Abstract
Objectives
Conventional synthetic DMARDs (csDMARDs) are the first-line treatment for PsA, but there is conflicting data regarding their efficacy and scarce reports describing the duration of use (drug retention) of csDMARD in this population. Their position in treatment recommendations is a matter of growing debate due to the availability of alternative treatment options with higher levels of evidence. We aimed to study drug retention and predictors for drug retention among PsA patients receiving first-line csDMARD monotherapy.
Methods
Retrospective cohort study in DMARD-naïve adult PsA patients in whom a first csDMARD was prescribed as monotherapy primarily to treat PsA-related symptoms. The main outcome was time to failure of the csDMARD (i.e. stopping the csDMARD or adding another DMARD).
Results
A total of 187 patients were included, who were mainly prescribed MTX (n = 163) or SSZ (n = 21). The pooled median drug retention time was 31.8 months (interquartile range 9.04–110). Drug retention was significantly higher in MTX (median 34.5 months; interquartile range 9.60–123) as compared with SSZ-treated patients (median 12.0 months; interquartile range 4.80– 55.7) (P =0.016, log-rank test). In multivariable Cox regression, the use of MTX and older age were associated with increased retention. The main reasons for treatment failure were inefficacy (52%) and side effects (28%). Upon failure, MTX treated patients were more commonly, subsequently treated with a biologic DMARD compared with SSZ (P < 0.05).
Conclusion
MTX outperforms SSZ as a first-line csDMARD in DMARD-naïve PsA patients with respect to monotherapy drug retention in daily clinical practice.
Keywords: PsA, csDMARDs, MTX, SSZ, outcome measures, quality of life, clinical practice
Rheumatology key messages
PsA patients initiating their first-line csDMARD have a monotherapy drug retention rate of ∼2.5 years.
First-line csDMARD monotherapy retention is higher in PsA patients that initiated methotrexate compared with sulfasalazine.
The main reasons for csDMARD retention failure in PsA are treatment inefficacy (52%) and side effects (28%).
Introduction
PsA is a chronic, inflammatory musculoskeletal disorder, which develops in approximately one in ten patients with psoriasis and often leads to a decreased quality of life and impaired function [1]. Currently, conventional synthetic DMARDs (csDMARDs) are the most commonly prescribed drugs as first-line treatment for peripheral arthritis in PsA, as recommended by the international EULAR and GRAPPA guidelines [2, 3]. These guidelines refer to MTX, SSZ, LEF and ciclosporin A as possible treatment options. However, previous studies found little or no effect of MTX on psoriatic synovitis, and higher effectiveness of TNF inhibitors compared with MTX in reducing radiographic progression in PsA patients [4–9]. Efficacy of LEF in PsA has been shown in one randomized controlled trial [10]. Regarding SSZ, clinical trials have found a modest favourable effect on musculoskeletal symptoms [11–13].
While there is a lack of high-level evidence to support the use of csDMARD efficacy in PsA, csDMARD drug retention rates can provide indirect evidence. One large study found a two-year retention rate of MTX therapy of ∼65% in both RA and PsA, suggestive of a beneficial effect of MTX in PsA [14]. Another comparable study observed mean MTX and LEF drug retention of 13 and 6 years, respectively [15]. More indirect evidence comes from the fact that PsA has historically been treated similar to RA, where high-level evidence supports the use of csDMARDs, and justifies this treatment in PsA patients. Other arguments to consider csDMARD therapy include well-described long-term safety outcomes and low costs; a factor considered by some (inter)national guidelines.
Despite these reasons to treat PsA with csDMARDs, their position is under pressure due to alternative treatment options with higher levels of evidence [1]. In line with this, a recent guideline recommends the use of TNF-inhibitors as a first-line treatment [16]. Furthermore, in daily practice csDMARD side effects are commonly reported that negatively impact drug retention.
This study aimed to evaluate the level of indirect evidence for csDMARD efficacy by describing first-line csDMARD monotherapy drug retention for treating PsA in daily clinical practice, comparing the retention rate of different csDMARDs and investigating possible predictors of drug retention.
Methods
Study design
This retrospective cohort study was performed at the University Medical Centre Utrecht, the Netherlands, and approved by the local institutional review board. The first selection of eligible patients was performed via electronic search based on diagnosis and diagnosis-related groups. Manual screening was performed twice to ensure eligibility. Inclusion criteria were clinical diagnosis of PsA, DMARD-naïve (no prior DMARD therapy for any cause, including psoriasis), and initiated DMARD as monotherapy after 1 January 2000. Patients were excluded if csDMARD therapy was primarily started for treating extra-articular manifestations (e.g. to treat psoriasis). All patient data was encrypted and saved using the online database CastorEDC.
Outcome measures
The main outcome was defined as the first-line csDMARD monotherapy drug retention time. The first-line csDMARD monotherapy cessation date (abbreviated ‘csDMARD monotherapy failure’) was set at the last recorded date during which the first-line csDMARD was prescribed as monotherapy. Thus, discontinuation of csDMARD monotherapy occurred upon cessation of the first-line csDMARD therapy or continuing the first-line csDMARD but adding a biological disease-modifying antirheumatic drug (bDMARD) or second csDMARD. Observations were considered ‘censored data’ if the patient was still on therapy at the last known medical record observation point or if the patient was lost to follow-up.
Using pre-defined categories, the research team retrospectively identified the main reason for first-line treatment cessation, as based on the reason recorded by the treating physician in the medical record. Categories included remission, inefficacy, side effects, (planned) pregnancy and other reasons. We registered the subsequent treatment prescribed within a window of six months after csDMARD monotherapy failure. A maximum tolerated ‘drug holiday’ of three months was allowed to mimic clinical care. Demographic, clinical and radiographic parameters were collected to identify predictors of treatment response.
Statistical analysis
Data analyses were performed using SPSS software (version 25.0). Data were represented as mean and standard deviation for normally distributed data and median and interquartile range (IQR) for non-normally distributed data. Baseline characteristics between MTX and SSZ groups were compared using the independent samples t test (normally distributed data), Mann–Whitney U test (non-normally distributed data) or χ2 test as appropriate. A P-value of <0.05 was considered statistically significant. Drug retention was described using Kaplan–Meier plots and statistically compared using the log-rank test. Potential predictors of drug retention were studied using a multivariable Cox model (described in Supplementary Material, section Methods, available at Rheumatology online).
Results
Cohort characteristics
In total, 187 patients with PsA met the inclusion criteria. Main demographics and disease activity characteristics are shown in Supplementary Table S1, available at Rheumatology online. The cohort consisted of 68% males with mean age 48 years (13.3 s.d.). The duration of disease was 0.4 years (IQR 0.1–1.0) and 7.5 years (IQR 2.1–18.1) for PsA and psoriasis, respectively. The most commonly prescribed first-line csDMARD was MTX (87%), followed by SSZ (11%) and LEF (2%). As compared with SSZ, patients initiating MTX had significantly higher age, body mass index and swollen and tender joint count. Also, there was a trend for erosive disease to be more common in the MTX than SSZ group.
csDMARD monotherapy drug retention
In total, 132 patients (71%) failed their first-line therapy during follow-up, while 55 patients (29%) had censored observation. The monotherapy drug retention showed a large drop in retention early after treatment initiation. In the entire study population, the median monotherapy drug retention was 31.8 months (95% CI 18.9, 44.6; IQR 9.04–110). At 12 months after treatment initiation, 70% of patients were still using the first-line csDMARD as monotherapy.
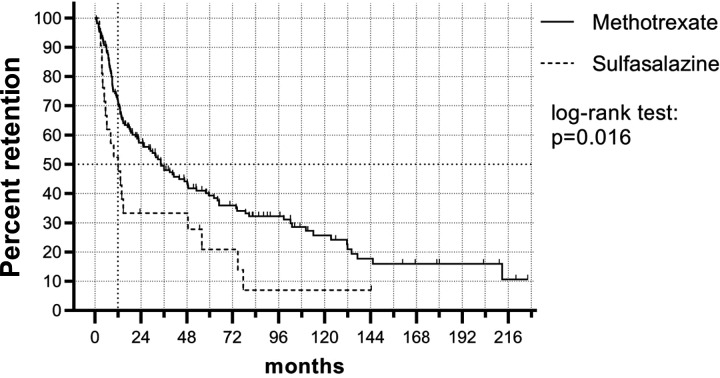
We next compared the different csDMARDs initiated, excluding LEF from further analysis due to low numbers. MTX had significantly higher monotherapy drug retention as compared with SSZ (P =0.016) (Fig. 1; Supplementary Fig. S1, available at Rheumatology online). For MTX, the median monotherapy drug retention was 34.5 months (95% CI 22.2, 46.8; IQR 9.60–123). For SSZ, the median monotherapy drug retention was 12.0 months (95% CI 4.32, 19.8; IQR 4.80–55.7). At 12 months, 72% of patients that initiated MTX were still using MTX as monotherapy, whereas 52% of patients that initiated SSZ were still using SSZ as monotherapy.
Fig. 1.
csDMARD monotherapy drug retention in PsA
Kaplan–Meier plot shows monotherapy drug retention rate of MTX or SSZ prescribed as first-line treatment in DMARD-naïve PsA patients. Ticks indicate censored data. MTX showed significantly higher monotherapy drug retention as compared to SSZ. csDMARD: conventional synthetic DMARD.
Based on univariable Cox regression analysis, the DMARD initiated was significantly associated with DMARD retention, where MTX-initiated patients had better retention as compared with SSZ-initiated patients (Hazard ratio (HR) 0.545 (95% CI 0.330, 0.899), P =0.017). In addition, older age increased csDMARD monotherapy retention (HR 0.985 per year age increase (95% CI 0.971, 0.998), P =0.026). When incorporating age and csDMARD initiated into the multivariable Cox regression model, there was a non-significant trend for longer drug retention in the MTX group [HR 0.630 (CI 0.372, 1.069), P =0.087] and older patients [per year age increase HR 0.988 (CI 0.974, 1.002), P =0.095]. We next screened for potential predictors of drug retention in a multivariable Cox model: the final model included csDMARD-initiated and age as the only predictors of drug retention (same HR as above).
Cause of csDMARD monotherapy failure
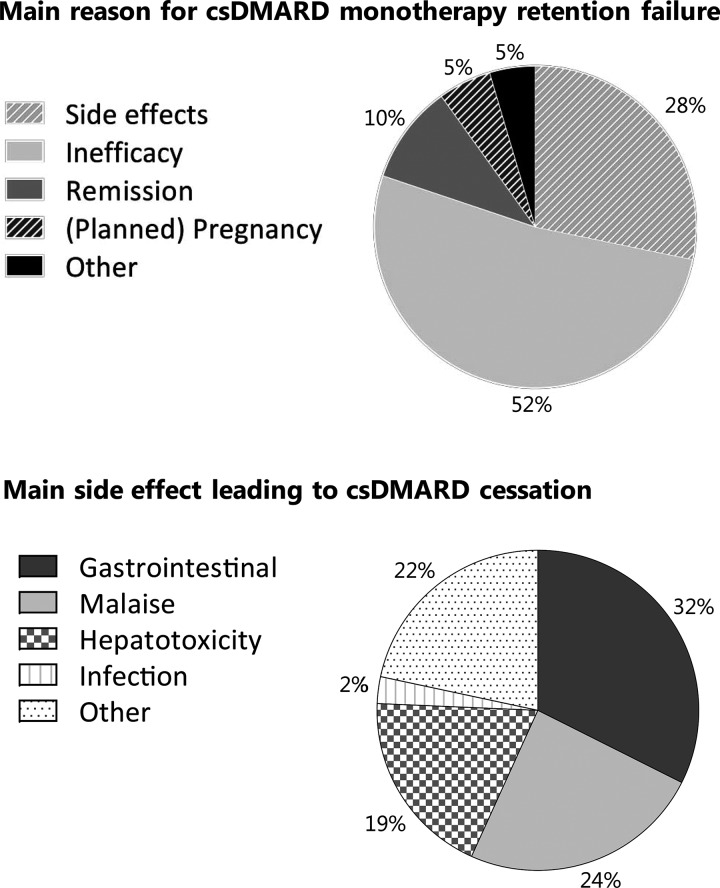
The main reason for csDMARD monotherapy treatment cessation was treatment inefficacy (52%), followed by side effects (28%) (Fig. 2). The main reasons for treatment cessation between MTX and SSZ were slightly different, with more patients that stopped MTX due to (planned) pregnancy and more patients that stopped SSZ due to inefficacy (Supplementary Table S2, available at Rheumatology online). Remission occurred in 11 patients that initiated MTX and two patients that initiated SSZ. Retention analysis remained similar when remission cases were excluded or considered censored. The most important side effects were gastrointestinal complaints (32%) and general malaise (24%) (Fig. 2). Patients treated with MTX reported more side effects than patients treated with SSZ (Supplementary Table S3 and S4, available at Rheumatology online). At time of csDMARD monotherapy failure, the patients that failed due to inefficacy had more active disease than the patients that failed due to other reasons (Supplementary Table S5, available at Rheumatology online).
Fig. 2.
csDMARD monotherapy retention failure
Top: Main reasons for csDMARD monotherapy retention failure (non-censored cases, n=132). Bottom: Main side effect reported at stop date for patients in whom the primary reason for csDMARD cessation was side effects (non-censored data, n=37). csDMARD: conventional synthetic DMARD.
Follow-up treatment upon csDMARD monotherapy failure
Upon csDMARD monotherapy failure, the first-line csDMARD was most commonly switched to a different csDMARD (27%) or a bDMARD was added (25%). However, the follow-up treatment regimen was significantly different between the MTX and SSZ groups: MTX-treated patients were more commonly prescribed a bDMARD upon failure (P <0.05). In addition, failure due to side effects vs inefficacy resulted in different follow-up treatment strategies: a bDMARD was prescribed in 55% of patients that failed due to inefficacy as compared with 11% of patients that failed due to side effects (P <0.05) (Supplementary Fig. S2 and Supplementary Tables S6–S8, available at Rheumatology online).
Discussion
This study shows that MTX as a first-line csDMARD for treating peripheral arthritis in PsA has higher monotherapy drug survival than SSZ. For all csDMARDs, monotherapy drug retention shows a large drop in the first year of treatment. Inefficacy is most commonly seen as the reason for drug cessation, followed by side effects. The results from this study are derived from a real-world setting and display a realistic clinical scenario of the first-line csDMARD monotherapy retention in csDMARD-naïve PsA patients.
A limited number of previous studies have evaluated csDMARD monotherapy drug retention [14, 15, 17]. We found a median csDMARD monotherapy retention of ∼2.5 years, but witnessed a large drop in drug retention within the first year of treatment. One previous study found a 10-year MTX retention rate of >50%, which largely exceeds our 10-year retention rate of around 25%. This difference may be partly explained by the concomitant steroid use [15]. Overall, the validity of our results are strengthened by those of other studies that found a similar drug retention rate [14, 17]. Our data also reveal that—even in the presence of potential efficacy—side effects were reported in >50% of patients at the moment of csDMARD monotherapy failure. Although not all of these side effects were deemed the principle cause of failure, they may have contributed to the modification of the treatment regimen.
With regard to predictors of csDMARD survival, one study described a larger PsA study cohort treated with MTX, but a shorter follow-up period with a maximum of 2 years. Their regression analysis showed age, disease duration and patient reported outcomes to be significant predictors of MTX drug retention [14]. We also found that older age was a predictor of longer drug retention, but did not identify other clinical parameters to be associated with drug retention. Additionally, we didn’t find sex or CRP levels to be significant predictors of drug retention, as proposed in earlier cohort studies [15, 18].
This study has a number of limitations. An important limitation is the retrospective nature of the study. Another factor that needs to be taken into account is the small number of subjects in the SSZ group compared with the MTX group. Also, drug retention is an assumed indirect measure of treatment efficacy, while drug adherence and treatment modifications are dependent on multiple factors in daily practice. Nonetheless, the use of real-world data also contains advantages over trial data by better portraying the setting in which DMARDs are initiated, as exemplified by the relative low joint count in our study cohort as compared with patients enlisted in PsA trials.
Overall, our results support the use of MTX as first-line therapy in treating peripheral arthritis in PsA, as recommended by current EULAR and GRAPPA guidelines [2, 3]. These data show that, at least compared with SSZ, MTX performs better with respect to monotherapy drug retention. Considering the emergence of numerous novel drugs for treating PsA, prospective studies (e.g. pragmatic randomized clinical trials [19]) are required to further elucidate the differential efficacy of specific csDMARDs as first-line treatment in PsA. The future research agenda should continue to focus on treatment challenges faced in the real-world setting, where the largest group of patients with PsA present with early, mono- or oligoarticular disease.
In conclusion, we found that MTX outperforms SSZ as first-line csDMARD in DMARD-naïve PsA patients with respect to monotherapy drug retention in daily clinical practice. Future prospective studies should further elucidate the efficacy of csDMARDs as first-line treatment for PsA.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: T.R.D.J.R. is currently an employee of AbbVie, with no conflicts of interest regarding the work of this manuscript. The other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1. Ritchlin CT, Colbert RA, Gladman DD.. Psoriatic arthritis. N Engl J Med 2017;376:957–70. [DOI] [PubMed] [Google Scholar]
- 2. Gossec L, Smolen JS, Ramiro S. et al. European League against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499–510. [DOI] [PubMed] [Google Scholar]
- 3. Coates LC, Kavanaugh A, Mease PJ. et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 2016;68:1060–71. [DOI] [PubMed] [Google Scholar]
- 4. Mahmood F, Coates LC, Helliwell PS.. Current concepts and unmet needs in psoriatic arthritis. Clin Rheumatol 2018;37:297–305. [DOI] [PubMed] [Google Scholar]
- 5. Eder L, Thavaneswaran A, Chandran V, Gladman DD.. Tumour necrosis factor α blockers are more effective than methotrexate in the inhibition of radiographic joint damage progression among patients with psoriatic arthritis. Ann Rheum Dis 2014;73:1007–11. [DOI] [PubMed] [Google Scholar]
- 6. Marchesoni A, Lubrano E, Cauli A, Ricci M, Manara M.. Psoriatic disease: update on traditional disease-modifying antirheumatic drugs. J Rheumatol 2015;93:61–4. [DOI] [PubMed] [Google Scholar]
- 7. Kingsley GH, Kowalczyk A, Taylor H. et al. A randomized placebo-controlled trial of methotrexate in psoriatic arthritis. Rheumatology 2012;51:1368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mease PJ, Gladman DD, Collier DH. et al. Etanercept and methotrexate as monotherapy or in combination for psoriatic arthritis: primary results from a randomized, controlled phase III trial. Arthritis Rheumatol 2019;71:1112–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilsdon TD, Whittle SL, Thynne TR, Mangoni AA.. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev 2017;2017:CD012722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaltwasser JP, Nash P, Gladman D. et al. Efficacy and safety of leflunomide in the treatment of psoriatic arthritis and psoriasis: a multinational, double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum 2004;50:1939–50. [DOI] [PubMed] [Google Scholar]
- 11. Clegg DO, Reda DJ, Mejias E. et al. Comparison of sulfasalazine and placebo in the treatment of psoriatic arthritis. A Department of Veterans Affairs Cooperative Study. Arthritis Rheum 1996;39:2013–20. [DOI] [PubMed] [Google Scholar]
- 12. Gupta AK, Grober JS, Hamilton TA. et al. Sulfasalazine therapy for psoriatic arthritis: a double blind, placebo controlled trial. J Rheumatol 1995;22:894–8. [PubMed] [Google Scholar]
- 13. Dougados M, Linden SVD, Leirisalo-Repo M. et al. Sulfasalazine in the treatment of spondylarthropathy. A randomized, multicenter, double-blind, placebo-controlled study. Arthritis Rheum 1995;38:618–27. [DOI] [PubMed] [Google Scholar]
- 14. Lie E, van der Heijde D, Uhlig T. et al. Effectiveness and retention rates of methotrexate in psoriatic arthritis in comparison with methotrexate-treated patients with rheumatoid arthritis. Ann Rheum Dis 2010;69:671–6. [DOI] [PubMed] [Google Scholar]
- 15. Zaffarana CA, Cerda O, Schneeberger EE. et al. Methotrexate and leflunomide survival in patients with psoriatic arthritis. Int J Clin Rheumtol 2018;13:102. [Google Scholar]
- 16. Singh JA, Guyatt G, Ogdie A. et al. Special article: 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol 2019;71:5–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ricci M, De Marco G, Desiati F. et al. Long-term survival of methotrexate in psoriatic arthritis. Reumatismo 2011;61:125–31. [DOI] [PubMed] [Google Scholar]
- 18. Glintborg B, Østergaard M, Dreyer L. et al. Treatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti–tumor necrosis factor α therapy: results from the nationwide Danish DANBIO registry. Arthritis Rheum 2011;63:382–90. [DOI] [PubMed] [Google Scholar]
- 19. Gamerman V, Cai T, Elsäßer A.. Pragmatic randomized clinical trials: best practices and statistical guidance. Heal Serv Outcomes Res Methodol 2019;19:23–35. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.