Abstract
Objectives
We sought to evaluate perceptions of biosimilar products among US rheumatologists who prescribe TNF-α inhibitors, given that 10 TNF-α inhibitor biosimilars and two rituximab biosimilars have Food and Drug Administration (FDA) approval.
Methods
A 19-question self-administered online survey was conducted from 6 May to 1 June 2019, and fielded by WebMD, LLC. Rheumatologists (n = 9050) who were members of Medscape.com and its partner panels were invited to participate. Likert and other rating scales were used to collect responses, which were summarized descriptively.
Results
Responses were obtained from 320 board-certified US rheumatologists, 85% of whom were fellows of the ACR. Nearly all respondents were familiar with the FDA definition of a biosimilar product and were aware that an infliximab biosimilar was FDA approved; fewer realized that adalimumab, etanercept and rituximab biosimilars were also FDA approved. Most respondents (84%) were aware that an approved biosimilar was not automatically deemed interchangeable by the FDA. Rheumatologists were more likely to initiate biosimilar treatment for a biologic treatment-naïve patient with RA (73%) than they were to switch to the biosimilar for a patient with RA doing well on the reference product (35%).
Conclusions
The results of this survey suggest that US rheumatologists have a good understanding and acceptance of biosimilar products, particularly for the initiation of treatment in biologic-naïve individuals. They were hesitant to switch from a reference product to a biosimilar for a patient doing well on the reference product. Additional education on biosimilars is required to help inform treatment decisions by rheumatologists.
A plain language summary of this article has been uploaded as supplementary material, available at Rheumatology online.
Keywords: biosimilars, education, rheumatologists, survey, United States
Rheumatology key messages
US rheumatologists understand and accept biosimilars.
Rheumatologists are hesitant to switch patients with low disease activity or remission to a biosimilar.
Additional education on biosimilars is required to help inform treatment decisions.
Introduction
TNF-α inhibitors adalimumab, infliximab and etanercept are the cornerstones of therapy for many chronic immune-mediated diseases, such as RA. However, these biologic agents are associated with high financial burden. In 2018, adalimumab and etanercept were two of the top three most expensive US prescription drugs by expenditure [1]. In an effort to provide lower-cost medication options, and thereby increase patient access, the development of biosimilar agents has occurred in recent years. As defined by the US Food and Drug Administration (FDA), a biosimilar is a biological product that is highly similar to and has no clinically meaningful differences (in terms of safety, purity and potency) from the original FDA-approved reference product [2]. In the USA, 27 biosimilars have been approved by the FDA (as of June 2020) [3], and >1000 potential biosimilars are in development [4]. FDA-approved biosimilars include 11 TNF-α inhibitors (Table 1), six of which were approved at the time of this survey report (May 2019), and two rituximab biosimilars, one of which was approved at the time of the survey [5]. Thus far, 18 biosimilars are marketed in the USA [3]; this is in contrast to the biosimilar landscape in Europe, where currently 64 biosimilar agents are on the market. Patent litigation by manufacturers of reference products has delayed the availability of biosimilar products in the USA.
Table 1.
FDA-approved biosimilars for TNF-α inhibitor agents and rituximab
| Name | Manufacturer | FDA approval | US launch |
|---|---|---|---|
| Infliximab (Remicade®) biosimilars | |||
| Inflectra® (infliximab-dyyb) | Pfizer, Inc. | Apr 2016 | Dec 2016 |
| Renflexis® (infliximab-abda) | Merck & Co., Inc. | Apr 2017 | Jul 2017 |
| Ixifi™ (infliximab-qbtx) | Pfizer, Inc. | Dec 2017 | (Will not be marketed in USA) |
| Avsola™ (infliximab-axxq) | Amgen, Inc | Dec 2019 | Jul 2020 |
| Adalimumab (Humira®) biosimilars | |||
| Amjevita™ (adalimumab-atto) | Amgen, Inc. | Sep 2016 | Not yet marketed |
| Cyltezo™ (adalimumab-adbm) | Boehringer Ingelheim Pharmaceuticals, Inc. | Aug 2017 | Not yet marketed |
| Hyrimoz™ (adalimumab-adaz) | Sandoz, Inc. | Oct 2018 | Not yet marketed |
| Hadlima™ (adalimumab-bwwd) | Samsung Bioepis | Jul 2019 | Not yet marketed |
| Abrilada™ (adalimumab-afzb) | Pfizer, Inc. | Nov 2019 | Not yet marketed |
| Etanercept (Enbrel®) biosimilars | |||
| Erelzi® (etanercept-szzs) | Sandoz, Inc. | Aug 2016 | Not yet marketed |
| Eticovo™ (etanercept-ykro) | Samsung Bioepis | Apr 2019 | Not yet marketed |
| Rituximab (Rituxan®) biosimilars | |||
| Truxima® (rituximab-abbs) | Teva/Celltrion | Nov 2018 | Not yet marketed |
| Ruxience® (rituximab-pvvr) | Pfizer, Inc. | Jul 2019 | Not yet marketed |
It is important to understand physicians’ awareness and attitudes toward biosimilars, as it is anticipated that they will require education about the biosimilars of these agents before they are comfortable offering them to their patients [6]. Previous surveys have assessed the knowledge and beliefs of physicians in the USA and Europe regarding biosimilarity [6, 7]. A recent systematic review of biosimilars survey literature from 2014 to 2018 reported that health-care providers in the USA and Europe remain cautious about using these agents, citing limited familiarity with biosimilars, contributing to safety and efficacy concerns and limited biosimilar prescribing [8].
The survey reported here sought to evaluate current perceptions of biosimilar products among US rheumatologists who prescribe TNF-α inhibitors, and their considerations for selecting biosimilars for the treatment of immune-mediated chronic diseases.
Methods
Respondents
Rheumatologists (n = 9050) who were members of Medscape.com and its partner panels were invited via email to complete a self-administered online survey. An online store gift card was offered as an incentive for survey participation. The target sample size (i.e. quota) was 320 board-certified US rheumatologists. To obtain regional representation across the USA, quotas were based on the geographical distribution of rheumatologists in the Medscape database (Midwest 18%, Northeast 25%, South 35%, West 22%). Once each quota was reached, no further surveys were administered to that region. Physicians from Vermont and Maine were excluded due to state laws that prohibit physicians from receiving incentive payments for participation in this type of research. Eligibility criteria required survey respondents to be a medical doctor self-identifying as rheumatologist, American Board of Internal Medicine (ABIM)-eligible or ABIM-certified, see patients for at least 75% of their working week and to have prescribed a TNF-α inhibitor for the treatment of an autoimmune disease.
Survey
The survey was developed by the authors and pilot-tested in April 2019 among 30 rheumatologists who were members of Medscape.com. Pilot survey data were evaluated for understanding and interpretation and, based on the results, two questions were revised. One question concerned biosimilar attributes (i.e. physicochemical properties, effectiveness, safety) and was modified from a Likert-scale response to a ranking question (i.e. which of these is most important). The second question related to the type of conditions physicians had treated in the last 6 months, and generalized pustular psoriasis was removed from the list.
The final survey consisted of 19 questions, and took ∼10–15 min to complete (Supplementary Data S1, available at Rheumatology online). The survey assessed the following: familiarity with the FDA definition of a biosimilar; familiarity with currently available biosimilar agents; reasons for selecting a biosimilar over its corresponding reference product; knowledge and attitudes regarding the terms ‘interchangeable’, ‘extrapolation’, ‘totality of evidence’ and ‘non-medical switching’; and the likelihood of initiating or switching to a biosimilar for different patient scenarios. Survey questions were multiple-choice format, and most used a 5-point Likert-type scale for responses. The survey was conducted from 6 May to 1 June 2019, and was fielded by WebMD, LLC (Atlanta, GA, United States).
Ethics approval and informed consent for survey participation
Participants were informed about the intentions of the research and how their personal information and responses would be used and their confidentiality protected; informed consent was obtained by having the participant check a box at the beginning of the survey. Because no medications or interventions were administered, institutional review board approval was not required.
Data analysis
Responses to each survey item were summarized and analysed descriptively. A Z-test was used for statistical comparisons. Calculations were performed using Decipher software (Decipher Software Solutions LLC, Clearwater, FL, USA).
Results
Respondents
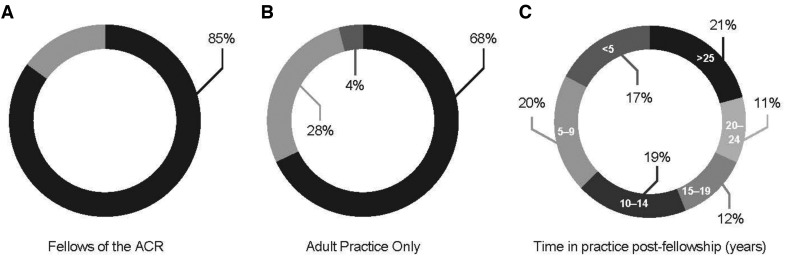
There were 421 physicians who responded to the survey. Of these, 21 responded after the regional quotas were reached, 32 did not meet screening eligibility criteria, 48 provided only partial responses and 30 responses were collected during the pilot testing phase. Thus, data are presented for 320 rheumatologists who met eligibility criteria and completed the entire final survey. The majority of respondents were fellows of the ACR (Fig. 1A), saw adult patients exclusively (Fig. 1B), and had practiced their specialty for 10 years or more post-fellowship (Fig. 1C).
Fig. 1.
Respondent details (n = 320)
Percentage of ACR fellows (85%) (A), percentage of those seeing adult patients all the time (68%), adult patients 90–99% of the time (28%), and paediatric patients up to 10% of the time (4%) (B), and post-fellowship time in practice, ranging from <5 years to >25 years (C).
Familiarity with biosimilar definition and availability of approved agents
The majority of respondents (83%) were very/extremely familiar with the FDA definition of a biosimilar product (‘A biosimilar is a biological product that is highly similar to and has no clinically meaningful differences from an existing FDA-approved reference product.’). Respondents were asked ‘Recognizing that there may be more than one biosimilar product available or in development for each biologic reference product, please choose the ONE answer that represents the most advanced stage of development for each listed reference product’. The choices were ‘FDA approved’, ‘Submitted for FDA registration’ and ‘In development’. Although 96% of respondents were aware that an infliximab biosimilar had FDA approval, fewer realized that adalimumab, etanercept and rituximab biosimilars were FDA approved (56%, 62% and 39%, respectively).
Criteria for selecting a biosimilar product
When asked to rank what factors are considered when selecting a biosimilar product vs its corresponding reference product (1, most important to 7, least important), responses were varied (Fig. 2): 77% ranked effectiveness as 1 or 2, and 51% ranked physicochemical/functional characteristics as 6 or 7.
Fig. 2.
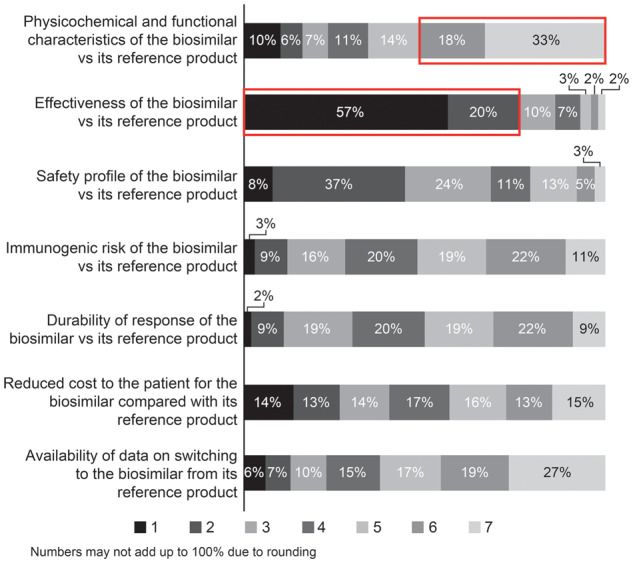
Factors considered when selecting a biosimilar
Ranking scale: 1 = most important; 7 = least important. The red boxes indicate that 51% ranked physicochemical/functional characteristics as 6 or 7, and 77% ranked effectiveness as 1 or 2.
Familiarity with biosimilar terminology
Most respondents (84%) were aware that an approved biosimilar was not automatically deemed interchangeable by the FDA; 86% felt it important/very important for interchangeable approval to be on the label. Overall, 80% were familiar with the term, ‘totality of evidence’.
Non-medical switching
About half (54%) of respondents were familiar with the term ‘non-medical switching’. Among those responding ‘yes’, half (50%; 80/158) had patients for whom this switch had been suggested. The main reasons for non-medical switching included pharmacy benefit insurance/formulary coverage (80%), hospital system formulary (67%) and requirement for a step therapy (63%).
Likelihood of biosimilar treatment initiation and switching
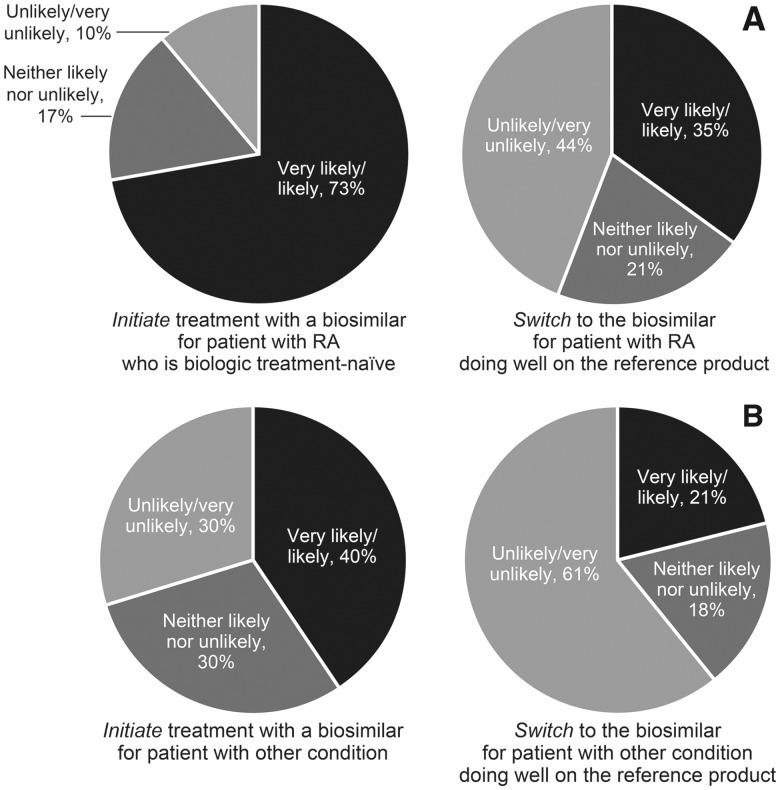
For a patient with the same rheumatological condition as the one on which the biosimilar approval was based (e.g. RA), 73% of respondents were very likely/likely to initiate biosimilar treatment for a patient with RA who is biologic treatment-naïve, whereas only 35% of respondents were very likely/likely to switch to a biosimilar for a patient with RA who was doing well on the reference product (Fig. 3A). For a patient with a different rheumatological condition than the one on which the biosimilar approval was based (e.g. extrapolation of RA to psoriatic arthritis), more respondents (40%) were very likely/likely to initiate biosimilar treatment for a biologic treatment-naïve patient than to switch to the biosimilar (21%) if the patient was doing well on the reference product (Fig. 3B). These findings suggest that US rheumatologists are more comfortable initiating treatment with biosimilars for patients who are biologic treatment-naïve, rather than switching treatment for patients who are doing well on the reference product.
Fig. 3.
Likelihood of biosimilar treatment initiation and switching
Biosimilar approval based on RA and patient having RA (A) and biosimilar approval based on RA but patient having a different rheumatological condition (e.g. psoriatic arthritis) (B).
Discussion
The present survey provides insights regarding US rheumatologists’ beliefs and knowledge surrounding biosimilars. With >1000 potential biosimilars in development in the USA and 24 FDA-approved at the time the survey was conducted, including seven agents to treat RA, it will become increasingly difficult for physicians to keep up with new agents. Of the seven biosimilars that are approved and indicated for the treatment of rheumatological conditions, six are TNF-α inhibitors and one is an anti-CD20 monoclonal antibody; only two TNF-α inhibitors and one rituximab biosimilar are currently marketed in the USA.
Although the majority of rheumatologists were familiar with the FDA definition of a biosimilar, awareness regarding availability of FDA-approved biosimilars was lacking. The majority of rheumatologists knew that an infliximab biosimilar had been approved, which was to be expected, with infliximab-dyyb and infliximab-abda approved in 2016 and 2017, respectively. Additionally, both agents have now been available to prescribe for >2 years. Fewer surveyed rheumatologists knew about adalimumab and etanercept biosimilars (a little more than half), and about one-third knew about a rituximab biosimilar being approved.
Effectiveness (77%) and safety (45%) of a biosimilar vs the reference product were the leading reasons for choosing a particular agent. The goal of biosimilar development is to provide cost savings for the patient by offering an alternative to more expensive treatment options; however, this was deemed important only by slightly more than a quarter of rheumatologists surveyed.
The majority of rheumatologists (≥80%) were familiar with the terms ‘interchangeability’ and ‘totality of evidence’, but only about half had heard of non-medical switching; this term is used when a patient whose disease is stable on a medication changes medications for reasons unrelated to the health of the patient [9]. Non-medical switches are often driven by health-care plans/policies or financial considerations. The results of this survey show that although the majority (73%) of rheumatologists were very likely/likely to initiate biosimilar treatment for a patient with RA who is biologic treatment-naïve, they were hesitant to switch to a biosimilar if the patient was doing well on the reference product (35%). Even fewer rheumatologists (21%) were likely to switch a patient with a different condition than RA (e.g. extrapolation to psoriatic arthritis) if the patient was doing well on the reference product. Interestingly, 19% and 15% of respondents were very likely/likely to switch a patient who was not doing well on the reference product to a biosimilar (patient had RA vs a different rheumatologic condition, respectively). This suggests that nearly one in five rheumatologists surveyed do not understand the underpinnings of biosimilarity; alternatively, they were confused by the question.
The limitations of this survey include the fact that the survey population was drawn from the Medscape.com database and that the sample size was relatively small and may not have been representative of all US rheumatologists. Additionally, survey participants self-identified as rheumatologists and ACR members.
Conclusion
This survey suggests that US rheumatologists have a good understanding and acceptance of biosimilar products, particularly for the initiation of treatment in biologic-naïve individuals. US rheumatologists were hesitant to switch from a reference product to a biosimilar for a patient doing well on the reference product. Additional education on biosimilars is required to help inform treatment decisions by rheumatologists.
Supplementary Material
Acknowledgements
This survey was fielded in partnership with WebMD, LLC. The authors thank Sam Badawi, PharmD, JD, from Boehringer Ingelheim Pharmaceuticals, Inc. for his contributions to the survey development. Medical writing assistance, supported financially by Boehringer Ingelheim Pharmaceuticals, Inc., was provided by Deborah Brocksmith, MB ChB, PhD, and Linda Merkel, PhD, of Elevate Scientific Solutions. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE) and were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and have approved the final version of the manuscript, which reflects the authors’ interpretations and conclusions. The authors received no direct compensation related to the development of the manuscript.
Funding: This work was supported by Boehringer Ingelheim Pharmaceuticals, Inc.
Disclosure statement: A.G. is a consultant/advisor for AbbVie Inc., Eli Lilly and Company, Horizon Pharma plc, Novartis Pharmaceuticals Corporation, Pfizer Inc., Sandoz and Samumed. He is on the speakers’ bureau for AbbVie Inc., Amgen, Horizon Pharma plc, Novartis Pharmaceuticals Corporation and Pfizer Inc., and has stock ownership in AbbVie Inc., Amgen, Bristol-Myers Squibb Company and Pfizer Inc. D.M. is a full-time employee of Boehringer Ingelheim Pharmaceuticals, Inc.
Data availability statement
The data underlying this article will be shared upon reasonable request to the corresponding author.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Schumock GT, Stubbings J, Hoffman JM. et al. National trends in prescription drug expenditures and projections for 2019. Am J Health Syst Pharm 2019;76:1105–21. [DOI] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration. Guidances (drugs): Biosimilars. https://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/guidances/ucm290967.htm (28 November 2019, date last accessed).
- 3.Biosimilars Review and Report. Biosimilar Approval Status in the US: FDA Filing Dates and actions. 2019. https://biosimilarsrr.com/us-biosimilar-filings/ (28 November 2019, date last accessed).
- 4.Biosimilar Development. Biosimilars Pipeline Shows Remarkable, Sustained Growth. 2019. https://www.biosimilardevelopment.com/doc/biosimilars-pipeline-shows-remarkable-sustained-growth-0001 (28 November 2019, date last accessed).
- 5.US Food and Drug Administration. Biosimilar Product Information: FDA-approved Biosimilar Products. 2019. https://www.fda.gov/drugs/biosimilars/biosimilar-product-information (28 November 2019, date last accessed).
- 6. Cohen H, Beydoun D, Chien D. et al. Awareness, knowledge, and perceptions of biosimilars among specialty physicians. Adv Ther 2017;33:2160–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Waller J, Sullivan E, Piercy J, Black CM, Kachroo S.. Assessing physician and patient acceptance of infliximab biosimilars in rheumatoid arthritis, ankylosing spondyloarthritis and psoriatic arthritis across Germany. Patient Prefer Adherence 2017;11:519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leonard E, Wascovich M, Oskouei S, Gurz P, Carpenter D.. Factors affecting health care provider knowledge and acceptance of biosimilar medicines: a systematic review. J Manag Care Spec Pharm 2019;25:102–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Teeple A, Ellis LA, Huff L. et al. Physician attitudes about non-medical switching to biosimilars: results from an online physician survey in the United States. Curr Med Res Opin 2019;35:611–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.