Abstract
Background
There is growing evidence that breast cancer survivors have higher cardiovascular disease (CVD) mortality relative to the general population. Information on temporal patterns for all-cause and CVD mortality among breast cancer survivors relative to cancer-free women is limited.
Methods
All-cause and CVD-related mortality were compared in 628 women with breast cancer and 3140 age-matched cancer-free women within CLUE II, a prospective cohort. We calculated adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) using Cox proportional hazards regression for all-cause mortality, and Fine and Gray models for CVD-related mortality to account for competing risks.
Results
Over 25 years of follow-up, 916 deaths occurred (249 CVD related). Breast cancer survivors had an overall higher risk of dying compared with cancer-free women (HR = 1.79, 95% CI = 1.53 to 2.09) irrespective of time since diagnosis, tumor stage, estrogen receptor status, and older age at diagnosis (≥70 years). Risk of death was greatest among older survivors at more than 15 years after diagnosis (HR = 2.69, 95% CI = 1.59 to 4.55). CVD (69.1% ischemic heart disease) was the leading cause of death among cancer-free women and the second among survivors. Survivors had an increase in CVD-related deaths compared with cancer-free women beginning at 8 years after diagnosis (HR = 1.65, 95% CI = 1.00 to 2.73), with the highest risk among older survivors (HR = 2.24, 95% CI = 1.29 to 3.88) and after estrogen receptor-positive disease (HR = 1.85, 95% CI = 1.06 to 3.20).
Conclusions
Breast cancer survivors continue to have an elevated mortality compared with the general population for many years after diagnosis. Preventing cardiac deaths, particularly among older breast cancer patients, could lead to reductions in mortality.
Advances in effective screening and treatment strategies have led to an increasing population of over 3.8 million breast cancer survivors in the United States (1). With 15-year survival rates now at 80% (2), understanding the long-term health of breast cancer survivors has become an issue of increasing importance. Cardiovascular disease (CVD) is the second leading cause of morbidity and mortality among breast cancer survivors overall (3). However, women diagnosed with early-stage breast cancer at an older age may be more likely to die from CVD than cancer (4,5). A number of recent studies have shown that breast cancer survivors have a greater incidence of CVD, including heart failure and cardiac arrhythmias, compared with the general population (6–8). A higher risk of CVD may result from cancer treatment and manifest either at the time of treatment or as a late effect (9–12). CVD risk may also be elevated due to shared risk factors between CVD and breast cancer, such as increasing age, early menopause, and lifestyle factors (9,13). To determine if breast cancer and its related treatment have an independent effect on CVD burden, it is important to examine CVD in survivors with a cancer-free comparison and to account for these shared factors.
Previous studies of CVD mortality in breast cancer survivors compared with the general population have been limited in scope (14–19). To date, only 2 studies have accounted for risk factors (18,19) and only 1 examined trends over time (18). Furthermore, although prognosis and treatment differ by clinical characteristics, whether associations vary by age at diagnosis, tumor stage, and estrogen receptor (ER) status remains unclear. To address these gaps in the literature, we prospectively examined all-cause and CVD-related mortality in women diagnosed with breast cancer by clinical characteristics and time since diagnosis relative to age-matched cancer-free women in a prospective community-based cohort with over 25 years of follow-up.
Methods
Study Participants and Design
The CLUE II (“Give Us a Clue to Cancer and Heart Disease”) cohort was formed in 1989 when 32 894 residents of Washington County, Maryland, and the surrounding area completed an enrollment questionnaire and provided blood samples (20). Written informed consent was obtained for all patients, and the study was approved by the institutional review board of Johns Hopkins Bloomberg School of Public Health.
For the present analysis, we identified women aged 18 years up to and including 80 years from CLUE II with no history of cancer (except for nonmelanoma skin cancer or cervical carcinoma in situ) at study enrollment who developed a first primary stage I-III breast cancer during follow-up (through December 31, 2015) (n = 628). For each woman diagnosed with breast cancer, we randomly selected 5 cancer-free women matched on age within 1 year (n = 3140). A ratio of 1:5 matching resulted in the closest matching on age at diagnosis while maintaining statistical efficiency. The index date was the date of breast cancer diagnosis for women with breast cancer and for their matched cancer-free women.
Ascertainment of Breast Cancer
Breast cancer diagnoses were ascertained through regular linkage to the Washington County hospital records and state cancer registry. In our study, all breast cancers were confirmed through pathology or medical records. Information on clinical and tumor characteristics included date of diagnosis, age at diagnosis, tumor stage, tumor size, and ER status.
Ascertainment of Death
Deaths were identified via hospital records, Maryland Vital Statistics, National Death Index, next of kin, and obituaries through December 31, 2015. Cause of death was ascertained through death certificates per Centers for Disease Control and Prevention guidelines (21). The following International Classification of Disease codes were used to identify CVD as the primary cause of death: 390-398, 402, 404, 410-429, I00-I09, I11, I13, and I20-I51. These codes were defined a priori to primarily represent deaths due to heart disease, including ischemic heart disease, hypertensive heart disease, pulmonary heart disease, and other heart diseases (eg, cardiomyopathy and heart failure).
Ascertainment of Covariates
Covariate information was obtained from the enrollment questionnaire in 1989. The questionnaire included information on date of birth, anthropometric factors (weight, height), lifestyle behaviors (smoking, alcohol use), reproductive or hormonal factors (oral contraceptive use, hormone use, and menopause status), medication use within the past 48 hours (eg, medication for blood pressure, cholesterol, CVD, diabetes), and socio-demographic indicators (race or ethnicity, education). In addition, resting blood pressure and plasma total cholesterol were measured at the time of study enrollment.
Statistical Analysis
Characteristics of breast cancer survivors and cancer-free women were compared with frequency distributions for categorical variables and means (SDs) for continuous variables. Breast cancer clinical characteristics were also summarized for breast cancer survivors.
For all-cause mortality, we calculated Kaplan-Meier failure curves and used Cox proportional hazards regression to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). Time since index date was used as the underlying time metric. Women contributed person-time from the index date to the date of death or December 31, 2015, whichever occurred first. The proportional hazards assumption was assessed graphically and with Schoenfeld residuals; there was no indication that the assumption of proportional hazards was violated. Results are presented overall, stratified by stage (I, II or III) and ER status (ER-positive, ER-negative), and restricted to older women at diagnosis (≥70 years, based on the age distribution in the cohort). To examine temporal trends, Cox proportional hazards regression models were stratified by time since diagnosis (0-5 years, >5-15 years, and >15 years). These cut points were determined a priori and based on clinically meaningful thresholds for risk of breast cancer recurrence (22). Temporal trends were examined by stage at diagnosis among ER-positive survivors and older survivors. We used multiplicative interaction terms between breast cancer status and time since diagnosis. Overall models were stratified by index date (<2005, ≥2005) to examine whether trends in mortality differed by year of diagnosis. We also examined initial breast cancer treatment subgroups (radiation, chemotherapy, and hormone therapy) among women with an index date of 1998 or later because breast cancer treatment had less than 10% missing during this time period.
We used inverse probability weighting (IPW) to standardize the distribution of variables between survivors and cancer-free women in both Kaplan Meier failure curves and regression models for all-cause mortality (23–26). Adjustment for covariates included age, menopausal status, education, smoking status, alcohol intake, body mass index (BMI), and history of oral hormone use. Further details on IPW and covariates are included in the Supplementary Methods (available online). To account for possible changes in covariates over time, we conducted a sensitivity analysis restricted to women diagnosed within 5 years of completing the enrollment questionnaire.
For CVD-related mortality, we used a competing risk approach to account for non-CVD mortality as a competing event (27–29). For these analyses, there were 3 potential outcomes: CVD-related death (event of interest); non–CVD-related death (competing event), and administrative censoring (alive at the end of follow-up). Subdistribution hazard ratios using Fine and Gray regression models are presented in the text. Cause-specific hazard ratios were similar and are reported in the Supplementary Methods (available online). Details on the competing risk approach are further described in the Supplementary Methods (available online). IPW was used to standardize the distribution of variables for cumulative incidence curves and regression models. Because the assumption of proportional hazards by breast cancer status was violated for CVD-related mortality, we also report associations stratified by follow-up time.
We conducted a similar series of stratified analyses for CVD-related mortality by stage, ER status, and older age at diagnosis. However, temporal trends were stratified by 0-8 years vs greater than 8 years of follow-up. These cut points were determined empirically based on the overall cumulative incidence curves. We restricted estimates up to 15 years when examining temporal trends due to a small number of events in survivors after this cut point. Temporal trends for stage II or III and results for treatment subgroups were not included due to the small number of CVD deaths. We further conducted several post hoc analyses to examine CVD-related mortality due to ischemic heart disease.
Analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC) and Stata version 14.0 (StataCorp LP, College Station, TX). All statistical tests were 2-sided, and P values less than .05 were considered statistically significant.
Results
Descriptive Characteristics
Table 1 describes characteristics of 3768 women by breast cancer status (628 breast cancer survivors, 3140 cancer-free women). The mean age at diagnosis was 64.5 years and the median year of diagnosis was 2002 (25th-75th percentile: 1996-2009). Breast cancers were primarily ER-positive and diagnosed at an early stage. Among those diagnosed with ER-positive disease, tumors were primarily stage I and 2 cm or less at diagnosis (Supplementary Table 1 available online). The mean time from enrollment into the cohort to index date was 13.3 years (SD = 7.3). Baseline characteristics were overall similar in survivors compared with cancer-free women (postmenopausal: 58.8% vs 56.6%; mean BMI: 26.5 vs 26.1 kg/m2; never smokers: 61.8% vs 61.1%). In addition, variables related to cardiovascular health, such as mean total cholesterol and blood pressure measurements, and the proportion of women taking heart disease and diabetes medications were also similar among breast cancer survivors and cancer-free women.
Table 1.
Baseline characteristics in breast cancer survivors and cancer-free women in CLUE II
| Characteristic | Cancer-free women (n = 3140) | Survivors (n = 628) |
|---|---|---|
| Age at index date, mean (SD), y | 64.4 (11.8) | 64.5 (11.8) |
| White, No. (%) | 3089 (98.4) | 622 (99.0) |
| Education, No. (%) | ||
| <12 y | 652 (20.8) | 118 (18.8) |
| 12 y | 1523 (48.5) | 290 (46.2) |
| >12 y | 964 (30.7) | 220 (35.0) |
| Missing | 1 (0.03) | 0 (0.0) |
| Postmenopausal, No. (%) | 1777 (56.6) | 369 (58.8) |
| Body mass index, mean (SD), kg/m2 | 26.1 (5.1) | 26.5 (5.4) |
| Smoking status, No. (%) | ||
| Never | 1919 (61.1) | 388 (61.8) |
| Former | 672 (21.4) | 149 (23.7) |
| Current | 549 (17.5) | 91 (14.5) |
| Missing | 0 (0.0) | 0 (0.0) |
| Alcohol intake, No. (%) | ||
| Never or <1 drink/mo | 1614 (51.4) | 323 (51.4) |
| 1-3 drinks/mo | 382 (12.2) | 82 (13.1) |
| ≥1 drinks/wk | 1027 (32.7) | 202 (32.2) |
| Missing | 117 (3.7) | 21 (3.3) |
| Oral contraceptive use, No. (%) | ||
| Never | 1910 (60.8) | 396 (63.1) |
| Former | 998 (31.8) | 183 (29.1) |
| Current | 196 (6.2) | 42 (6.7) |
| Missing | 36 (1.1) | 7 (1.1) |
| Hormone use, No. (%) | ||
| Never | 2515 (80.1) | 503 (80.1) |
| Former | 132 (4.2) | 24 (3.8) |
| Current estrogen only | 286 (9.1) | 49 (7.8) |
| Current estrogen + progesterone or progesterone only | 100 (3.2) | 26 (4.1) |
| Missing | 107 (3.4) | 26 (4.1) |
| Systolic blood pressure, mean (SD), mmHg | 125.9 (35.6) | 126.3 (38.6) |
| Diastolic blood pressure, mean (SD), mmHg | 79.2 (34.1) | 79.9 (37.9) |
| Plasma total cholesterol, mean (SD), mg/dL | 228.0 (119.3) | 223.5 (96.8) |
| Current medication use, No. (%) | ||
| High–blood pressure medication | 603 (19.2) | 117 (18.6) |
| High-cholesterol medication | 139 (4.4) | 24 (3.8) |
| CVD medication | 784 (25.0) | 149 (23.7) |
| Diabetes medication | 66 (2.1) | 14 (2.2) |
| Year of breast cancer diagnosis, median (25th-75th percentile) | —a | 2002 (1996-2009) |
| Breast cancer stage, No. (%) | ||
| I | — | 375 (59.7) |
| II or III | — | 244 (38.9) |
| Unknown or missing | — | 9 (1.4) |
| Estrogen receptor status, No. (%) | ||
| Positive | — | 463 (73.7) |
| Negative | — | 105 (16.7) |
| Unknown or missing | — | 60 (9.6) |
| Vital status at end of study follow-up,b,c,d No. (%) | ||
| Death from any cause | 699 (22.3) | 217 (34.6) |
| Death from breast cancer | 0 (0.0) | 64 (10.2) |
| Death from cancer (non-breast cancer) | 118 (3.8) | 25 (4.0) |
| Death from CVD | 205 (6.5) | 44 (7.0) |
| Death from non-CVD | 494 (15.7) | 173 (27.6) |
| Administrative censoring (alive at end of follow-up) | 2441 (77.7) | 411 (65.4) |
Not applicable to cancer-free women. CVD = cardiovascular disease.
Vital status as of December 31, 2015.
Among 699 deaths in cancer-free women, 29% due to CVD-related disease (n = 205) and 17% due to non-breast cancer (n = 118).
Among 217 deaths in breast cancer survivors, 29% due to breast cancer (n = 64), 20% due to CVD-related disease (n = 44), and 12% due to non-breast cancer (n = 25).
All-Cause Mortality
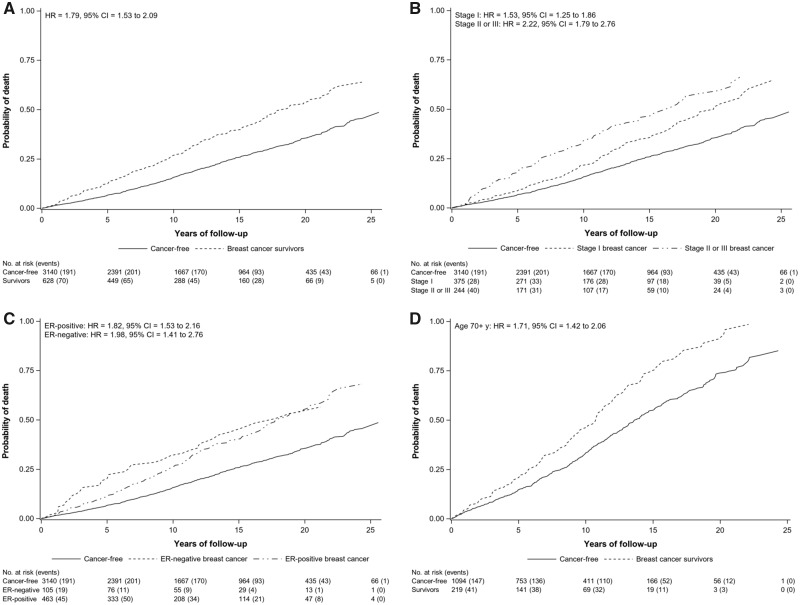
Over a median follow-up of 10.4 years (maximum, 26 years), there were 916 deaths from any cause (699 in cancer-free women, 217 in survivors) (Table 1; Supplementary Table 2 available online). All-cause mortality was consistently higher among breast cancer survivors relative to cancer-free women after adjusting for age, menopausal status, education level, smoking status, alcohol intake, BMI, and hormone use (Figure 1A). Overall, survivors had an almost 2-fold higher risk of dying compared with cancer-free women (HR = 1.79, 95% CI = 1.53 to 2.09). Similar results were observed for survivors when stratified by tumor stage, ER status, and older age at diagnosis compared with cancer-free women (Figure 1, B-D). Results restricted to women diagnosed within 5 years of completing the enrollment questionnaire were slightly attenuated (HR = 1.72, 95% CI = 1.29 to 2.29). For treatment subgroups, results were similar but also attenuated compared to overall estimates (Supplementary Table 3 available online).
Figure 1.
Adjusted Kaplan-Meier failure curves and hazard ratios (HRs) with 95% confidence intervals (CIs) for all-cause mortality in breast cancer survivors compared with cancer-free women. Figures are presented overall (A) and by stage at diagnosis (B), estrogen receptor (ER) status (C), and restricted to women aged 70 years or older at diagnosis (D). Results are adjusted for age (years), menopausal status (premenopausal, postmenopausal), education (<12, 12, >12 years), smoking status (never, former, current), alcohol intake (<3 drinks/mo, ≥1 drinks/wk), body mass index (<25, 25 to <30, ≥30 kg/m2), and oral hormone use (ever, never) using inverse probability weighting. P values from log-rank tests were less than .001 for figures A–D.
Risk of death from any cause was not statistically different over follow-up (0-5 years: HR = 1.91, 95% CI = 1.45 to 2.52; >5-15 years: HR = 1.70, 95% CI = 1.37 to 2.11; and >15 years: HR = 1.84, 95% CI = 1.28 to 2.66, Pinteraction = .91) (Table 2). Patterns of association were similar by stage (Pinteraction: stage I = .17; stage II or III = .47) and for women with ER-positive tumors (Pinteraction = .36). Among older women, risk of all-cause mortality differed by time with a 44% increased risk of all-cause mortality within the first 5 years after diagnosis (HR = 1.44, 95% CI = 1.01 to 2.04) and an almost 3-fold higher risk of all-cause mortality at more than 15 years after diagnosis (HR = 2.69, 95% CI = 1.59 to 4.55) (Pinteraction = .05).
Table 2.
Adjusted hazard ratios and 95% confidence intervals for all-cause mortality according to time since diagnosis and clinical characteristics at breast cancer diagnosis
| Cancer-free |
Survivors |
|||
|---|---|---|---|---|
| Time since breast cancer diagnosis | Events/PT | HR (95% CI)a | Events/PT | HR (95% CI)a |
| Overall | ||||
| 0-5 y | 191/13 900 | 1.00 (ref) | 70/2694 | 1.91 (1.45 to 2.52) |
| >5-15 y | 371/16 726 | 1.00 (ref) | 110/2924 | 1.70 (1.37 to 2.11) |
| >15 y | 137/4620 | 1.00 (ref) | 37/716 | 1.84 (1.28 to 2.66) |
| Pinteractionb | — | .91 | ||
| Clinical characteristics | ||||
| Stage I | ||||
| 0-5 y | 191/13 900 | 1.00 (ref) | 28/1619 | 1.27 (0.85 to 1.90) |
| >5-15 y | 371/16 726 | 1.00 (ref) | 61/1766 | 1.55 (1.18 to 2.04) |
| >15 y | 137/4620 | 1.00 (ref) | 23/418 | 1.93 (1.24 to 3.00) |
| Pinteractionb | — | .17 | ||
| Stage II or III | ||||
| 0-5 y | 191/13 900 | 1.00 (ref) | 40/1035 | 2.81 (1.99 to 3.96) |
| >5-15 y | 371/16 726 | 1.00 (ref) | 48/1106 | 1.98 (1.45 to 2.70) |
| >15 y | 137/4620 | 1.00 (ref) | 14/269 | 1.93 (1.09 to 3.40) |
| Pinteractionb | — | .47 | ||
| ER-positive | ||||
| 0-5 y | 191/13 900 | 1.00 (ref) | 45/1993 | 1.66 (1.20 to 2.31) |
| >5-15 y | 371/16 726 | 1.00 (ref) | 84/2114 | 1.81 (1.42 to 2.30) |
| >15 y | 137/4620 | 1.00 (ref) | 29/507 | 2.11 (1.42 to 3.15) |
| Pinteractionb | — | .36 | ||
| Age ≥70 y | ||||
| 0-5 y | 147/4649 | 1.00 (ref) | 41/902 | 1.44 (1.01 to 2.04) |
| >5-15 y | 246/4292 | 1.00 (ref) | 70/717 | 1.76 (1.35 to 2.30) |
| >15 y | 64/635 | 1.00 (ref) | 14/54 | 2.69 (1.59 to 4.55) |
| Pinteractionb | — | .05 | ||
Adjusted for age (years), menopausal status (premenopausal, postmenopausal), education (<12, 12, >12 years), smoking status (never, former, current), alcohol intake (<3 drinks/mo, ≥1 drinks/wk), body mass index (<25, 25 to <30, ≥30 kg/m2), and oral hormone use (ever, never) using inverse probability weighting. CI = confidence interval; ER = estrogen receptor; HR = hazard ratio; PT = person-time in years.
P value for interaction between breast cancer status and time.
CVD-Related Mortality
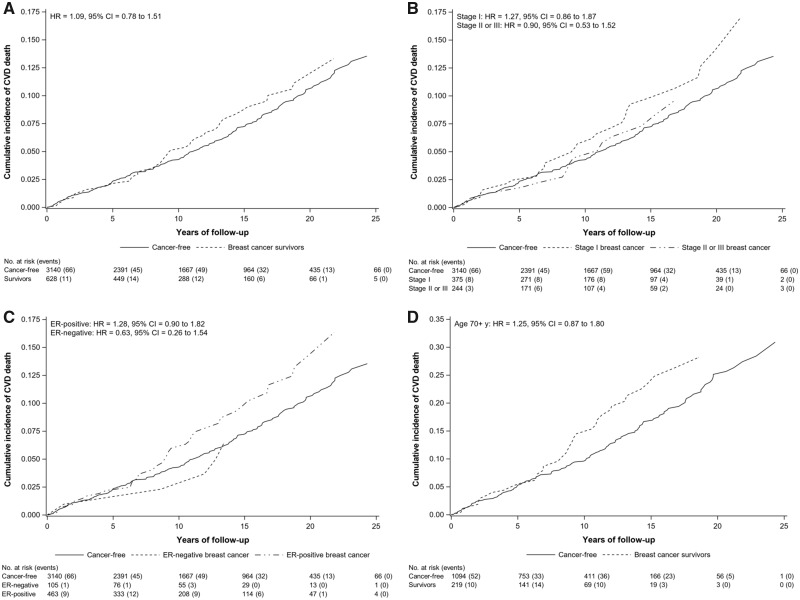
CVD-related mortality (n = 249 deaths) was the second most common cause of death among survivors (20% of deaths; n = 44 CVD-related deaths) and the most frequent cause among cancer-free women (29% of deaths; n = 205 CVD-related deaths). Ischemic heart disease was the leading cause of CVD death (Supplementary Table 2 available online). Based on adjusted cumulative incidence curves, an increase in CVD-related deaths among survivors was observed after approximately 8 years of follow-up (Figure 2A). Similarly, an elevated risk of CVD-related mortality only became apparent after several years of follow-up among stage I, ER-positive, and older breast cancer survivors (Figure 2, B-D).
Figure 2.
Adjusted cumulative incidence function and subdistribution hazard ratios (HRs) with 95% confidence intervals (CIs) for cardiovascular disease (CVD)-related mortality, both of which account for competing risks, in breast cancer survivors compared with cancer-free women. Figures are presented overall (A) and by stage at diagnosis (B), estrogen receptor (ER) status (C), and restricted to women aged 70 years or older at diagnosis (D). Results are adjusted for age (years), menopausal status (premenopausal, postmenopausal), education (<12, 12, >12 years), smoking status (never, former, current), alcohol intake (<3 drinks/mo, ≥1 drinks/wk), body mass index (<25, 25 to <30, ≥30 kg/m2), and oral hormone use (ever, never) using inverse probability weighting. Subdistribution hazard ratios are estimated from Fine and Gray models.
The adjusted hazard ratio comparing CVD-related deaths in breast cancer survivors with cancer-free women was 0.94 (95% CI = 0.56 to 1.58) at 0-8 years and 1.65 (95% CI = 1.00 to 2.73) after 8 years of follow-up (Pinteraction = .13) (Table 3). Results for ER-positive breast cancer survivors were similar (0-8 years: HR = 1.06, 95% CI = 0.60 to 1.86; >8 years: HR = 1.85, 95% CI = 1.06 to 3.20; Pinteraction = .17). Stage I survivors did not have a statistically significant higher risk of CVD-related mortality compared with cancer-free women even after 8 years of follow-up. Among older women, results differed by time since diagnosis with an over 2-fold higher risk of CVD-related mortality after 8 years in breast cancer survivors relative to cancer-free women (HR = 2.24, 95% CI = 1.29 to 3.88) and no statistically significant association before 8 years (HR = 1.10, 95% CI = 0.64 to 1.88) (Pinteraction = .07). In these analyses, non–CVD-related mortality was most commonly due to cancer (51% and 24% of non–CVD-related deaths in survivors and cancer-free women, respectively). Although survivors had a statistically significant higher risk of non–CVD-related mortality, within the first 8 years of follow-up, risk declined 8 years after diagnosis. Cause-specific hazard ratios were similar and are presented in Supplementary Table 4 (available online).
Table 3.
Adjusted subdistribution hazard ratios and 95% confidence intervals for CVD-related and non-CVD mortality according to time since diagnosis and clinical characteristics at breast cancer diagnosis
| Time since breast cancer diagnosis | CVD-related mortality |
Non-CVD mortalitya |
||||||
|---|---|---|---|---|---|---|---|---|
| Cancer-free |
Survivors |
Cancer-free |
Survivors |
|||||
| Events/PT | sdHR(95% CI)b | Events/PT | sdHR(95% CI)b | Events/PT | sdHR(95% CI)b | Events/PT | sdHR(95% CI)b | |
| Overall | ||||||||
| 0-8 y | 92/20 398 | 1.00 (ref) | 17/3875 | 0.94 (0.56 to 1.58) | 210/20 398 | 1.00 (ref) | 93/3875 | 2.36 (1.85 to 3.02) |
| >8 y | 68/10 228 | 1.00 (ref) | 20/1744 | 1.65 (1.00 to 2.73) | 192/10 228 | 1.00 (ref) | 50/1744 | 1.48 (1.09 to 2.03) |
| Pinteractionc | — | .13 | .02 | |||||
| Clinical characteristicsd | ||||||||
| Stage I | ||||||||
| 0-8 y | 92/20 398 | 1.00 (ref) | 12/2327 | 1.16 (0.63 to 2.12) | 210/20 398 | 1.00 (ref) | 34/2327 | 1.42 (0.98 to 2.04) |
| >8 y | 68/10 228 | 1.00 (ref) | 12/1054 | 1.61 (0.87 to 3.00) | 192/10 228 | 1.00 (ref) | 31/1054 | 1.52 (1.03 to 2.23) |
| Pinteractionc | — | .45 | .80 | |||||
| ER-positive | ||||||||
| 0-8 y | 92/20 398 | 1.00 (ref) | 14/2861 | 1.06 (0.60 to 1.86) | 210/20 398 | 1.00 (ref) | 62/2861 | 2.13 (1.60 to 2.83) |
| >8 y | 68/10 228 | 1.00 (ref) | 16/1246 | 1.85 (1.06 to 3.20) | 192/10 228 | 1.00 (ref) | 37/1246 | 1.55 (1.09 to 2.21) |
| Pinteractionc | — | .17 | .17 | |||||
| Age ≥70 y | ||||||||
| 0-8 y | 73/6577 | 1.00 (ref) | 16/1248 | 1.10 (0.64 to 1.88) | 152/6577 | 1.00 (ref) | 48/1248 | 1.68 (1.21 to 2.33) |
| >8 y | 48/2364 | 1.00 (ref) | 18/370 | 2.24 (1.29 to 3.88) | 120/2364 | 1.00 (ref) | 29/370 | 1.39 (0.93 to 2.08) |
| Pinteractionc | — | .07 | .48 | |||||
Non–CVD-related mortality is defined as the competing event for CVD-related mortality and includes death due to breast cancer, non-breast cancer, and all other causes. CI = confidence interval; CVD = cardiovascular disease; ER = estrogen receptor; PT = person-time in years; sdHR = subdistribution hazard ratios.
Subdistribution hazard ratios adjusted for age (years), menopausal status (premenopausal, postmenopausal), education (<12, 12, >12 years), smoking status (never, former, current), alcohol intake (<3 drinks/mo, ≥1 drinks/wk), body mass index (<25, 25 to <30, ≥30 kg/m2), and oral hormone use (ever, never) using inverse probability weighting. Subdistribution hazard ratios are estimated from Fine and Gray models, which account for competing events.
P value for interaction between breast cancer status and time.
Results for women diagnosed with stage II or III breast cancer are not presented due to a small number of CVD events.
Because cardiotoxicity of cancer treatments have changed over time, analyses were stratified by year of diagnosis (Supplementary Table 5 available online). A statistically non-significant elevated risk of CVD-related mortality was observed in women diagnosed in 2005 or later but not in women diagnosed before 2005 (HR = 1.66, 95% CI = 0.78 to 3.55; HR = 1.00, 95% CI = 0.69 to 1.43, respectively).
Analyses examining risk of ischemic heart disease mortality found that survivors overall had a statistically non-significant increased risk of dying from ischemic heart disease compared with cancer-free women (HR = 1.22, 95% CI = 0.84 to 1.79) (Supplementary Table 6 available online). However, we observed a statistically significant increased risk of ischemic heart disease death in ER-positive (HR = 1.51, 95% CI = 1.02 to 2.25) and older survivors (HR = 1.55, 95% CI = 1.02 to 2.34).
Discussion
After 25 years of follow-up, we observed that breast cancer survivors had a 79% higher risk of death from any cause relative to cancer-free women. All-cause mortality was consistently higher in survivors regardless of tumor stage, ER status, and older age at diagnosis, whereas CVD deaths were increased only after 8 years since diagnosis, particularly among women diagnosed at an older age or with an ER-positive tumor.
Although CVD mortality has been commonly reported in case-only studies among breast cancer survivors (3–5,30–34) and compared with the general population in a few studies (14–19), these studies, however, lacked information on risk factors and trends over time. To date, only 2 studies have examined all-cause and CVD-related mortality in breast cancer survivors relative to cancer-free women after accounting for shared risk factors (18,19) and only 1 examined temporal trends (18). Neither study examined mortality by clinical characteristics. The first of these studies, conducted in the Long Island Breast Cancer Study Project from 1996 to 2009, found that breast cancer survivors had an 80% increased risk of all-cause mortality compared with cancer-free women (HR = 1.8, 95% CI = 1.5 to 2.1) even after accounting for age, menopause, and other potential confounding factors (18). This study also observed that CVD-related mortality was almost 2-fold higher in breast cancer survivors relative to cancer-free women only after 7 years since diagnosis (18). Although results were consistent with our findings, the study had several limitations, including a short period of ascertainment for cancer cases (1996-1997), which did not extend into the more recent treatment era, lack of follow-up for incident cancers, minimal information on tumor characteristics, and a shorter duration of follow-up through 2009 (maximum follow-up, 13.5 years). The second study, conducted in the Women’s Health Initiative (WHI) from 1993 to 2010, reported a higher total mortality rate in women with localized breast cancer compared with cancer-free women. The study also found that CVD was the leading cause of death among women aged 70-79 years at breast cancer diagnosis (19). Results were restricted to 10 years postdiagnosis and may be less generalizable because WHI participants were postmenopausal women aged 60-79 years who had a predicted survival of more than 3 years at enrollment. Notably, the WHI study did not stratify by time since diagnosis, which may have attenuated any increase in CVD mortality among breast cancer survivors compared with cancer-free women.
Our results suggest that ischemic heart disease is a major cause of CVD death among breast cancer survivors. Primary mechanisms that have been proposed for CVD risk among survivors include both a higher prevalence of CVD risk factors (eg, older age, obesity, hypertension, diabetes, and physical inactivity) (35) and cardiotoxic effects from breast cancer treatment (36). Radiotherapy, particularly to the left side of the chest wall, has been associated with both cardiomyopathy and ischemic heart disease (37). Radiation-related ischemic heart disease has been shown to develop within a few years after exposure or as a late-effect up to 20 years after treatment (38). The development of cardiomyopathy and subsequent heart failure has also been associated with the use of specific chemotherapeutic agents (eg, anthracyclines) and trastuzumab, a targeted therapy used to treat human epidermal growth factor receptor 2-positive breast cancers. Finally, aromatase inhibitors used to treat postmenopausal ER-positive breast cancer have been associated with an increased risk of CVD events (39) as well as hypertension, vascular dysfunction, and unfavorable lipid changes (40–42).
Adding to the literature, our findings support that CVD may manifest as a late toxicity and that older women and women diagnosed with ER-positive tumors are most at risk, particularly for ischemic heart disease death. It is plausible that hormone therapy may be driving the elevated mortality in women with ER-positive tumors. Survivors treated with aromatase inhibitors may have increased CVD relative to those treated with tamoxifen therapy (43), and this may be of particular importance among older women (44). However, the effect of hormone therapy when compared with no treatment is still uncertain. The underlying mechanisms of higher CVD-related mortality in older breast cancer survivors may be due to treatment-related cardiotoxicity (9) and higher prevalence of shared risk factors or comorbidities compared with the general population (45).
Strengths of our study include the fact that both women with breast cancer and cancer-free women were from the same community-based cohort, which had data on risk factors and tumor characteristics and over 25 years of follow-up. There are also several limitations of our study. First, power was limited to detect more modest associations and interactions. Further, we were unable to conduct subgroup analyses by breast cancer treatment for CVD-related mortality. However, we did examine associations by tumor characteristics, which can be considered proxies for hormone therapy treatment because ER status is highly correlated with hormone therapy (46) and early-stage ER-positive tumors may be treated with hormone therapy alone. Finally, our study consisted of primarily White participants (>98%) and, although population based, was restricted to women living in Washington County, Maryland. Therefore, results may not be generalizable to women of other racial groups or women outside of Washington County. Future studies are needed to examine these associations over time among more diverse populations.
In conclusion, our results show that breast cancer survivors continue to have an elevated long-term risk of mortality compared with the general population, supporting the need for novel approaches to reduce mortality in these women. Survivors, particularly those diagnosed at an older age or with an ER-positive tumor, may have a higher risk of dying from CVD than comparable cancer-free women and therefore would benefit from targeted approaches to CVD prevention. Large prospective studies are needed to evaluate specific breast cancer treatments on CVD incidence and mortality compared with the general population.
Funding
This work was supported by the Breast Cancer Research Foundation and grants from the National Cancer Institute at the National Institutes of Health (T32-CA009314 to CR and MLS) and an NCI Cancer Center Support Grant (P30 CA006973).
Notes
Role of the funder: The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: The authors have no conflicts of interest to disclose.
Role of the authors: CR: Conceptualization; Data curation; Methodology; Writing - review and editing. MLS: Data curation; Writing—review and editing. ZZ: Data curation; Writing—review and editing. AEC: Writing—review and editing. JH-B: Data curation; Writing—review and editing. BL: Methodology; Writing—review and editing. KV: Conceptualization; Funding acquisition; Methodology; Supervision; Writing—review and editing.
Acknowledgments: Cancer data was provided by the Maryland Cancer Registry, Center for Cancer Prevention and Control, Department of Health and Mental Hygiene, with funding from the State of Maryland and the Maryland Cigarette Restitution Fund. The collection and availability of cancer registry data is also supported by the Cooperative Agreement NU58DP006333, funded by the Centers for Disease Control and Prevention. We thank the participants of CLUE II and appreciate the continued efforts of the staff at the Johns Hopkins George W. Comstock Center for Public Health Research and Prevention in the conduct of the CLUE Cohort Studies.
Disclaimers: The manuscript contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services..
Data Availability
The data underlying this article may be made available upon application to the CLUE II study (Principal Investigator, Kala Visvanathan).
Supplementary Material
References
- 1. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019;69(5):363–385. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Breast Cancer Facts & Figures 2019-2020. Atlanta, GA: American Cancer Society, Inc; 2019. [Google Scholar]
- 3. Schairer C, Mink PJ, Carroll L, et al. Probabilities of death from breast cancer and other causes among female breast cancer patients. J Natl Cancer Inst. 2004;96(17):1311–1321. [DOI] [PubMed] [Google Scholar]
- 4. Abdel-Qadir H, Austin PC, Lee DS, et al. A population-based study of cardiovascular mortality following early-stage breast cancer. JAMA Cardiol. 2017;2(1):88–93. [DOI] [PubMed] [Google Scholar]
- 5. Patnaik JL, Byers T, DiGuiseppi C, et al. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13(3):R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abdel-Qadir H, Thavendiranathan P, Austin PC, et al. The risk of heart failure and other cardiovascular hospitalizations after early stage breast cancer: a matched cohort study. J Natl Cancer Inst. 2019;111(8):854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buddeke J, Gernaat SAM, Bots ML, et al. Trends in the risk of cardiovascular disease in women with breast cancer in a Dutch nationwide cohort study. BMJ Open. 2019;9(5):e022664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Strongman H, Gadd S, Matthews A, et al. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet. 2019;394(10203):1041–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mehta LS, Watson KE, Barac A, et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. 2018;137(8):e30–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carver JR, Shapiro CL, Ng A, et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25(25):3991–4008. [DOI] [PubMed] [Google Scholar]
- 11. Chargari C, Kirov KM, Bollet MA, et al. Cardiac toxicity in breast cancer patients: from a fractional point of view to a global assessment. Cancer Treat Rev. 2011;37(4):321–330. [DOI] [PubMed] [Google Scholar]
- 12. Curigliano G, Cardinale D, Dent S, et al. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J Clin. 2016;66(4):309–325. [DOI] [PubMed] [Google Scholar]
- 13. Koene RJ, Prizment AE, Blaes A, et al. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hooning MJ, Aleman BMP, van Rosmalen AJM, et al. Cause-specific mortality in long-term survivors of breast cancer: a 25-year follow-up study. Int J Radiat Oncol Biol Phys. 2006;64(4):1081–1091. [DOI] [PubMed] [Google Scholar]
- 15. Schonberg MA, Marcantonio ER, Ngo L, et al. Causes of death and relative survival of older women after a breast cancer diagnosis. J Clin Oncol. 2011;29(12):1570–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riihimäki M, Thomsen H, Brandt A, et al. Death causes in breast cancer patients. Ann Oncol. 2012;23(3):604–610. [DOI] [PubMed] [Google Scholar]
- 17. Sturgeon KM, Deng L, Bluethmann SM, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40(48):3889–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bradshaw PT, Stevens J, Khankari N, et al. Cardiovascular disease mortality among breast cancer survivors. Epidemiology. 2016;27(1):6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park N-J, Chang Y, Bender C, et al. Cardiovascular disease and mortality after breast cancer in postmenopausal women: results from the Women’s Health Initiative. PLoS ONE. 2017;12(9):e0184174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. George W. Comstock Center for Public Health Research and Prevention, CLUE studies. https://www.jhsph.edu/research/centers-and-institutes/george-w-comstock-center-for-public-health-research-and-prevention/clue_research_activities.html. Accessed December 16, 2019.
- 21.National Center for Health Statistics, National Vital Statistics System. SuperMICAR data entry instructions. NCHS instruction manual; Part 2S. 2005. https://www.cdc.gov/nchs/nvss/instruction_manuals.htm. Accessed June 30, 2017.
- 22. Carlson RW. Surveillance of patients following primary therapy In: Harris JR, Lippman ME, Morrow M, et al. , eds. Diseases of the Breast. 4th ed Philadelphia, PA: Lippincott Williams & Wilkins; 2010;823-831. [Google Scholar]
- 23. Hernan M, Brumback B, Robins JM.. Marginal structural models to estimate the causal effect of Zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11(5):561–570. [DOI] [PubMed] [Google Scholar]
- 24. Robins JM, Hernan M, Brumback B.. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. [DOI] [PubMed] [Google Scholar]
- 25. Sato T, Matsuyama Y.. Marginal structural models as a tool for standardization. Epidemiology. 2003;14(6):680–686. [DOI] [PubMed] [Google Scholar]
- 26. Cole SR, Hernán MA.. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coviello V, Boggess M.. Cumulative incidence estimation in the presence of competing risks. Stata J. 2004;4(2):103–112. [Google Scholar]
- 28. Fine JP, Gray RJ.. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 29. Lau B, Cole SR, Gange SJ.. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chapman J-A, Meng D, Shepherd L, et al. Competing causes of death from a randomized trial of extended adjuvant endocrine therapy for breast cancer. J Natl Cancer Inst. 2008;100(4):252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Colzani E, Liljegren A, Johansson ALV, et al. Prognosis of patients with breast cancer: causes of death and effects of time since diagnosis, age, and tumor characteristics. J Clin Oncol. 2011;29(30):4014–4021. [DOI] [PubMed] [Google Scholar]
- 32. Du XL, Fox EE, Lai D.. Competing causes of death for women with breast cancer and change over time from 1975 to 2003. Am J Clin Oncol. 2008;31(2):105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hanrahan EO, Gonzalez-Angulo AM, Giordano SH, et al. Overall survival and cause-specific mortality of patients with stage T1a, bN0M0 breast carcinoma. J Clin Oncol. 2007;25(31):4952–4960. [DOI] [PubMed] [Google Scholar]
- 34. Roychoudhuri R, Robinson D, Putcha V, et al. Increased cardiovascular mortality more than fifteen years after radiotherapy for breast cancer: a population-based study. BMC Cancer. 2007;7(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weaver KE, Foraker RE, Alfano CM, et al. Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecologic cancers: a gap in survivorship care? J Cancer Surviv. 2013;7(2):253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gernaat SAM, Ho PJ, Rijnberg N, et al. Risk of death from cardiovascular disease following breast cancer: a systematic review. Breast Cancer Res Treat. 2017;164(3):537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taunk NK, Haffty BG, Kostis JB, et al. Radiation-induced heart disease: pathologic abnormalities and putative mechanisms. Front Oncol. 2015;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–998. [DOI] [PubMed] [Google Scholar]
- 39. Khosrow-Khavar F, Filion KB, Al-Qurashi S, et al. Cardiotoxicity of aromatase inhibitors and tamoxifen in postmenopausal women with breast cancer: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol. 2017;28(3):487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Esteva FJ, Hortobagyi GN.. Comparative assessment of lipid effects of endocrine therapy for breast cancer: implications for cardiovascular disease prevention in postmenopausal women. Breast. 2006;15(3):301–312. [DOI] [PubMed] [Google Scholar]
- 41. Amir E, Seruga B, Niraula S, et al. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011;103(17):1299–1309. [DOI] [PubMed] [Google Scholar]
- 42. Blaes A, Beckwith H, Florea N, et al. Vascular function in breast cancer survivors on aromatase inhibitors: a pilot study. Breast Cancer Res Treat. 2017;166(2):541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goldvaser H, Barnes TA, Šeruga B, et al. Toxicity of extended adjuvant therapy with aromatase inhibitors in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2018;110(1):31–39. [DOI] [PubMed] [Google Scholar]
- 44. Chlebowski RT, Haque R, Hedlin H, et al. Benefit/risk for adjuvant breast cancer therapy with tamoxifen or aromatase inhibitor use by age, and race/ethnicity. Breast Cancer Res Treat. 2015;154(3):609–616. [DOI] [PubMed] [Google Scholar]
- 45. Schonberg MA, Marcantonio ER, Li D, et al. Breast cancer among the oldest old: tumor characteristics. J Clin Oncol. 2010;28(12):2038–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–5312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article may be made available upon application to the CLUE II study (Principal Investigator, Kala Visvanathan).