Abstract
Background
Patients with hematological malignancies (HM) are known to carry an increased risk of invasive pneumococcal disease (IPD). However, temporal variations in IPD risks following a cancer diagnosis remain poorly characterized. To inform vaccine guidelines and patient management, we assessed the IPD incidence among patients with HM and other malignancies.
Methods
The study population included all individuals aged ≥15 years during 2000–2016 in Denmark. Variations in incidences of IPD over time and between different types of hematological malignancies and diagnoses were assessed by Poisson regression.
Results
During 85 002 224 person-years of observation, 13 332 episodes of a first IPD were observed, of which 765 (5.7%) occurred among individuals with HM. Among HM patients, the IPD incidence rate decreased continuously during the study period (rate ratio per year, 0.91; 95% confidence interval, .90–.92). The risk of IPD in patients with HM was up to 39 times higher when compared to the background population and was highest for multiple myeloma, acute lymphoblastic leukemia, and chronic lymphocytic leukemia. Unlike other malignancies, the increased IPD risk did not wane with the time since HM diagnosis. We found a vaccination uptake of only ≤2% in patients with HM and ≤1% for those with other types of malignancies.
Conclusions
Adults with HM in general and patients with lymphoid malignancies in particular have an increased risk for IPD, compared with patients with other types of cancer and with individuals free of cancer. The pneumococcal vaccination uptake is extremely low in this at risk-population. Efforts to prevent IPD in HM patients are continuously warranted.
Keywords: invasive pneumococcal disease, hematological malignancies, risk factors, mortality, pneumococcal serotypes
The risk of invasive pneumococcal disease (IPD) is nearly 40 times higher in adults with hematological malignancies, especially those with lymphoid malignancies. Given the high IPD risk, efforts to prevent IPD in hematological malignancies patients are continuously warranted.
Streptococcus pneumoniae is part of the normal nasopharyngeal flora but may also cause severe and potentially fatal diseases, such as pneumonia, bacteremia, and meningitis [1]. The risk of IPD is increased among immunocompromised individuals [2]. Patients with hematological malignancies (HM) are among those with the highest risk of IPD, with incidences ranging between 13–50 times higher when compared with the background population, and with HM patients accounting for up to 10% of all IPD episodes in adults [1–5].
The epidemiology of IPD and specific pneumococcal serotypes is complex due to serotype replacement following vaccine introduction, seasonal variation, and cyclic serotype patterns [6, 7]. After the introduction of pneumococcal conjugate vaccines (PCV) in the childhood vaccination program, the incidence of IPD overall decreased markedly in Denmark [6]. However, it is not known whether these changes observed in the general population also apply to high-risk groups, including patients with HM. Such knowledge is important for the continued risk assessments and vaccination recommendations for this group of patients.
Pneumococcal vaccination is strongly recommended for the prevention of IPD in individuals with HM, but the evidence for vaccine effectiveness remains low [8]. Among patients with HM, vaccination coverage remains low despite multiple encounters with the health-care system [9]. Although the national vaccination guidelines recommend vaccinations to patients with immunosuppression, some current Danish hematological guidelines do not recommend pneumococcal vaccines as standard of care [10, 11]. A general lack of knowledge about vaccination, concerns about the immunogenicity of vaccines, and a poor understanding of the risk of IPD for this group of patients may play a role.
To report precise incidence rates and examine temporal patterns of IPD incidences among HM patients, as well as in the background population, we undertook this nationwide cohort study.
We explored temporal changes in the risk of IPD and associated mortality in adults with HM, compared to the risk in patients with non-hematological cancers and cancer-free individuals during 16 years in Denmark. We also studied the differences in risk according to the type of malignancy and the time at which patients are at highest risk after a diagnosis. Pneumococcal serotype-specific changes were also explored in relation to the general introduction of PCV in the childhood immunization program.
METHODS
We conducted a nationwide, population-based cohort study including all individuals in Denmark during 2000–2016. Nationwide registers were linked using the unique personal identification numbers, registered in the Civil Registration System, that have been given to all residents since 1968 [12].
National Surveillance Data on Invasive Pneumococcal Disease
We obtained information on IPD from the Danish laboratory surveillance system [13]. We defined IPD when isolates were obtained from cerebrospinal fluid, blood, pleura, or another sterile anatomical site. IPD isolates have routinely been serotyped at the reference laboratory at Statens Serum Institut since 1938 [6].
On 1 October 2007, the 7-valent PCV (PCV7) was introduced into the Danish childhood immunization program in a 2 + 1 schedule. The vaccine was offered free of charge to all children, with doses at 3, 5, and 12 months of age. In April 2010, PCV7 was replaced by the 13-valent PCV (PCV13) [14]. Sequential pneumococcal vaccination (PCV13 followed by pneumococcal polysaccharide vaccine [PPV] 23) has been recommended for at-risk groups outside the childhood vaccination program since 2014 [15]. Current recommendations include the vaccination of immunocompromised patients and patients with certain underlying conditions. Consequently, we categorized pneumococcal serotypes according to those included in PPV23 and different PCV vaccine formulations: PCV7 (serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F); additional PCV13 serotypes (1, 3, 5, 6A, 7F, and 19A), PPV23 non-PCV13 serotypes (2, 8, 9N, 10A, 11A, 12F, 15B, 17F, 20, 22F, and 33F), and non-vaccine serotypes (non-VT), defined as all serotypes not included in any of the mentioned vaccines.
Danish Cancer Register
The Danish Cancer Register contains information on all incident cancers in Denmark since 1943 [16]. We identified all individuals registered with Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), multiple myeloma (MM), acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), acute myeloid leukemia, chronic myeloid leukemia, other leukemias, myelodysplastic syndrome, or myeloproliferative neoplasm (MPN), as well as individuals with any non-hematological cancer in the period between 1995 and 2016 (Supplementary Table 1).
Danish National Patient Register
Since 1978, all admissions to Danish hospitals (including diagnoses, time of admission, and time of discharge) have been registered in the Danish National Patient Register [17]. We obtained information on the following morbidities: congestive heart failure, chronic pulmonary disease, hemiplegia, dementia, rheumatic disease, diabetes with complications, moderate/severe renal disease, moderate and severe liver disease, human immunodeficiency virus/AIDS, and splenectomy after 1995 and during follow-up [18, 19] (Supplementary Table 2).
The Danish National Health Service Register and the Danish Prescription Register
To identify vaccinations in our cohort, we used the service code 8940 for vaccination from the Danish National Health Service Register, the procedure code BPNA92 from the Danish National Patient Register, and the anatomical therapeutic classification code J07AL from the Danish Prescription Register, which identified all individuals receiving a pneumococcal vaccine at a hospital, a reimbursed vaccination at the general practitioner, or a prescription for a pneumococcal vaccine, respectively [20]. Reimbursed vaccinations at the general practitioner cover vaccination with PPV23 and, since October 2012, also cover vaccination with PCV13 to individuals at a high risk of IPD. Importantly, the code for reimbursed vaccinations at the general practitioner also covers hepatitis B vaccines to persons with trisomy 21 or following needle stick injuries. None of the codes provide information on whether PPV23 or PCV13 was administrated.
Statistical Methods
We followed all residents in Denmark from age 15 years or the year 2000, whichever came last, until IPD, the end of 2015, date of emigration, death, or age 100 years, whichever came first. Individuals with IPD prior to the study entry and individuals previously treated for childhood cancer were excluded from the cohort. Population incidence rates were calculated for each sex, calendar year, and 5-year age group (ie, 15–19, 20–24, …, 95–99 years). Calendar years were grouped into 4 successive periods, corresponding to the PCV introduction (ie, 2000–2006; 2007–2009; 2010–2012; 2013–2015). The individual follow-up time and corresponding population rates were stratified according to the presence of the selected morbidities and by vaccination status, with both treated as time-dependent covariates. The case fatality rate (CFR) was defined based on deaths within 30 days of IPD. Incidence rates are shown as IPD events per 100 000 person-years (PY) at risk.
Follow-up data were analyzed using Poisson regression models, with IPD episodes as the outcome and log person-years as offset [21]. The association between a vaccine and IPD was tested within the specific HM, adjusted for sex, age, calendar year, and comorbidities. We did a sensitivity analysis including only data from the Danish National Patient Register, the Danish National Health Service Register, or the Danish Prescription Register, or from pairwise combinations of the 3 registers, to examine how the vaccination effect changed. Models were compared by means of the log likelihood ratio tests. All effect estimates are presented as rate ratios (RRs) with 95% confidence intervals (CI).
All data management was performed using SAS Statistical Software 9.4 using the stratify macro (SAS Institute, Inc., Cary, NC) [21]. All statistical analyses were performed in R version 3.2 (R Development Core Team, Vienna, Austria). The study was approved by the Danish Data Protection Agency (2015–57–0102). The data that support the findings of this study are archived at governmental institutions in Denmark, and can be obtained through applications to relevant data agencies.
RESULTS
During 85 002 224 person-years of observation, 13 332 first IPD episodes were observed among 6 971 406 individuals from 2000 to 2016. Of all IPD episodes, 765 (5.7%) episodes occurred among individuals with HM. The overall IPD incidence rate for the first episode was 15.7 IPDs/100 000 PY across all periods and age groups.
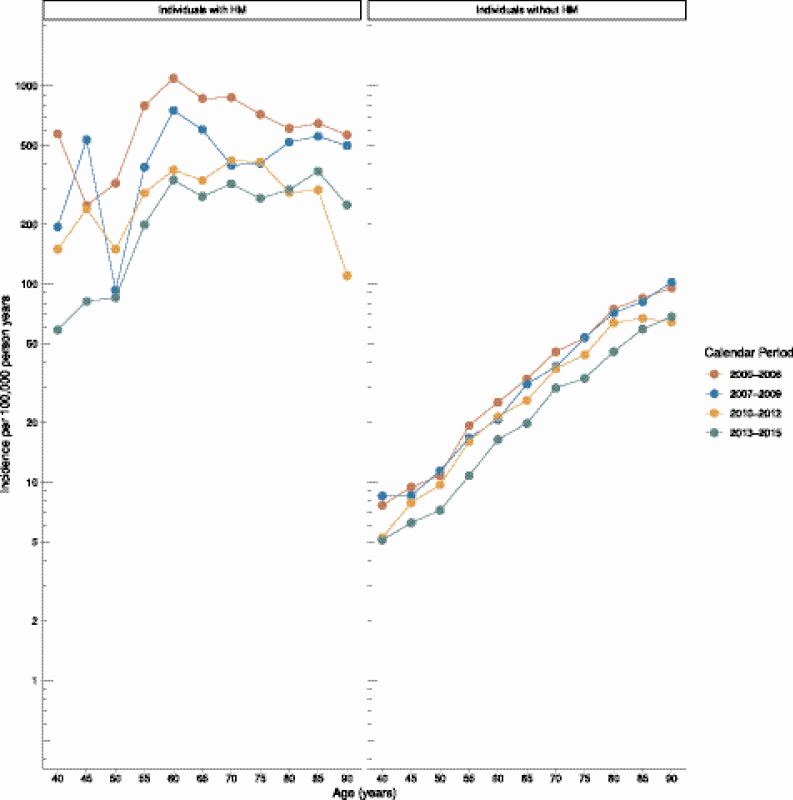
IPD incidence rates were higher for individuals with HM, compared with individuals without HM (421.1 vs 12.7/100 000 PY, respectively). The risk of IPD increased with age both in individuals without HM (RRper year = 1.06; 95% CI, 1.05–1.06) and in patients with HM (RRper year = 1.01; 95% CI, 1.01–1.02; Figure 1). The overall incidence rate of IPD decreased continuously during the study period (RRper year = 0.96; 95% CI, .96−.97; Figure 1). The decrease in the IPD incidence rate was more pronounced among patients with HM (RRper year = 0.91; 95% CI, .90–.92) than among individuals without (RRper year = 0.97; 95% CI, .96−.97; Figure 1).
Figure 1.
Incidence of invasive pneumococcal disease in the Danish population. The y-axis shows the number of cases per 100 000 PY, and the x-axis shows age stratified by the presence of a hematological malignancy and grouped in 4 calendar periods. Abbreviations: HM, hematological malignancies; PY, person years.
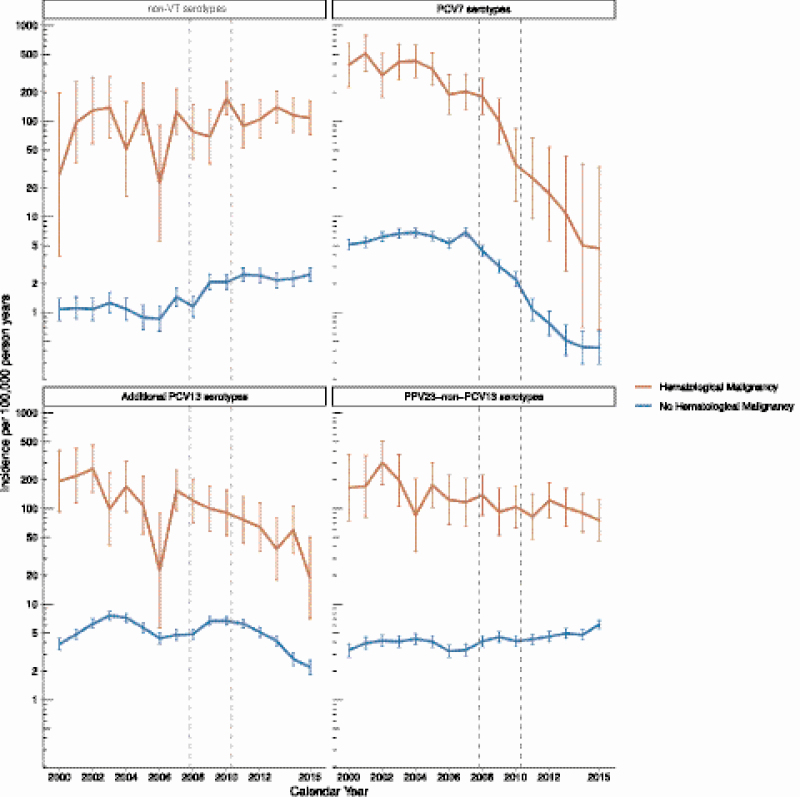
During the study period, the incidence of IPD caused by PCV7 serotypes decreased both for individuals with and without HM (Figure 2). The incidence rates of IPD caused by additional PCV13 serotypes also decreased slightly after PCV13 introduction, while no changes were seen for PPV23 non-PCV13 serotypes. The decreases in PCV7 and additional PCV13 serotypes coincided with the introduction of PCV7 and PCV13 into the childhood vaccination program. The incidence of IPD caused by non-VT increased during the study period (RRper year = 1.07; 95% CI, 1.06–1.09; Figure 2; for details on each serotype, see Supplementary Figure 1).
Figure 2.
Incidence rates of invasive pneumococcal disease in the Danish population for individuals with and without a hematological malignancy according to serotypes included in pneumococcal vaccines. The vaccine groups were PCV7 (PCV7 serotypes), additional PCV13 serotypes (serotypes covered by PCV13 but not PCV7), PPV23 non-PCV13 serotypes (serotypes that are covered by PPV23 but not by PCV13), and non-VT (non-VT, defined as all serotypes that are not covered by PCV7, PCV13, or PPV23). Abbreviations: PCV7, 7-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; PPV23, 23-valent pneumococcal polysaccharide vaccine; VT, vaccine serotype.
Incidence of Invasive Pneumococcal Disease According to Type of Hematological Malignancy
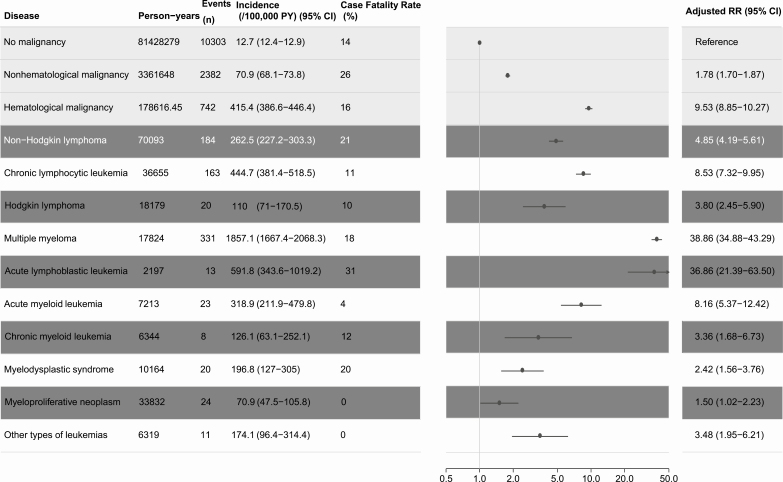
The crude incidence rate of a first IPD was lowest for individuals without any malignancy (12.7; 95% CI, 12.4–12.9) followed by the combined group of non-hematological cancers (70.9; 95% CI, 68.1–73.8) and the combined group of HM patients (421.1; 95% CI, 392.3–452.0). CFR also varied greatly (range 0–26%) between patient groups, with the highest CFR observed among individuals with a non-hematological malignancy and the lowest among individuals diagnosed with MPN, other types of leukemia, and acute myeloid leukemia (Table 1; Supplementary Table 3).
Table 1.
Incidence and Case Fatality Rates of Invasive Pneumococcal Diseases in Patients With and Without Hematological Malignancies, Denmark 2000–2016
PY, number of events, crude incidence rates, and case-fatality rates are shown for all groups. RR for IPD in individuals with a hematological malignancy are adjusted for age, gender, calendar year, morbidity, and type of malignancy. Persons with more than one malignancy count multiple times for person years, events, and incidences.
Abbreviations: CI, confidence interval; IPD, invasive pneumococcal disease; PY, person years; RR, rate ratio.
The incidences of IPD varied markedly between different types of HM. Incidence rates were lowest for MPN (70.9; 95% CI, 47.5–105.8) and HL (110.0; 95% CI, 71.0–170.0) and highest for individuals with CLL (444.7; 95% CI, 381.4–518.5) and MM (1857.1; 95% CI, 1667.4–2068.3; Table 1).
In the regression model adjusted for calendar year, age, sex, and the presence of comorbidities, MM patients had the highest risk of IPD, with an RR of 38.9 (95% CI, 34.9–43.3), followed by ALL (RR = 36.9; 95% CI, 21.4–63.5) and CLL (RR = 8.5; 95% CI, 7.3–10.0; Table 1).
Risk of Invasive Pneumococcal Disease by Time Since Malignancy
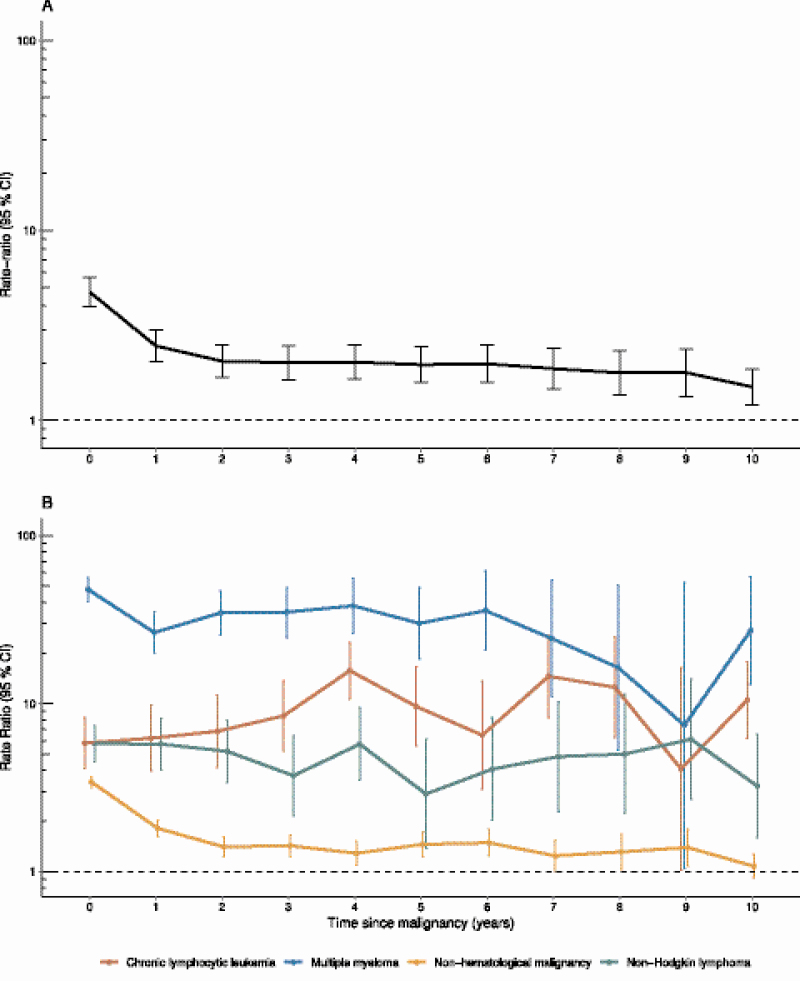
The risk of IPD among patients with any malignancy was highest during the first 2 years after the cancer diagnosis, with RRs of 4.7 (95% CI, 4.0–5.7) during the first year and 2.5 (95% CI, 2.0–3.0) during the second year (Figure 3A). The risk of IPD over time varied by underlying malignancy: that is, among patients diagnosed with non-hematological cancer, the RR decreased rapidly after the first year of diagnosis, but for patients diagnosed with CLL, NHL, and MM, the RR was uniformly elevated or even increased over time (Figure 3B).
Figure 3.
Time to invasive pneumococcal disease since time of diagnosis of malignancy. A, Individuals with any nonhematological malignancy. B, Individuals with and without hematological malignancies. RR estimates are adjusted for age, gender, calendar year, comorbidity, and type of malignancy. The y-axis shows the RR, and the x-axis shows the time since malignancy, with 0 being the year of diagnosis. Abbreviations: CI, confidence interval; CLL, chronic lymphocytic leukemia; IPD, invasive pneumococcal disease; RR, rate ratio.
Use of Pneumococcal Vaccine Among Patients with Malignancies
During 74 537 person-years at risk among 12 977 vaccinated individuals, 35 episodes of first IPDs were observed. The Danish National Patient Register, Danish National Health Service Register, and Danish Prescription Register contributed 1505, 5011, and 6461 registrations of vaccination, respectively. This corresponded to 0.17% of the study population without malignancies receiving a pneumococcal vaccine, compared with 1.64% among those with HM and 0.13% among those with non-hematological cancers (Supplementary Figure 2).
Incidence Rate Among Vaccinated Individuals
Among vaccinated individuals, the mean annual incidence of IPD was 56.2/100 000 PY. Compared with unvaccinated individuals, vaccinated individuals without malignancy had a lower incidence rate of IPD (12.7 vs 10.6/100 000 PY, respectively) as did vaccinated MM patients compared with unvaccinated MM patients (1430.3 vs 1857.1/100.000 PY, respectively). In contrast, vaccinated patients with other hematological or non-hematological malignancies had a higher incidence compared with unvaccinated patients (Supplementary Table 3; Supplementary Figure 2).
Distribution of Serotypes in Unvaccinated and Vaccinated Individuals
The distribution of specific serotypes varied somewhat between vaccinated and unvaccinated individuals. Among unvaccinated individuals, 1593 (12.0%) IPD cases were caused by non-VT serotypes, 3359 (25.2 %) by PCV7 serotypes, 4526 (34.0%) by additional PCV13 serotypes, and 3819 (28.7%) by PPV23 non-PCV13 serotypes. In vaccinated individuals, the corresponding serotype distribution was 4 (11.4%) cases caused by non-VT serotypes, 12 (34.3%) by PCV7 serotypes, 5 (14.3%) by additional PCV13 serotypes, and 14 (40.0%) by PPV23 non-PCV13 serotypes.
DISCUSSION
In this nationwide cohort study, adult individuals with HM had up to a 39-fold increased risk of IPD when compared with the background population. The risk of IPD varied considerably by type of HM and by time after the cancer diagnosis. We observed a 3.5% annual decrease in the incidence of IPD in the adult population in conjunction with the introduction of PCV in the childhood vaccination program. The decrease in the IPD incidence rate was more pronounced among individuals with HM; specifically, 9% annually. This suggests a strong and differential indirect effect of pneumococcal vaccination in this highly susceptible population, but changes in patient demographics and/or health care, as well as advances in cancer and supportive care, may all have contributed to the observed temporal variations [5]. Specifically, during the study period there have been enormous developments in the treatment of HM, most markedly in CLL, MM, chronic myeloid leukemia, and NHL. In the same period, PCV was introduced to the childhood vaccination program, which may also have changed the risk of IPD significantly for these patients. Moreover, the new treatment regimens have increased the prevalence of HM; namely, through patients living after or with HM in the population.
We observed an IPD incidence rate of 421.1 per 100 000 person years among individuals with HM. This is comparable with previous reports from the United States, Canada, Scotland, and Sweden [1–5]. The risk of IPD differed greatly between different types of HM, but also by the time of diagnosis. The risk of IPD was highest in patients with MM, ALL, and CLL. This was true also when the time since diagnosis was taken into consideration; that is, the relative risk of IPD was increased during the first 5 years after a CLL diagnosis. This is consistent with our current understanding of the immune dysfunction in many HM cases. In CLL, the immune function becomes progressively impaired as the disease evolves and as patients receive treatment [22, 23]. In contrast, in many solid tumors (and possibly also in some curable HMs) the immune function recovers following successful treatment of the tumor. Thus, the mechanisms underlying the increased IPD risk shortly after a cancer diagnosis are not identical to those influencing the IPD risk among long-term cancer survivors, adding another layer of complexity to the direct comparison of reported incidence rates and risk estimates. The acute and progressive immune dysfunction may emphasize the importance of early vaccination immediately after a diagnosis. However, to complicate matters, the immune response may be impaired in patients with malignancies [24–28].
We found remarkably low levels of adult pneumococcal vaccination, both among the background population and in those with a cancer diagnosis. We did not find evidence of protection after pneumococcal vaccination among vaccinated individuals, except for patients with MM and cancer-free individuals. This may reflect the poor immune responses to vaccination in general, but also elements of confounding by indication. Since many patients are not routinely vaccinated, vaccination is based on a clinical assessment of the risk of IPD, and the markedly increased IPD occurrence among vaccinated patients may suggest that treating physicians were able to accurately identify particularly susceptible cancer patients and vaccinate them [9]. Although pneumococcal vaccines are recomended and reimbursed for high-risk patients in Denmark, many current guidelines for the management of hematological patients do not recommend sequential pneumococcal vaccination as a standard of care [10, 11]. This may explain the low vaccine uptake found in our study.
Our study had several limitations. First, we identified those individuals receiving a pneumococcal vaccine in three different registers, but we did not have any information on the type of pneumococcal vaccine, nor did we include information on revaccination. Furthermore, the completeness of these registers for assessing pneumococcal vaccination coverage is not known, and there might be sources of errors, such as the misclassification of the vaccine administrated or missing registrations. Therefore, we interpret these results with caution. Secondly, the definition of IPD is highly specific, including only laboratory-confirmed cases, which may grossly lead to underestimations of the real burden of the disease. Thirdly, we did not have any information on important lifestyle factors associated with an increased risk of IPD, such as smoking, alcohol consumption, and crowding [29, 30]. Lastly, we did not have any information on specific treatments for the different malignancies, and different thresholds for blood culture sampling between medical specialties may also have contributed to our findings by introducing a surveillance bias. Patients with HM are often more intensively monitored and may have more active health care–seeking behaviours, compared with other patients, and a microbiological diagnosis therefore more likely precedes the prescription of antibiotics in these patients. This interpretation would also explain the difference in CFRs we found, and this has also been described among human immunodeficiency virus patients [31].
We could not assess the reasons for not vaccinating patients for whom the vaccine was indicated, and the opportunity for pneumococcal vaccination is often missed during health-care encounters. Physicians may have deemed that patients would not respond to pneumococcal vaccination due to their underlying disease or therapy, and some patients may have received the vaccination without an appropriate registration. However, excluding vaccination from the Danish National Health Service Register showed no different results.
Acknowledging these limitations, this is the largest study to date reporting IPD rates in adults with HM. We show that adults with HM in general and patients with lymphoid malignancies in particular are at an increased risk of IPD, compared both with patients with other types of cancer and with individuals free of cancer. We found very low uptakes of pneumococcal vaccination in this group. Efforts to prevent IPD in patients with HM are continuously warranted.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. M. A. A. performed the statistical analysis and drafted the manuscript. Z. B. H., H. H., R. S., and M. A. A. generated the idea and concept for the study, contributed to the data analysis and data interpretation, and revised the manuscript. K. R. oversaw the conduct of the statistical analysis. K. R. and C. U. N. contributed to the data analysis and data interpretation and revised the manuscript. T. D. and D. M. W. contributed to the data interpretation and revised the manuscript.
Financial support. This work was supported by the Research Foundation of Rigshospitalet and Danish National Research Foundation (grant number 126).
Potential conflicts of interest. C. U. N. received funding from the Danish Cancer Society and the Novo Nordisk Foundation (grant number NNF16OC0019302) and has received grants/consultancy fees from AbbVie, Janssen, Sunesis, Gilead, Astra-Zeneca, Acerta, CSL Behring Roche, and Novartis, outside of this study. D. M. W. is supported by the National Institute of Allergy and Infectious Diseases (grant number R01AI123208) and has received consulting fees from Pfizer, Glaxosmithkline (GSK), and Affinivax. Z. B. H. received travel grants and consultancy fees from GSK and Pfizer. R. S. has been an employee at Novo Nordisk A/S during the limited time of the study. The employment did not contribute to this study, as of the time of submission. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Backhaus E, Berg S, Andersson R, et al. Epidemiology of invasive pneumococcal infections: manifestations, incidence and case fatality rate correlated to age, gender and risk factors. BMC Infect Dis 2016; 16:1471–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wong A, Marrie TJ, Garg S, Kellner JD, Tyrrell GJ; SPAT Group. Increased risk of invasive pneumococcal disease in haematological and solid-organ malignancies. Epidemiol Infect 2010; 138:1804–10. [DOI] [PubMed] [Google Scholar]
- 3. Kyaw MH, Christie P, Clarke SC, et al. Invasive pneumococcal disease in Scotland, 1999–2001: use of record linkage to explore associations between patients and disease in relation to future vaccination policy. Clin Infect Dis 2003; 37:1283–91. [DOI] [PubMed] [Google Scholar]
- 4. Kyaw MH, Rose CE Jr, Fry AM, et al. ; Active Bacterial Core Surveillance Program of the Emerging Infections Program Network The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis 2005; 192:377–86. [DOI] [PubMed] [Google Scholar]
- 5. Lee YJ, Huang YT, Kim SJ, et al. Trends in invasive pneumococcal disease in cancer patients after the introduction of 7-valent pneumococcal conjugate vaccine: a 20-year longitudinal study at a major urban cancer center. Clin Infect Dis 2018; 66:244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harboe ZB, Benfield TL, Valentiner-Branth P, et al. Temporal trends in invasive pneumococcal disease and pneumococcal serotypes over 7 decades. Clin Infect Dis 2010; 50:329–37. [DOI] [PubMed] [Google Scholar]
- 7. Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011; 378:1962–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rubin LG, Levin MJ, Ljungman P, et al. ; Infectious Diseases Society of America 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:e44–100. [DOI] [PubMed] [Google Scholar]
- 9. Kyaw MH, Greene CM, Schaffner W, et al. ; Active Bacterial Core Surveillance Program of the Emerging Infections Program Network Adults with invasive pneumococcal disease: missed opportunities for vaccination. Am J Prev Med 2006; 31:286–92. [DOI] [PubMed] [Google Scholar]
- 10. Danish Myeloma Group. Diagnostik og behandling af myelomatose. Retningslinje 2017. Dansk Myelomatose Studie Gruppe (DMSG) [Danish]. Available at: www.myeloma.dk, Accessed 14 November 2019. [Google Scholar]
- 11. Danish Lymphoma Group. Danish Lymphoma Guidelines: Diagnostik og behandling af lymfomer i Danmark Available at: http://www.lymphoma.dk/retningslinjer/ Accessed 14 November 2019.
- 12. Pedersen CB The Danish civil registration system. Scand J Public Health 2011; 39:22–5. [DOI] [PubMed] [Google Scholar]
- 13. Harboe ZB, Valentiner-Branth P, Benfield TL, et al. Estimated effect of pneumococcal conjugate vaccination on invasive pneumococcal disease and associated mortality, Denmark 2000–2005. Vaccine 2008; 26:3765–71. [DOI] [PubMed] [Google Scholar]
- 14. Harboe ZB, Dalby T, Weinberger DM, et al. Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clin Infect Dis 2014; 59:1066–73. [DOI] [PubMed] [Google Scholar]
- 15. Statens Serum Institut. Epi-News, No 40 - 2014 Available at: https://en.ssi.dk/news/epi-news/2014/no-40---2014. Accessed 26 November 2019.
- 16. Gjerstorff ML The Danish cancer registry. Scand J Public Health 2011; 39:42–5. [DOI] [PubMed] [Google Scholar]
- 17. Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health 2011; 39:30–3. [DOI] [PubMed] [Google Scholar]
- 18. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173:676–82. [DOI] [PubMed] [Google Scholar]
- 19. Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sørensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol 2011; 11:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kildemoes HW, Sørensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health 2011; 39:38–41. [DOI] [PubMed] [Google Scholar]
- 21. Rostgaard K Methods for stratification of person-time and events - a prerequisite for Poisson regression and SIR estimation. Epidemiol Perspect Innov 2008; 5:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Andersen MA, Eriksen CT, Brieghel C, et al. Incidence and predictors of infection among patients prior to treatment of chronic lymphocytic leukemia: a Danish nationwide cohort study. Haematologica 2018; 103:e300–e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rozman C, Montserrat E, Viñolas N. Serum immunoglobulins in B-chronic lymphocytic leukemia. Natural history and prognostic significance. Cancer 1988; 61:279–83. [DOI] [PubMed] [Google Scholar]
- 24. Karlsson J, Hogevik H, Andersson K, Roshani L, Andréasson B, Wennerås C. Pneumococcal vaccine responses in elderly patients with multiple myeloma, Waldenstrom’s macroglobulinemia, and monoclonal gammopathy of undetermined significance. Trials Vaccinol 2013; 2:31–8. [Google Scholar]
- 25. Hinge M, Ingels HA, Slotved HC, Mølle I. Serologic response to a 23-valent pneumococcal vaccine administered prior to autologous stem cell transplantation in patients with multiple myeloma. APMIS 2012; 120:935–40. [DOI] [PubMed] [Google Scholar]
- 26. Sinisalo M, Aittoniemi J, Oivanen P, Käyhty H, Olander RM, Vilpo J. Response to vaccination against different types of antigens in patients with chronic lymphocytic leukaemia. Br J Haematol 2001; 114:107–10. [DOI] [PubMed] [Google Scholar]
- 27. Renaud L, Schraen S, Fouquet G, et al. Impact of pneumococcal vaccination on multiple myeloma. Blood 2017; 130:1871–1871.29051155 [Google Scholar]
- 28. Lindström V, Aittoniemi J, Salmenniemi U, et al. Antibody persistence after pneumococcal conjugate vaccination in patients with chronic lymphocytic leukemia. Hum Vaccines Immunother 2018; 14:1471–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harboe ZB, Thomsen RW, Riis A, et al. Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study. PLOS Med 2009; 6:e1000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nuorti JP, Mcgeer A. Cigarette smoking and invasive pneumococcal disease. N Engl J Med 2000:681–9. [DOI] [PubMed] [Google Scholar]
- 31. Harboe ZB, Larsen MV, Ladelund S, et al. Incidence and risk factors for invasive pneumococcal disease in HIV-infected and non-HIV-infected individuals before and after the introduction of combination antiretroviral therapy: persistent high risk among HIV-infected injecting drug users. Clin Infect Dis 2014; 59:1168–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.