Abstract
Background
Metformin has been associated with lower breast cancer (BC) risk and improved outcomes in observational studies. Multiple biologic mechanisms have been proposed, including a recent report of altered sex hormones. We evaluated the effect of metformin on sex hormones in MA.32, a phase III trial of nondiabetic BC subjects who were randomly assigned to metformin or placebo.
Methods
We studied the subgroup of postmenopausal hormone receptor-negative BC subjects not receiving endocrine treatment who provided fasting blood at baseline and at 6 months after being randomly assigned. Sex hormone-binding globulin, bioavailable testosterone, and estradiol levels were assayed using electrochemiluminescence immunoassay. Change from baseline to 6 months between study arms was compared using Wilcoxon sum rank tests and regression models.
Results
312 women were eligible (141 metformin vs 171 placebo); the majority of subjects in each arm had T1/2, N0, HER2-negative BC and had received (neo)adjuvant chemotherapy. Mean age was 58.1 (SD=6.9) vs 57.5 (SD=7.9) years, mean body mass index (BMI) was 27.3 (SD=5.5) vs 28.9 (SD=6.4) kg/m2 for metformin vs placebo, respectively. Median estradiol decreased between baseline and 6 months on metformin vs placebo (−5.7 vs 0 pmol/L; P < .001) in univariable analysis and after controlling for baseline BMI and BMI change (P < .001). There was no change in sex hormone-binding globulin or bioavailable testosterone.
Conclusion
Metformin lowered estradiol levels, independent of BMI. This observation suggests a new metformin effect that has potential relevance to estrogen sensitive cancers.
Metformin has garnered attention as a potential anticancer agent across a range of cancers, including breast cancer (BC); potential effects on BC outcomes are being studied in the Canadian Cancer Trials Group MA.32, an ongoing phase 3 adjuvant trial comparing metformin 850 mg twice a day vs placebo twice a day (each given for 5 years) in subjects receiving standard breast cancer therapy (1). It has been postulated that metformin may impact BC directly (eg, via intratumoral LKB1-mediated AMP activated protein kinase leading to suppression of mTORC1 signaling) and/or indirectly (eg, via inhibition of hepatic gluconeogenesis with subsequent reduction in circulating insulin levels, reducing PI3K/Akt/mTOR signaling in cancer cells expressing the insulin receptor) (2). Data from neoadjuvant clinical trials have provided some support for both direct and indirect mechanisms (3,4). Recent research has suggested metformin may also act indirectly via an effect on sex hormones (SHs), although findings have been inconsistent (5–8).
SHs are of relevance to both BC risk and prognosis, particularly in postmenopausal women (9–12). In a case-control study nested in the Women’s Healthy Eating and Living Study, BC patients who recurred had higher levels of estrogens than those who did not recur (22.7 vs 10.8 pg/mL; P = .05) (10). The importance of SHs in hormone receptor-positive BC is highlighted by the therapeutic effectiveness of aromatase inhibitors, which reduce estrogen production in postmenopausal women.
Campagnoli et al. (5,6) studied the effect of metformin (1500 mg/day vs 1000 mg/day after a 1-month run-in of 1000 mg/day) on SHs in 96 nondiabetic, postmenopausal BC patients (50% of whom were on tamoxifen) with prebaseline testosterone above 0.28 ng/mL. Metformin reduced estradiol (−38%; P < .02) and free testosterone (−29%; P < .01); these differences remained statistically significant after controlling for baseline body mass index (BMI) and weight change.
Patterson et al. (7) used a 2 × 2 factorial design to randomly assign 313 overweight or obese postmenopausal BC patients to metformin 1500 mg/daily vs placebo and a lifestyle weight-loss program vs control. Metformin (vs placebo) lowered estradiol (-10%, 95% confidence interval [CI] = -18.5% to -1.5%) and testosterone (-9.5%, 95% CI = -15.2% to -3.8%) and increased sex hormone-binding globulin (SHBG) (+7.5%, 95% CI = 2.4% to 12.6%) levels. However, estradiol appeared to be reduced only in those receiving both metformin and the lifestyle intervention (8). In a final study conducted in 382 overweight, glucose-intolerant patients without BC enrolled onto the Diabetes Prevention Program (13), metformin had no impact on SHBG, estradiol, testosterone, or dehydroepiandrosterone.
We investigated the SHs in postmenopausal women with hormone receptor-negative BC (selected to avoid use of endocrine therapies that may impact SHs) enrolled onto the MA.32 trial. We also explored effects of the minor allele (C) of the rs11212617 SNP that has been associated with greater metabolic response to metformin in type 2 diabetes (14) and increased pathologic response to neoadjuvant metformin in early stage HER2-positive BC (15).
Methods
Design
We conducted a substudy of patients enrolled onto the MA.32 randomized phase III clinical trial (1). The focus on SHs was not part of the original trial protocol; as evidence of an effect of metformin on SHs emerged, this work was approved by the trial steering committee as part of a priori plan to investigate potential mechanisms of metformin action. The primary objective of this substudy was to compare change in levels of prespecified SHs (estradiol, SHBG, and bioavailable testosterone [BT]) from baseline to 6 months between metformin and placebo arms; if a difference between study arms was found, secondary objectives were to explore two possible pathways of metformin action, namely BMI and insulin change, as well as the impact of the SNP rs11212617 on any SH change.
Study Population
The Canadian Cancer Trials Group MA.32 Clinical Trial (Clinical Trials.gov identifier: NCT01101438, and EudraCT number: 2011-005230-18) (1) is a phase III, double-blind trial that randomly assigned 3649 nondiabetic patients with T1c-3 (any estrogen receptor [ER], progesterone receptor [PR], HER2), N0-3, M0 BC who received standard treatment to receive metformin 850 mg by mouth twice a day or placebo by mouth twice a day for 5 years (including a 4-week ramp up of 1 tablet per day) between 2010 and 2013. In May 2012, after 2382 women were enrolled, eligibility criteria were amended: those with T1cN0 disease had to have triple-negative BC (ER negative, PR negative, HER2 negative) to enter the trial, and those with T2 N0 BC were eligible only if they had at least 1 of the following risk factors: histologic grade III, presence of lymphovascular invasion, negative ER and PR receptors, HER2 positivity, an oncotype recurrence score of at least 25, or Ki-67 above 14%. Exclusion criteria included fasting glucose above 7.0 mmol/L (126 mg/dL); known diabetes or current use of diabetes medication; hypersensitivity to or intolerance of metformin; history of lactic acidosis; participation in trials of weight-loss or exercise interventions; recurrence of BC or prior BC; excessive alcohol intake; or marked hepatic, kidney, or cardiac dysfunction.
All patients provided written informed consent to participate in the MA.32 clinical trial in keeping with approval by relevant institutional human subjects committees.
The SH substudy was conducted in postmenopausal women (to avoid cyclical changes in endogenous hormone production) with hormone receptor negative BC, who were not receiving endocrine treatment (to avoid effects of hormonal agents used to treat BC). Postmenopausal was defined as prior bilateral oophorectomy or more than 12 months since last menses without prior hysterectomy. Baseline blood was obtained before study treatment was initiated; patients were required to be on study treatment at the time of the 6-month blood draw.
At baseline, information was collected on age and tumor characteristics (stage, histological type, immunohistochemical profile) and treatment. Height and weight were measured at baseline, and weight at 6 months. BMI was calculated as weight/height2 (kg/m2).
Blood Assays
The serum samples were collected into heparin tubes, aliquoted, and stored at −80°C after an overnight fast of at least 12 hours. Paired specimens from each patient were retrieved, thawed, and analyzed in the same batch by technicians blinded to patient treatment. The biologically most active estrogen (17β estradiol), SHGB, and total testosterone levels were determined using competitive electrochemiluminescence immunoassay on cobas e602 and insulin using Roche electrochemiluminescence immunoassay (catalogue #12017547122) at a CAP/CLIA–accredited clinical laboratory (Mount Sinai Services). Intra-assay coefficients of variability were 7%, 2.4%, 4.6.%, and 3%, respectively. Albumin was assayed to allow calculation of BT from total testosterone, SHBG, and albumin. The automated platform cobas e602 provides a lower detection limit for estradiol of 18.4 pmol/L and for total testosterone of 0.025 ng/mL. The measurement range for SHBG was 0.350 nmol/L to 200 nmol/L (defined as the limit of detection and the maximum of the master curve).
SNP Analysis
Genomic DNA was extracted from whole blood, and samples were genotyped for the rs11212617 SNP [Chr11(GRCh38):g.108412434C>A] at the Centre for Applied Genomics, the Hospital for Sick Children, Toronto, Canada, using a QIAsymphony magnetic bead DNA extractor (Qiagen, Germany) and a TaqMan PCR assay with dual-label MGB probes (Applied Biosystems, ThermoFisher Scientific, Waltham, MA, USA).
Statistical Analysis
Patient and tumor characteristics at diagnosis were summarized. Because the distributions of the SHs were skewed, medians were used as measures of central tendency and 25th and 75th percentiles as measures of dispersion. Descriptive summaries were tabulated for baseline and change in the SH levels, where change was calculated as the 6-month value minus the baseline value for each patient. Estradiol, testosterone, and SHBG were considered equally important main outcomes.
The prespecified method of comparing the degree of change between arms was the Wilcoxon rank sum test. Ten baseline and 7-month 6 SHBG results (3%) were recorded as more than 200 nmol/L; these were replaced by 200 nmol/L. Overall, 25% of baseline and 33% of month 6 estradiol assay results were below the assay’s limit of detection (LOD); change calculations, summary statistics, and the Wilcoxon test were performed after replacing these results by half the LOD. Because of criticism of this method (16), we performed an additional sensitivity analysis using survival methods for left-censored data. The data can be analyzed using right-censored survival methods (product limit estimator and the log-rank test) by subtracting the left-censored observations from a large constant, a process called “flipping” (17). With only one point of censoring (the LOD), the product-limit method simplifies to the construction of exceedance curves, where for a defined set of levels e*, one calculates the proportion of patients in each arm whose estradiol at month 6 was higher than each of the e*.
For SHs with a statistically significant difference between arms, we explored 2 possible pathways of metformin action by fitting multivariable linear regression models controlling for baseline BMI and BMI change and insulin change. This was done by log-transforming all continuous variables to reduce skewness and then fitting a model with SH change as dependent variable and with treatment status and baseline assay level as explanatory variables. Baseline and change in BMI, or insulin change, was then added to this model. We back-transformed the coefficient for treatment status to get the percent difference between the metformin and placebo arms. Finally, using a regression model for change that included an interaction term for treatment by SNP, coded as any C vs AA, we examined whether a metformin effect was restricted to patients with the C allele of the rs11212617 SNP. Reported P values are nominal and not adjusted for multiple comparisons.
A priori power calculations indicated we had 80% power with 150 patients per arm to detect differences in change between the arms of 12%, 14%, and 20% for SHBG, testosterone, and estradiol, respectively, using a 2-sided alpha of 0.05 without adjustment for multiple comparisons.
Results
Patient Population
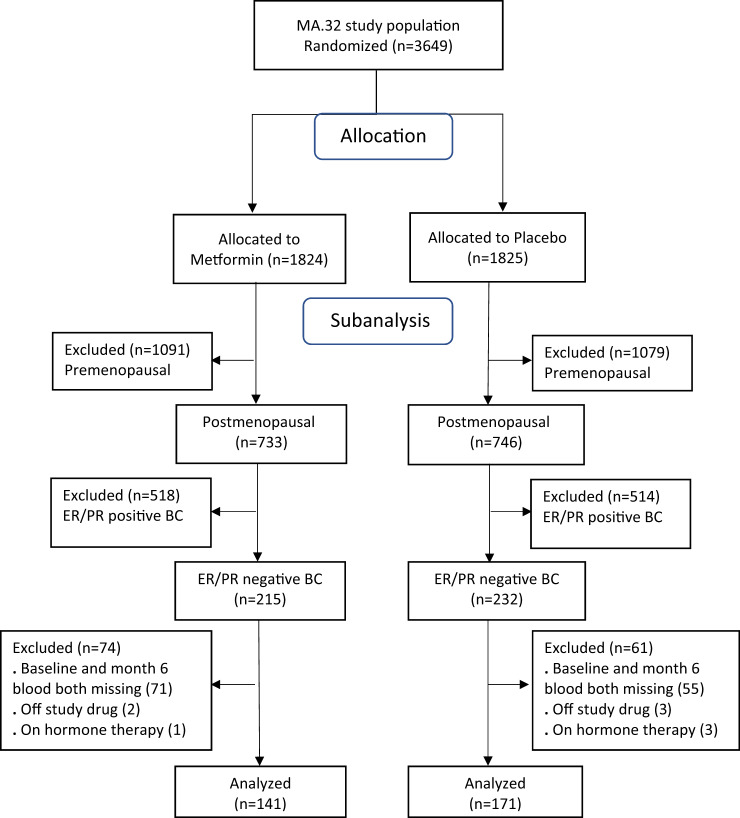
Three hundred twelve women were eligible based on postmenopausal status, ER- and PR-negative invasive BC not receiving hormonal treatment, and availability of blood at baseline and 6 months (the latter on study treatment): 141 on metformin and 171 on placebo arm (Figure 1, Consort Diagram). Patient and tumor characteristics are shown in Table 1. At baseline, mean age was 58.1 (SD=6.9) vs 57.5 (SD=7.9) years, mean weight 72.6 (SD=14.1) vs 76.6 (SD=17.5) kg, and mean BMI 27.3 (SD=5.2) vs 28.9 (SD=6.4) kg/m2 in metformin vs placebo arms, respectively. Combining clinical stage (in patients receiving neoadjuvant therapy) and pathologic stage, the majority of patients had T1 or T2 (39.0% T1 and 54.6% T2 in the metformin arm, 42.7% T1 and 46.8% T2 in the placebo arm) and node-negative breast cancer (66.7% and 61.4% in the metformin and placebo arms, respectively). HER2 amplification was observed in 22.7% and 15.8% of the women in the metformin and placebo arms, respectively. Of the women, 98% had received neoadjuvant or adjuvant chemotherapy.
Figure 1.
Consort diagram. BC = breast cancer; ER = estrogen receptor; PR = progesterone receptor.
Table 1.
Patient and tumor characteristics at baselinea
| Characteristics | Metformin (n = 141) | Placebo (n = 171) |
|---|---|---|
| Age, mean (SD), y | 58.1 (6.9) | 57.5 (7.9) |
| Weight, mean (SD), kg | 72.6 (14.1) | 76.6 (17.5) |
| BMI, mean (SD), kg/m2 | 27.3 (5.2) | 28.9 (6.4) |
| Postmenopausal, No. (%) | 141 (100) | 171 (100) |
| Receptor status, No. (%) | ||
| ER/PR negative | 141 (100) | 171 (100) |
| HER2 status, No. (%) | ||
| HER2 positive | 32 (22.7) | 27 (15.8) |
| HER2 negative | 109 (77.3) | 144 (84.2) |
| Any (neo)adjuvant chemotherapy, No. (%) | ||
| Yes | 139 (98.6) | 168 (98.2) |
| No | 2 (1.4) | 3 (1.8) |
| T stage, No. (%) | ||
| T1 | 55 (39.0) | 73 (42.7) |
| T2 | 77 (54.6) | 80 (46.8) |
| T3 | 9 (6.4) | 18 (10.5) |
| N stage, No. (%) | ||
| N0 | 94 (66.7) | 105 (61.4) |
| N1 | 34 (24.1) | 40 (23.4) |
| N2 | 8 (5.7) | 18 (10.5) |
| N3 | 5 (3.5) | 8 (4.7) |
BMI = body mass index; ER = estrogen receptor; PR = progesterone receptor.
Baseline SH Measurements
Estradiol levels were below the assay’s lower detection limit of 18.4 pmol/L in 24.1% of metformin and 26.2% of placebo patients (χ2 test P = .68). As noted previously, in the primary analysis, the estradiol levels of these cases were set to half the lower detection limit (9.2 pmol/L).
Baseline SH measurements are shown in Table 2. At baseline, the median estradiol was 32.2 vs 33.3 pmol/L, SHBG was 76.4 vs 72.8 nmol/L, and BT was 0.02 vs 0.03 nmol/L for metformin vs placebo, respectively.
Table 2.
Baseline sex hormones measurements and change from baseline to month 6 on metformin vs placebo arms
| Sex hormones | Baseline |
Changea |
|||
|---|---|---|---|---|---|
| Median (Q1, Q3*) |
Median (Q1, Q3) |
||||
| Metformin (n = 140) | Placebo (n = 170) | Metformin (n = 136) | Placebo (n = 168) | P b | |
| Estradiol, pmol/L | 32.2 (19.0, 45.5) | 33.3 (9.2, 56.4) | −5.7 (−18.6, 0) | 0 (−12.6, 14.7) | <.001 |
| BT, nmol/L | 0.02 (0.01, 0.05) | 0.03 (0.02,0.06) | 0 (−0.01, 0.01) | 0 (−0.01, 0.02) | .02 |
| SHBG, nmol/L | 76.4 (60.9, 112) | 72.8 (48.8, 105) | −5.9 (−15.6, 3.0) | −5.9 (−17.4, 1.5) | .43 |
Q1 and Q3 are the 25th and 75th percentiles. BT = bioavailable testosterone; SHBG =sex hormone-binding globulin.
Change calculated within patient as month 6 value minus baseline value.
P value from 2-sided Wilcoxon rank sum test comparing change between the two arms.
Change in SHs at 6 Months
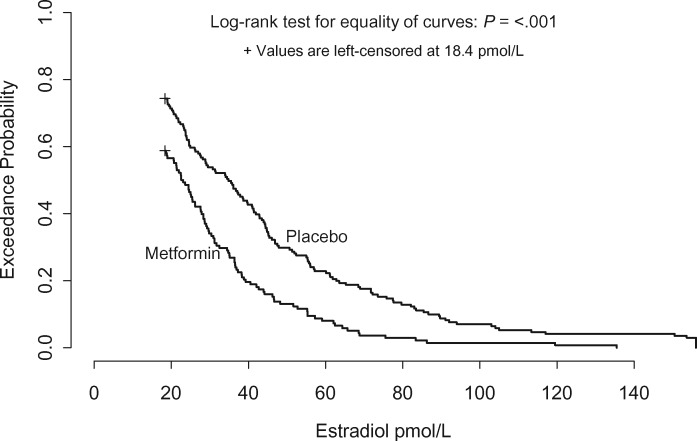
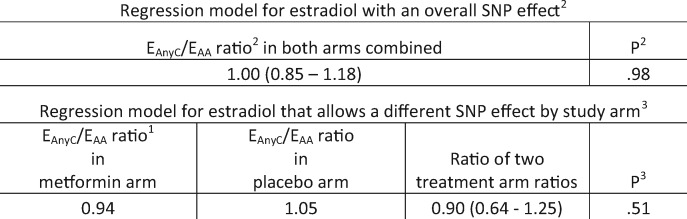
In the main analysis, the median change between baseline and month 6 in estradiol was 5.7 pmol/L in the metformin arm vs 0 in the placebo arm (Wilcoxon test P < .001; Table 2). The supplementary analysis supported this result. Figure 2 shows that the estradiol exceedance probabilities were consistently lower in the metformin than the placebo group (log-rank P < .001), for example, 56.9% vs 71.0% had estradiol levels above 20 pmol/L, 19.7% vs 42.6% had estradiol levels above 40 pmol/L, and 8.0% vs 23.1% had estradiol levels above 60 pmol/L, respectively. The multivariable regression model estimated that month 6 estradiol was approximately 30.1% (95% CI = 19.0% to 39.7%) lower in the metformin vs placebo groups. When adjustment for baseline BMI and BMI change was added to the basic model, the reduction in estradiol in metformin vs placebo subjects was 25.7% (95% CI = 13.2% to 36.4%), and when adjustment for change in insulin was added to the basic model, it was 30.1% (95% CI = 18.7% to 39.8%). In addition, examination of the interaction term in the regression model did not find evidence that the reduction in estradiol associated with the metformin arm was affected by the C allele of the SNP rs11212617 (Figure 3).
Figure 2.
Exceedance curves for estradiol at 6 months, by study arm. The curves give the proportion of patients whose estradiol was higher than any chosen cutoff on the x-axis, for example, 20% of metformin patients vs 43% of placebo patients had estradiol scores above 40 pmol/L.
Figure 3.
Linear regression model for change in estradiol, adjusted for baseline estradiol, with treatment (metformin vs placebo) and single nucleotide polymorphism (SNP; any C vs AA) as explanatory variables. 1EAnyC/EAA denotes the ratio of estradiol levels at month 6 for the 2 SNP levels, adjusted to the same baseline estradiol. 2Main effects model: log(month 6 estradiol)—log(baseline estradiol) as outcome and treatment status, SNP status, and baseline assay level as covariates. The P value is for the null hypothesis of no SNP effect, which in terms of back-transformed results is that the ratio EAnyC/EAA = 1. 3Interaction model: A treatment status by SNP status interaction was added to the main effects model. The P value is for the null hypothesis of no interaction effect, which in terms of back-transformed results is that EAnyC/EAA in the metformin arm equals EAnyC/EAA in the placebo arm.
In contrast to estradiol, median changes in SHBG and BT were similar in the metformin vs placebo arms (SHBG, −5.9 vs −5.9 nmol/L; P = .43; BT, 0 vs 0 nmol/L; P = .24), as observed in Table 2.
Discussion
Our observation of a statistically significant decrease in estradiol between baseline and month 6 in the metformin arm as compared with placebo is consistent with the work published by Campagnoli et al. (5, 6), who studied a selected postmenopausal nondiabetic BC population (50% of whom were receiving tamoxifen) who were required to have high baseline testosterone levels. In that study, a decrease in estradiol (−38%; P < .02) and in testosterone (−29%; P < .02) was seen in those receiving metformin 1500 mg/day (close to the 1700 mg/day administered in MA.32) vs metformin 1000 mg/day. The 30% reduction in estradiol we identified was similar to that seen by Campagnoli et al. Our population differs from the studies by Campagnoli et al. in that our subjects were not selected for high baseline levels of testosterone; this difference in entry criteria may account for our failure to identify changes in SHBG or BT with metformin. These observations suggest that the estradiol change we observed is independent of testosterone or of a potential effect of metformin on liver synthesis of SHBG.
Patterson et al. (7) reported reductions in estradiol, testosterone, and SHBG in overweight or obese BC patients receiving metformin; however, it was not clear whether the small reductions in estradiol that were observed (-10%, 95% CI = -18.5% to -1.5%) in those receiving metformin were due to co-administration of the lifestyle-based weight loss intervention. Small changes were also seen in testosterone and SHBG. As noted previously, metformin had no impact on SHBG, estradiol, testosterone, and dehydroepiandrosterone in 382 overweight, glucose-intolerant patients enrolled into the Diabetes Prevention Program (13).
Our study is the first to report the independent effect of metformin on estradiol in a placebo-controlled trial without co-intervention (tamoxifen or lifestyle intervention). The reduction we observed (approximately 30%) is substantial and of potential clinical relevance in breast cancer and possibly in women with hormone receptor-positive BC, although we did not study whether similar effects would have occurred in women receiving hormonal therapies for their BC. Our findings are also potentially relevant to other estrogen sensitive cancers, notably endometrial cancer for which observational data suggest strong associations of metformin with both risk and prognosis. Should beneficial effects of metformin be seen in our primary efficacy analysis in hormone receptor-positive BC, we plan to investigate effects of metformin on SHs, including estradiol, in this population. The independence of the observed reduction in estradiol from baseline BMI, BMI change, and insulin change suggests it did not occur as a result of loss of fat mass, nor is it associated with an insulin effect.
The mechanism by which metformin lowered estradiol remains unclear. Preclinical data have suggested that metformin may inhibit aromatase activity, potentially accounting for our observed reduction and also suggesting an additional mechanism of anticancer action of metformin. Both ER-positive breast cancer cells and breast adipose stromal cells exhibited reductions in aromatase mRNA levels in response to metformin treatment via mechanisms involving the suppression of promoter (PII) and P1.3-specific transcripts as well as activation of AMPK (18, 19).
Strengths of our study include its conduct in the setting of a placebo-controlled randomized trial in carefully selected postmenopausal women (thereby excluding menstrual cycle variability in sex hormones) who were not receiving hormonal therapy (thereby excluding potential confounding by these treatments). Limitations include the use of a nonhighly sensitive estradiol assay; just under 30% of estradiol assays yielded results below the lower detection limit. In our primary analyses, we assigned an estradiol level of half the lower detection limit. We performed an additional sensitivity analysis to generate exceedance curves that provided results similar to those obtained in our primary analysis. The similarity of our findings using 2 different methods of analysis, one designed specifically for censored values, reduces the likelihood that use of a more sensitive assay would have led to different findings. Furthermore, the selection criteria used in this substudy, particularly the requirement that subjects be on study medication for the 6-month blood draw, may have led to some imbalances between study arms. Additionally, the P values reported are not adjusted for multiple comparisons; thus the possibility of false-positives cannot be excluded.
In conclusion, metformin lowered estradiol levels, independent of BMI and insulin in nondiabetic, postmenopausal women with ER- and PR-negative BC enrolled onto MA.32 trial. This observation suggests a new mechanism of metformin action that may be relevant in breast and other estrogen-mediated cancers.
Funding
This work was supported by the Canadian Cancer Society Research Institute, National Cancer Institute (US), Breast Cancer Research Foundation (New York), Canadian Breast Cancer Foundation, Hold’Em for Life, and Apotex Canada (in kind donation of placebo and metformin).
Notes
Role of the funder: The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.Disclosures: Dr Goodwin reports research funding from the Breast Cancer Research Foundation, Epic Sciences, and Hold’Em for Life charities. Dr Mayer reports research funding from Genentech/Roche, Novartis, and Pfizer; consulting with Genentech/Roche, Novartis, Pfizer, Lilly, Abvie, GSK, Immunomedics, Macrogenics, Seattle Genetics, and PUMA. Dr Rastogi reports travel and accommodation support from Roche, AstraZeneca, and Lilly. Dr Rea reports research funding from Roche, Biotheranostics, RNA diagnostics, and Celgene; honoraria from Roche, Novartis, Pfizer, and Diachii-Sanyo; travel support from Diachii-Sanhyo, Novartis, and Eisai. Dr Bedard reports research funding from Bristol-Myers Squibb, Sanofi, AstraZeneca, Genentech/Roche, Servier, GSK, Novartis, SignalChem, PTC Therapeutics, Nektar, Merck, Seattle Genetics, Mersana, Immunomedics, and Lilly; consulting with BristolMyersSquibb, Sanofi, and Pfizer . The other authors have no conflicts of interest to declare.
Data Availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Author contributions: BEC: Formal analysis; Investigation; Methodology; Writing—original draft; Writing—review & editing. AEL: Writing—original draft; Writing—review & editing. ME: Formal analysis; Methodology; Writing—original draft; Writing—review & editing. JL: Writing—original draft; Writing—review & editing. LS: Writing—original draft. DLH: Writing—original draft; Writing—review & editing. TW: Writing—original draft; Writing—review & editing. VS: Writing—original draft; Writing—review & editing. IM: Writing—original draft; Writing—review & editing. TH: Writing—original draft; Writing—review & editing. JL: Writing—original draft; Writing—review & editing. AT: Writing—original draft; Writing—review & editing. PR: Writing—original draft; Writing—review & editing. KG: Writing—original draft; Writing—review & editing. DR: Writing—original draft; Writing—review & editing. MR: Writing—original draft; Writing—review & editing. SE: Writing—original draft. MM: Writing—original draft; Writing—review & editing. PB: Writing—original draft; Writing—review & editing. LP: Writing—original draft; Writing—review & editing. TV: Writing—original draft; Writing—review & editing. RJOD: Writing—original draft; Writing—review & editing. WP: Writing—original draft; Writing—review & editing. PJG: Investigation; Methodology; Writing—original draft; Writing—review & editing.
Acknowledgments: Canadian Clinical Trials Group.
References
- 1.Canadian Cancer Trials Group. MA.32 Clinical Trial: A Phase III Randomized Trial of Metformin vs Placebo in Early Stage Breast Cancer. https://clinicaltrials.gov/ct2/show/NCT01101438.
- 2. Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N.. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67(22):10804–10812. [DOI] [PubMed] [Google Scholar]
- 3. Niraula S, Dowling RJ, Ennis M, et al. Metformin in early breast cancer: a prospective window of opportunity neoadjuvant study. Breast Cancer Res Treat. 2012;135(3):821–830. [DOI] [PubMed] [Google Scholar]
- 4. Dowling RJ, Niraula S, Chang MC, et al. Changes in insulin receptor signaling underlie neoadjuvant metformin administration in breast cancer: a prospective window of opportunity neoadjuvant study. Breast Cancer Res. 2015;17(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campagnoli C, Pasanisi P, Abbà C, et al. Effect of different doses of metformin on serum testosterone and insulin in non-diabetic women with breast cancer: a randomized study. Clin Breast Cancer. 2012;12(3):175–182. [DOI] [PubMed] [Google Scholar]
- 6. Campagnoli C, Berrino F, Venturelli E, et al. Metformin decreases circulating androgen and estrogen levels in nondiabetic women with breast cancer. Clin Breast Cancer. 2013;13(6):433–438. [DOI] [PubMed] [Google Scholar]
- 7. Patterson RE, Marinac CR, Sears D, et al. The effects of metformin and weight-loss on biomarkers associated with breast cancer. J Natl Cancer Inst. 2018;110(11):1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lohmann AE, Pimentel I, Goodwin PJ.. Novel insights into the impact of lifestyle-based weight loss and metformin on obesity-associated biomarkers in breast cancer. J Natl Cancer Inst. 2018;110(11):1161–1162. [DOI] [PubMed] [Google Scholar]
- 9. Folkerd E, Dowsett M.. Sex hormones and breast cancer risk and prognosis. Breast. 2013;22(Suppl 2):S38–43. [DOI] [PubMed] [Google Scholar]
- 10. Rock CL, Flatt SW, Laughlin GA, et al. Reproductive steroid hormones and recurrence-free survival in women with a history of breast cancer. Cancer Epidemiol Biomarkers. 2008;17(3):614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zeleniuch-Jacquotte A, Shore RE, Koenig KL, et al. Postmenopausal levels of oestrogen, androgen, and SHBG and breast cancer: long-term results of a prospective study. Br J Cancer. 2004;90(1):153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hetemäki N, Savolainen-Peltonen H, Tikkanen MJ, et al. Estrogen metabolism in abdominal subcutaneous and visceral adipose tissue in postmenopausal women. J Clin Endocrinol Metabol. 2017;102(12):4588–4595. [DOI] [PubMed] [Google Scholar]
- 13. Kim C, Kong S, Laughlin GA, et al. Endogenous sex hormone changes in postmenopausal women in the diabetes prevention program. J Clin Endocrinol Metab. 2012;97(8):2853–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou K, Bellenguez C, Spencer CC, MAGIC investigators, et al. Common variants near ATM are associated with glycemic response to metformin in type 2 diabetes. Nat Genet. 2011;43(2):117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cuyàs E, Buxó M, Ferri Iglesias MJ, et al. The C Allele of ATM rs11212617 associates with higher pathological complete remission rate in breast cancer patients treated with neoadjuvant metformin. Front Oncol. 2019;9:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Helsel DR. Fabricating data: how substituting values for nondetects can ruin results, and what can be done about it. Chemosphere. 2006;65(11):2434–2439. [DOI] [PubMed] [Google Scholar]
- 17. Helsel DR. Nondetects and Data Analysis: Statistics For Censored Environmental Data Hoboken: Wiley-Interscience; 2005. [Google Scholar]
- 18. Samarajeewa NU, Ham S, Yang F, Simpson ER, Brown KA.. Promoter-specific effects of metformin on aromatase transcript expression. Steroids. 2011;76(8):768–771. [DOI] [PubMed] [Google Scholar]
- 19. Brown KA, Hunger NI, Docanto M, Simpson ER.. Metformin inhibits aromatase expression in human breast adipose stromal cells via stimulation of AMP-activated protein kinase. Breast Cancer Res Treat. 2010;123(2):591–596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Author contributions: BEC: Formal analysis; Investigation; Methodology; Writing—original draft; Writing—review & editing. AEL: Writing—original draft; Writing—review & editing. ME: Formal analysis; Methodology; Writing—original draft; Writing—review & editing. JL: Writing—original draft; Writing—review & editing. LS: Writing—original draft. DLH: Writing—original draft; Writing—review & editing. TW: Writing—original draft; Writing—review & editing. VS: Writing—original draft; Writing—review & editing. IM: Writing—original draft; Writing—review & editing. TH: Writing—original draft; Writing—review & editing. JL: Writing—original draft; Writing—review & editing. AT: Writing—original draft; Writing—review & editing. PR: Writing—original draft; Writing—review & editing. KG: Writing—original draft; Writing—review & editing. DR: Writing—original draft; Writing—review & editing. MR: Writing—original draft; Writing—review & editing. SE: Writing—original draft. MM: Writing—original draft; Writing—review & editing. PB: Writing—original draft; Writing—review & editing. LP: Writing—original draft; Writing—review & editing. TV: Writing—original draft; Writing—review & editing. RJOD: Writing—original draft; Writing—review & editing. WP: Writing—original draft; Writing—review & editing. PJG: Investigation; Methodology; Writing—original draft; Writing—review & editing.
Acknowledgments: Canadian Clinical Trials Group.