Abstract
Background
Early antiretroviral therapy (ART) is recommended for infants with human immunodeficiency virus (HIV) infection. However, few antiretroviral options are available for neonates.
Methods
The Early Infant Treatment Study in Botswana tested HIV-exposed infants within 96 hours of birth, and HIV-infected infants started nevirapine (NVP) 6 mg/kg twice daily, zidovudine (ZDV), and lamivudine (3TC) at age < 7 days. NVP trough concentrations were tested at 1 and 2 weeks. NVP was switched to ritonavir-boosted lopinavir (LPV/r) at week 2, 3, 4, or 5 according to delivery gestational age.
Results
Forty HIV-infected infants started ART at median age 2 days (range, 1–5 days). NVP trough concentrations were highly variable and below therapeutic target (3000 ng/mL) for 50% of 2-week measurements; concentrations did not correlate with viral decline at weeks 2, 4, or 12. Two deaths unrelated to ART occurred through 24 weeks. Only 1 unscheduled treatment modification was required. Within 4 weeks of transition to LPV/r, 9 (22.5%) had transient HIV RNA increases, likely due to poor LPV/r palatability. At 12 weeks, 22 (55%) of 40 were <40 copies/mL (93% <400 copies/mL); by 24 weeks, 27 of 38 (71%) were < 40 copies/mL (84% < 400 copies/mL). HIV-1 RNA response at 12 and 24 weeks did not differ by baseline HIV RNA or other factors.
Conclusions
NVP/ZDV/3TC started in the first week of life was safe and effective, even when trough NVP levels were below target. Transient viral increases occurred following transition to LPV/r, but by 12 and 24 weeks most children achieved and maintained viral suppression.
Clinical Trials Registration
Keywords: early infant treatment, combination antiretroviral drugs, safety, efficacy, Botswana
Despite limited neonatal HIV treatment options, 71% of infants who initiated nevirapine/zidovudine/lamivudine in the first week of life and transitioned to ritonavir-boosted lopinavir/zidovudine/lamivudine at 2–5 weeks achieved viral suppression < 40 copies/mL at 24 weeks, with few adverse events.
(See the Editorial Commentary by Kuhn on pages 394–5.)
Early infant human immunodeficiency virus (HIV) diagnosis and early initiation of antiretroviral therapy (ART) are critical in the global fight against HIV in children [1–3], as high mortality and rapid disease progression occur in untreated HIV-infected infants [1–4]. World Health Organization [5] and other international pediatric guidelines [6–8] recommend prompt initiation of ART regardless of clinical, immunologic, and virological status [7–9]. However, HIV testing immediately following birth does not occur in most countries [10, 11], available drug formulations are limited in the first month of life [10–13], and there are limited pharmacokinetic (PK), safety, and efficacy data for neonatal ART [12, 13].
Nevirapine (NVP), zidovudine (ZDV), and lamivudine (3TC) have been used most frequently in early infancy [1, 14–16]. While all are extensively used for prophylaxis [16, 17], the optimal treatment dose for NVP in particular is not established [15, 17]. Ritonavir-boosted lopinavir (LPV/r) is approved beginning at 2 weeks of age or the equivalent of 40 weeks’ gestation, but it is not approved for younger neonates because of concerns about toxicities and endocrine effects in preterm infants [18–20].
In 2015, we launched the Early Infant Treatment Study (EIT) in Botswana to diagnose and treat HIV-infected infants starting in the first week of life. The primary objective of this analysis was to demonstrate that ART with NVP, ZDV, and 3TC can be initiated safely very early in life, with early transition to LPV/r-based ART, resulting in rapid viral decay for most infants.
METHODS
Study Design
EIT was an open-label clinical trial of very early ART in HIV-infected infants conducted by the Botswana-Harvard AIDS Institute Partnership (BHP) in Botswana. The antepartum cohort consisted of EIT-enrolled children ≥ 35 weeks’ gestation and ≥ 2000 g who tested HIV positive < 96 hours after birth and were able to initiate ART < 7 days after birth. The study was implemented at 2 BHP clinical research sites in Botswana (Princess Marina Hospital in Gaborone and Nyangabgwe Hospital in Francistown), but screening was conducted more widely at selected maternity wards (within a radius of 200 km from Gaborone or Francistown).
Screening, Enrollment, Dosing, and Follow-up
Trained research assistants approached HIV-infected women after delivery, introduced the study, and offered infant HIV testing. Following the mother’s consent for screening, infants were tested for HIV as soon as possible and no later than 96 hours after birth. Blood was collected by heel-stick, and dried blood spots were sent to national HIV laboratories in Gaborone and Francistown for DNA polymerase chain reaction (PCR) testing. HIV infection was defined as DNA PCR–positive or indeterminate on an initial screening specimen, followed by a positive confirmatory specimen.
Following maternal consent, infants with positive HIV screening initiated ART at enrollment with treatment doses of NVP (6 mg/kg twice daily [BID]), ZDV (4 mg/kg BID), and 3TC (2 mg/kg BID) as the initial regimen. Per protocol, the regimen was switched to LPV/r, ZDV, and 3TC at week 2, 3, 4, or 5 according to delivery gestational age (≥ 38, 37, 36, or 35 weeks’ gestation, respectively). This regimen was the recommended ART regimen for infants in Botswana throughout the study period. LPV/r was dosed based on recommended Botswana/World Health Organization (WHO) weight bands, updated at each study visit [6]. At 4 weeks of age, ZDV was increased to 8 mg/kg BID, and 3TC was increased to 4 mg/kg BID; from age 6 weeks onward, Botswana/WHO weight bands were used.
Infants were followed longitudinally and evaluated regularly for safety, adherence, and viral suppression. This analysis focuses on the short-term outcomes through 24 weeks of ART, including HIV type 1 (HIV-1) RNA, and laboratory and clinical safety evaluations at weeks 1, 2, 4, 8, 12, and 24 of ART. PK samples and associated dosing information were collected at 1 and 2 weeks of study treatment (all on initial ART regimen), sampled just prior to morning ART dosing to represent a trough concentration. Protocol-directed treatment modification was required for confirmed symptomatic hematologic toxicities grade 3 or higher, and for rashes grade 3 or higher on NVP.
Laboratory Methods
Samples were tested for HIV-1 DNA utilizing Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 Qualitative PCR (Roche Diagnostics, Mannheim, Germany) on dried blood spot at the Botswana-Harvard HIV Reference Laboratory in Gaborone and the National HIV Reference Laboratory in Francistown, Botswana. Plasma HIV-1 RNA was tested by Coulter Abbott m2000sp/m2000rt (Abbott Molecular, Des Plaines, Illinois). Quantitative cell-associated HIV-1 DNA was by amplification of LTR-gag segment by digital-droplet PCR. Genotyping was performed for suspected virologic failure as previously described [21].
Trough NVP concentrations were evaluated for the first 30 infants at the Division of Clinical Pharmacology and Developmental Therapeutics, University of California, San Diego. Plasma NVP concentrations were determined by a validated liquid chromatography–mass spectrometry assay [22] because of small volumes. The lower limit of quantification of the assay was 43 ng/mL for NVP.
Statistical Methods
For primary outcomes, 95% confidence intervals were calculated using the exact Clopper-Pearson method. Analyses were intent-to-treat and include all follow-up through 24 weeks. Exact logistic regression evaluated associations with virologic outcome. Wilcoxon rank-sum tests compared HIV-1 RNA measures between specified groups. The level of statistical significance was .05 (2-sided). SAS version 9.4 software (SAS Institute, Cary, North Carolina) was used for all statistical analyses.
Ethical Review and Monitoring
The clinical trial was approved by the ethics committees in Botswana and at the Harvard T. H. Chan School of Public Health. The study was reviewed at least annually by the National Institute of Allergy and Infectious Diseases Africa Data and Safety Monitoring Board.
RESULTS
ART Initiation
Between April 2015 and July 2018, 10 622 infants were screened, 42 (0.4%) were confirmed as HIV infected, and 40 enrolled in the study. The median age at HIV screening was 1 day after birth (range, 0–4 days), and median age at start of ART was 2 days after birth (range, 1–5 days). Table 1 provides a summary of selected baseline and HIV characteristics for the 40 mothers and 40 infants enrolled in the antepartum cohort of EIT. Similar to other cohorts of HIV-infected children [23, 24], there was a female predominance; 28 of 40 (70%) enrolled children were female.
Table 1.
Baseline Maternal and Infant Characteristics
| Baseline Characteristicsa | No. (%) |
|---|---|
| Maternal characteristics (n = 40) | |
| Age, y, median (IQR) | 27 (22–30) |
| CD4 count, cells/μL, median (IQR) | 348 (222–567) |
| HIV-1 RNA, log10 copies/mL, median (IQR) | 4.38 (2.77–4.91) |
| ART regimen in pregnancy | |
| None | 17 (43) |
| EFV/TDF/FTC | 10 (25) |
| DTG/TDF/FTC | 11 (28) |
| Other | 2 (5) |
| Education, highest level attained | |
| None/primary | 4 (10.0) |
| Secondary | 33 (82.5) |
| Tertiary | 3 (7.5) |
| Employment status | |
| Salaried | 4 (10.0) |
| Domestic work | 1 (2.5) |
| Self-employed/temporary | 7 (17.5) |
| Unemployed | 28 (70.0) |
| Infant characteristics (n = 40) | |
| Female sex | 28 (70) |
| Gestational age at birth, wk | |
| 35 | 5 (12.5) |
| 36 | 7 (17.5) |
| 37 | 4 (10.0) |
| 38–41 | 24 (60.0) |
| Birthweight, kg, median (IQR) | 3.0 (2.6–3.1) |
| HIV-1 RNA, log10 copies/mL, median (IQR) | 4.05 (2.79–4.86) |
| CD4%, median (IQR) | 50 (38–56) |
| Age at HIV screening, d, median (range) | 1 (0–4) |
| Age at ART start, d, median (range) | 2 (1–5) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; DTG, dolutegravir; EFV, efavirenz; FTC, emtricitabine; HIV-1, human immunodeficiency virus type 1; IQR, interquartile range; TDF, tenofovir disoproxil fumarate.
aUnless otherwise noted, “baseline” indicates day of enrollment in the study.
Prior to enrollment, all HIV-exposed children had received ZDV and at least a single dose of NVP, and infants considered high-risk for HIV infection may have also received 3TC. At enrollment into EIT, all infants were converted to treatment doses of NVP, ZDV, and 3TC. Infants were then switched to LPV/r, ZDV, and 3TC at a median of 19.5 days of life (range, 15–41 days of life), per protocol. Children were switched to LPV/r during the scheduled week for all but 1 child, born at 37 weeks’ gestation but switched at 2.7 weeks of age.
NVP Pharmacokinetics
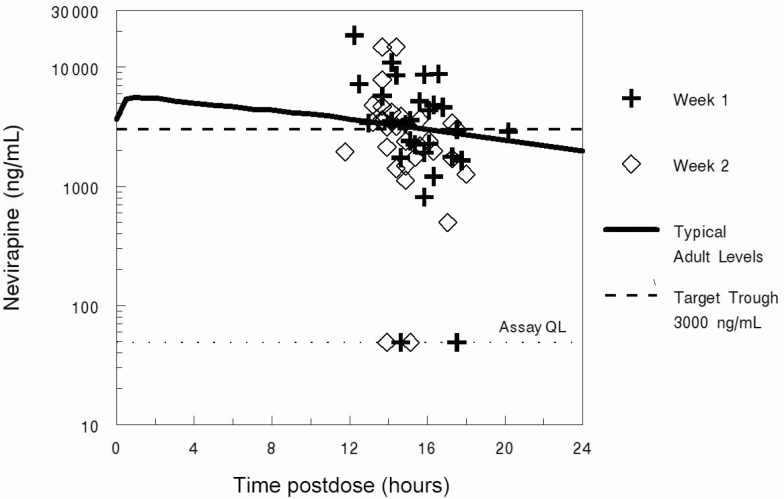
Median NVP trough concentration was 3300 (interquartile range [IQR], 1777–5160) ng/mL at 1 week and 2700 (IQR, 1719–3638) ng/mL at 2 weeks on treatment (at a median of 15.6 and 14.5 hours from last dose, respectively). At 1 week, 14 of 29 (48.3%) infants tested were below the ideal therapeutic target for NVP of 3000 ng/mL, and at 2 weeks, 15 of 30 (50.0%) infants tested were below the ideal therapeutic target. This included 5 troughs below the limit of quantification (3 at week 1, 2 at week 2), indicating nonadherence. However, 87% of troughs were > 1000 ng/mL, and overall these values fell in the range of typical values seen for most studies of NVP in adults [25, 26] (Figure 1). In addition, there was no significant association between target vs subtarget NVP concentrations (above or below 3000 ng/mL) at 2 weeks and the change in log10 HIV-1 RNA at 2 weeks (P = .97), 4 weeks (P = 1.00), or 12 weeks (P = .87).
Figure 1.
Nevirapine pharmacokinetics. Abbreviation: QL, quantification limit.
Safety
Table 2 shows treatment modifications and adverse events through week 24. By week 24, 2 deaths had occurred (1 from acute diarrheal illness at week 23, and the other from suspected pneumonia at week 21), and none were lost to follow-up. There were 2 grade 3 and 4 grade 4 neutropenias, and 1 grade 3 thrombocytopenia, in the first 24 weeks of study treatment, for a total of 7 (18%) infants with grade 3 or higher hematologic events. All but 1 hematologic event resolved without need for ART modification; 1 child with persistent neutropenia switched from ZDV to abacavir at week 24, with resolution. There were a total of 10 grade 3 or higher diagnoses (grade 2 or higher for rash) reported among 7 (17.5%) infants in the first 24 weeks of study follow-up. Of these events, 5 (50%) were diagnosed before the study-specified switch to LPV/r, but none occurred in the first 2 weeks of NVP treatment.
Table 2.
Treatment Modification and Adverse Events Through 24 Weeks
| Event | No. (%) |
|---|---|
| Modification of NVP/ZDV/3TC regimen due to AE prior to transition to LPV/r at 2–5 wk | 0 (0) |
| Modification of LPV/r/ZDV/3TC due to AE from LPV/r start through 24 wk | 1 (2.5)a |
| Deaths | 2 (5) |
| Hematologic toxicity (grade 3/4) | |
| Anemia | 0 (0) |
| Neutropenia | 6 (15) |
| Thrombocytopenia | 1 (2.5) |
| Diagnosesb (grade 2 rash, otherwise grade 3/4) | 10 |
Abbreviations: 3TC, lamivudine; AE, adverse event; LPV/r, ritonavir-boosted lopinavir; NVP, nevirapine; ZDV, zidovudine.
aIncludes all 40 children; no ART modification occurred among the 2 children who died prior to 24 weeks.
bTen diagnoses were among 7 children. One child had 2 instances of grade 2 rash (while on NVP at weeks 3–4) and 1 instance of grade 3 adenoid hypertrophy with chronic/severe undernutrition. All remaining diagnoses were in 7 unique children: 1 grade 2 rash, 2 instances of grade 3 sepsis, 1 instance of grade 3 electrolyte imbalance (grade 3 hyperkalemia), 1 instance of grade 3 pneumonia (suspected), 1 instance of grade 3 pneumonia (chest radiograph confirmed), and 1 instance of grade 3 diarrhea or gastroenteritis.
Efficacy
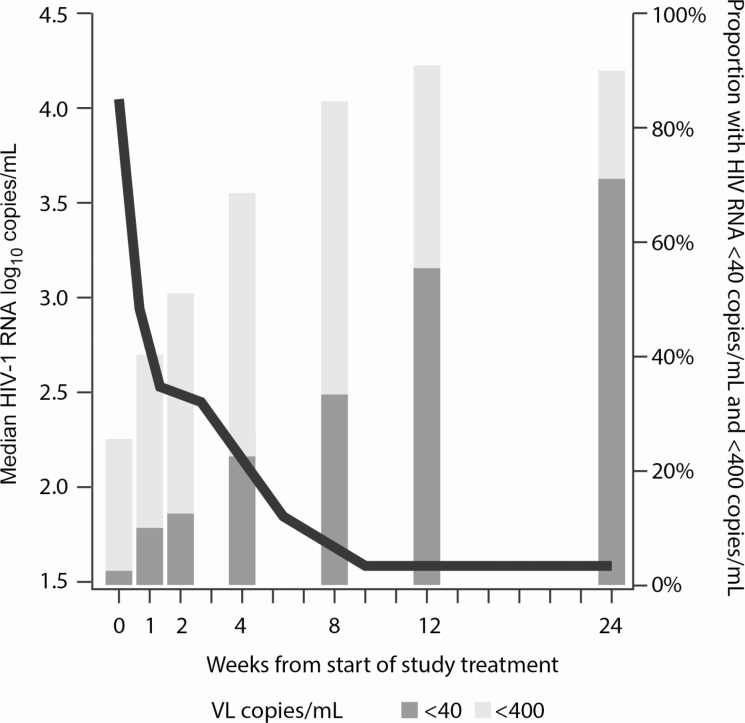
Infant plasma HIV RNA declined from a median of 4.05 (IQR, 2.79–4.86) log10 copies/mL at baseline to 2.54 (IQR, 1.86–3.21) log10 copies/mL at 2 weeks, and fell to < 1.60 (IQR, < 1.60–1.93) log10 copies/mL at 12 weeks and remained < 1.60 (IQR, < 1.60–2.16) log10 copies/mL at 24 weeks. During the first 2 weeks of NVP-based treatment, 37 of 40 (92.5%) had an HIV RNA decline; of the remaining 3 children, 2 were thought to be nonadherent to ART (including 1 of the 2 deaths by week 24) and the third went from a baseline HIV RNA of 113 copies/mL to a maximum of 133 copies/mL at week 2 with subsequent decline to < 40 copies/mL by week 12. At 12 weeks of ART, 22 (55.0%) had HIV RNA < 40 copies/mL, and 37 (92.5%) had HIV RNA < 400 copies/mL (Figure 2). At 24 weeks, among the 38 infants still alive, 27 (71%) had HIV RNA < 40 copies/mL, and 32 (84%) had HIV RNA < 400 copies/mL.
Figure 2.
Median HIV RNA and proportion suppressed to <40 copies/mL and <400 copies/mL, by treatment week. Abbreviations: HIV, human immunodeficiency virus; VL, viral load.
Although limited by the available number of children in the cohort, we evaluated risk factors for HIV RNA suppression to < 40 copies/mL at 12 weeks and 24 weeks. We did not identify differences in suppression at either time point by week 2 NVP trough concentration, sex, or maternal or infant log10 HIV-1 RNA at baseline, whether the mother was on ART during pregnancy (either started prior to or during the pregnancy), or other factors. The timing of the switch from NVP-based to LPV/r-based ART (and the gestational age at birth) also did not predict the likelihood of suppression at either time point. Clinical genotyping was available for sustained virologic failure, but it was rarely performed within the first 24 weeks while adherence measures were being attempted; of the 6 infants genotyped by 24 weeks while on LPV/r-based ART, 3 failed to amplify, and of the other 3 that amplified, 1 had M184V, 1 had M184V and K103N, and the last had no clinically significant mutations.
Adherence
Reported adherence in the first 24 weeks was high, with only 5 (13%) infants missing 1 or more complete days of ART by maternal report (2 missed 1 complete day, 2 missed 2 complete days, and 1 missed > 2 complete days of ART). However, adherence evaluation did not account for infants spitting out doses because of poor palatability, which was widely reported by mothers and observed by study nurses following the switch to LPV/r. When complete doses were spit out, mothers were advised to redose the infant, but redosing could not be confirmed. When we evaluated the 4-week period following transition to LPV/r-based ART for each child, 9 (22.5%) had transient increases in HIV-1 RNA, thought to be related to spitting out LPV/r.
DISCUSSION
We performed a comprehensive study of HIV treatment starting from the first week of life. Our 3 major findings were that starting NVP, ZDV, and 3TC at treatment dosages was safe and effective; that all infants could be successfully transitioned to LPV/r despite its poor initial tolerability; and that most children achieved and maintained HIV-1 RNA suppression by 12 and 24 weeks of treatment.
This is the largest assessment of nevirapine trough concentrations in infants at 1 and 2 weeks using the treatment dose of 6 mg/kg twice daily. This dosage is several-fold higher than used for prophylaxis and is greater on a milligram per kilogram basis than the adult treatment dose. Trough concentrations were highly variable and somewhat below the values predicted by Cressey et al [27], but similar to the expected range based on adult studies [28]. Although 50% fell below the recommended therapeutic target of 3000 ng/mL [25, 28, 29], the late collection of troughs (averaging 15.2 hours postdose) likely contributed to these lower observed values. More importantly, we did not detect association of NVP concentrations with viral suppression at 2, 4, or 12 weeks, suggesting good NVP antiviral activity across the study population. This finding is in contrast to expectations based on the consensus dosing recommendation for adults, where troughs > 3000 ng/mL have been associated with a higher likelihood of therapeutic response [30, 31]. Of note, the half-maximal inhibitory concentration (IC50) for NVP is 24 ng/mL [31], which is far below the target threshold, and all infants with quantifiable NVP levels were at least 20-fold above this IC50; these considerations, combined with our favorable safety and virologic suppression data, suggest that the 3000 ng/mL target may be unnecessarily high (and perhaps difficult to achieve without added toxicity) for infant treatment, and that 6 mg/kg BID dosing appears to be adequate.
Prior studies for older infants and children have reported superior efficacy starting LPV/r-based ART rather than NVP-based ART [32, 33]. Our study began in 2015, when the only 3-drug ART option for neonates was NVP, ZDV, 3TC, and we demonstrate that starting with NVP and switching to LPV/r at the earliest possible time was a successful strategy. Safety data were reassuring for our study cohort, with only 1 child requiring a treatment modification through 24 weeks. Despite the fact that 25% of mothers had received EFV-based ART in pregnancy, we noted nearly universal early responses to the NVP-based regimen, followed by a generally successfully transition to LPV/r-based ART at or after 2 weeks. For preterm infants, this transition was earlier than current labeling for LPV/r (recommended at 42 weeks’ gestational age equivalent), but we noted no adverse events. However, caretakers widely reported poor taste tolerability and frequent spitting out of LPV/r doses, especially in the first few weeks following the transition, and 9 (22.5%) infants had transient increase in HIV-1 RNA in the 4 weeks following the transition to LPV/r. Fortunately, with intensive adherence counseling and teaching caretakers to place the LPV/r dose in the cheek rather than the tongue, HIV-1 RNA resumed its decline for almost all infants following this early transition period.
Our 24-week virologic suppression findings were higher than those observed in other early infant treatment studies, though few have been performed from the first week of life, limiting comparability. A recent South African study that initiated very early ART reported just over 50% with HIV RNA < 400 copies/mL at 6 months, and > 10% mortality [34]. Similarly, in several earlier pediatric cohorts in Johannesburg, only 25%–49% achieved 6-month viral suppression to < 50 copies/mL [35], and only 29% achieved 6-month viral suppression to < 50 copies/mL among infants starting ART < 3 months of age in a cohort in Cape Town [36]. In contrast, 71% of infants in the EIT study had HIV-1 RNA < 40 copies/mL at the 24-week visit. We attribute the high rate of suppression to our 100% study retention, to extensive adherence counseling and training given to caregivers, and possibly to starting ART from the first week of life before clinical decline and extensive viral reservoir seeding could occur.
Our study had several limitations. We studied only 1 sequence of ART regimens (NVP- to-LPV/r-based), and the transition to LPV/r-based ART in the neonatal period depended on gestational age at birth. Newer antiretrovirals such as raltegravir are now available for neonates, and despite formulation complexities [37, 38], these may offer additional options for infant ART that do not require drug switches. We had limited PK assessments (trough values only), rather than a comprehensive PK assessment of each dose. We did not have baseline resistance testing available in the study and could not assess for transmitted drug resistance, which was especially possible for the 25% whose mothers received the nonnucleoside reverse transcriptase inhibitor efavirenz in pregnancy. We were unable to identify specific risk factors for lack of virologic suppression at 12 or 24 weeks, but our cohort size allowed little power to evaluate risk factors and larger studies will be required to identify specific determinants of successful early treatment. Finally, our study was a closely monitored, resource-intensive clinical trial and therefore may not reflect the likelihood of treatment success in nonstudy settings.
Accumulating data support the benefits of early infant treatment. Our study demonstrates the feasibility of advancing the initiation of diagnosis and treatment to the very first week of life (with repeat HIV testing later in infancy to detect intrapartum transmissions). Starting ART in the first week has several proven and potential benefits. First, in the setting where hospital delivery occurs, it reduces loss to follow-up by providing a diagnosis and treatment before leaving the hospital in most cases. Second, clinical progression is rapid in the first year of life, and even a 6-week head start on treatment can make a difference [1–4]. Third, very early ART reduces reservoir seeding, which may have long-term benefits [39]. Finally, preservation of immune responses may be more likely among very early treated infants [40], with possible long-term effects on immune development and even HIV control in later life.
In conclusion, a pediatric HIV treatment strategy starting NVP, ZDV, and 3TC in the first week of life and then transitioning to LPV/r, ZDV, and 3TC, was safe and effective in Botswana. Although half of infants had trough NVP levels below the standard therapeutic PK target, there was no apparent association with subsequent virologic success, and our study supports a NVP treatment dose of 6 mg/kg BID from the first week of life. All infants were successfully transitioned to LPV/r-based ART at 2–5 weeks, although subsequent transient viral increases were noted that were thought to be related to spitting out LPV/r. By 12 and 24 weeks, most children achieved and maintained HIV RNA declines to < 40 copies/mL, setting the stage for successful HIV treatment throughout infancy and for the years to follow.
Notes
Acknowledgments. The authors thank the Early Infant Treatment Study (EIT) Study participants and their families, and additional members of the EIT Study team: Tlhabologo Baitseme, Thelma Ketshabile, Maduo Oabona, Mogomotsi Modikwa, Salome Othusitse, Obakeng Makalane, Rabana Thuto Aeshar, Maipelo Kegakilwe, Nametsegang Tshosa, Dorcus Babuile, Jamie Ramaabya, Simon Mosopa, Muchaneta Bhondai, Kebaiphe Moabi, Tebogo Mokotedi, Boitumelo Tebape, Kesegofetse Agisanang, Masego Rabantetse, Disaro Jack, Tumalano Sekoto, Thabani Ncube, Lorato Esele, Princess Bobo Mapenshi, Comfort Maphorisa, Tshepho Frank, Laura Vaughan, Chloe Aluetta-Young, Morgan Packer, Ria Madison, Mompati Mmalane, Ron Bosch, Max Essex, Timothy Henrich, Mogomotsi Matshaba, Loeto Mazhani, Kenneth McIntosh, Madisa Mine, Chipo Petlo, Kathleen Powis, Xu Yu, Lendsey Melton, and Lucia Ricci. The authors also thank the Botswana Ministry of Health and Wellness, the Botswana Human Research Development Committee, the Harvard Office of Human Research Administration, the EIT data and safety monitoring board members, and the National Institutes of Health (Judi Miller, Ellen Decarlo, and Sheryl Zwerski).
Financial support. This work was supported by National Institute of Allergy and Infectious Diseases (grant number U01AI14235). S. L. was supported by Harvard Center For AIDS Research grant (grant numbers U01 AI14235 and P30 AI060354).
Potential conflicts of interest. K. B. reports personal fees for biostatistical analysis from Harvard T. H. Chan School of Public Health. E. C. reports personal fees from Nabriva, Celltrion, and Rempex, outside the submitted work. M. H. reports grants from NIH. M. H. also reports that his wife receives HIV-related grant support, paid to her institution. D. K. reports grant support from AbbVie, Gilead, and Merck, and personal fees from Gilead, GlaxoSmithKline, Janssen, Merck, and ViiV, outside the submitted work. All other authors have no potential conflicts to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Cotton MF, Holgate S, Nelson A, et al. The last and first frontier—emerging challenges for HIV treatment and prevention in the first week of life with emphasis on premature and low birth weight infants. J Intern AIDS Society 2015; 18 (Suppl 6):20271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cotton MF, Violari A, Otwombe K, et al. CHER Study Team Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet 2013; 382:1555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lilian RR, Kalk E, Technau KG, Sherman GG. Birth diagnosis of HIV infection in infants to reduce infant mortality and monitor for elimination of mother-to-child transmission. Pediatr Infect Dis J 2013; 32:1080–5. [DOI] [PubMed] [Google Scholar]
- 4. Desmonde S, Coffie P, Aka E, et al. Severe morbidity and mortality in untreated HIV-infected children in a paediatric care programme in Abidjan, Côte d’Ivoire, 2004-2009. BMC Infect Dis 2011; 11:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treatment and prevention HIV infection: recommendations for a public health approach. 2nd ed2016. Available at: http://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed 20 April 2019. [PubMed] [Google Scholar]
- 6. Botswana Ministry of Health. National HIV and AIDS treatment guidelines, 2012 version Available at: http://www.moh.gov.bw. Accessed 23 April 2019.
- 7. South Africa Department of Health. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. South Africa: National Department of Health, 2015. Available at: https://aidsfree.usaid.gov/sites/default/files/tx_south-africa_pmtc t_2015.pdf . Accessed 16 March 2019. [Google Scholar]
- 8. Bamford A, Turkova A, Lyall H, et al. PENTA Steering Committee Paediatric European Network for Treatment of AIDS (PENTA) guidelines for treatment of paediatric HIV-1 infection 2015: optimizing health in preparation for adult life. HIV Med 2018; 19:e1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barlow-Mosha L, Musiime V, Davies MA, et al. Universal antiretroviral therapy for HIV-infected children: a review of the benefits and risks to consider during implementation. J Int AIDS Soc 2017; 20:21552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jean-Philippe P, Spiegel H, Gnanashanmugam D, et al. HIV birth testing and linkage to care for HIV-infected infants. AIDS 2017; 31:1797–807. [DOI] [PubMed] [Google Scholar]
- 11. Abrams EJ Birth testing for HIV diagnosis in children: considerations and controversies. Available at: https://aidsfree.usaid.gov/sites/default/files/birth_testing.pdf. Accessed 19 August 2019. [Google Scholar]
- 12. Abrams EJ, Ananworanich J, Archary M, Ngongondo M, Brouwers P. Propelling the pediatric HIV therapeutic agenda with science, innovation, and collaboration. J Acquir Immune Defic Synd 2018; 78(Suppl 1):S32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Penazzato M, Gnanashanmugam D, Rojo P, et al. Paediatric Antiretroviral Working Group (PAWG) Optimizing research to speed up availability of pediatric antiretroviral drugs and formulations. Clin Infect Dis 2017; 64:1597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mirochnick M, Nielsen-Saines K, Pilotto JH, et al. NICHD HPTN 040/PACTG 1043 PROTOCOL Team Nelfinavir and lamivudine pharmacokinetics during the first two weeks of life. Pediatr Infect Dis J 2011; 30:769–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fillekes Q, Mulenga V, Kabamba D, et al. Pharmacokinetics of nevirapine in HIV-infected infants weighing 3 kg to less than 6 kg taking paediatric fixed dose combination tablets. AIDS 2012; 26:1795–800. [DOI] [PubMed] [Google Scholar]
- 16. Luzuriaga K, McManus M, Mofenson L, Britto P, Graham B, Sullivan JL; PACTG 356 Investigators A trial of three antiretroviral regimens in HIV-1-infected children. N Engl J Med 2004; 350:2471–80. [DOI] [PubMed] [Google Scholar]
- 17. Fillekes Q, Mulenga V, Kabamba D, et al. CHAPAS-1 Trial Team Is nevirapine dose-escalation appropriate in young, African, HIV-infected children? AIDS 2013; 27:2111–5. [DOI] [PubMed] [Google Scholar]
- 18. US Food and Drug Administration. Kaletra (lopinavir/ritonavir) oral solution label changes related to toxicity in preterm neonates 2011. Available at: http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm244639.htm. Accessed 2 August 2019.
- 19. Simon A, Warszawski J, Kariyawasam D, et al. ANRS French Perinatal Cohort Study Group Association of prenatal and postnatal exposure to lopinavir-ritonavir and adrenal dysfunction among uninfected infants of HIV-infected mothers. JAMA 2011; 306:70–8. [DOI] [PubMed] [Google Scholar]
- 20. Kariyawasam D, Peries M, Foissac F, et al. Lopinavir-ritonavir impairs adrenal function in infants [manuscript published online ahead of print 21 October 2019]. Clin Infect Dis2019. doi:10.1093/cid/ciz888.
- 21. World Health Organization. Global action plan on HIV drug resistance 2017–2021 Available at: https://www.who.int/hiv/pub/drugresistance/hivdr-action-plan-2017–2021/en/. Accessed 12 November 2019.
- 22. Nikanjam M, Kabamba D, Cressey TR, et al. Nevirapine exposure with WHO pediatric weight band dosing: enhanced therapeutic concentrations predicted based on extensive international pharmacokinetic experience. Antimicrob Agents Chemother 2012; 56:5374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. European Collaborative Study. Are girls more at risk of intrauterine acquired HIV infection than boys? AIDS 2004; 18:344–47. [DOI] [PubMed] [Google Scholar]
- 24. Galli L, Puliti D, Chiappini E, et al. Writing Commitee Lower mother-to-child HIV-1 transmission in boys is independent of type of delivery and antiretroviral prophylaxis: the Italian Register for HIV infection in children. J Acquir Immune Defic Syndr 2005; 40:479–85. [DOI] [PubMed] [Google Scholar]
- 25. Nevirapine (Viramune) [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc, 2018. [Google Scholar]
- 26. de Maat MM, Huitema AD, Mulder JW, Meenhorst PL, van Gorp EC, Beijnen JH. Population pharmacokinetics of nevirapine in an unselected cohort of HIV-1-infected individuals. Br J Clin Pharmacol 2002; 54:378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cressey TR, Punyawudho B, Le Coeur S, et al. PHPT-5 Study Team Assessment of nevirapine prophylactic and therapeutic dosing regimens for neonates. J Acquir Immune Defic Syndr 2017; 75:554–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Capparelli EV, Aweeka F, Hitti J, et al. PACTG 1026S Study Team; PACTG P1022 Study Team Chronic administration of nevirapine during pregnancy: impact of pregnancy on pharmacokinetics. HIV Med 2008; 9:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Veldkamp AI, Weverling GJ, Lange JM, et al. High exposure to nevirapine in plasma is associated with an improved virological response in HIV-1-infected individuals. AIDS 2001; 15:1089–95. [DOI] [PubMed] [Google Scholar]
- 30. US Department of Health and Human Services Panel on Antiretroviral Therapy and Medical Management of Children Living With HIV—a Working Group of the Office of AIDS Research Advisory Council. Guidelines for the use of antiretroviral agents in pediatric HIV infection Available at: https://aidsinfo.nih.gov/guidelines. Accessed 9 September 2019.
- 31. Parkin NT, Hellmann NS, Whitcomb JM, Kiss L, Chappey C, Petropoulos CJ. Natural variation of drug susceptibility in wild-type human immunodeficiency virus type 1. Antimicrob Agents Chemother 2004; 48:437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barlow-Mosha L, Angelidou K, Lindsey J, et al. Nevirapine-versus lopinavir/ritonavir-based antiretroviral therapy in HIV-infected infants and young children: long-term follow-up of the IMPAACT P1060 randomized trial. Clin Infect Dis 2016; 63:1113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Violari A, Lindsey JC, Hughes MD, et al. Nevirapine versus ritonavir‐boosted lopinavir for HIV‐infected children.. N Engl J Med 2012; 366:2380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Technau K, Strehlau R, Patel F, et al. 12-month outcomes of HIV-infected infants identified at birth at one maternity site in Johannesburg, South Africa: an observational cohort study. Lancet HIV 2018; 5:e706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shiau S, Strehlau R, Technau KG, et al. Early age at start of antiretroviral therapy associated with better virologic control after initial suppression in HIV-infected infants. AIDS 2017; 31:355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Teasdale CA, Sogaula N, Yuengling KA, et al. HIV viral suppression and longevity among a cohort of children initiating antiretroviral therapy in Eastern Cape, South Africa. J Int AIDS Soc 2018; 21:e25168. doi: 10.1002/jia2.25168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lommerse J, Clarke D, Kerbusch T, et al. Maternal-neonatal raltegravir population pharmacokinetics modeling: implications for initial neonatal dosing. CPT Pharmacometrics Syst Pharmacol 2019; 8:643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Clarke DF, Acosta EP, Rizk ML, et al. International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1097 Study Team Raltegravir pharmacokinetics in neonates following maternal dosing. J Acquir Immune Defic Syndr 2014; 67:310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shapiro RL, Lichterfeld M, Hughes MD, et al. Low HIV reservoir at 84 weeks in very early treated HIV-infected children in Botswana [abstract 136]. In: Conference on Retroviruses and Opportunistic Infections, Boston, MA, 4–7 March 2018. Available at: http://www.croiconference.org/sessions/low-hiv-reservoir-84-weeks-very-early-treated-hiv-infected-children-botswana. Accessed 19 September 2019. [Google Scholar]
- 40. Luzuriaga K, McManus M, Catalina M, et al. Early therapy of vertical human immunodeficiency virus type 1 (HIV-1) infection: control of viral replication and absence of persistent HIV-1-specific immune responses. J Virol 2000; 74:6984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]