSD, Nanobiotix, Novartis, and Roche, all outside the submitted work. J Dinis has nothing to disclose. LF Licitra: Honoraria and consultant/advisory role: Eisai, Bristol-Myers Squibb, Merck Sharp & Dohme, Boehringer Ingelheim, Novartis, AstraZeneca, Roche, Bayer, Debiopharm, Sobi, Kura Oncology, Health & Life, Ipsen Innovation, Immuno-Oncology Hub, Incyte Biosciences Italy, Doxapharma, SRI International, Amgen, and Nanobiotix. Research funding: Eisai, Merck Sharp & Dohme, Boehringer Ingelheim, Novartis, AstraZeneca, Roche, Bristol-Myers Squibb, Celgene International, Exelixis, Hoffmann-La Roche, IRX Therapeutics, Medpace, and Pfizer. Travel accommodations/expenses: Merck Serono, Bristol-Myers Squibb, Merck Sharp & Dohme, Bayer, and Debiopharm. M-J Ahn has nothing to disclose. A Soria: Consultant/advisory role and speakers bureau: Merck Sharp & Dohme, Roche, Novartis, Bristol-Myers Squibb, Pierre Fabre. Expert testimony and travel accommodations/expenses: Merck Sharp & Dohme, Novartis, Roche, and Bristol-Myers Squibb. J-PH Machiels: Personal fees: Roche, AstraZeneca, Bayer, Innate, Merck Serono, Boehringer Ingelheim, Bristol-Myers Squibb, Novartis, Janssen, Incyte, Cue Biopharma, ALX Oncology, and Pfizer. Grant support from BMS. Other: Amgen, MSD, PsiOxus, Debiopharm, and Nanobiotix. N Mach has nothing to disclose. R Mehra: Consultant/advisory role: Bristol-Myers Squibb, Genentech, and Bayer. Research funding to the institution: AstraZeneca and Merck. B Burtness: Research funding to the institution. Personal fees for serving on a steering committee for the work under consideration for publication and research funding to the institution from Advaxis and Bristol-Myers Squibb; for serving on a data safety monitoring committee for IDDI; for serving on an advisory board for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, and Genentech. Travel support: Boehringer Ingelheim. All were outside the submitted work. MC Ellison, JD Cheng, DR Chirovsky, and RF Swaby: Employees of Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ, USA, and stockholders of Merck & Co, Inc, Kenilworth, NJ, USA. EEW Cohen: Consultant/advisory role: Eisai, Pfizer, Merck, AstraZeneca, Bristol-Myers Squibb, and Human Longevity.
Author contributions: Dr Harrington had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Harrington, Burtness, Cheng, Chirovsky, Cohen. Provision of study materials or patients: Harrington, Le Tourneau, Dinis, Burtness. Collection and assembly of data: Harrington, Souliéres, Licitra, Soria, Mach, Mehra, Burtness, Swaby, Cohen. Data analysis and interpretation: Harrington, Souliéres, Le Tourneau, Dinis, Licitra, Ahn, Soria, Machiels, Mehra, Ellison, Cheng, Chirovsky, Swaby, Cohen. Statistical analysis: Ellison, Chirovsky. Drafting of the manuscript: Chirovsky. Critical revision of the manuscript for important intellectual content: All authors. Final approval of manuscript: All authors.
Acknowledgments: We thank the patients and their families and caregivers for participating in this trial; all the investigators and site personnel; Pingye (Eric) Zhang and Fang Liu (Merck Sharp & Dohme, Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ, USA) for assistance with the HRQoL statistical analyses; Josephine Norquist (Merck Sharp & Dohme, Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ, USA) for assistance with interpretation of the HRQoL results; and Gerardo Diaz and Rita Bardi (Merck Sharp & Dohme, Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ, USA) for contributions to study conduct. Medical writing was provided by Jacqueline Kolston, PhD, and Matthew Grzywacz, PhD, of ApotheCom (Yardley, PA). This assistance was funded by Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ, USA.
Data sharing statement: The data sharing policy of Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ, USA, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
Supplementary Material
References
- 1. Velikova G, Coens C, Efficace F, et al. Health-related quality of life in EORTC clinical trials - 30 years of progress from methodological developments to making a real impact on oncology practice. EJC Supplements. 2012;10(1):141–149. [Google Scholar]
- 2. Rhoten BA, Murphy B, Ridner SH.. Body image in patients with head and neck cancer: a review of the literature. Oral Oncol. 2013;49(8):753–760. [DOI] [PubMed] [Google Scholar]
- 3. Melo Filho MR, Rocha BA, Pires MB, et al. Quality of life of patients with head and neck cancer. Braz J Otorhinolaryngol. 2013;79(1):82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network I. NCCN Clinical Practice Guidelines in Oncology - Head and Neck Cancers, v1.2020. National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed on May 18, 2020. [DOI] [PubMed]
- 5. Vermorken JB, Herbst RS, Leon X, Amellal N, Baselga J.. Overview of the efficacy of cetuximab in recurrent and/or metastatic squamous cell carcinoma of the head and neck in patients who previously failed platinum-based therapies. Cancer. 2008;112(12):2710–2719. [DOI] [PubMed] [Google Scholar]
- 6. Argiris A, Ghebremichael M, Gilbert J, et al. Phase III randomized, placebo-controlled trial of docetaxel with or without gefitinib in recurrent or metastatic head and neck cancer: an eastern cooperative oncology group trial. J Clin Oncol. 2013;31(11):1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gregoire V, Lefebvre JL, Licitra L, Felip E, EHNS-ESMO-ESTRO Guidelines Working Group. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v184–v186. [DOI] [PubMed] [Google Scholar]
- 8. Lala M, Chirovsky D, Cheng JD, Mayawala K.. Clinical outcomes with therapies for previously treated recurrent/metastatic head-and-neck squamous cell carcinoma (RM HNSCC): a systematic literature review. Oral Oncol. 2018;84:108–120. [DOI] [PubMed] [Google Scholar]
- 9. Machiels JP, Haddad RI, Fayette J, et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trial. Lancet Oncol. 2015;16(5):583–594. [DOI] [PubMed] [Google Scholar]
- 10. Mesia R, Rivera F, Kawecki A, et al. Quality of life of patients receiving platinum-based chemotherapy plus cetuximab first line for recurrent and/or metastatic squamous cell carcinoma of the head and neck. Ann Oncol. 2010;21(10):1967–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Urba S, van Herpen CM, Sahoo TP, et al. Pemetrexed in combination with cisplatin versus cisplatin monotherapy in patients with recurrent or metastatic head and neck cancer: final results of a randomized, double-blind, placebo-controlled, phase 3 study. Cancer. 2012;118(19):4694–4705. [DOI] [PubMed] [Google Scholar]
- 12. Murphy BA. To treat or not to treat: balancing therapeutic outcomes, toxicity and quality of life in patients with recurrent and/or metastatic head and neck cancer. J Support Oncol. 2013;11(4):149–159. [DOI] [PubMed] [Google Scholar]
- 13. Stewart JS, Cohen EE, Licitra L, et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected]. J Clin Oncol. 2009;27(11):1864–1871. [DOI] [PubMed] [Google Scholar]
- 14. Cohen EE, Kane MA, List MA, et al. Phase II trial of gefitinib 250 mg daily in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2005;11(23):8418–8424. [DOI] [PubMed] [Google Scholar]
- 15. Licitra L, Mesia R, Keilholz U.. Individualised quality of life as a measure to guide treatment choices in squamous cell carcinoma of the head and neck. Oral Oncol. 2016;52:18–23. [DOI] [PubMed] [Google Scholar]
- 16. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harrington KJ, Ferris RL, Blumenschein G Jr, et al. Nivolumab versus standard, single-agent therapy of investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 2017;18(8):1104–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956–965. [DOI] [PubMed] [Google Scholar]
- 19.Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol 2017;35(14):1542–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cohen EEW, Soulieres D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393(10167):156–167. [DOI] [PubMed] [Google Scholar]
- 21. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. [DOI] [PubMed] [Google Scholar]
- 22. Bjordal K, de Graeff A, Fayers PM, et al. A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. EORTC Quality of Life Group. Eur J Cancer. 2000;36(14):1796–1807. [DOI] [PubMed] [Google Scholar]
- 23.The EuroQol Group. EuroQol. A new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 24. Cocks K, King MT, Velikova G, et al. Evidence-based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur J Cancer. 2012;48(11):1713–1721. [DOI] [PubMed] [Google Scholar]
- 25. Cocks K, King MT, Velikova G, Martyn St-James M, Fayers PM, Brown JM.. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol. 2011;29(1):89–96. [DOI] [PubMed] [Google Scholar]
- 26. Osoba D, Rodrigues G, Myles J, Zee B, Pater J.. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–144. [DOI] [PubMed] [Google Scholar]
- 27. Pickard AS, Neary MP, Cella D.. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anota A, Hamidou Z, Paget-Bailly S, et al. Time to health-related quality of life score deterioration as a modality of longitudinal analysis for health-related quality of life studies in oncology: do we need RECIST for quality of life to achieve standardization? Qual Life Res. 2015;24(1):5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kenward MG, White IR, Carpenter JR.. Should baseline be a covariate or dependent variable in analyses of change from baseline in clinical trials? By G. F. Liu, K. Lu, R. Mogg, M. Mallick and D. V. Mehrotra. Stat Med. 2010;29(13):1455–1456. author reply 7. [DOI] [PubMed] [Google Scholar]
- 30. Liu GF, Lu K, Mogg R, Mallick M, Mehrotra DV.. Should baseline be a covariate or dependent variable in analyses of change from baseline in clinical trials? Stat Med. 2009;28(20):2509–2530. [DOI] [PubMed] [Google Scholar]
- 31. Mehrotra DV, Liu F, Permutt T.. Missing data in clinical trials: control-based mean imputation and sensitivity analysis. Pharm Stat. 2017;16(5):378–392. [DOI] [PubMed] [Google Scholar]
- 32. Mann HB, Whitney DR.. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat. 1947;18(1):50–60. [Google Scholar]
- 33. Hettmansperger TP, McKean JW.. A geometric interpretation of inferences based on ranks in the linear model. J Am Stat Assoc. 1983;78(384):885–893. [Google Scholar]
- 34. Brahmer JR, Rodriguez-Abreu D, Robinson AG, et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol. 2017;18(12):1600–1609. [DOI] [PubMed] [Google Scholar]
- 35. Vaughn DJ, Bellmunt J, Fradet Y, et al. Health-related quality-of-life analysis from KEYNOTE-045: a phase III study of pembrolizumab versus chemotherapy for previously treated advanced urothelial cancer. J Clin Oncol. 2018;36(16):1579–1587. [DOI] [PubMed] [Google Scholar]
- 36. Schadendorf D, Dummer R, Hauschild A, et al. Health-related quality of life in the randomised KEYNOTE-002 study of pembrolizumab versus chemotherapy in patients with ipilimumab-refractory melanoma. Eur J Cancer. 2016;67:46–54. [DOI] [PubMed] [Google Scholar]
- 37. Gruenwald V, Chirovsky D, Cheung W, et al. Pcn11 - global longitudinal assessment of treatment outcomes in squamous cell carcinoma of the head and neck (Glance-H&N) study. Value Health. 2018;21:S17. [Google Scholar]
- 38. Laban S, Kimmeyer J, Knecht R, et al. Palliative treatment standards for head and neck squamous cell carcinoma: survey of clinical routine in German-speaking countries. HNO. 2016;64(7):487–493. [DOI] [PubMed] [Google Scholar]
- 39. van der Linden N, Buter J, Pescott CP, Lalisang RI, et al. Treatments and costs for recurrent and/or metastatic squamous cell carcinoma of the head and neck in the Netherlands. Eur Arch Otorhinolaryngol. 2016;273(2):455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. La EM, Smyth EN, Talbird SE, et al. Treatment patterns and health care resource use in patients receiving multiple lines of therapy for metastatic squamous cell carcinoma of the head and neck in the United Kingdom. Eur J Cancer Care. 2018;27(5):e12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nadler E, Joo S, Boyd M, Black-Shinn J, Chirovsky D.. Treatment patterns and outcomes among patients with recurrent/metastatic squamous cell carcinoma of the head and neck. Future Oncol. 2019;15(7):739–751. [DOI] [PubMed] [Google Scholar]
- 42. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. [DOI] [PubMed] [Google Scholar]
- 43. Barlesi F, Garon E, Kim DW, et al. Assessment of health-related quality of life (HRQoL) in KEYNOTE-010: a phase 2/3 study of pembrolizumab (pembro) vs docetaxel in patients (pts) with previously treated advanced NSCLC. Ann Oncol. 2016;27:416–454. [Google Scholar]
- 44. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reck M, Taylor F, Penrod JR, et al. Impact of nivolumab versus docetaxel on health-related quality of life and symptoms in patients with advanced squamous non-small cell lung cancer: results from the CheckMate 017 study. J Thor Oncol. 2018;13(2):194–204. [DOI] [PubMed] [Google Scholar]
- 46. Saba NF, Mody MD, Tan ES, et al. Toxicities of systemic agents in squamous cell carcinoma of the head and neck (SCCHN); a new perspective in the era of immunotherapy. Crit Rev Oncol Hematol. 2017;115:50–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.