Abstract
Background: Systemic therapy for hepatocellular carcinoma (HCC) consisting of the tyrosine kinase inhibitor sorafenib has remained unchanged for over a decade, although results from phase III targeted therapy trials have recently emerged. This review considers available phase III evidence on the use and sequencing of targeted therapy for intermediate and advanced non-locoregional therapy (LRT) eligible HCC and discusses implications for clinical practice. Methods: Published and presented literature on phase III data reporting on targeted therapy for advanced HCC that was not eligible for loco-regional therapies was identified using the key search terms “hepatocellular cancer” AND “advanced” AND “targeted therapy” AND “phase III” OR respective aliases (PRISMA). Results: Ten phase III trials assessed targeted therapy first-line and eight following sorafenib. In the first-line, atezolizumab plus bevacizumab statistically significantly improved overall survival (OS) and patient-reported outcomes (PROs) compared with sorafenib, while lenvatinib demonstrated non-inferior OS. Following progression on sorafenib, statistically significant OS improvements over placebo were seen for cabozantinib and regorafenib in unselected patients and for ramucirumab in those with baseline α-fetoprotein≥400 ng/mL. Based on improved OS and PROs, atezolizumab plus bevacizumab appears to be a preferred first-line treatment option for intermediate or advanced non-LRT eligible HCC. Phase III data informing sequencing of later lines of treatment is lacking. Therefore, sequencing principles are proposed that can be used to guide treatment selection. Conclusions: Ongoing trials will continue to inform optimal therapy. Multiple targeted therapies have improved OS in intermediate or advanced non-LRT eligible HCC, although optimal sequencing is an area of ongoing investigation.
Liver cancer is one of the most common malignancies worldwide, with approximately 840 000 new cases and 780 000 deaths resulting from this type of cancer in 2018 (1). The majority of primary liver cancers (75%-85%) are hepatocellular carcinoma (HCC) (1,2), which is often associated with well-known risk factors such as hepatitis B/C infection, alcohol, diabetes, and other metabolic diseases (3,4). HCC is unique in that the majority of cases (70%-90%) occur within a background of chronic liver disease and cirrhosis (3), and approximately two-thirds of diagnosed HCC cases are not eligible for curative options (5). HCC represents a growing health threat with annual mortality rates increasing by approximately 2%-3% per year from 2003 to 2012 (6) and a 43% increase in the rate of death from HCC from 2000 to 2016 in the United States (7). Scoring systems have been developed to predict outcomes for HCC with some based on the Child-Pugh score (8-10), which uses the clinical parameters of encephalopathy and ascites as well as biochemical indicators (11). These can help predict potential efficacy of response based on hepatic reserve in addition to morphological features (12,13), both being factors for determining overall prognosis.
Treatment strategies in Western countries center around the Barcelona Clinic Liver Cancer (BCLC) staging system, which informs treatment approach based on disease burden presentation and underlying hepatic function (14). Initial curative options include transplant, resection, and/or ablation (BCLC 0 and A), with a progression to palliative locoregional therapies (LRT), with or without embolization, followed by systemic therapy in LRT-ineligible patients or those progressing on LRT (BCLC B and C) (11,14-16). Although initial LRT is considered, up to two-thirds of patients may become ineligible due to tumor burden or liver decompensation (15,17). Although conventional chemotherapy has not improved survival in this setting (18), initial (first-line systemic therapy) and subsequent lines of therapy (second- or third-line systemic therapy) using targeted agents have been evaluated.
Tyrosine kinases are key targets for HCC therapy because they catalyze transfer of the gamma phosphate group from adenosine triphosphate to target proteins that can drive tumor cell progression, proliferation, neovascularization, and metastasis while reducing apoptosis (19). A number of multi-target tyrosine kinase inhibitors (TKIs) exert antitumor activity by targeting components of the mitogen-activated protein kinase pathway (Raf/MEK/ERK) (20), which partially controls cellular processes as diverse as division, differentiation, movement, and apoptosis (21). TKIs also target factors involved in neovascularization, which otherwise stimulate tumor growth (20). The first-generation, oral, multi-TKI sorafenib was approved by the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in 2007 (22) and became a convenient standard of care for the first-line systemic treatment of intermediate or advanced non-LRT–eligible HCC (16). Second- and subsequent-generation TKIs have also been investigated, including sunitinib (23), brivanib (24), linifanib (25), and lenvatinib (26) as first-line systemic therapy. Brivanib (27), regorafenib (28), tivantinib (29), and cabozantinib (30) have also been evaluated following progression on sorafenib.
In addition to TKIs, novel targeted agents have also been evaluated for advanced HCC in the last decade, including those targeting the mechanistic target of rapamycin (mTOR) (31), the immune system (32–34), and tumor vasculature (35,36), with the latter 2 most promising. Immune checkpoints modulate immune responses to reduce collateral damage to healthy tissues (37), in part through interactions between the programmed cell death protein 1 (PD-1) on activated T-cells and the programmed death ligand 1 (PD-L1) in peripheral tissues (37,38). Tumor cells also express PD-L1 to avoid immune attack (39), so recombinant monoclonal antibody (MoAb) immune checkpoint inhibitors (ICIs) that disrupt PD-1 and PD-L1 interactions have been extensively investigated as cancer therapies. The anti-PD-1 agents nivolumab and pembrolizumab and the anti-PD-L1 drug atezolizumab have been assessed either alone (33,34) or in combination with other targeted agents (32) for intermediate or advanced non-LRT–eligible HCC.
Solid tumors, through their inherent metabolism, generate a hypoxic environment that is presumed to drive the angiogenic pathway, resulting in increased tumor vascularity (40). Cooption of hepatic arterial inflow rather than sprouting is believed to be 1 possible cause of acquired sorafenib resistance, among others (41–44). Vascular endothelial growth factors (VEGFs) and their receptors (VEGFRs) are key regulators of angiogenesis, with VEGFR-2 expressed on almost all endothelial cells and VEGF-A integral to the formation and branching of new vasculature (40,45). Two MoAb inhibitors of angiogenesis (V-MoAbs), bevacizumab, which targets VEGF-A (45), and ramucirumab, which targets VEGFR-2 (20), have also been assessed in intermediate or advanced non-LRT–eligible HCC (32,35,36).
Many phase III trials in the past decade have evaluated newer targeted agents and immunotherapies alone or in combinations, although many have not been practice-changing (23–25,27,29,31,33,34,36,46,47). Positive trials were recently reported, however (26,28,30,32,35), requiring a careful review of new data and reevaluation of best treatment sequences and pathways. This review provides updated guidance on targeted therapies for intermediate or advanced non-LRT–eligible HCC based on randomized phase III data.
Methods
Targeted therapy was defined as small-molecule drugs or MoAbs that selectively target specific molecules on tumor cells or their microenvironment, thereby inhibiting tumor growth or spread. A search of published and presented literature identified phase III trials reporting efficacy outcomes on targeted therapy to treat advanced HCC patients not eligible for LRT. PubMed (all time to November 7, 2019), the proceedings from the American Society of Clinical Oncology, the European Society for Medical Oncology 2018 and 2019 annual meetings, and the European Society for Medical Oncology-Asia 2018 and 2019 Congress were searched for phase III trials assessing targeted therapy using the key search terms “hepatocellular cancer” AND “advanced” AND “targeted therapy” AND “phase III” OR respective aliases. A supplemental bibliographic search of review articles and pooled or meta-analyses was also conducted.
English language records were vetted at abstract level and confirmed at full text as needed. Excluded studies included those that were nonoriginal research; preclinical; correlative science; not specific to HCC; outside the intermediate or advanced non-LRT–eligible setting; retrospective; prospective phase I, II, IV, or undefined phase; duplicate or prior reports; studies without reported outcomes; and those that assessed combinations of targeted therapy and LRT. Categorization of studies by line of therapy was based on the number of lines of prior systemic therapy delivered in the advanced setting.
Findings
Literature Search Results
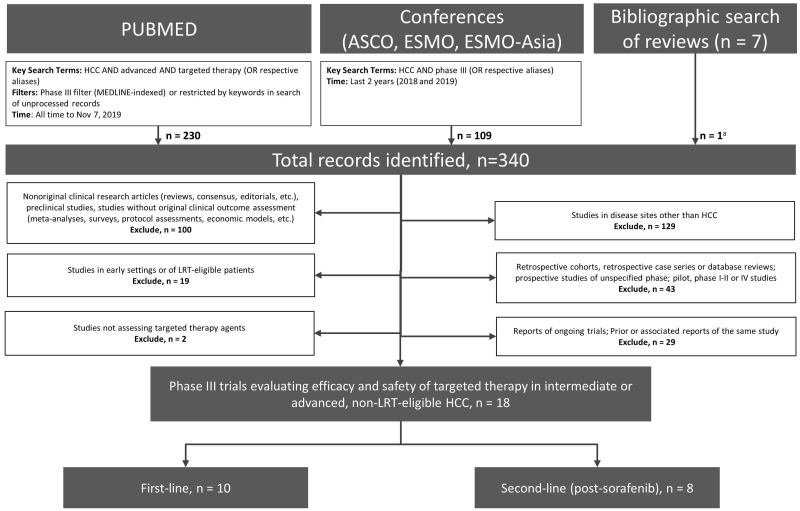
The literature search identified a total of 340 records, resulting in a total of 18 eligible phase III trials (Preferred Reporting Items for Systematic Reviews and Meta-Analyses; Figure 1) (23–36,46–49). Ten trials assessed targeted agents as first-line systemic therapy (23–26,32,34,46–49), and 8 trials evaluated these agents in patients who had progressed on sorafenib (27–31,33,35,36).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram. aPrimary reports of eligible studies that were not identified through database search. ASCO = American Society of Clinical Oncology; ESMO = European Society for Medical Oncology; HCC = hepatocellular carcinoma; LRT = locoregional therapy.
First-Line Systemic Therapy
Among studies assessing systemic first-line targeted agents, research has primarily focused on 2 major classes of drugs, TKIs and, more recently, ICIs.
Tyrosine Kinase Inhibitors
Eight phase III trials assessed TKIs as first-line systemic therapy either alone or in combination for intermediate or advanced non-LRT–eligible HCC (Table 1) (23-26,46-49). The benefits of TKIs in unresectable HCC were first confirmed in 2 pivotal phase III studies in 2008-2009, which demonstrated statistically significant improvements in overall survival (OS) for sorafenib compared with placebo (48,49). SHARP (n = 602) and Sorafenib Asia-Pacific (n = 226) showed statistically significant improvements in median OS (10.7 vs 7.9 months, hazard ratio [HR] = 0.69, 95% confidence interval [CI] = 0.55 to 0.87, P < .001; and 6.5 vs 4.2 months, HR = 0.68, 95% CI = 0.50 to 0.93, P = .014, respectively) and median time to radiologic progression (TTP, 5.5 vs 2.8 months, HR = 0.58, 95% CI = 0.45 to 0.74, P < .001; and 2.8 vs 1.4 months, HR = 0.57, 95% CI = 0.42 to 0.79, P = .0005), respectively, for sorafenib vs placebo (Table 1) (48,49). Rates of treatment discontinuation due to adverse events (AEs) were similar between treatments in SHARP and Sorafenib Asia-Pacific (38% vs 37% and 19.5% vs 13.3%, respectively), and the most common grade 3 or 4 AEs for sorafenib compared with placebo in the respective trials were hand-foot skin reactions (8% vs <1%, P < .001 and 10.7% vs 0), diarrhea (8% vs 2%, P < .001 and 6.0% vs 0), fatigue (approximately 4% vs <4%, P = 1.0 and 3.4% vs 1.3%), and hypertension (2% vs 1%, P = .28 and 2.0% vs 0). Drug-related deaths were not reported in SHARP (49) or in either arm of Sorafenib Asia-Pacific (48).
Table 1.
Efficacy outcomes of phase III trials assessing targeted therapy in intermediate and advanced non-LRT–eligible HCC
| Trial name (reference) |
Regimen(s) | No. | Median follow-up, mo (range) |
ORR,a % OR (95% CI) |
Median DoR, mo (95% CI) [range] |
Median PFS,a mo HR (95% CI) |
Median OS, mo HR (95% CI) |
|---|---|---|---|---|---|---|---|
| First-line therapy | |||||||
| SHARP (49) | Sorafenib 400 mg BID | 299 | NR | NR |
10.7HR = 0.69(0.55 to 0.87)P < .001 |
||
| Placebo | 303 | 1b,c | NR | 2.8 (TTP)b,c | 7.9 | ||
| Sorafenib Asia-Pacific (48) | Sorafenib 400 mg BID | 150 | NR | 3.3 | NR |
2.8 (TTP)HR = 0.57(0.42 to 0.79)P = .0005 |
6.5HR = 0.68(0.50 to 0.93)P = .014 |
| Placebo | 76 | 1.3 | NR | 1.4 (TTP) | 4.2 | ||
| A6181170 (23) | Sunitinib 37.5 mg/d | 530 | 7.4 | 6.6 | NR |
3.6 (TTP)HR = 1.13(0.99 to 1.30)P = .23 |
7.9HR = 1.30(1.13 to 1.50)P = .0014 |
| Sorafenib 400 mg BID | 544 | 7.8 | 6.1 | NR | 3.0 (TTP) |
10.2 |
|
| BRISK-FL (24) | Brivanib 800 mg/d | 577 | NR |
12.0OR = 1.45(0.99 to 2.13)P = .057 |
NR |
4.2 (TTP)HR = 1.01(0.88 to 1.16)P = .85 |
9.5HR = 1.07(0.94 to 1.23)P = .31 |
| Sorafenib 400 mg BID | 578 | 8.8 | NR | 4.1 (TTP) | 9.9 | ||
| M10-963 (25) | Linifanib 17.5 mg/d | 514 | NR |
13.0P = .018 |
NR |
5.4 (TTP)HR = 0.76(0.64 to 0.90)P = .001 |
9.1HR = 1.05(0.90 to 1.22)P = NR |
| Sorafenib 400 mg BID | 521 | 6.9 | NR | 4.0 (TTP) | 9.8 | ||
| SEARCH (46) | Sorafenib 400 mg BID plus erlotinib 150 mg/d | 362 | NR |
6.6P = .10 |
NR |
3.2 (TTP)HR = 1.14(0.94 to 1.37)P = .18 |
9.5HR = 0.93(0.78 to 1.11)P = .41 |
| Sorafenib 400 mg BID plus placebo | 358 | 3.9 | NR | 4.0 (TTP) | 8.5 | ||
| REFLECT (26) | Lenvatinib 12 mg or 8 mg/dd | 478 | 27.7 |
24.1eOR = 3.13(2.15 to 4.56)P < .0001 |
NR |
7.4eHR = 0.66(0.57 to 0.77)P < .0001 |
13.6HR = 0.92(0.79 to 1.06)(noninferior) |
| Sorafenib 400 mg BID | 476 | 27.2 | 9.2e | NR | 3.7e | 12.3 | |
| CheckMate 459 (34) | Nivolumab 240 mg Q2W | 371 | 22.8f | 15c |
23.3[3.1-34.5+] |
3.7c |
16.4HR = 0.85(0.72 to 1.02)P = .075 |
| Sorafenib 400 mg BID | 372 | 7c |
23.4[1.9+-28.7+] |
3.8c | 14.7 | ||
| IMbrave150 (32,50) | Atezolizumab 1200 mg plus bevacizumab 15 mg/kg Q3W | 336 | 8.6 | NEc,e |
6.8cHR = 0.59(0.47 to 0.76)P < .001 |
NEHR = 0.58(0.42 to 0.79)P < .001 |
|
| Sorafenib 400 mg BID | 165 | 13.3c,e | 4.3c | 13.2 | |||
| CALGB 80802 (47) | Sorafenib 400 mg BID plus doxorubicin Q3W | 180 | 36.1 |
10.0P = .52 |
NR |
4.0HR = 0.93(0.75 to 1.16)P = .54 |
9.3HR = 1.05(0.83 to 1.31)P = .68 |
| Sorafenib 400 mg BID | 176 | 5.4 | NR | 3.7 | 9.4 | ||
| Second-line therapy | |||||||
| BRISK-PS (27) | Brivanib 800 mg/d plus BSC | 263 | NR |
9.9OR = 5.72(1.41 to 23.25)P = .003 |
NR |
4.2 (TTP)HR = 0.56(0.42 to 0.76)P < .001 |
9.4HR = 0.89(0.69 to 1.15)P = .33 |
| Placebo plus BSC | 132 | 1.5 | NR | 2.7 (TTP) | 8.2 | ||
| EVOLVE-1 (31) | Everolimus 7.5 mg/d plus BSC | 362 |
24.6(14.8-36.6) |
2.2 | NR |
3.0 (TTP)HR = 0.93(0.75 to 1.15)P = NR |
7.6HR = 1.05(0.86 to 1.27)P = .68 |
| Placebo plus BSC | 184 | 1.6 | NR | 2.6 (TTP) | 7.3 | ||
| REACH (36) | Ramucirumab 8 mg/kg Q2W plus BSC | 283 | 8.3 |
7.1P < .0001 |
NR |
2.8HR = 0.63(0.52 to 0.75)P < .0001 |
9.2HR = 0.87(0.72 to 1.05)P = .14 |
| Placebo Q2W plus BSC | 282 | 7.0 | 0.71 | NR | 2.1 | 7.6 | |
| RESORCE (28) | Regorafenib 160 mg/d plus BSC wk 1-3 Q4W | 379 | 7.0 |
10.6eP = .005 |
3.5(1.9 to 4.5) |
3.1eHR = 0.46(0.37 to 0.56)P < .0001 |
10.6HR = 0.63(0.50 to 0.79)P < .0001 |
| Placebo daily plus BSC wk 1-3 Q4W | 194 | 4.1e |
2.7(1.9 to NE) |
1.5e | 7.8 | ||
| METIV-HCC (29) | Tivantinib 120 mg BID | 226 | 18.1 | 0 | NR |
2.1HR = 0.96(0.75 to 1.22)P = .72 |
8.4HR = 0.97(0.75 to 1.25)P = .81 |
| Placebo | 114 | 0 | NR | 2.0 | 9.1 | ||
| CELESTIAL (30) | Cabozantinib 60 mg/d | 470 | NR |
3.8P = .009 |
NR |
5.2HR = 0.44(0.36 to 0.52)P < .001 |
10.2HR = 0.76(0.63 to 0.92)P = .005 |
| Placebo daily | 237 | 0.4 | NR | 1.9 | 8.0 | ||
| REACH-2 (35) | Ramucirumab 8 mg/kg Q2W plus BSC | 197 | 7.6 |
4.6P = .17 |
NR |
2.8HR = 0.45(0.34 to 0.60) P < .0001 |
8.5HR = 0.71(0.53 to 0.95)P = .02 |
| Placebo Q2W plus BSC | 95 | 1.1 | NR | 1.6 | 7.3 | ||
| KEYNOTE-240 (33) | Pembrolizumab 200 mg Q3W plus BSC | 278 | NR |
18.3cP = .00007 |
13.8[1.5+-23.6+] |
3.0cHR = 0.72(0.57 to 0.90)P < .002 |
13.9HR = 0.78(0.61 to 1.0)P = .024g |
| Placebo Q3W plus BSC | 135 | 4.4c |
NYR[2.8-20.4+] |
2.8c | 10.6 | ||
Based on investigator assessment by RECIST 1.1 or otherwise footnoted. BID = twice daily; BSC = best supportive care; CI = confidence interval; DoR = duration of response; HCC = hepatocellular carcinoma; HR = hazard ratio; LRT = locoregional therapy; NE = not estimable; NR = not reported; NYR = not yet reached; OR = odds ratio; ORR = overall response rate; OS = overall survival; PFS = progression free survival; QXW = every X weeks; TTP = time to progression.
RECIST 2000.
Independent review.
12 mg/d for bodyweight 60 kg and over, and 8 mg/d for bodyweight less than 60 kg.
mRECIST.
Minimum follow-up.
Did not reach statistical significance according to prespecified criteria.
Since then, 5 phase III trials have evaluated, without success, either alternative TKIs or the addition of other agents to first-line sorafenib compared with sorafenib alone (23-25,46,47). Three trials, A6181170, BRISK-FL, and M10-963, compared sunitinib (23), brivanib (24), or linifanib (25), respectively, with sorafenib. In A6181170 (n = 1074), at a median follow-up of approximately 7.5 months, sunitinib showed no improvement in the primary endpoint of OS (HR = 1.30, P = 1.0) or TTP (HR = 1.13, P = .83) (Table 1) (23). In BRISK-FL (n = 1155) (24) and M10-963 (n = 1035) (25), neither brivanib nor linifanib statistically significantly improved the primary endpoint of OS compared with sorafenib (HR = 1.07, P = .31; HR = 1.05, P = not reported, respectively) (24,25), although improved TTP for linifanib was shown in M10-963 (HR = 0.76, 95% CI = 0.64 to 0.90, P = .001) (Table 1) (25). Two trials assessed the addition of either chemotherapy or targeted therapy to sorafenib (46,47). At a median follow-up of 36.1 months, CALGB 80802 (n = 356) showed no improvement in the primary endpoint of OS or TTP and greater toxicity with the addition of doxorubicin (47), and SEARCH (n = 720) showed a similar lack of improvement with the addition of the first-generation epidermal growth factor receptor TKI, erlotinib (Table 1) (46).
The phase III REFLECT trial compared lenvatinib with sorafenib in 954 patients. At a median follow-up of 27.7 months, lenvatinib was shown to be noninferior to sorafenib for the primary endpoint of median OS (13.6 vs 12.3 months, HR = 0.92, 95% CI = 0.79 to 1.06, upper limit of 2-sided 95% CI < 1.08) and demonstrated statistically significant improvements in TTP (8.9 vs 3.7 months, HR = 0.63, 95% CI = 0.53 to 0.73, P < .0001), progression-free survival (PFS = 7.4 vs 3.7 months, HR = 0.66, 95% CI = 0.57 to 0.77, P < .0001), and modified response evaluation criteria in solid tumors (mRECIST) overall response rate (ORR = 24.1% vs 9.2%, P < .0001) (Table 1) (26). AEs leading to treatment discontinuation occurred in 13.2% vs 9.1% of patients, grade 3 or greater treatment-related AEs (TRAEs) occurred in 56.7% vs 48.6% of patients (Table 2), and grade 3 or 4 treatment-emergent AEs that occurred in more than 15% of patients for lenvatinib vs sorafenib included hypertension (23.3% vs 14.3%), decreased weight (7.6% vs 2.9%), decreased appetite (4.6% vs 1.3%), diarrhea (4.2% vs 4.2%), and fatigue (3.8% vs 3.6%). Deaths attributed to AEs occurred in 2.3% vs 0.8% of patients in the lenvatinib vs sorafenib arms, respectively.
Table 2.
Safety outcomes of select phase III trials assessing targeted therapy in intermediate and advanced non-LRT–eligible HCC
| Trial name (reference) |
Treatment | Safety population | Treatment discontinuation due to TRAEs, % | Overall TRAEs, % |
Deaths due to TRAEs, % | |
|---|---|---|---|---|---|---|
| Any grade | Grade 3 or 4 | |||||
| First-line therapy | ||||||
| REFLECT (26) | Lenvatinib | 476 |
13.2(Any AE) |
93.9 | 56.7 | 2.3 |
| Sorafenib | 475 |
9.1(Any AE) |
95.2 | 48.6 | 0.8 | |
| CheckMate 459 (34) | Nivolumab | 367 | 4.4 | NR | 22.1 | 0.3 |
| Sorafenib | 363 | 8.0 | NR | 49.3 | 0.3 | |
| IMbrave150 (32,50) | Atezolizumab plus bevacizumab | 329 |
15.5(Any AE) |
83.9 | 35.6 | 1.8 |
| Sorafenib | 156 |
10.3(Any AE) |
94.2 | 45.5 | 0.6 | |
| Second-line therapy | ||||||
| REACH (36) | Ramucirumab plus BSC | 277 | 10.1 | 97.4a | 62.3a | 2.5 |
| Placebo plus BSC | 276 | 2.9 | 94.2a | 48.0a | 1.4 | |
| RESORCE (28) | Regorafenib | 374 | 10.4 | 92.5 | 50.0 | 1.9 |
| Placebo plus BSC | 193 | 3.6 | 51.8 | 16.6 | 1.0 | |
| CELESTIAL (30) | Cabozantinib | 467 | 16.3 |
98.5(Any AE) |
67.7(Any AE) |
1.3 |
| Placebo | 237 | 3.0 |
92.4(Any AE) |
36.3(Any AE) |
0.4 | |
| REACH-2 (35) | Ramucirumab | 197 | 10.7 | 10.7 | NR |
3.0(Any AE) |
| Placebo plus BSC | 95 | 3.2 | 5.3 | NR |
3.2(Any AE) |
|
| KEYNOTE-240 (33,51) | Pembrolizumab plus BSC | 279 | 6.5 | 60.9 | 18.3 | 0.4 |
| Placebo plus BSC | 134 | 0.7 | 48.5 | 7.5 | 0 | |
Treatment-emergent AEs. AE = adverse event; BSC = best supportive care; HCC = hepatocellular carcinoma; LRT = locoregional therapy; NR = not reported; TRAE = treatment-related adverse event.
Immune Checkpoint Inhibitors
Two phase III trials evaluated ICIs used either alone or with targeted therapy compared with sorafenib as first-line therapy for intermediate or advanced non-LRT–eligible HCC (32,34). At median follow-ups of 15.2 and 13.4 months for nivolumab and sorafenib, respectively, CheckMate 459 showed no statistically significant improvement in the primary endpoint of median OS (16.4 vs 14.7 months, HR = 0.85, 95% CI = 0.72 to 1.02, P = .075), PFS (3.7 vs 3.8 months, HR = 0.93, 95% CI = 0.79 to 1.10, P = not reported), or duration of response (23.3 vs 23.4 months) for nivolumab vs sorafenib (Table 1) (34). Nivolumab showed improvements over sorafenib in overall health-related quality-of-life (HRQoL) with fewer TRAEs leading to discontinuation (4.4% vs 7.9%) and grade 3 or 4 TRAEs overall (22.1% vs 49.3%) (Table 2). Approximate rates of the most common grade 3 or 4 TRAEs reported in the nivolumab and sorafenib arms, respectively, were aspartate aminotransferase (AST) increase (7% vs 4%), diarrhea (1% vs 5%), fatigue (1% vs 2%), pruritis (1% vs <1%), and HFSR (<1% vs 15%). Treatment-related deaths were reported in 0.3% of patients in each arm.
IMbrave150 randomly assigned 501 patients 2:1 to receive atezolizumab plus bevacizumab or sorafenib. At a median follow-up of 8.6 months, the coprimary endpoints of median OS (not estimable vs 13.2 months, HR = 0.58, 95% CI = 0.42 to 0.79, P < .001) and median PFS (6.8 vs 4.3 months, HR = 0.59, 95% CI = 0.47 to 0.76, P < .001) were statistically significantly improved for atezolizumab plus bevacizumab vs sorafenib, as was mRECIST ORR (33.2% vs 13.3%, P < .001) (Table 1) (32). The median time to deterioration in HRQoL also favored the atezolizumab plus bevacizumab arm (11.2 vs 3.6 months, HR = 0.63, 95% CI = 0.46 to 0.85). AEs leading to treatment discontinuation occurred in 15.5% vs 10.3% and grade 3 or 4 TRAEs occurred in 35.6% vs 45.5% of patients (Table 2) (50). Approximate rates of the most common grade 3 or 4 TRAEs reported for atezolizumab plus bevacizumab vs sorafenib were hypertension (10.3% vs 9.0%), AST increase (4.3% vs 2.6%), proteinuria (2.7% vs 0.6%), platelet count decrease (2.4% vs 0.6%), and infusion-related reactions or alanine aminotransferase increase (2.1% vs 0 for each) (32). Deaths due to TRAEs occurred in 1.8% vs 0.6% of patients who received atezolizumab plus bevacizumab and sorafenib, respectively (50).
Second-Line Systemic Therapy
Studies assessing targeted agents as second-line systemic therapy for intermediate or advanced non-LRT–eligible HCC have evaluated mTOR and VEGF-2 inhibitors (31,35,36) in addition to TKIs and ICIs (27–30,33).
VEGF-2 and mTOR and Inhibitors
Two phase III studies assessed the VEGF-2 inhibitor ramucirumab (35,36), with both agents compared with placebo for the second-line treatment of intermediate or advanced non-LRT–eligible HCC, and 1 phase III trial evaluated the mTOR inhibitor everolimus (31). REACH evaluated ramucirumab vs placebo in 565 patients and after a median follow-up of 8.3 and 7.0 months for ramucirumab and placebo, respectively, showed no statistically significant improvements in the primary endpoint of median OS (9.2 vs 7.6 months, HR = 0.87, 95% CI = 0.72 to 1.05, P = .14) (Table 1), although statistically significant OS improvements were seen in a prespecified analysis of 250 patients with baseline α-fetoprotein levels of at least 400 ng/mL (7.8 vs 4.2 months, HR = 0.67, 95% CI = 0.51 to 0.90, P = .006) (36). Based on these findings, REACH-2 randomly assigned 292 patients with baseline α-fetoprotein of at least 400 ng/mL 2:1 to receive ramucirumab or placebo. At a median follow-up of 7.6 months, statistically significant improvements were seen for ramucirumab vs placebo in the primary endpoint of median OS (8.5 vs 7.3 months, HR = 0.71, 95% CI = 0.53 to 0.95, P = .02) and median PFS (2.8 vs 1.6 months, HR = 0.45, 95% CI = 0.34 to 0.60, P < .0001) (Table 1) (35). TRAEs leading to discontinuation occurred in 10.7% vs 3.2% of patients receiving ramucirumab vs placebo, respectively, and overall rates of grade 3 or 4 TRAEs were not reported (Table 2). The most common TRAEs in the ramucirumab and placebo arms, respectively, were hypertension (7.6% vs 2.1%), proteinuria (2.0% vs 0), liver injury or failure (1.5% vs 0), peripheral edema (1.0% vs 0), and fatigue (1.0% vs 0). Deaths due to TRAEs occurred in 1.5% of patients receiving ramucirumab and in no patients receiving placebo. EVOLVE-1 randomly assigned 546 patients 2:1 to receive everolimus or placebo and at a median follow-up of 24.6 months showed no statistically significant differences between arms for OS (HR = 1.05, P = .68) or TTP (HR = 0.93, P = not tested; Table 1) (31).
Tyrosine Kinase Inhibitors
TKIs were also assessed for second-line intermediate or advanced non-LRT–eligible HCC in 4 phase III trials (27–30). In BRISK-PS (n = 395) (27) and METIV-HCC (n = 340) (29), brivanib and tivantinib, respectively, were compared with placebo either with (BRISK-PS) or without (METIV-HCC) best supportive care (BSC). With median follow-ups not reported for BRISK-PS and 18.1 months for METIV-HCC, neither trial showed statistically significant improvements in their primary end-points of OS (HR = 0.89, P = .33 and HR = 0.97, P = .81, respectively) (27,29), although brivanib showed statistically significantly improved TTP compared with placebo (HR = 0.56, 95% CI = 0.42 to 0.76, P < .001) (Table 1) (27).
RESORCE randomly assigned 573 patients 2:1 to regorafenib or placebo plus BSC. At a median follow-up of 7.0 months, regorafenib demonstrated statistically significant improvements in the primary endpoint of median OS (10.6 vs 7.8 months, HR = 0.63, 95% CI = 0.50 to 0.79, P < .0001), median PFS (3.1 vs 1.5 months, HR = 0.46, 95% CI = 0.37 to 0.56, P < .0001) (Table 1), and median TTP (3.2 vs 1.5 months, HR = 0.44, 95% CI = 0.36 to 0.55, P < .0001) compared with placebo (Table 1) (28). TRAEs leading to treatment discontinuation occurred in 10.4% vs 3.6% of patients, grade 3 or 4 TRAEs occurred in 50.0% vs 16.6% of patients (Table 2), and grade 3 or 4 TRAEs included hypertension (13.1% vs 3.1%), HFSR (12.6% vs 0.5%), increased blood bilirubin (6.7% vs 2.1%), fatigue (6.4% vs 1.6%), and increased AST (5.1% vs 5.2%). Deaths attributed to AEs occurred in 1.9% vs 1.0% of patients in the regorafenib vs placebo arms. CELESTIAL randomly assigned 707 patients 2:1 to receive cabozantinib or placebo. At the second interim analysis, cabozantinib also demonstrated statistically significant improvements over placebo in the primary endpoint of median OS (10.2 vs 8.0 months, HR = 0.76, 95% CI = 0.63 to 0.92, P = .005) and median PFS (5.2 vs 1.9 months, HR = 0.44, 95% CI = 0.36 to 0.52, P < .001) (Table 1) (30). TRAEs leading to discontinuation occurred in 16.3% vs 3.0% of patients, and any grade 3 or 4 AEs occurred in 67.7% vs 36.3% of patients in the cabozantinib vs placebo arms, respectively (Table 2). The most common grade 3 or 4 AEs included HFSR (16.9% vs 0), hypertension (15.8% vs 1.7%), increased AST (11.8% vs 6.8%), fatigue (10.5% vs 4.2%), and diarrhea (9.9% vs 1.7%). Treatment-related deaths occurred in 1.3% and 0.4% of patients in the cabozantinib and placebo arms, respectively.
An ICI has also been evaluated for second-line HCC following sorafenib in 1 phase III trial. KEYNOTE-240 randomly assigned 413 patients 2:1 to receive pembrolizumab or placebo plus BSC. At a median follow-up of 13.8 months for pembrolizumab and 10.6 months for placebo, numerical improvements were seen in the coprimary endpoints of median OS (13.9 vs 10.6 months, HR = 0.78, 95% CI = 0.61 to 1.0, P = .024) and PFS (3.0 vs 2.8 months, HR = 0.72, 95% CI = 0.57 to 0.90, P = .002; Table 1), which did not achieve statistical significance according to prespecified boundaries (OS, P = .017 at the final analysis and PFS, P = .002 at the first interim analysis) (33). TRAEs leading to discontinuation occurred in 6.5% vs 0.7% of patients (51) and grade 3 or 4 TRAEs occurred in 18.3% vs 7.5% of patients receiving pembrolizumab and placebo, respectively (Table 2) (33). Grade 3 or 4 TRAEs in the pembrolizumab vs placebo arms included increased AST (5.4% vs 1.5%), increased alanine aminotransferase (3.6% vs 1.5%), fatigue (1.1% vs 0.7%), and increased blood bilirubin and decreased appetite (1.1% vs 0 for each). Treatment-related deaths occurred in 0.4% of patients receiving pembrolizumab, with none reported in the placebo arm.
Discussion
With respect to the clinical benefit of first-line systemic therapy, statistically significant improvements in OS and/or HRQoL must be shown over standard of care in a phase III trial to warrant a change in clinical practice. During the last decade, multiple phase III trials have sought to improve OS and HRQoL using alternative strategies in this setting without success, including sunitinib, brivanib, and linifanib (23–25); the addition of erlotinib or chemotherapy to sorafenib (46,47); and the ICI nivolumab (34). Recently, however, 2 phase III trials have shown promise; 1 assessed lenvatinib (26) and the other the ICI atezolizumab plus the VEGF-A inhibitor bevacizumab (32). REFLECT demonstrated a statistically significant 34% reduced risk of progression for lenvatinib vs sorafenib (net 3.7 months, P < .0001) and over a 2.5-fold improvement in mRECIST ORR (24.1% vs 9.2%, P < .0001), although with only a noninferior median OS (HR = 0.92) (26). However, despite slightly higher rates of grade 3 or 4 TRAEs for lenvatinib compared with sorafenib (56.7% vs 48.6%), higher rates of overall AEs were mainly related to hypertension (any grade, 42.2% vs 30.3%; grade 3 or 4, 23.3% vs 14.3%), which is generally manageable, with decreased rates of HSFR (any grade, 26.9% vs 52.4%; grade 3 or 4, 2.9% vs 11.4%) suggesting a more favorable toxicity profile. IMbrave150 showed a statistically significant 42% reduction in the risk of death (HR = 0.58, P < .001), a 41% reduction in the risk of progression (HR = 0.59, P < .001), a 2.5-fold improved mRECIST ORR (33.2% vs 13.3%, P < .001), clear HRQoL benefits (HR = 0.63), and lower rates of grade 3 or 4 TRAEs (35.6% vs 45.5%) for the combination of atezolizumab plus bevacizumab compared with sorafenib (32). Approximate rates of treatment-related hypertension were similar (any grade, 30% vs 24%; grade 3 or 4, 15% vs 12%), and HSFR rates were substantially lower (any grade, 1% vs 48%; grade 3 or 4, 0 vs 9%) for atezolizumab plus bevacizumab vs sorafenib, respectively. Given the statistically significant improvements in OS, PFS, and ORR in addition to the favorable safety profile of atezolizumab plus bevacizumab, this regimen represents a new systemic first-line standard for intermediate or advanced non-LRT–eligible HCC and was approved by the FDA in this setting in 2020 (52). Lenvatinib received FDA approval in 2018 for first-line use (53), and lenvatinib or sorafenib could be considered in patients after liver transplant or in those who are ineligible for atezolizumab plus bevacizumab or infusion therapy. The benefits of LRT in patients with a morphologic response from systemic therapy are currently unknown.
Second-line systemic therapy for intermediate or advanced non-LRT–eligible HCC has historically been BSC following progression on sorafenib, and statistically significant OS and/or HRQoL improvements in a phase III trial would be needed to establish a new standard of care. Over the last decade, several phase III studies attempted to improve OS in this setting without success, including those assessing brivanib (27), tivantinib (29), everolimus (31), ramucirumab (36), and pembrolizumab (33). More promising results have been reported for ramucirumab in select patients (35), in addition to regorafenib and cabozantinib (28,30). Despite the lack of demonstrated OS benefit for ramucirumab compared with placebo among unselected patients in REACH (36), a follow-up trial in poor prognosis patients with α-fetoprotein concentrations of 400 ng/mL or greater (REACH-2) showed a statistically significant 29% reduction in the risk of death (net 1.2 months, P = .02), providing a safe alternative to TKIs for select patients in this setting (discontinuation due to TRAEs, 10.7% vs 10.4%-16.3%) (35). RESORCE and CELESTIAL also demonstrated clinically meaningful OS gains for both regorafenib and cabozantinib compared with placebo, respectively (28,30). Regorafenib was associated with a statistically significant 37% reduction in risk of death (net 2.8 months, P < .0001) in patients with demonstrated tolerance to sorafenib (28), and cabozantinib showed a statistically significant 24% reduced risk of death (net 2.2 months, P = .005) in both sorafenib tolerant and intolerant patients (30). Patterns of toxicity were comparable for both TKIs (28,30). Although regorafenib showed a fourfold increase (50.0% vs 16.6%) (28) and cabozantinib a twofold increase (67.7% vs 36.3%) (30) in overall grade 3 or 4 TRAEs, rates of grade 3 or 4 HSFR were low for both agents (regorafenib vs placebo, treatment-related, 12.6% vs 0.5% and cabozantinib vs placebo, any cause, 16.9% vs 0%) (28,30). Given the clinically meaningful OS gains for regorafenib and cabozantinib in unselected patients plus their comparable toxicity profiles and oral delivery, either agent is recommended in patients who have progressed on sorafenib. Regorafenib received FDA (54) and EMA (55) approval in 2017 and cabozantinib received EMA approval in 2018 (56) and FDA approval in 2019 (57) for patients with HCC previously treated with sorafenib. Ramucirumab received FDA and EMA approvals in 2019 (58,59) and may be a tolerable intravenous option for patients with α-fetoprotein concentrations of at least 400 ng/mL. Nivolumab (2017) (60) and pembrolizumab (2018) (61) also received accelerated FDA approval for HCC following treatment with sorafenib, although neither agent has demonstrated statistically significantly improved survival compared with controls in a phase III trial (62,63).
Patient or disease characteristics and biomarkers would be helpful for selecting patients with intermediate or advanced non-LRT–eligible HCC who would benefit from a given therapy because multiple treatment options exist. Exploratory analyses of OS outcomes for subpopulations of interest from positive trials are summarized in Table 3. Predictive factors were not evident, although this may be due to the variability in patient populations and the reduced power of subgroup analyses to detect differences. Further research is needed to identify predictive factors that will help guide therapy selection for intermediate or advanced non-LRT–eligible HCC.
Table 3.
OS outcomes for select subgroups for positive phase III trials assessing targeted therapy in intermediate and advanced non-LRT–eligible HCCa
| Trial | Patient exclusions | BCLC Stage B % of patients HR (95% CI) |
No MVI/EHS or both % of patients HR (95% CI) |
Nonviral etiology % of patients HR (95% CI) |
ECOG PS 0 % of patients HR (95% CI) |
AFP at baseline <400 μg/L % of patients HR (95% CI) |
|---|---|---|---|---|---|---|
|
First-line | ||||||
| REFLECT (26) |
ResectableChild-Pugh B,CECOG PS 2-5 |
20.5%0.91 (0.65 to 1.28) |
30.3%1.05 (0.79 to 1.40) |
Alcohol, other, unknown:5.9% 1.03 (0.47 to 2.28) |
63.4%0.88 (0.73 to 1.06) |
<200 μg/L56.7% 0.91 (0.74 to 1.12) |
| IMbrave150 (32) |
ResectableChild-Pugh B,CECOG PS 2-5 |
15.6%1.09 (0.33 to 3.53) |
24.6%0.69 (0.29 to 1.65) |
30.5%0.91 (0.52 to 1.60) |
62.3%0.67 (0.43 to 1.06) |
62.7%0.52 (0.34 to 0.81) |
|
Second-line | ||||||
| RESORCE (28) |
ResectableChild-Pugh B,CECOG PS 2-5 |
12.7%NR |
18.1%0.98 (0.58 to 1.66) |
No hepatitis B:60.4% 0.73 (0.56 to 0.95)No hepatitis C:76.8%0.65 (0.51 to 0.82) |
63.8%0.61 (0.47 to 0.80) |
54.8%0.67 (0.50 to 0.90) |
| CELESTIAL (30) |
ResectableChild-Pugh B,CECOG PS 2-5 |
NR |
15.4%0.99 (0.59 to 1.65) |
40.2%0.72 (0.54 to 0.96) |
53.2%0.69 (0.53 to 0.89) |
58.6%0.81 (0.62 to 1.04) |
| REACH-2 (35) |
ResectableChild-Pugh B,CECOG PS 2-5 |
18.5%0.69 (0.35 to 1.35) |
No MVI:64.7%0.60 (0.42 to 0.87)No EHS:27.7%0.84 (0.48 to 1.48) |
33.6%0.63 (0.38 to 1.06) |
57.5%0.71 (0.49 to 1.04) |
n/a |
AFP = α-fetoprotein; BCLC = Barcelona Clinic Liver Cancer; CI = confidence interval; ECOG = Eastern Cooperative Oncology Group; EHS = extrahepatic spread; HCC = hepatocellular carcinoma; HR = hazard ratio; LRT = locoregional therapy; MVI = macroscopic portal vein invasion; n/a = not applicable; NR = not reported; OS = overall survival; PS = performance status.
Treatment of advanced HCC involves care from a multi-disciplinary team of specialists. Systemic therapy is indicated for patients with intermediate disease (BCLC B) who are ineligible for or have progressed on LRT, as well as for those with advanced disease (BCLC C) (16). Sorafenib has been the preferred systemic therapy for patients with Child-Pugh A disease and an Eastern Cooperative Oncology Group performance status of 2 or less for over a decade (64). Two additional classes of agents, ICIs and V-MoAbs, as well as next-generation TKIs have now demonstrated OS benefits in phase III trials compared with controls (28,30,32,35). These new treatments should not be given to patients with Child-Pugh B or Eastern Cooperative Oncology Group performance status 2 scores, although they can be administered to patients with active hepatitis B infection with the administration of antiviral therapy. Although the number of options is encouraging when systemic therapy is indicated, treatment selection can be challenging due to the lack of randomized data informing sequencing decisions. However, some insight might be gained by applying a few key sequencing principles for the selection of systemic therapy. Whenever possible, it is important to select therapies: 1) in a manner that optimizes survival or quality of life, 2) with consideration of clinical trial eligibility, and 3) to allow for exposure to all 3 active classes of agents: TKIs, an ICI, and a V-MoAb. Application of these principles could result in any number of sequences, preferably beginning with atezolizumab plus bevacizumab in suitable patients followed by a TKI (ICI + V-MoAb → TKI → Figure 2). The exact sequencing of subsequent therapies is unclear, and selection should be informed by demonstrated survival benefit following progression on sorafenib, TRAE risk, prior sorafenib tolerance [regorafenib was only assessed in sorafenib tolerant patients (28)], and α-fetoprotein levels [demonstrated OS benefit for ramucirumab in patients with baseline α-fetoprotein ≥400 ng/mL (35)]. A reasonable sequence in unselected patients following atezolizumab plus bevacizumab could consist of lenvatinib or sorafenib followed by cabozantinib or regorafenib (TKI → TKI). In some instances, the use of atezolizumab plus bevacizumab as first-line therapy may not be suitable, such as in patients with autoimmune disease or those who received a first-line TKI before atezolizumab plus bevacizumab availability. An alternate sequence to consider in these instances would be a first-line TKI (lenvatinib or sorafenib) followed by subsequent TKIs such as cabozantinib or regorafenib, ramucirumab in patients with baseline α-fetoprotein 400 ng/mL or greater, or atezolizumab plus bevacizumab in appropriate patients.
Figure 2.
Potential systemic therapy treatment sequencing for advanced HCC. aPatients who are unsuitable for first-line ATEZO+BEV or those who started a TKI before ATEZO+BEV availability. bPatients with demonstrated ability to tolerate sorafenib. cPatients with baseline α-fetoprotein ≥400 ng/mL only. ATEZO = atezolizumab; BEV = bevacizumab; CABO = cabozantinib; ECOG = Eastern Cooperative Oncology Group; HCC = hepatocellular carcinoma; HRQoL = health-related quality of life. dPhase III subgroup data exists for this third-line option post-sorafenib, although not following the ICI combination; ICI = immune checkpoint inhibitor; LEN = lenvatinib; LRT = locoregional therapy; PS = performance status; RAM = ramucirumab; REG = regorafenib; SOR = sorafenib; TKI = tyrosine kinase inhibitor; V-MoAb = anti-VEGF(R) monoclonal antibody.
First-line systemic treatment for intermediate or advanced non-LRT–eligible HCC is rapidly evolving. Moreover, phase III trials in patients who are LRT ineligible or have failed LRT continue to assess the merits of targeted therapies either alone (65,66), such as BGB-A317 (primary completion date [PCD], June 2021) (66), or as dual targeted therapy combinations (67–74). Results of multiple trials assessing the role of adding ICIs to a TKI are expected within the year, including COSMIC-312 (PCD, August 2020) (69), SHR-1210-III-310 (PCD, December 2021) (70), and LEAP-002 (PCD, May 2022) (Table 4) (71). Combinations of PD-1 inhibitors plus cytotoxic T-lymphocyte-associated protein 4 inhibitors are also an area of ongoing investigation. Nivolumab plus ipilimumab received accelerated FDA approval in patients who progressed on sorafenib in 2020 based on results from the phase I/II CheckMate 040 study (90,91), and phase III trials assessing dual ICIs such as HIMALAYA (PCD, March 2020) (67) and Checkmate 9DW (PCD, September 2023) (68) are ongoing. Combinations of PD-1 inhibitor plus a V-MoAb are also being assessed in ORIENT-32 (PCD, December 2022) (72).
Table 4.
Ongoing phase III clinical trials of combination targeted therapy in unresectable HCCa
| Experimental agent(s) or approach | Trial ID (NCT No.) (reference) |
Experimental regimen | Comparator | Primary endpoint(s) | Estimated PCD |
|---|---|---|---|---|---|
| Dual targeted therapy combinations | |||||
| Dual ICI combinations | |||||
| Durvalumab (PD-L1), tremelimumab (CTLA-4) | HIMALAYA (NCT03298451) (67) | Durvalumab with or without tremelimumab | Sorafenib | OS | Mar 2020 |
| Nivolumab (PD-1), ipilimumab (CTLA-4) | CheckMate 9DW (NCT04039607) (68) | Nivolumab plus ipilimumab | Sorafenib or Lenvatinib | OS | Sep 2023 |
| Checkpoint inhibitor plus TKI or V-MoAb combinations | |||||
| Cabozantinib (c-MET/VEGFR-2), atezolizumab (PD-L1) | COSMIC-312 (NCT03755791) (69) | Atezolizumab with or without cabozantinib | Sorafenib | PFS/OS | Aug 2020 |
| SHR-1210 (PD-1), apatinib (VEGFR-2) | SHR-1210-III-310 (NCT03764293) (70) | SHR-1210 plus apatinib | Sorafenib | PFS/OS | Dec 2021 |
| Pembrolizumab (PD-1) | LEAP-002 (NCT03713593) (71) | Pembrolizumab plus lenvatinib | Placebo plus Lenvatinib | PFS/OS | May 2022 |
| Sintilimab (PD-1), IBI305 (VEGF-A) | ORIENT-32 (NCT03794440) (72) | Sintilimab plus IBI305 | Sorafenib | ORR/OS | Dec 2022 |
| CS1003 (PD-1), lenvatinib (VEGFR1-3) | CS1003-305 (NCT04194775) (73) | CS1003 plus lenvatinib | Placebo plus Lenvatinib | PFS/OS | Jun 2023 |
| AK105 (PD-1), anlotinib (VEGFR/FGFR/PDGFR/c-kit) | ALTN-AK105-III-02 (NCT04344158) (74) | AK105 plus anlotinib | Sorafenib | OS | Jun 2024 |
| LRT (or TACE or SBRT) in combination with TT | |||||
| SBRT | RTOG-1112 (NCT01730937) (75) | SBRT plus sorafenib | Sorafenib | OS | Jun 2020 |
| TACE |
SELECT (NCT01906216) (76) |
TACE plus sorafenib | Sorafenib | OS | Sep 2020 |
| TACE, SBRT | HEPIC2001 (NCT04387695) (77) | SBRT plus TACE plus sorafenib | Sorafenib | PFS | Jun 2021 |
| TAI | B2019-076-01 (NCT04053985) (78) | TAI plus lenvatinib | Lenvatinib | PFS/OS | Dec 2022 |
| TACE |
TACE (NCT03905967) (79) |
TACE plus lenvatinib | Lenvatinib | OS | Apr 2023 |
| TKI (TKI, ICI, V-MoAb) in combination with LRT | |||||
| HAIC | HCC-S022 (NCT02856126) (80) | Sorafenib plus HAIC | Sorafenib plus TACE | OS | Feb 2020 |
| Sorafenib (VEGFR) |
E1208 (NCT01004978) (81) |
Sorafenib plus TACE | Placebo plus TACE | PFS | Sep 2020 |
| Durvalumab (PD-L1), bevacizumab (VEGF-A) | EMERALD-1 (NCT03778957) (82) | Durvalumab with or without bevacizumab plus TACE | Placebos plus TACE | PFS | Aug 2021 |
| Sorafenib (VEGFR) |
TREAT (NCT04103398) (83) |
Sorafenib plus TACE | TACE | OS | Oct 2021 |
| Sintilimab (PD-1) | ISBRT01 (NCT04167293) (84) | Sintilimab plus SBRT | SBRT | PFS | Nov 2021 |
| Lenvatinib (VEGFR1-3) | HCC-S055 (NCT03775395) (85) | Lenvatinib plus HAIC | Sorafenib plus HAIC | OS | Dec 2021 |
| PD-1 Inhibitor (PD-1), lenvatinib (VEGFR1-3) |
DEEP (NCT04229355) (86) |
PD-1 inhibitor or lenvatinib plus DEB-TACE | Sorafenib plus DEB-TACE | PFS | Dec 2022 |
| Pembrolizumab (PD-1), lenvatinib (VEGFR1-3) | LEAP-012 (NCT04246177) (87) | Pembrolizumab plus lenvatinib plus TACE | Placebo plus TACE | PFS/OS | Apr 2025 |
| Nivolumab (PD-1), ipilimumab (CTLA-4) |
CheckMate 74W (NCT04340193) (88) |
Nivolumab with or without ipilimumab plus TACE | Placebo plus TACE | TTTP/OS | Jun 2025 |
| Nivolumab (PD-1) |
TACE-3 (NCT04268888) (89) |
Nivolumab plus DEB-TACE | DEB-TACE | OSa | Jun 2025 |
TTTP was the primary endpoint of phase II stage of the trial. BSC = best supportive care; c-MET = tyrosine-protein kinase Met; CTLA-4 = cytotoxic T-lymphocyte-associated protein 4; DEB-TACE = drug-eluting beads transarterial chemoembolization; FGFR - human fibroblast growth factor receptor; FOLFOX = oxaliplatin = fluorouracil = and leucovorin; HAIC = hepatic artery infusion chemotherapy; HCC = hepatocellular carcinoma; ICI = immune checkpoint inhibitor; LRT = locoregional therapy; ORR = overall response rate; OS = overall survival; PCD = primary completion date; PD-1 = programmed cell death protein 1; PDGFR - platelet-derived growth factor receptor; PD-L1 = programmed death ligand 1; PFS = progression-free survival; SBRT = stereotactic body radiation therapy; TACE = transarterial chemoembolization; TAI = transarterial chemoinfusion; TKI = tyrosine kinase inhibitor; TTTP = time to TACE progression; VEGF = vascular endothelial growth factor; VEGFR = vascular endothelial growth factor receptor; V-MoAb = anti-VEGF(R) monoclonal antibody.
There is an increasing recognition of the benefits of using targeted therapy in earlier stage disease as reflected by the Asian Pacific Consensus, which recommends use of targeted therapy before transarterial chemoembolization as a means of downstaging or achieving best overall response in high-burden intermediate HCC (92–95). There is also ongoing research into the benefits of combining targeted therapy with LRT in eligible patients either through adding LRT to first-line TKIs such as sorafenib or lenvatinib (Table 4) (75–79) or adding targeted therapy to LRT such as transarterial chemoembolization, hepatic artery infusion chemotherapy, or stereotactic body radiation (81–84,87–89).
Atezolizumab in combination with bevacizumab statistically significantly improved OS with clinically meaningful improvements in patient-reported outcomes compared with sorafenib as systemic first-line therapy in intermediate or advanced non-LRT–eligible HCC, and cabozantinib and regorafenib statistically significantly improved OS compared with BSC in unselected patients progressing on sorafenib. Atezolizumab plus bevacizumab appears to represent a new, preferred, first-line treatment in this setting, and there is a paucity of phase III data informing the sequencing of later lines of systemic therapy. The use of sequencing principles that optimize survival benefit and allow for exposure to all 3 active classes of agents, TKIs, ICIs, and V-MoAbs, is recommended. Research into additional first-line targeted therapy combinations and targeted therapy combined with LRT in earlier settings is ongoing.
Funding
This work was supported by unrestricted educational grants from Hoffmann La-Roche Canada, Eisai Limited, Ipsen Biopharmaceuticals Canada Inc, Merck Canada Inc, Bayer Canada, Inc, and Eli Lilly Canada Inc.
Notes
Role of the funder: The sponsors had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Role of the authors: Howard Lim: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing — review & editing. Ravi Ramjeesingh: Investigation, Resources, Validation, Writing — review & editing. Dave Liu: Investigation, Resources, Validation, Writing — review & editing. Vincent C. Tam: Investigation, Resources, Validation, Writing — review & editing. Jennifer J. Knox: Investigation, Resources, Validation, Writing — review & editing. Paul B. Card: Data curation, Formal Analysis, Investigation, Project administration, Resources, Validation, Visualization, Writing — original draft, Writing — review & editing. Brandon M. Meyers: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing — review & editing. Ilidio Martins: Data curation, Formal Analysis, Methodology, Resources. Deanna McLeod: Conceptualization, Funding acquisition, Supervision.
Disclosures: Howard Lim has served as a consultant or on an advisory board for Roche, Amgen, Ipsen, Eisai, BMS, Lilly, and Taiho, has received honoraria from Roche, Amgen, Ipsen, Eisai, BMS, Lilly, and Taiho and has received research funding from Roche, BMS, Bayer, and Astellas. Ravi Ramjeesingh has served as a consultant or on an advisory board for Eisai and Ipsen, has received honoraria from Eisai, BMS, Ipsen and Bayer and has received research funding from Eisai. Dave Liu has served as a consultant or on an advisory board for Sirtex Medical and Ethicon Endocare, has received honoraria from Esai Pharmaceuticals (speaker) and has received research funding from Boston Scientific. Vincent C. Tam has served as a consultant or on an advisory board for BMS, Eisai, Ipsen, and Roche, has received honoraria from BMS, Eisai, Ipsen, and Roche, has received research funding from AstraZeneca, Bayer, BMS, Eisai, Exelixis, Ipsen and Merck and has given expert testimony for Ipsen. Jennifer J. Knox has served as a consultant or on an advisory board for Merck, has received honoraria from Merck and AstraZeneca and has received research funding from Astra Zeneca, Merck, Ibsen and Roche. Paul B. Card has nothing to disclose and Brandon M. Meyers has served as a consultant or on an advisory board for Merck, Eisai, Ipsen, BMS and Roche, has received honoraria from Merck, Eisai, Ipsen, BMS and Roche, has received research funding from Sillajen, Merck, Exelisis, GSK and Astra Zeneca, has given expert testimony for Eisai and Roche and has other disclosures from Eisai and Merck.
Acknowledgments: We would like to thank Ilidio Martins and Deanna McLeod from Kaleidoscope Strategic Inc. for their research and editorial support.
This review was prepared according to International Committee of Medical Journal Editors standards with editorial assistance from Kaleidoscope Strategic Inc. No discussion or viewing of review content was permitted with sponsors at any stage of review development.
Data Availability
No new data were generated or analyzed in support of this research.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A.. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Gelband H, Jha P, Sankaranarayanan R, Horton S.. Disease Control Priorities, Volume 3: Cancer 3rd ed. Washington, DC: The World Bank; 2015. [PubMed]
- 3. El-Serag HB, Rudolph KL.. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. [DOI] [PubMed] [Google Scholar]
- 4. Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD.. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol. 2008;14(27):4300–4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Llovet JM, Villanueva A, Lachenmayer A, Finn RS.. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol. 2015;12(8):436–436. [DOI] [PubMed] [Google Scholar]
- 6. Ryerson AB, Eheman CR, Altekruse SF, et al. Annual report to the nation on the status of cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122(9):1312–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. [DOI] [PubMed] [Google Scholar]
- 8. Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85. [PubMed] [Google Scholar]
- 9. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R.. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646. [DOI] [PubMed] [Google Scholar]
- 10. Hiraoka A, Kumada T, Michitaka K, Kudo M.. Newly proposed ALBI grade and ALBI-T score as tools for assessment of hepatic function and prognosis in hepatocellular carcinoma patients. Liver Cancer. 2019;8(5):312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Hepatobiliary Cancers. Version 3 2019. https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed December 18, 2019. [Google Scholar]
- 12. Wang YY, Zhao XH, Ma L, et al. Comparison of the ability of Child-Pugh score, MELD score, and ICG-R15 to assess preoperative hepatic functional reserve in patients with hepatocellular carcinoma. J Surg Oncol. 2018;118(3):440–445. [DOI] [PubMed] [Google Scholar]
- 13. Levy I, Sherman M.. Liver Cancer Study Group of the University of Toronto. Staging of hepatocellular carcinoma: assessment of the CLIP, Okuda, and Child-Pugh staging systems in a cohort of 257 patients in Toronto. Gut. 2002;50(6):881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Richani M, Kolly P, Knoepfli M, et al. Treatment allocation in hepatocellular carcinoma: assessment of the BCLC algorithm. Ann Hepatol. 2016;15(1):82–90. [DOI] [PubMed] [Google Scholar]
- 15. Saffo S, Taddei TH.. Systemic management for advanced hepatocellular carcinoma: a review of the molecular pathways of carcinogenesis, current and emerging therapies, and novel treatment strategies. Dig Dis Sci. 2019;64(4):1016–1029. [DOI] [PubMed] [Google Scholar]
- 16. Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(suppl 4):iv238–iv55. [DOI] [PubMed] [Google Scholar]
- 17. Wege H, Li J, Ittrich H.. Treatment lines in hepatocellular carcinoma. Visc Med. 2019;35(4):266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lopez PM, Villanueva A, Llovet JM.. Systematic review: evidence-based management of hepatocellular carcinoma—an updated analysis of randomized controlled trials. Aliment Pharmacol Ther. 2006;23(11):1535–1547. [DOI] [PubMed] [Google Scholar]
- 19. Arora A, Scholar EM.. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther. 2005;315(3):971–979. [DOI] [PubMed] [Google Scholar]
- 20. Kudo M. Targeted and immune therapies for hepatocellular carcinoma: predictions for 2019 and beyond. World J Gastroenterol. 2019;25(7):789–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schaeffer HJ, Weber MJ.. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19(4):2435–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wörns MA, Galle PR.. Sorafenib for the treatment of hepatocellular carcinoma. Hepat Oncol. 2014;1(2):189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31(32):4067–4075. [DOI] [PubMed] [Google Scholar]
- 24. Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31(28):3517–3524. [DOI] [PubMed] [Google Scholar]
- 25. Cainap C, Qin S, Huang WT, et al. Linifanib versus sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33(2):172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. [DOI] [PubMed] [Google Scholar]
- 27. Llovet JM, Decaens T, Raoul JL, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31(28):3509–3516. [DOI] [PubMed] [Google Scholar]
- 28. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. [DOI] [PubMed] [Google Scholar]
- 29. Rimassa L, Assenat E, Peck-Radosavljevic M, et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018;19(5):682–693. [DOI] [PubMed] [Google Scholar]
- 30. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhu AX, Kudo M, Assenat E, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA. 2014;312(1):57–67. [DOI] [PubMed] [Google Scholar]
- 32. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. [DOI] [PubMed] [Google Scholar]
- 33. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202. [DOI] [PubMed] [Google Scholar]
- 34. Yau T, Park J, Finn R, et al. CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2019;30(suppl 5):v874–v875. [Google Scholar]
- 35. Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296. [DOI] [PubMed] [Google Scholar]
- 36. Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859–870. [DOI] [PubMed] [Google Scholar]
- 37. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Melosky B, Chu Q, Juergens R, Leighl N, McLeod D, Hirsh V.. Pointed progress in second-line advanced non-small-cell lung cancer: the rapidly evolving field of checkpoint inhibition. J Clin Oncol. 2016;34(14):1676–1688. [DOI] [PubMed] [Google Scholar]
- 39. Harvey RD. Immunologic and clinical effects of targeting PD-1 in lung cancer. Clin Pharmacol Ther. 2014;96(2):214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morse MA, Sun W, Kim R, et al. The role of angiogenesis in hepatocellular carcinoma. Clin Cancer Res. 2019;25(3):912–920. [DOI] [PubMed] [Google Scholar]
- 41. Kuczynski EA, Yin M, Bar-Zion A, et al. Co-option of liver vessels and not sprouting angiogenesis drives acquired sorafenib resistance in hepatocellular carcinoma. J Natl Cancer Inst. 2016;108(8):djw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhu YJ, Zheng B, Wang HY, Chen L.. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacol Sin. 2017;38(5):614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu K, Zhang X, Xu W, et al. Targeting the vasculature in hepatocellular carcinoma treatment: starving versus normalizing blood supply. Clin Transl Gastroenterol. 2017;8(6):e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Méndez-Blanco C, Fondevila F, García-Palomo A, González-Gallego J, Mauriz JL.. Sorafenib resistance in hepatocarcinoma: role of hypoxia-inducible factors. Exp Mol Med. 2018;50(10):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maj E, Papiernik D, Wietrzyk J.. Antiangiogenic cancer treatment: the great discovery and greater complexity. Int J Oncol. 2016;49(5):1773–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhu AX, Rosmorduc O, Evans TR, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33(6):559–566. [DOI] [PubMed] [Google Scholar]
- 47. Abou-Alfa GK, Shi Q, Knox JJ, et al. Assessment of treatment with sorafenib plus doxorubicin vs sorafenib alone in patients with advanced hepatocellular carcinoma: phase 3 CALGB 80802 randomized clinical trial. JAMA Oncol. 2019;5(11):1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. [DOI] [PubMed] [Google Scholar]
- 49. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. [DOI] [PubMed] [Google Scholar]
- 50. Cheng A-L, Qin S, Ikeda M, et al. IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo)+ bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30(suppl 9):ix186–ix187. [Google Scholar]
- 51. Finn RS, Ryoo B-Y, Merle P, et al. Results of KEYNOTE-240: Phase 3 Study of Pembrolizumab (Pembro) vs best Supportive Care (BSC) for Second Line Therapy in Advanced Hepatocellular Carcinoma (HCC) Presented at the 55th American Society of Clinical Oncology annual meeting; 31 May–04 June 2019 J Clin Oncol. 2019;37:4004. [Google Scholar]
- 52.Food and Drug Administration. FDA approves atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-plus-bevacizumab-unresectable-hepatocellular-carcinoma#:∼:text = On%20May%2029%2C%202020%2C%20the, not%20received%20prior%20systemic%20therapy. Accessed July 1, 2020.
- 53.Food and Drug Administration. FDA approves lenvatinib for unresectable hepatocellular carcinoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lenvatinib-unresectable-hepatocellular-carcinoma. Accessed March 17, 2020.
- 54.Food and Drug Administration. FDA expands approved use of stivarga to treat liver cancer. https://www.fda.gov/news-events/press-announcements/fda-expands-approved-use-stivarga-treat-liver-cancer Accessed April 22, 2020.
- 55.European Medicines Agency. EMA recommends extensions of therapeutic indications for regorafenib. https://www.esmo.org/oncology-news/EMA-Recommends-Extensions-of-Therapeutic-Indications-for-Regorafenib. Accessed April 16, 2020.
- 56.European Medicines Agency (EMA). Cabometyx (cabozantinib). https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-cabometyx_en.pdf . Accessed April 16, 2020.
- 57.Food and Drug Administration. FDA approves cabozantinib for hepatocellular carcinoma. https://www.fda.gov/drugs/fda-approves-cabozantinib-hepatocellular-carcinoma. Accessed March 17, 2020.
- 58.Food and Drug Administration. FDA approves ramucirumab for hepatocellular carcinoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ramucirumab-hepatocellular-carcinoma. Accessed March 17, 2020.
- 59.European Medicines Agency (EMA). Cyramza (ramucirumab). https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-cyramza-ii-27_en.pdf . Accessed April 16, 2020.
- 60.Food and Drug Administration. FDA grants accelerated approval to nivolumab for HCC previously treated with sorafenib. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-hcc-previously-treated-sorafenib. Accessed January 20, 2020.
- 61.Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for hepatocellular carcinoma. https://www.fda.gov/drugs/fda-grants-accelerated-approval-pembrolizumab-hepatocellular-carcinoma. Accessed January 20, 2020.
- 62. Crocenzi TS, El-Khoueiry AB, Yau T, et al. Nivolumab in sorafenib-naive and-experienced patients with advanced hepatocellular carcinoma: CheckMate 040 study. J Clin Oncol. 2017;35(suppl 15):4013–4013. [DOI] [PubMed] [Google Scholar]
- 63. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–952. [DOI] [PubMed] [Google Scholar]
- 64. Marisi G, Cucchetti A, Ulivi P, et al. Ten years of sorafenib in hepatocellular carcinoma: are there any predictive and/or prognostic markers? World J Gastroenterol. 2018;24(36):4152–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. http://ClinicalTrials.gov. Infusion of toripalimab via hepatic arterial versus vein for immunotherapy of advanced hepatocellular carcinoma. https://clinicaltrials.gov/ct2/show/NCT03949231. Accessed June 26, 2020.
- 66. http://ClinicalTrials.gov. Phase 3 study of BGB-A317 versus sorafenib in patients with unresectable HCC. https://clinicaltrials.gov/ct2/show/NCT03412773. Accessed June 26, 2020.
- 67. http://ClinicalTrials.gov. Study of durvalumab and tremelimumab as first-line treatment in patients with advanced hepatocellular carcinoma (HIMALAYA). https://clinicaltrials.gov/ct2/show/NCT03298451. Accessed March 18, 2020.
- 68. http://ClinicalTrials.gov. A study of nivolumab in combination with ipilimumab in participants with advanced hepatocellular carcinoma (CheckMate 9DW). https://clinicaltrials.gov/ct2/show/NCT04039607. Accessed March 18, 2020.
- 69. http://ClinicalTrials.gov. Study of cabozantinib in combination with atezolizumab versus sorafenib in subjects with advanced HCC who have not received previous systemic anticancer therapy (COSMIC-312). https://clinicaltrials.gov/ct2/show/NCT03755791. Accessed March 18, 2020.
- 70. http://ClinicalTrials.gov. A study to evaluate SHR-1210 in combination with apatinib as first-line therapy in patients with advanced HCC. https://clinicaltrials.gov/ct2/show/NCT03764293. Accessed March 18, 2020.
- 71. http://ClinicalTrials.gov. Safety and efficacy of lenvatinib (E7080/MK-7902) in combination with pembrolizumab (MK-3475) versus lenvatinib as first-line therapy in participants with advanced hepatocellular carcinoma (MK-7902-002/E7080-G000-311/LEAP-002). https://clinicaltrials.gov/ct2/show/NCT03713593. Accessed March 18, 2020.
- 72. http://ClinicalTrials.gov. A study to evaluate the efficacy and safety of sintilimab in combination with IBI305 (Anti-VEGF Monoclonal Antibody) compared to sorafenib as the first-line treatment for advanced hepatocellular carcinoma. https://clinicaltrials.gov/ct2/show/NCT03794440. Accessed March 18, 2020.
- 73. http://ClinicalTrials.gov. A study of CS1003 in subjects with advanced hepatocellular carcinoma. https://clinicaltrials.gov/ct2/show/NCT04194775. Accessed March 18, 2020.
- 74. http://ClinicalTrials.gov. A phase III clinical trial of AK105 injection combined with anlotinib hydrochloride capsules versus sorafenib in subjects with advanced hepatocellular carcinoma (HCC). https://clinicaltrials.gov/ct2/show/NCT04344158. Accessed June 24, 2020.
- 75. http://ClinicalTrials.gov. Sorafenib tosylate with or without stereotactic body radiation therapy in treating patients with liver cancer. https://clinicaltrials.gov/ct2/show/NCT01730937. Accessed March 18, 2020.
- 76. http://ClinicalTrials.gov. Sorafenib chemoembolization evaluation controlled trial (SELECT). https://clinicaltrials.gov/ct2/show/NCT01906216. Accessed March 18, 2020.
- 77. http://ClinicalTrials.gov. SBRT+TACE+sorafenib vs sorafenib in the treatment of uHCC with PVTT. https://clinicaltrials.gov/ct2/show/NCT04387695. Accessed June 26, 2020.
- 78. http://ClinicalTrials.gov. The efficacy of transarterial chemoinfusion (TAI) combine lenvatinib in advanced hepatocellular carcinoma (HCC). https://clinicaltrials.gov/ct2/show/NCT04053985. Accessed March 18, 2020.
- 79. http://ClinicalTrials.gov. TACE with lenvatinib versus lenvatinib alone in in first-line treatment of advanced HCC (TACE). https://clinicaltrials.gov/ct2/show/NCT03905967. Accessed March 18, 2020.
- 80. http://ClinicalTrials.gov. HAIC plus sorafenib versus TACE plus sorafenibfor advanced HCC. https://clinicaltrials.gov/ct2/show/NCT02856126. Accessed March 18, 2020.
- 81. http://ClinicalTrials.gov. Chemoembolization with or without sorafenib tosylate in treating patients with liver cancer that cannot be removed by surgery. https://clinicaltrials.gov/ct2/show/NCT01004978. Accessed March 18, 2020.
- 82. http://ClinicalTrials.gov. A global study to evaluate transarterial chemoembolization (TACE) in combination with durvalumab and bevacizumab therapy in patients with locoregional hepatocellular carcinoma (EMERALD-1). https://clinicaltrials.gov/ct2/show/NCT03778957. Accessed March 18, 2020.
- 83. http://ClinicalTrials.gov. TACE plus sorafenib versus TACE alone for recurrent unresectable hepatocellular carcinoma (TREAT). https://clinicaltrials.gov/ct2/show/NCT04103398. Accessed March 18, 2020.
- 84. http://ClinicalTrials.gov. Combination of sintilimab and stereotactic body radiotherapy in hepatocellular carcinoma (ISBRT01) (ISBRT01). https://clinicaltrials.gov/ct2/show/NCT04167293. Accessed March 18, 2020.
- 85. http://ClinicalTrials.gov. HAIC plus lenvatinib vs HAIC plus sorafenib for advanced HCC. https://clinicaltrials.gov/ct2/show/NCT03775395. Accessed March 18, 2020.
- 86. http://ClinicalTrials.gov. DEB-TACE plus lenvatinib or sorafenib or PD-1 inhibitor for unresectable hepatocellular carcinoma. https://clinicaltrials.gov/ct2/show/NCT04229355. Accessed March 18, 2020.
- 87. http://ClinicalTrials.gov. Safety and efficacy of lenvatinib (E7080/MK-7902) with pembrolizumab (MK-3475) in combination with transarterial chemoembolization (TACE) in participants with incurable/non-metastatic hepatocellular carcinoma (MK-7902-012/E7080-G000-318/LEAP-012). https://clinicaltrials.gov/ct2/show/NCT04246177. Accessed March 18, 2020.
- 88. http://ClinicalTrials.gov. A study of nivolumab and ipilimumab in combination with transarterial chemoembolization (TACE) in participants with intermediate stage liver cancer (CheckMate 74W). https://clinicaltrials.gov/ct2/show/NCT04340193. Accessed June 26, 2020.
- 89. http://ClinicalTrials.gov. Nivolumab in combination with TACE for patients with intermediate stage HCC (TACE-3). https://clinicaltrials.gov/ct2/show/NCT04268888. Accessed March 18, 2020.
- 90.Food and Drug Administration. FDA grants accelerated approval to nivolumab and ipilimumab combination for hepatocellular carcinoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-and-ipilimumab-combination-hepatocellular-carcinoma. Accessed April 23, 2020.
- 91. Yau T, Kang Y-K, Kim T-Y, et al. Nivolumab (NIVO)+ ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040. J Clin Oncol. 2019;37:4012–4012. [Google Scholar]
- 92. Kudo M, Han K-H, Ye S-L, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-Pacific primary liver cancer expert consensus statements. Liver Cancer. 2020;9(3):245–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li J, Liu W, Zhu W, Wu Y, Wu B.. Transcatheter hepatic arterial chemoembolization and sorafenib for hepatocellular carcinoma: a meta-analysis of randomized, double-blind controlled trials. Oncotarget. 2017;8(35):59601–59608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Li H, Li S, Geng J, et al. Efficacy evaluation of the combination therapy of sorafenib and transarterial chemoembolization for unresectable HCC: a systematic review and meta-analysis of comparative studies. Ann Transl Med. 2020;8(8):540–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.